Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy
Abstract
1. Introduction
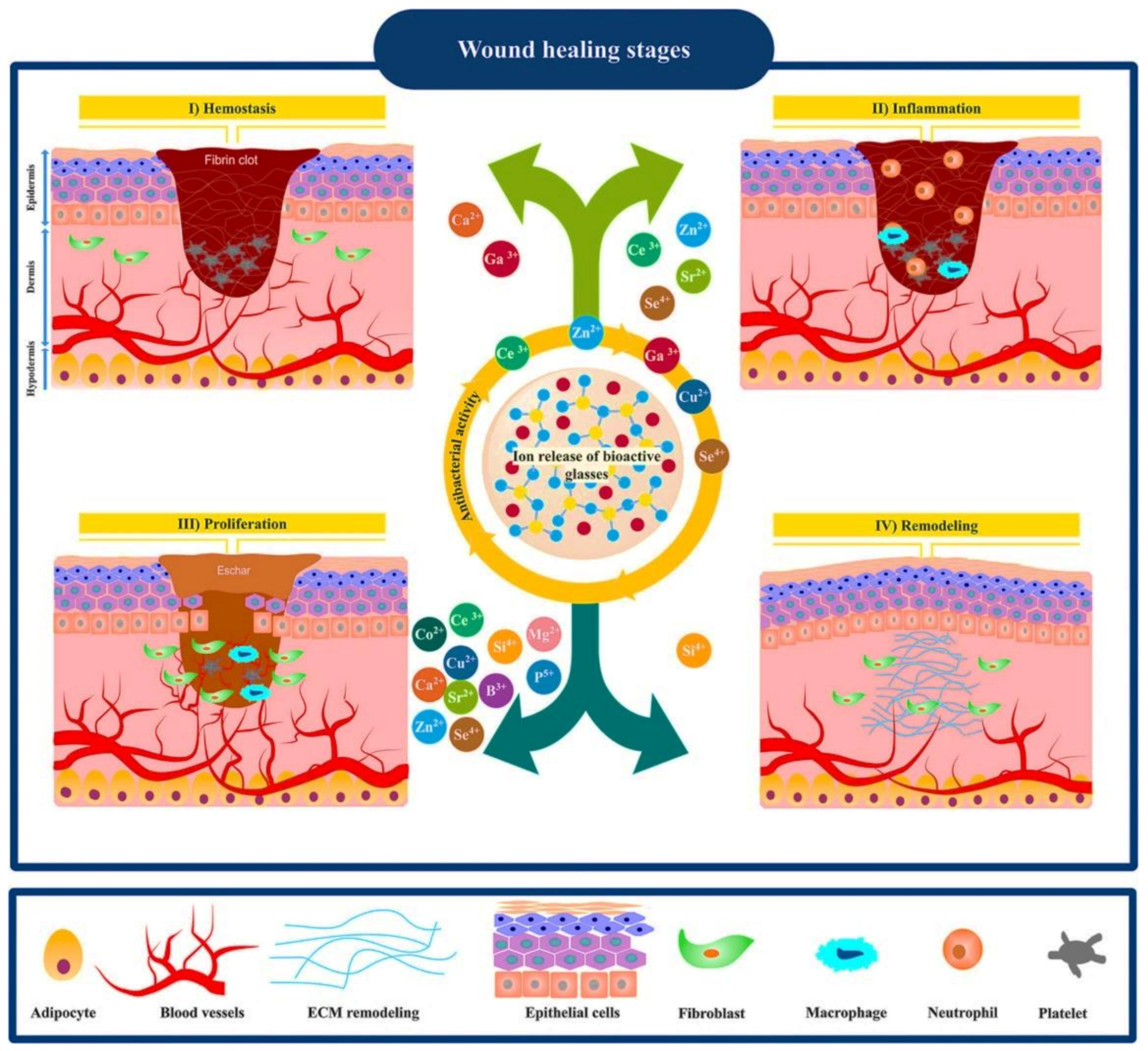
2. Wound Healing Process
3. Current Therapies in Managing Wounds and the Potential of Mesoporous Materials
4. MSNs: Classification, Preparation Methods, and Biocompatibility
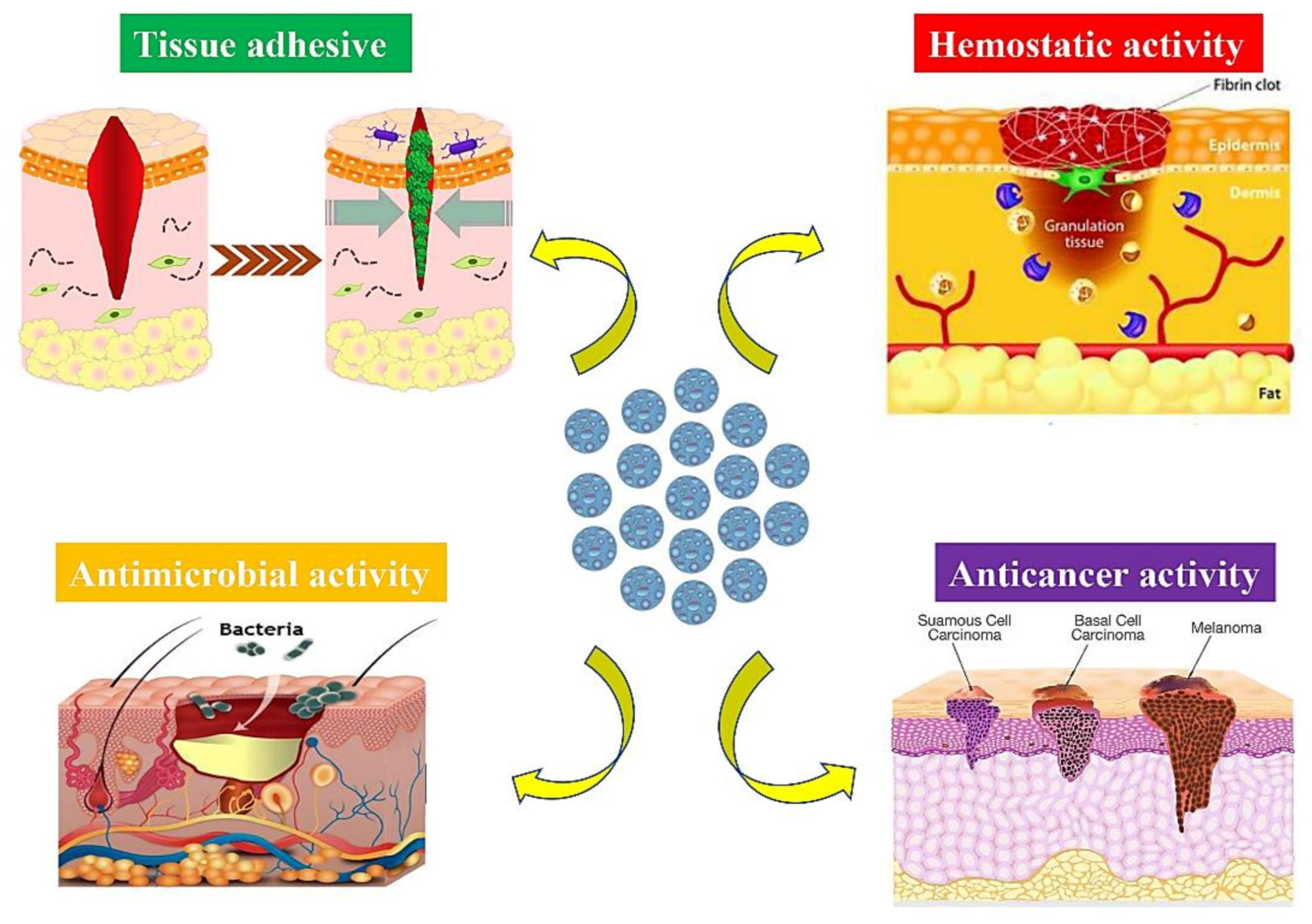
5. Multiple Roles of MSNs in Skin Wound Healing
5.1. Hemostatic Wound Care
5.2. Antibacterial and Antifungal Strategies
5.3. MSNs as Tissue Adhesives for Wound Closure
5.4. MSNs in Skin Cancer Therapy
6. Mesoporous Bioactive Glasses (MBGs)
7. MBGs for Wound Healing and Skin Regeneration
8. MBGs for Skin Cancer Therapy
8.1. MBGs for Hemostatic Applications
8.2. MBGs for Antibacterial Applications
8.3. MBGs for Angiogenesis
9. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; Chapter 1; pp. 1–14. [Google Scholar]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Hoseini, S.J.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Eng. C 2021, 121, 111741. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the surface functionalization on the fate and performance of mesoporous silica nanoparticles. Nanomaterials 2020, 10, 916. [Google Scholar] [CrossRef]
- Yan, T.; He, J.; Liu, R.; Liu, Z.; Cheng, J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr. Polym. 2020, 231, 115706. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.; Choi, Y.; Lee, D.S.; Kim, J.; Yi, G.-R. Colloidal Mesoporous Silica Nanoparticles as Strong Adhesives for Hydrogels and Biological Tissues. ACS Appl. Mater. Interfaces 2017, 9, 31469–31477. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses (MBGs) in cancer therapy: Full of hope and promise. Mater. Lett. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Banijamali, S.; Mozafari, M. Synthesis and physico-chemical characterization of fluoride (F)-and silver (Ag)-substituted sol-gel mesoporous bioactive glasses. Biomed. Glasses 2019, 5, 185–192. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Baino, F. Copper-doped ordered mesoporous bioactive glass: A promising multifunctional platform for bone tissue engineering. Bioengineering 2020, 7, 45. [Google Scholar] [CrossRef]
- Kermani, F.; Mollazadeh Beidokhti, S.; Baino, F.; Gholamzadeh-Virany, Z.; Mozafari, M.; Kargozar, S. Strontium-and cobalt-doped multicomponent mesoporous bioactive glasses (MBGs) for potential use in bone tissue engineering applications. Materials 2020, 13, 1348. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, Y.; Zhang, Z.; Liu, Y.; Zhai, D.; Zhuang, H.; Li, Q.; Yuye, J.; Wu, C.; Chang, J. Multifunctional bioactive Nd-Ca-Si glasses for fluorescence thermometry, photothermal therapy, and burn tissue repair. Sci. Adv. 2020, 6, eabb1311. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Madden, J.W.; Peacock, E.E., Jr. Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann. Surg. 1971, 174, 511. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3, e99911. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Stashak, T.S.; Farstvedt, E.; Othic, A. Update on wound dressings: Indications and best use. Clin. Tech. Equine Pract. 2004, 3, 148–163. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Salehi, M.; Niyakan, M.; Ehterami, A.; Haghi-Daredeh, S.; Nazarnezhad, S.; Abbaszadeh-Goudarzi, G.; Vaez, A.; Hashemi, S.F.; Rezaei, N.; Mousavi, S.R. Porous electrospun poly(ε-caprolactone)/gelatin nanofibrous mat containing cinnamon for wound healing application: In vitro and in vivo study. Biomed. Eng. Lett. 2020, 10, 149–161. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Baino, F.; Kim, H.-W.; Webster, T.J.; Kargozar, S. Electrospun Nanofibers for Improved Angiogenesis: Promises for Tissue Engineering Applications. Nanomaterials 2020, 10, 1609. [Google Scholar] [CrossRef]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.M.; Khastar, H.; Rezaei, N.; Mousavi, S.R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Cruz, J.D.; Park, J.S. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Kargozar, S.; Singh, R.K.; Kim, H.-W.; Baino, F. “Hard” ceramics for “Soft” tissue engineering: Paradox or opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef]
- Cao, C.; Ge, W.; Yin, J.; Yang, D.; Wang, W.; Song, X.; Hu, Y.; Yin, J.; Dong, X. Mesoporous silica supported silver–bismuth nanoparticles as photothermal agents for skin infection synergistic antibacterial therapy. Small 2020, 16, 2000436. [Google Scholar] [CrossRef]
- Lio, D.C.S.; Liu, C.; Oo, M.M.S.; Wiraja, C.; Teo, M.H.Y.; Zheng, M.; Chew, S.W.T.; Wang, X.; Xu, C. Transdermal delivery of small interfering RNAs with topically applied mesoporous silica nanoparticles for facile skin cancer treatment. Nanoscale 2019, 11, 17041–17051. [Google Scholar] [CrossRef]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef]
- Shi, M.; Xia, L.; Chen, Z.; Lv, F.; Zhu, H.; Wei, F.; Han, S.; Chang, J.; Xiao, Y.; Wu, C. Europium-doped mesoporous silica nanosphere as an immune-modulating osteogenesis/angiogenesis agent. Biomaterials 2017, 144, 176–187. [Google Scholar] [CrossRef]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional copper-containing mesoporous glass nanoparticles as antibacterial and proangiogenic agents for chronic wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef]
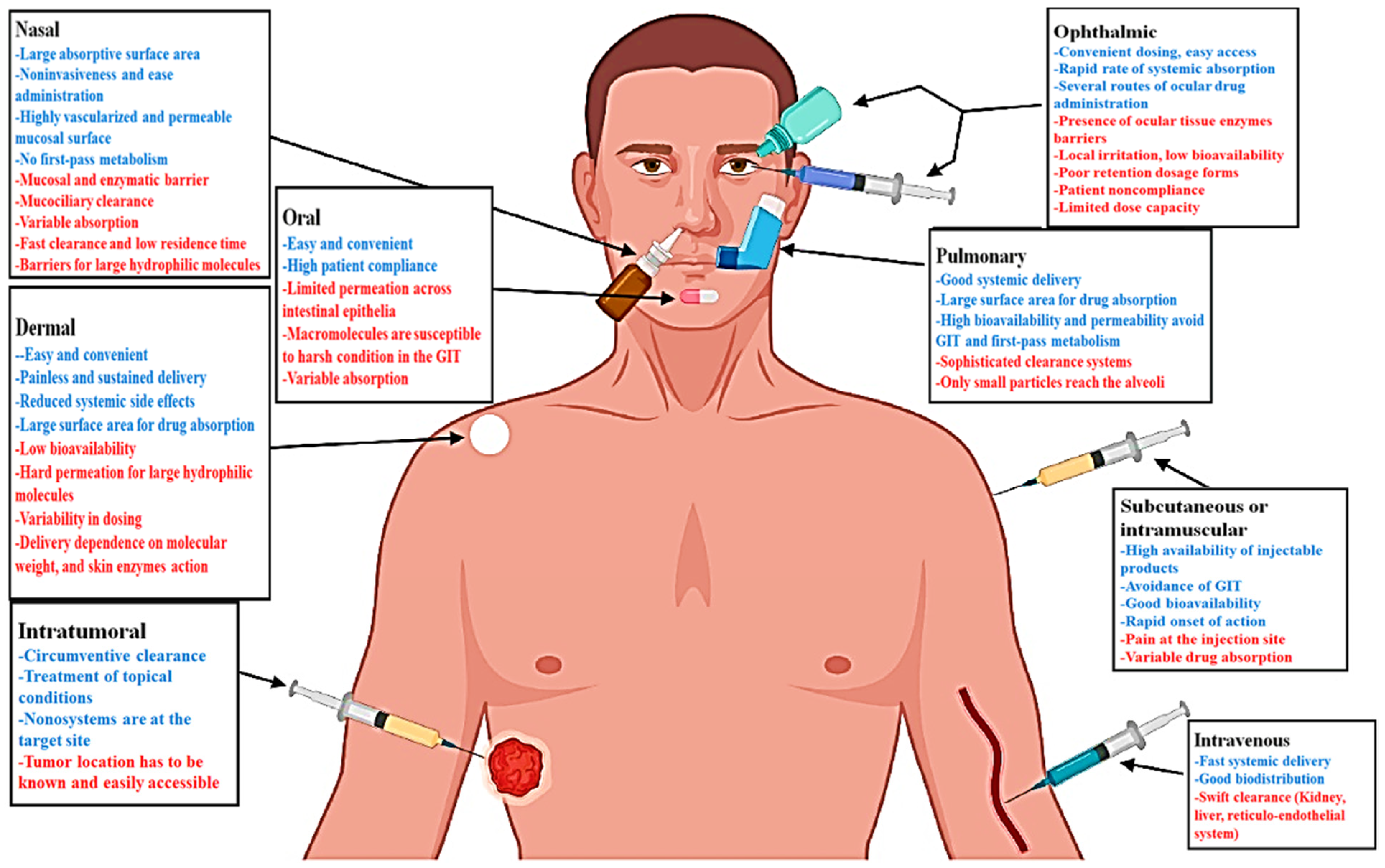
- Sábio, R.M.; Meneguin, A.B.; Martins dos Santos, A.; Monteiro, A.S.; Chorilli, M. Exploiting mesoporous silica nanoparticles as versatile drug carriers for several routes of administration. Microporous Mesoporous Mater. 2021, 312, 110774. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Baino, F. Using bioactive glasses in the management of burns. Front. Bioeng. Biotechnol. 2019, 7, 62. [Google Scholar] [CrossRef]
- Sergi, R.; Cannillo, V.; Boccaccini, A.R.; Liverani, L. A New Generation of Electrospun Fibers Containing Bioactive Glass Particles for Wound Healing. Materials 2020, 13, 5651. [Google Scholar] [CrossRef]
- Dong, X.; Chang, J.; Li, H. Bioglass promotes wound healing through modulating the paracrine effects between macrophages and repairing cells. J. Mater. Chem. B 2017, 5, 5240–5250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Łapa, A.; Cresswell, M.; Campbell, I.; Jackson, P.; Goldmann, W.H.; Detsch, R.; Parsons, A.; Ahmed, I.; Boccaccini, A.R. Ga and Ce ion-doped phosphate glass fibres with antibacterial properties and their composite for wound healing applications. J. Mater. Chem. B 2019, 7, 6981–6993. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tang, Y.; Pang, L.; Lin, C.; Huang, W.; Wang, D.; Jia, W. Angiogenesis and Full-Thickness Wound Healing Efficiency of a Copper-Doped Borate Bioactive Glass/Poly(lactic-co-glycolic acid) Dressing Loaded with Vitamin E in Vivo and in Vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.K.; Lali, F.V.; Autefage, H.; Agarwal, S.; Nommeots-Nomm, A.; Metcalfe, A.D.; Stevens, M.M.; Jones, J.R. Bioactive glasses and electrospun composites that release cobalt to stimulate the HIF pathway for wound healing applications. Biomater. Res. 2021, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-M.; Wang, W.-K.; Hsiung, P.-A.; Shyu, S.-G. Light-sensitive intelligent drug delivery systems of coumarin-modified mesoporous bioactive glass. Acta Biomater. 2010, 6, 3256–3263. [Google Scholar] [CrossRef]
- Shoaib, M.; ur Rahman, M.S.; Saeed, A.; Naseer, M.M. Mesoporous bioactive glass-polyurethane nanocomposites as reservoirs for sustained drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 806–811. [Google Scholar] [CrossRef]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef]
- Kresge, C.; Leonowicz, M.; Roth, W.J.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Durand, J.-O. Large pore mesoporous silica nanomaterials for application in delivery of biomolecules. Nanoscale 2015, 7, 2199–2209. [Google Scholar] [CrossRef]
- Park, H.; Cha, K.-H.; Hong, S.H.; Abuzar, S.M.; Lee, S.; Ha, E.-S.; Kim, J.-S.; Baek, I.-H.; Kim, M.-S.; Hwang, S.-J. Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type. Pharmaceutics 2020, 12, 333. [Google Scholar] [CrossRef]
- Alkafajy, A.M.; Albayati, T.M. High performance of magnetic mesoporous modification for loading and release of meloxicam in drug delivery implementation. Mater. Today Commun. 2020, 23, 100890. [Google Scholar] [CrossRef]
- Janfada, A.; Asefnejad, A.; Khorasani, M.T.; Joupari, M.D. Reinforcement of electrospun polycaprolacton scaffold using KIT-6 to improve mechanical and biological performance. Polym. Test. 2020, 84, 106391. [Google Scholar] [CrossRef]
- Saleh, K.A.; Aldulmani, S.A.; Awwad, N.S.; Ibrahium, H.A.; Asiri, T.H.; Hamdy, M.S. Utilization of lithium incorporated mesoporous silica for preventing necrosis and increase apoptosis in different cancer cells. BMC Chem. 2019, 13, 8. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhu, C.; Guo, T.; Xia, Q.; Hou, X.; Liu, W.; Feng, N. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int. J. Pharm. 2020, 586, 119576. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, J.; Gou, K.; Guo, Y.; Xu, X.; Li, S.; Li, H. Amino functionalized mesoporous silica with twisted rod-like shapes: Synthetic design, in vitro and in vivo evaluation for ibuprofen delivery. Microporous Mesoporous Mater. 2020, 294, 109896. [Google Scholar] [CrossRef]
- Ruthstein, S.; Schmidt, J.; Kesselman, E.; Popovitz-Biro, R.; Omer, L.; Frydman, V.; Talmon, Y.; Goldfarb, D. Molecular level processes and nanostructure evolution during the formation of the cubic mesoporous material KIT-6. Chem. Mater. 2008, 20, 2779–2792. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wei, H.-J.; Xia, Z.-N. Advances in microwave assisted synthesis of ordered mesoporous materials. Trans. Nonferrous Met. Soc. China 2009, 19, s656–s664. [Google Scholar]
- Khanna, S.; Paneliya, S.; Ray, A.; Mukhopadhyay, I.; Banerjee, R. Controlled etching of silica nanospheres monolayer for template application: A systematic study. Appl. Surf. Sci. 2020, 500, 144050. [Google Scholar]
- Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Colilla, M.; Feito, M.J.; Ramírez-Santillán, C.; Portolés, M.T.; Vallet-Regí, M. Inhibition of bacterial adhesion on biocompatible zwitterionic SBA-15 mesoporous materials. Acta Biomater. 2011, 7, 2977–2985. [Google Scholar] [CrossRef]
- Kargozar, S.; Kermani, F.; Mollazadeh Beidokhti, S.; Hamzehlou, S.; Verné, E.; Ferraris, S.; Baino, F. Functionalization and surface modifications of bioactive glasses (BGs): Tailoring of the biological response working on the outermost surface layer. Materials 2019, 12, 3696. [Google Scholar] [CrossRef]
- Shao, D.; Lu, M.-m.; Zhao, Y.-w.; Zhang, F.; Tan, Y.-f.; Zheng, X.; Pan, Y.; Xiao, X.-a.; Wang, Z.; Dong, W.-f. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef]
- Garrido-Cano, I.; Candela-Noguera, V.; Herrera, G.; Cejalvo, J.M.; Lluch, A.; Marcos, M.D.; Sancenon, F.; Eroles, P.; Martínez-Máñez, R. Biocompatibility and internalization assessment of bare and functionalised mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 310, 110593. [Google Scholar] [CrossRef]
- Liman, R.; Acikbas, Y.; Ciğerci, İ.H.; Ali, M.M.; Kars, M.D. Cytotoxic and Genotoxic Assessment of Silicon Dioxide Nanoparticles by Allium and Comet Tests. Bull. Environ. Contam. Toxicol. 2020, 104, 215–221. [Google Scholar] [CrossRef]
- Park, M.V.; Verharen, H.W.; Zwart, E.; Hernandez, L.G.; van Benthem, J.; Elsaesser, A.; Barnes, C.; McKerr, G.; Howard, C.V.; Salvati, A. Genotoxicity evaluation of amorphous silica nanoparticles of different sizes using the micronucleus and the plasmid lacZ gene mutation assay. Nanotoxicology 2011, 5, 168–181. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Moos, P.J.; Dobrovolskaia, M.A.; Ghandehari, H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 106–125. [Google Scholar] [CrossRef]
- Beňová, E.; Bergé-Lefranc, D.; Zeleňák, V.; Almáši, M.; Huntošová, V.; Hornebecq, V. Adsorption properties, the pH-sensitive release of 5-fluorouracil and cytotoxicity studies of mesoporous silica drug delivery matrix. Appl. Surf. Sci. 2020, 504, 144028. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Zhong, W.; Xiao, H.; Liu, X.; Yu, T.; Zhou, C. Tuning biodegradability and biocompatibility of mesoporous silica nanoparticles by doping strontium. Ceram. Int. 2020, 46, 11762–11769. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Haynes, C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Shi, M.; Liu, G.; Chen, Z.; Chang, J.; Wu, C.; Xiao, Y. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: From nanoimmunotoxicity to nanoimmunotherapy. Appl. Mater. Today 2018, 10, 184–193. [Google Scholar] [CrossRef]
- Kanniyappan, H.; Venkatesan, M.; Panji, J.; Ramasamy, M.; Muthuvijayan, V. Evaluating the inherent osteogenic and angiogenic potential of mesoporous silica nanoparticles to augment vascularized bone tissue formation. Microporous Mesoporous Mater. 2021, 311, 110687. [Google Scholar] [CrossRef]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef]
- Jia, L.; Shen, J.; Li, Z.; Zhang, D.; Zhang, Q.; Duan, C.; Liu, G.; Zheng, D.; Liu, Y.; Tian, X. Successfully tailoring the pore size of mesoporous silica nanoparticles: Exploitation of delivery systems for poorly water-soluble drugs. Int. J. Pharm. 2012, 439, 81–91. [Google Scholar] [CrossRef]
- Karimi, M.; Mirshekari, H.; Aliakbari, M.; Sahandi-Zangabad, P.; Hamblin, M.R. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnol. Rev. 2016, 5, 195–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-sensitive delivery vehicle based on folic acid-conjugated polydopamine-modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Omar, H.; Croissant, J.G.; Alamoudi, K.; Alsaiari, S.; Alradwan, I.; Majrashi, M.A.; Anjum, D.H.; Martins, P.; Laamarti, R.; Eppinger, J. Biodegradable magnetic silica@ iron oxide nanovectors with ultra-large mesopores for high protein loading, magnetothermal release, and delivery. J. Control. Release 2017, 259, 187–194. [Google Scholar] [CrossRef]
- Li, X.; Tang, T.; Zhou, Y.; Zhang, Y.; Sun, Y. Applicability of enzyme-responsive mesoporous silica supports capped with bridged silsesquioxane for colon-specific drug delivery. Microporous Mesoporous Mater. 2014, 184, 83–89. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, Z.-K.; Zhu, Q.-L.; Rong, X.-H.; Liang, C.-L.; Wang, J.; Ma, D.; Sun, J.; Wang, G.-H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B Biointerfaces 2017, 155, 41–50. [Google Scholar] [CrossRef]
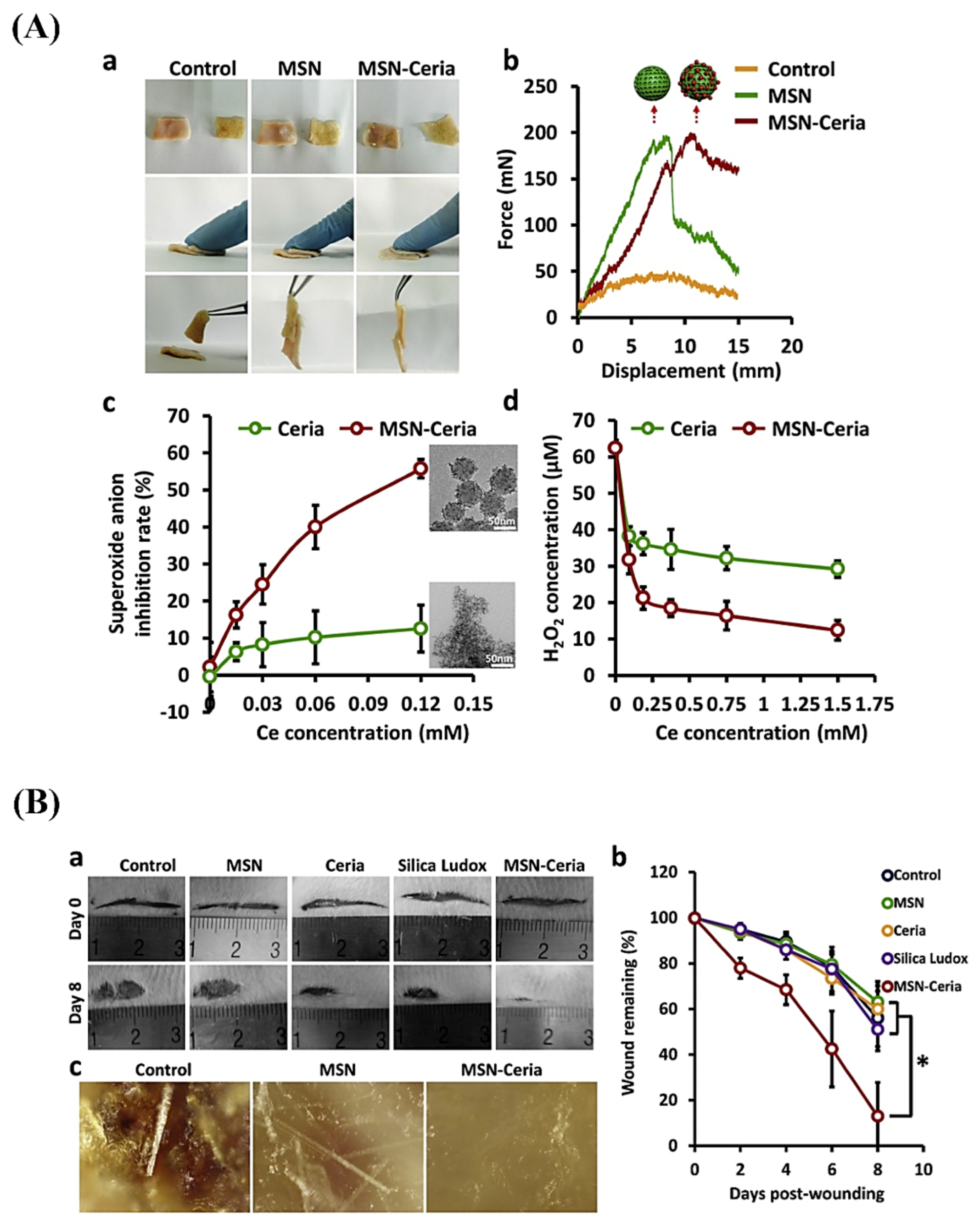
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Zhang, P.; Cai, L.; Yang, X.; Chen, Y.; Jing, Y.; Kong, J.; Yang, X.; Sun, F.L. Sustained Delivery Growth Factors with Polyethyleneimine-Modified Nanoparticles Promote Embryonic Stem Cells Differentiation and Liver Regeneration. Adv. Sci. 2016, 3, 1500393. [Google Scholar] [CrossRef]
- Pradhan, M.; Srivastava, S.; Singh, D.; Saraf, S.; Saraf, S.; Singh, M.R. Perspectives of lipid-based drug carrier systems for transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 331–367. [Google Scholar] [CrossRef]
- Nigro, A.; Pellegrino, M.; Greco, M.; Comandè, A.; Sisci, D.; Pasqua, L.; Leggio, A.; Morelli, C. Dealing with skin and blood-brain barriers: The unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics 2018, 10, 250. [Google Scholar] [CrossRef]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef]
- Zaccariello, G.; Back, M.; Zanello, M.; Canton, P.; Cattaruzza, E.; Riello, P.; Alimonti, A.; Benedetti, A. Formation and controlled growth of bismuth titanate phases into mesoporous silica nanoparticles: An efficient self-sealing nanosystem for UV filtering in cosmetic formulation. ACS Appl. Mater. Interfaces 2017, 9, 1913–1921. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.; Müller, R.; Bégu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Lademann, J.; Knorr, F.; Richter, H.; Jung, S.; Meinke, M.; Rühl, E.; Alexiev, U.; Calderón, M.; Patzelt, A. Hair follicles as a target structure for nanoparticles. J. Innov. Opt. Health Sci. 2015, 8, 1530004. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderón, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2017, 9, 172–182. [Google Scholar] [CrossRef]
- Li, X.; Pang, K.Y.; Ng, T.W.; Leung, P.C.; Zhang, C.F.; Leung, K.C.-F.; Jin, L. Cellular interactions and formation of an epithelial “nanocoating-like barrier” with mesoporous silica nanoparticles. Nanomaterials 2016, 6, 192. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent advances in skin penetration enhancers for transdermal gene and drug delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef]
- Champion, H.R.; Bellamy, R.F.; Roberts, C.P.; Leppaniemi, A. A profile of combat injury. J. Trauma Acute Care Surg. 2003, 54, S13–S19. [Google Scholar]
- Holcomb, J.B.; McMullin, N.R.; Pearse, L.; Caruso, J.; Wade, C.E.; Oetjen-Gerdes, L.; Champion, H.R.; Lawnick, M.; Farr, W.; Rodriguez, S. Causes of death in US Special Operations Forces in the global war on terrorism: 2001–2004. Ann. Surg. 2007, 245, 986. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Djordjevic, I.; Kadri, N.A.; Towler, M.R. Inorganic hemostats: The state-of-the-art and recent advances. Mater. Sci. Eng. C 2016, 58, 1255–1268. [Google Scholar] [CrossRef]
- Granville-Chapman, J.; Jacobs, N.; Midwinter, M. Pre-hospital haemostatic dressings: A systematic review. Injury 2011, 42, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A comprehensive review of topical hemostatic agents: Efficacy and recommendations for use. Ann. Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nie, W.; Chen, L.; Miao, Y.; Zhang, X.; Chen, F.; Yu, B.; Ao, R.; Yu, B.; He, C. Fabrication of curcumin-loaded mesoporous silica incorporated polyvinyl pyrrolidone nanofibers for rapid hemostasis and antibacterial treatment. RSC Adv. 2017, 7, 7973–7982. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, H.; Niu, H.; Ma, X.; Yuan, Y.; Hong, H.; Liu, C. Tannic acid-loaded mesoporous silica for rapid hemostasis and antibacterial activity. Biomater. Sci. 2018, 6, 3318–3331. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, T.; Lin, F.-C.; Zhang, B.; Zink, J.I. Supramolecular assemblies of heterogeneous mesoporous silica nanoparticles to co-deliver antimicrobial peptides and antibiotics for synergistic eradication of pathogenic biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef]
- Balaure, P.C.; Boarca, B.; Popescu, R.C.; Savu, D.; Trusca, R.; Vasile, B.Ș.; Grumezescu, A.M.; Holban, A.M.; Bolocan, A.; Andronescu, E. Bioactive mesoporous silica nanostructures with anti-microbial and anti-biofilm properties. Int. J. Pharm. 2017, 531, 35–46. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.-M.; Berger, D.; Pavel, I.Z.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Wijesiri, N.; Yu, Z.; Tang, H.; Zhang, P. Antifungal photodynamic inactivation against dermatophyte Trichophyton rubrum using nanoparticle-based hybrid photosensitizers. Photodiagnosis Photodyn. Ther. 2018, 23, 202–208. [Google Scholar] [CrossRef]
- Sattary, M.; Amini, J.; Hallaj, R. Antifungal activity of the lemongrass and clove oil encapsulated in mesoporous silica nanoparticles against wheat’s take-all disease. Pestic. Biochem. Physiol. 2020, 170, 104696. [Google Scholar] [CrossRef]
- Mas, N.; Galiana, I.; Hurtado, S.; Mondragón, L.; Bernardos, A.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Abril-Utrillas, N.; Martínez-Máñez, R.; et al. Enhanced antifungal efficacy of tebuconazole using gated pH-driven mesoporous nanoparticles. Int. J. Nanomed. 2014, 9, 2597–2606. [Google Scholar]
- Paramanantham, P.; Antony, A.P.; Lal, S.S.; Sharan, A.; Syed, A.; Ahmed, M.; Alarfaj, A.A.; Busi, S.; Maaza, M.; Kaviyarasu, K. Antimicrobial photodynamic inactivation of fungal biofilm using amino functionalized mesoporus silica-rose bengal nanoconjugate against Candida albicans. Sci. Afr. 2018, 1, e00007. [Google Scholar] [CrossRef]
- Montazeri, M.; Razzaghi-Abyaneh, M.; Nasrollahi, S.; Maibach, H.; Nafisi, S. Enhanced topical econazole antifungal efficacy by amine-functionalized silica nanoparticles. Bull. Mater. Sci. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Taboada, G.M.; Yang, K.; Pereira, M.J.N.; Liu, S.S.; Hu, Y.; Karp, J.M.; Artzi, N.; Lee, Y. Overcoming the translational barriers of tissue adhesives. Nat. Rev. Mater. 2020, 5, 310–329. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1902232. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Klimo, P., Jr.; Khalil, A.; Slotkin, J.R.; Smith, E.R.; Scott, R.M.; Goumnerova, L.C. Wound complications associated with the use of bovine serum albumin-glutaraldehyde surgical adhesive in pediatric patients. Oper. Neurosurg. 2007, 60, ONS-305–ONS-309. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Schmittling, Z.C.; Coselli, J.S.; Ündar, A.; Deady, B.A.; Clubb, F.J., Jr.; Fraser, C.D., Jr. BioGlue surgical adhesive impairs aortic growth and causes anastomotic strictures. Ann. Thorac. Surg. 2002, 73, 1500–1506. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, K.-R.; Gao, H.-L.; Zhou, Y.; Yan, B.-B.; Yang, C.; Zhang, Z.-Y.; Dong, L.; Chen, S.-M.; Xu, R. Activating proper inflammation for wound-healing acceleration via mesoporous silica nanoparticle tissue adhesive. Nano Res. 2020, 13, 373–379. [Google Scholar] [CrossRef]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.E. Global burden of skin disease: Inequities and innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mieras, L.F.; Taal, A.T.; Post, E.B.; Ndeve, A.G.; Van Hees, C.L. The development of a mobile application to support peripheral health workers to diagnose and treat people with skin diseases in resource-poor settings. Trop. Med. Infect. Dis. 2018, 3, 102. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Abadeer, N.; Haynes, C.L. Stability of small mesoporous silica nanoparticles in biological media. Chem. Commun. 2011, 47, 532–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol. Pharm. 2012, 9, 505–513. [Google Scholar] [CrossRef]
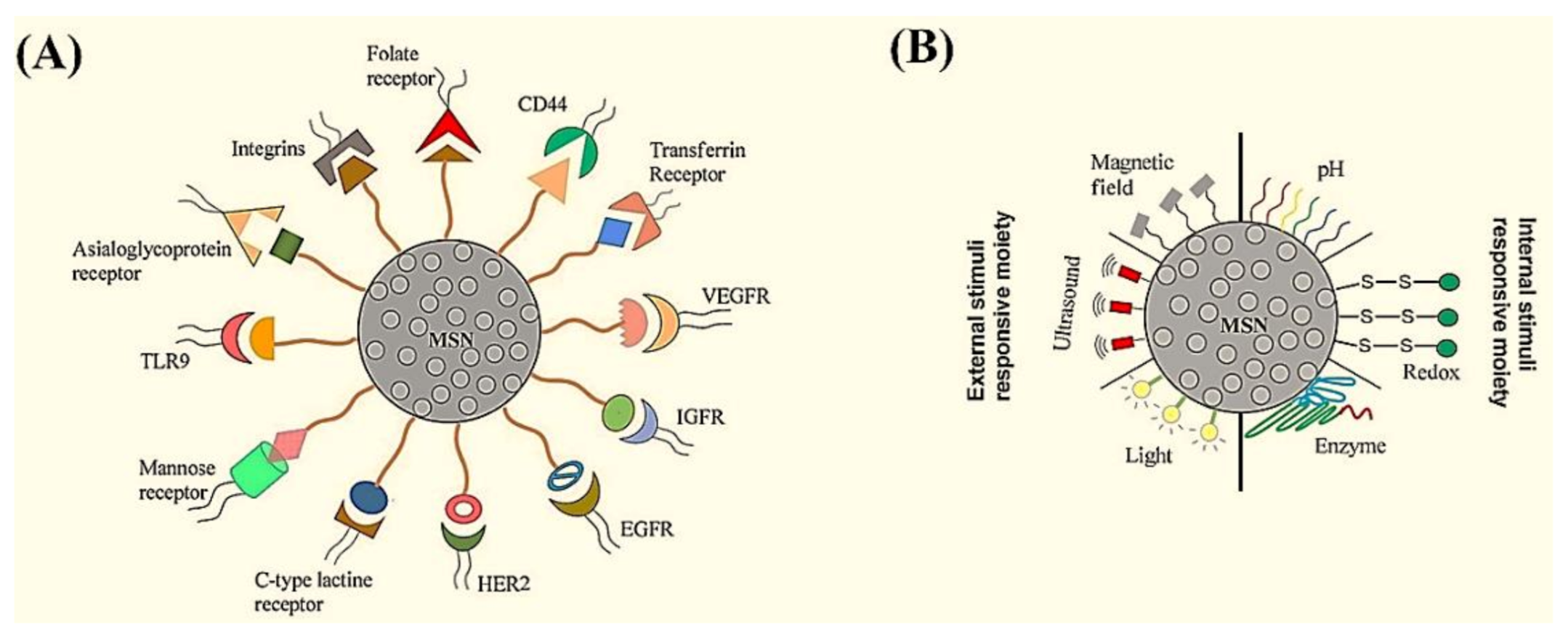
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef]
- Anirudhan, T.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Han, R.; Tang, K.; Hou, Y.; Yu, J.; Wang, C.; Wang, Y. Ultralow-intensity near infrared light synchronously activated collaborative chemo/photothermal/photodynamic therapy. Biomater. Sci. 2020, 8, 607–618. [Google Scholar] [CrossRef]
- Wu, Z.; Lim, H.K.; Tan, S.J.; Gautam, A.; Hou, H.W.; Ng, K.W.; Tan, N.S.; Tay, C.Y. Potent-By-Design: Amino Acids Mimicking Porous Nanotherapeutics with Intrinsic Anticancer Targeting Properties. Small 2020, 16, 2003757. [Google Scholar] [CrossRef]
- Clemente, N.; Miletto, I.; Gianotti, E.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-loaded mesoporous silica nanoparticles inhibit mouse melanoma proliferation in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2019, 197, 111533. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
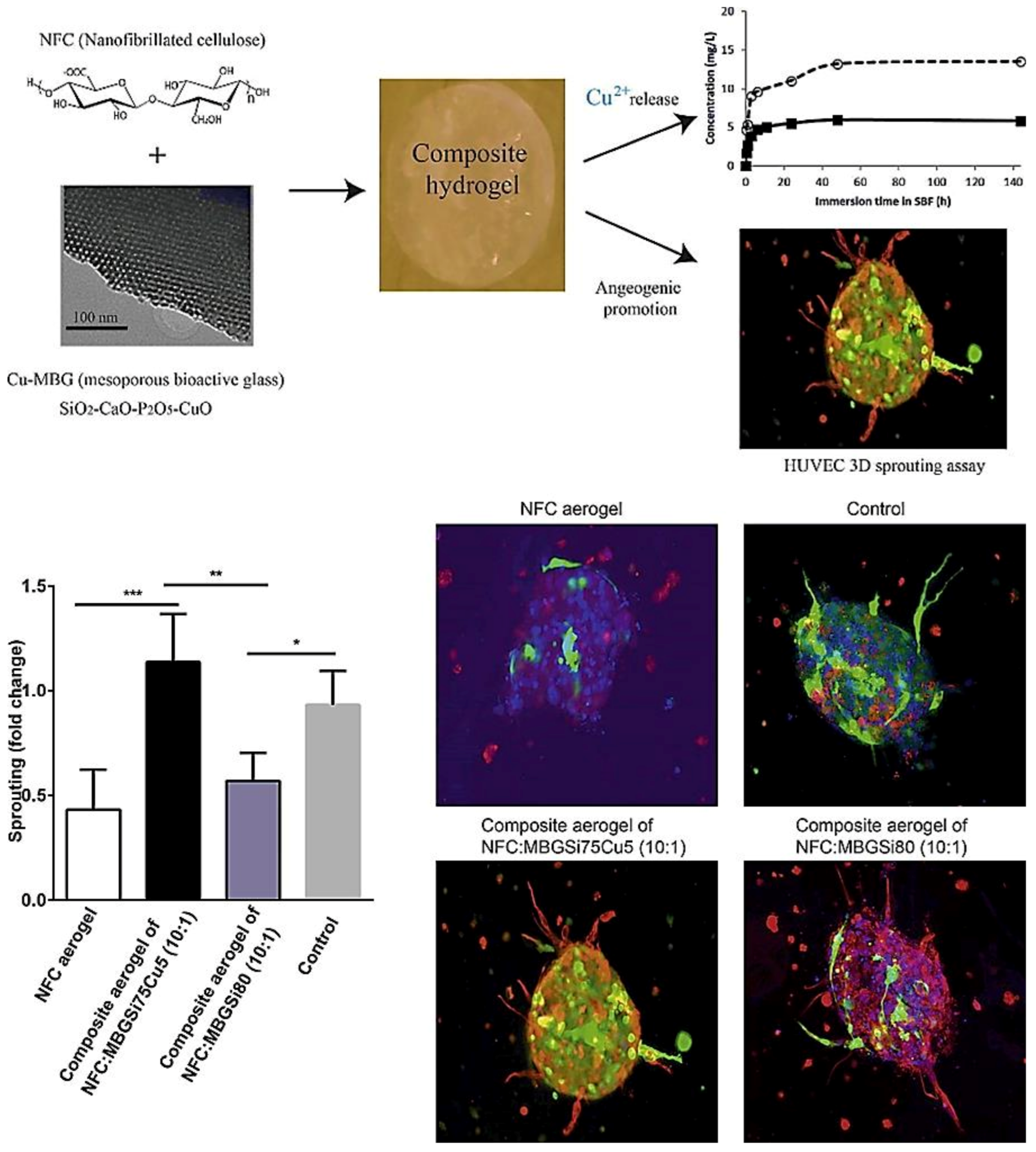
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Jindal, H.M.; Naveen, S.V.; Sekaran, S.D.; Kamarul, T.; Towler, M.R. Potency and cytotoxicity of a novel gallium-containing mesoporous bioactive glass/chitosan composite scaffold as hemostatic agents. ACS Appl. Mater. Interfaces 2017, 9, 31381–31392. [Google Scholar] [CrossRef]
- Mendonca, A.; Rahman, M.S.; Alhalawani, A.; Rodriguez, O.; Gallant, R.C.; Ni, H.; Clarkin, O.M.; Towler, M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon–calcium–phosphorous mesoporous bioactive glasses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2229–2237. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sameni, M.; Hashemi, A.; Zamani, F.; Rostami, A.; Mozafari, M. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: Activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns 2016, 42, 131–140. [Google Scholar] [CrossRef]
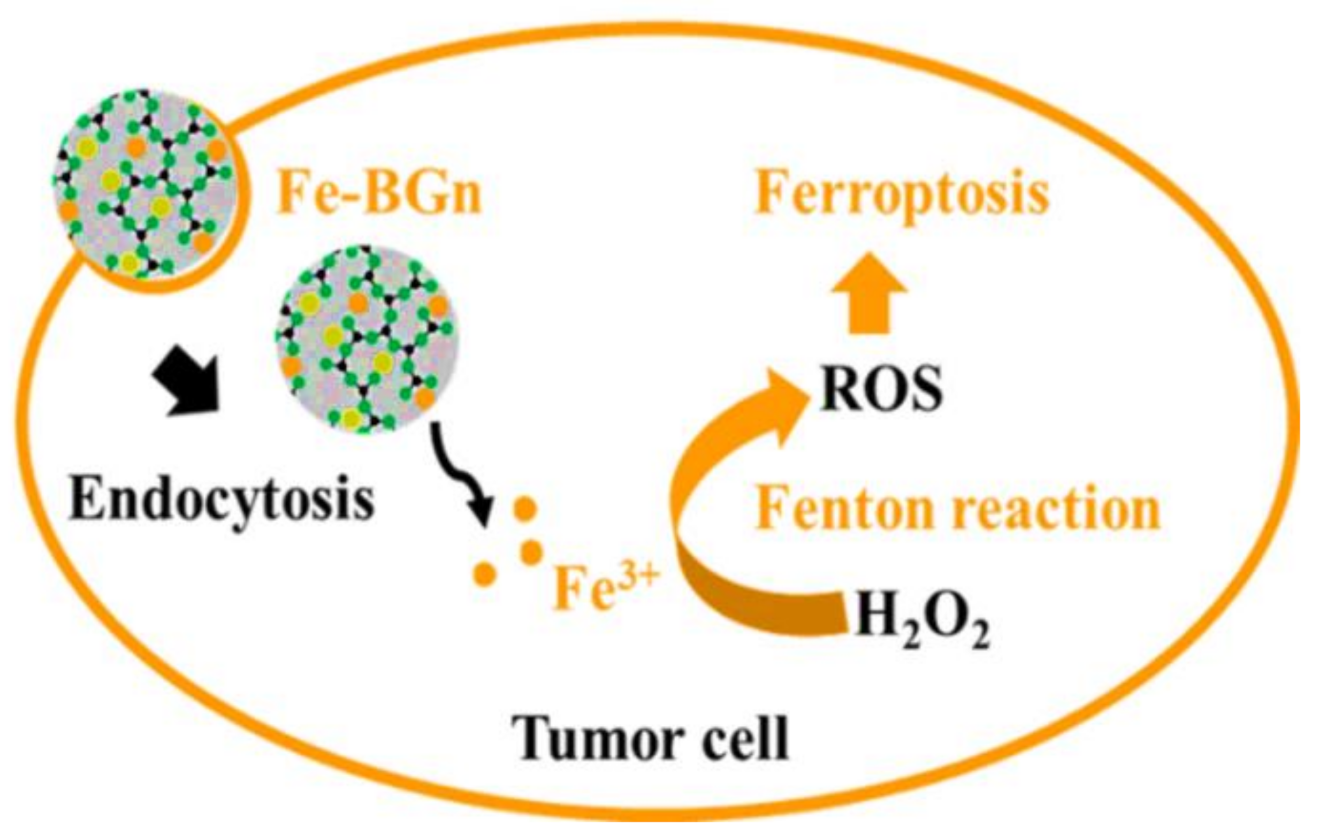
- El-Fiqi, A.; Kim, H.-W. Iron ions-releasing mesoporous bioactive glass ultrasmall nanoparticles designed as ferroptosis-based bone cancer nanotherapeutics: Ultrasonic-coupled sol–gel synthesis, properties and iron ions release. Mater. Lett. 2021, 294, 129759. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, D.; Ma, D.; Ji, C.; Wu, W.; Xu, L.; Zhang, L. Ferroptosis suppressed the growth of melanoma that may be related to DNA damage. Dermatol. Ther. 2019, 32, e12921. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Pingguan-Murphy, B.; Kadri, N.A.; Rothan, H.A.; Yusof, R.; Towler, M.R.; Djordjevic, I. Fabrication and characterization of poly (octanediol citrate)/gallium-containing bioglass microcomposite scaffolds. J. Mater. Sci. 2015, 50, 2189–2201. [Google Scholar] [CrossRef]
- Goodley, P.H.; Rogosnitzky, M. The effect of gallium nitrate on arresting blood flow from a wound. Case Rep. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Goncalves, J.; Wasif, N.; Esposito, D.; Coico, J.M.; Schwartz, B.; Higgins, P.J.; Bockman, R.S.; Staiano-Coico, L. Gallium nitrate accelerates partial thickness wound repair and alters keratinocyte integrin expression to favor a motile phenotype. J. Surg. Res. 2002, 103, 134–140. [Google Scholar] [CrossRef][Green Version]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Solids 2016, 432, 9–14. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, S.; Malavasi, G.; Salinas, A.J.; Lusvardi, G.; Rigamonti, L.; Menabue, L.; Vallet-Regi, M. Highly-bioreactive silica-based mesoporous bioactive glasses enriched with gallium (III). Materials 2018, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Naveen, S.V.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Almansoori, A.A.; Hwang, C.; Lee, S.-H.; Kim, B.; Kim, H.-E.; Lee, J.-H. Tantalum–poly (L-lactic acid) nerve conduit for peripheral nerve regeneration. Neurosci. Lett. 2020, 731, 135049. [Google Scholar] [CrossRef]
- van Koppen, C.J.; Hartmann, R.W. Advances in the treatment of chronic wounds: A patent review. Expert Opin. Ther. Pat. 2015, 25, 931–937. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Blome-Eberwein, S.A.; Twomey, J.A.; Herndon, D.N.; Luterman, A.; Silverstein, P.; Antimarino, J.R.; Bauer, G.J. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J. Burn Care Res. 2006, 27, 298–309. [Google Scholar] [CrossRef]
- Sharma, B. Infection in patients with severe burns: Causes and prevention thereof. Infect. Dis. Clin. N. Am. 2007, 21, 745–759. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Ciraldo, F.E.; Schnepf, K.; Goldmann, W.H.; Boccaccini, A.R. Development and Characterization of Bioactive Glass Containing Composite Coatings with Ion Releasing Function for Antibiotic-Free Antibacterial Surgical Sutures. Materials 2019, 12, 423. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Jindal, H.M.; Hasikin, K.; Naveen, S.V.; Sekaran, S.D.; Kamarul, T. Elastomeric biocomposite of silver-containing mesoporous bioactive glass and poly(1,8-octanediol citrate): Physiochemistry and in vitro antibacterial capacity in tissue engineering applications. Mater. Sci. Eng. C 2019, 98, 1022–1033. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Dahm, H. Silver Nanoparticles in Wound Infections: Present Status and Future Prospects. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Rai, M., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 151–168. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Durham, J.T.; Herman, I.M. Wound Healing Angiogenesis: Innovations and Challenges in Acute and Chronic Wound Healing. Adv. Wound Care 2012, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Thakur, S.; Neogi, S.; Dhara, S. Carbon nano dot decorated copper nanowires for SERS-Fluorescence dual-mode imaging/anti-microbial activity and enhanced angiogenic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117669. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Mulligan, A.M.; Wilson, M.; Knowles, J.C. Effect of increasing silver content in phosphate-based glasses on biofilms of Streptococcus sanguis. J. Biomed. Mater. Res. Part A 2003, 67A, 401–412. [Google Scholar] [CrossRef]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol–gel method. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef]
- Li, J.; Zhai, D.; Lv, F.; Yu, Q.; Ma, H.; Yin, J.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016, 36, 254–266. [Google Scholar] [CrossRef]
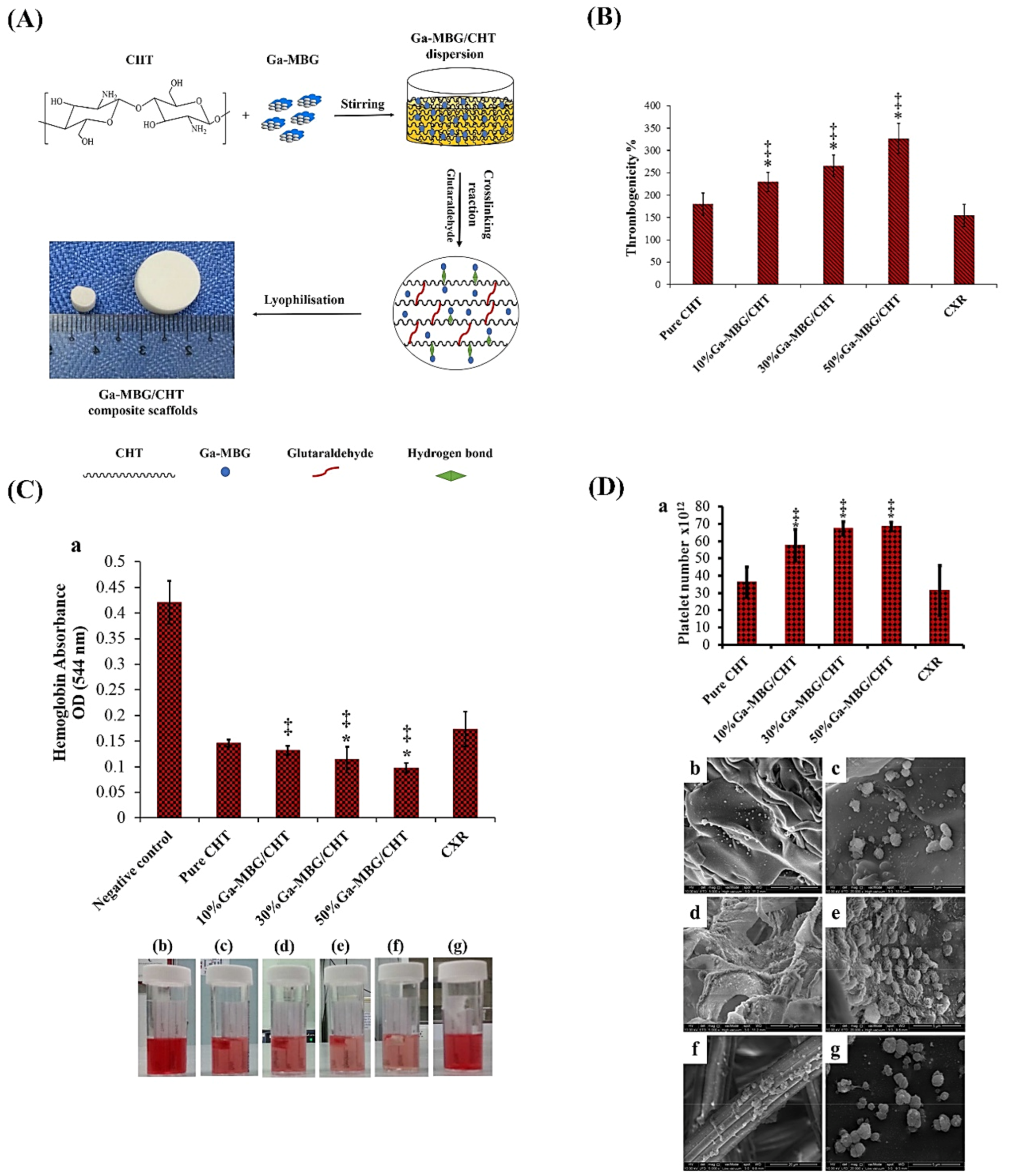
| Composition | Synthesis Method | Dopant | Application | Remarks | Ref |
|---|---|---|---|---|---|
| 1%Ga-MBG (79SiO2–15CaO–5P2O5–1Ga2O3) (10, 30, and 50 wt%) with CHT | Sol–gel using EISA with freeze-drying | Ga | Hemostatic and antibacterial |
| [139] |
| (80 − x) SiO2 − 15CaO − 5P2O5 − xTa2O5, where x = 0, 0.5, 1, 5, 10 | Sol–gel using EISA | Ta | Hemostatic |
| [140] |
| ([1 − (x + y)] (58SiO2 − 33P2O5 − 9CaO) − xCaF2 − yAg2O), where 0 ≤ x ≤ 20, 0 ≤ y ≤ 2 | Sol–gel | Ag | Antibacterial |
| [141] |
| MBGN (96.60SiO2-3.40CaO) and MBGN with Ag | Sol–gel using EA-CTAB-water micro-emulsion droplets | Ag | Antibacterial |
| [52] |
| MBGSi80 (molar ratio Si/Ca/P = 80/15/5)&&&MBGSi78Cu2 (molar ratio Si/Cu/Ca/P = 78/2/15/5) and MBGSi75Cu5 (molar ratio Si/Cu/Ca/P = 75/5/15/5) | Sol–gel using EISA | Cu | Angiogenic and antibacterial |
| [138] |
| 85SiO2–13CaO–2CuO | Ultra-sound-assisted base catalyzed sol–gel method | Cu | Angiogenic and antibacterial |
| [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hooshmand, S.; Mollazadeh, S.; Akrami, N.; Ghanad, M.; El-Fiqi, A.; Baino, F.; Nazarnezhad, S.; Kargozar, S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials 2021, 14, 3337. https://doi.org/10.3390/ma14123337
Hooshmand S, Mollazadeh S, Akrami N, Ghanad M, El-Fiqi A, Baino F, Nazarnezhad S, Kargozar S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials. 2021; 14(12):3337. https://doi.org/10.3390/ma14123337
Chicago/Turabian StyleHooshmand, Sara, Sahar Mollazadeh, Negar Akrami, Mehrnoosh Ghanad, Ahmed El-Fiqi, Francesco Baino, Simin Nazarnezhad, and Saeid Kargozar. 2021. "Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy" Materials 14, no. 12: 3337. https://doi.org/10.3390/ma14123337
APA StyleHooshmand, S., Mollazadeh, S., Akrami, N., Ghanad, M., El-Fiqi, A., Baino, F., Nazarnezhad, S., & Kargozar, S. (2021). Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials, 14(12), 3337. https://doi.org/10.3390/ma14123337








