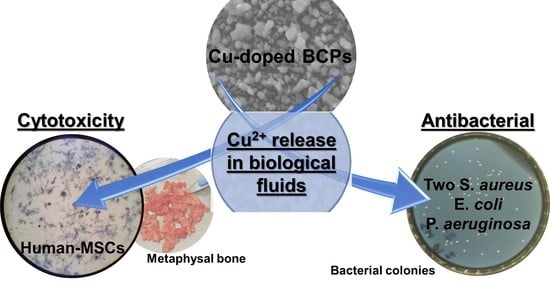
Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sol-Gel Synthesis of Copper-Doped BCP Samples
2.2. Cytotoxicity Evaluation
2.2.1. Human Mesenchymal Stem Cells (h-MSCs): Isolation and Culture
2.2.2. Cytotoxicity Evaluation of Cu-Doped Powders with MTT Assay
2.2.3. Cytotoxicity Evaluation of Cu2+ Ions in Standard Marrow Cell Culture Medium with MTT Assay
2.3. Antibacterial Properties
2.3.1. Bacterial Strains and Growth Conditions
2.3.2. Antibacterial Activity of Cu2+ ions in 1:500 Diluted TS Medium
2.3.3. Antibacterial Activity of Cu-Doped BCP Powders in 1:500 Diluted TS Medium
2.3.4. Antibacterial Activity against MSSA of Cu2+ Ions in Standard Marrow Cell Culture Medium (i.e., a Non-Diluted Medium)
2.4. Measurement of Cu2+ Copper Ions Concentration
2.5. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Cu-Doped BCP Powders
3.2. Cytotoxicity Measurements
3.2.1. Cytotoxicity Measurements on Cu-doped BCP Powders
3.2.2. Cytotoxicity Measurements in Copper Containing Solutions
3.3. Antibacterial Activity of BCP Samples
3.3.1. Antibacterial Activity of Cu-Doped BCP Powders against MSSA in 1:500 Diluted TS Medium
3.3.2. Antibacterial Activity of Cu-Doped BCP Powders against the Three other Strains in 1:500 Diluted TS Medium
3.3.3. Antibacterial Activity of Copper Containing 1:500 Diluted TS Medium
3.3.4. Antibacterial Activity against MSSA of Copper Containing Non-Diluted Media (Standard Marrow Cell Culture Medium)
4. Discussion
4.1. Cytotoxicity Evaluation
4.2. Antibacterial Evaluation
4.2.1. Antibacterial Activity of Cu-Doped BCP Powders in 1:500 Diluted TS Medium
4.2.2. Antibacterial Activity of Cu2+ Ions in 1:500 Diluted TS Medium
4.2.3. Antibacterial Activity against MSSA of Copper Containing Non-Diluted Standard Marrow Cell Culture Medium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomaterials 2011, 1, 3–56. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Daculsi, G.; LeGeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramicsin vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.-M.; Renaudin, G. Cu-doping of calcium phosphate bioceramics: From mechanism to the control of cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, J.H.; Manring, M.; Shirtliff, M. Osteomyelitis of the Long Bones. Semin. Plast. Surg. 2009, 23, 59–72. [Google Scholar] [CrossRef]
- ter Boo, G.-J.A.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006, 34, S3–S10. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. The elements of life and medicines. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140182. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Fang, Y.; Hooper, T.J.N.; Kelly, N.L.; Gupta, D.; Balani, K.; Manna, I.; Baikie, T.; Bishop, P.T.; White, T.J.; et al. Crystal Chemistry and Antibacterial Properties of Cupriferous Hydroxyapatite. Materials 2019, 12, 1814. [Google Scholar] [CrossRef]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.-M.; Descamps, S. Biological properties of copper-doped biomaterials for orthopedic applications: A review of antibacterial, angiogenic and osteogenic aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef]
- Bazin, T.; Magnaudeix, A.; Mayet, R.; Carles, P.; Julien, I.; Demourgues, A.; Gaudon, M.; Champion, E. Sintering and biocompatibility of copper-doped hydroxyapatite bioceramics. Ceram. Int. 2021, 47, 13644–13654. [Google Scholar] [CrossRef]
- Charbonnier, B.; Manassero, M.; Bourguignon, M.; Decambron, A.; El-Hafci, H.; Morin, C.; Leon, D.; Bensidoum, M.; Corsia, S.; Petite, H.; et al. Custom-made macroporous bioceramic implants based on triply-periodic minimal surface for bone defects in load-bearing sites. Acta Biomater. 2020, 109, 254–266. [Google Scholar] [CrossRef]
- Marquès, C.; Franceschi, C.; Collin, V.; Laurent, F.; Chatellier, S.; Forestier, C. Genome Sequence of a Clinical Staphylococcus aureus Strain from a Prosthetic Joint Infection. Genome Announc. 2016, 4. [Google Scholar] [CrossRef]
- Renaudin, G.; Gomes, S.; Nedelec, J.-M. First-Row Transition Metal Doping in Calcium Phosphate Bioceramics: A Detailed Crystallographic Study. Materials 2017, 10, 92. [Google Scholar] [CrossRef]
- Li, Y.; Ho, J.; Ooi, C.P. Antibacterial efficacy and cytotoxicity studies of copper (II) and titanium (IV) substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Marques, C.F.; Olhero, S.; Abrantes, J.; Marote, A.; Ferreira, S.; Vieira, S.I.; Ferreira, J.M. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Sahithi, K.; Swetha, M.; Prabaharan, M.; Moorthi, A.; Saranya, N.; Ramasamy, K.; Srinivasan, N.; Partridge, N.C.; Selvamurugan, N. Synthesis and Characterization of NanoscaleHydroxyapatite-Copper for Antimicrobial Activity Towards Bone Tissue Engineering Applications. J. Biomed. Nanotechnol. 2010, 6, 333–339. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Qiao, Y.; Liu, X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elements Med. Biol. 2019, 55, 127–135. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Karlov, A.V.; Khlusov, I.A.; Pontak, V.A.; Ignatov, V.P.; Ivin, M.A.; Zinatulina, S.Y. Adhesion of Staphylococcus Aureus to Implants with Different Physicochemical Characteristics. Bull. Exp. Biol. Med. 2002, 134, 277–280. [Google Scholar] [CrossRef]
- Çetiner, U.; Rowe, I.; Schams, A.; Mayhew, C.; Rubin, D.; Anishkin, A.; Sukharev, S. Tension-activated channels in the mechanism of osmotic fitness in Pseudomonas aeruginosa. J. Gen. Physiol. 2017, 149, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Multiple Mechanisms of Antimicrobial Resistance in Pseudomonas aeruginosa: Our Worst Nightmare? Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fraser, W.D.; Jackson, M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002, 61, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, W.; Zeronian, H.; Ellison, M.; Lewis, D.; Rucker, R.B.; Riggins, R.S. Role of Copper in Collagen Cross-linking and Its Influence on Selected Mechanical Properties of Chick Bone and Tendon. J. Nutr. 1982, 112, 708–716. [Google Scholar] [CrossRef]
- Barralet, J.E.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in Calcium Phosphate Scaffolds by Inorganic Copper Ion Release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef]
- Kong, N.; Lin, K.; Li, H.; Chang, J. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J. Mater. Chem. B 2014, 2, 1100–1110. [Google Scholar] [CrossRef]
- Ewald, A.; Käppel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. Part A 2012, 100, 2392–2400. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, M.; Gu, W.; Shen, Z.; Ma, X.; Lu, F.; Yang, X.; Zheng, Y.; Gou, Z. Zinc-/copper-substituted dicalcium silicate cement: Advanced biomaterials with enhanced osteogenesis and long-term antibacterial properties. J. Mater. Chem. B 2020, 8, 1060–1070. [Google Scholar] [CrossRef]







| Sample Series | Specific Surface Area (m2/g) | Composition (wt %) * | |
|---|---|---|---|
| HAp | β-TCP | ||
| XCu-600 | ~14.5 | ~88 | ~12 |
| 00Cu-600 | 14.8 | 86 | 14 |
| 05Cu-600 | 16.7 | 88 | 12 |
| 10Cu-600 | 12.4 | 90 | 10 |
| 20Cu-600 | 13.7 | 90 | 10 |
| XCu-900 | ~3.5 | ~94 | ~6 |
| 00Cu-900 | 3.3 | 96 | 4 |
| 05Cu-900 | 3.5 | 93 | 7 |
| 10Cu-900 | 4.1 | 94 | 6 |
| 20Cu-900 | 3 | 93 | 7 |
| XCu-1200 | ~1.0 | ~98 | ~2 |
| 00Cu-1200 | 1.2 | 99 | 1 |
| 05Cu-1200 | 0.5 | 98 | 2 |
| 10Cu-1200 | 1 | 98 | 2 |
| 20Cu-1200 | 0.6 | 97 | 3 |
| Samples | Cu2+ Concentration (ppm) | ||
|---|---|---|---|
| Day 3 | Day 7 | Day 15 * | |
| 05Cu-600 | 2.4 | 2.8 | 0.9 (3.7) |
| 10Cu-600 | 5.1 | 4.7 | 2.3 (7.0) |
| 20Cu-600 | 11.5 | 12.0 | 3.5 (15.5) |
| 05Cu-900 | 2.5 | 2.4 | 0.6 (3.0) |
| 10Cu-900 | 5.2 | 5.5 | 1.2 (6.7) |
| 20Cu-900 | 10.0 | 9.4 | 2.4 (11.8) |
| 05Cu-1200 | 0.5 | 0.7 | 0.4 (1.1) |
| 10Cu-1200 | 0.7 | 0.8 | 0.4 (1.2) |
| 20Cu-1200 | 1.5 | 1.7 | 0.6 (2.3) |
| Samples | Cu2+ Concentration (ppm) | |
|---|---|---|
| 5 h | 24 h | |
| 05Cu-600 | 2.2 | 2.2 |
| 10Cu-600 | 2.4 | 2.7 |
| 20Cu-600 | 2.7 | 2.8 |
| 05Cu-900 | 1.7 | 2.1 |
| 10Cu-900 | 1.5 | 2.2 |
| 20Cu-900 | 1.9 | 2.8 |
| 05Cu-1200 | 0.7 | 1.0 |
| 10Cu-1200 | 0.9 | 1.3 |
| 20Cu-1200 | 1.2 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, A.; Renaudin, G.; Charbonnel, N.; Nedelec, J.-M.; Forestier, C.; Descamps, S. Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties. Materials 2021, 14, 2393. https://doi.org/10.3390/ma14092393
Jacobs A, Renaudin G, Charbonnel N, Nedelec J-M, Forestier C, Descamps S. Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties. Materials. 2021; 14(9):2393. https://doi.org/10.3390/ma14092393
Chicago/Turabian StyleJacobs, Aurélie, Guillaume Renaudin, Nicolas Charbonnel, Jean-Marie Nedelec, Christiane Forestier, and Stéphane Descamps. 2021. "Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties" Materials 14, no. 9: 2393. https://doi.org/10.3390/ma14092393
APA StyleJacobs, A., Renaudin, G., Charbonnel, N., Nedelec, J.-M., Forestier, C., & Descamps, S. (2021). Copper-Doped Biphasic Calcium Phosphate Powders: Dopant Release, Cytotoxicity and Antibacterial Properties. Materials, 14(9), 2393. https://doi.org/10.3390/ma14092393