Thermodilatometric Study of the Decay of Zeolite-Bearing Building Materials
Abstract
:1. Introduction
2. Materials and Methods
- Campanian Ignimbrite from Dugenta (outskirts of Caserta, Campania region, Southern Italy);
- Ignimbrite from Sorano (outskirts of Grosseto, Toscana region, Central Italy);
- Campanian Ignimbrite from Comiziano (outskirts of Naples, Campania region, Southern Italy);
- Neapolitan Yellow Tuff from Chiaiano (outskirts of Naples, Campania region, Southern Italy);
- Neapolitan Yellow Tuff from Toledo subway station (center of Naples, Campania region, Southern Italy);
- Sardinian Epiclastite from Anela (outskirts of Sassari, Sardinia region, Insular Italy);
- Gray Campanian Ignimbrite from Faicchio (outskirts of Benevento, Campania region, Southern Italy).
- In the cases of rocks 1, 2 and 3 (the various ignimbrites), these processes led to the crystallization of a higher amount of chabazite, together with a lower amount of phillipsite and a small amount of analcime.
- In the cases of rocks 3 and 4 (the Neapolitan Yellow Tuff), the phillipsite and chabazite contents are usually similar, although, in some cases, the former largely prevailed on the latter (see rock 4, TG Chiaiano); moreover, in the Neapolitan Yellow Tuff, analcime is present only to very low extent, if any.
- In the case of rock 6 (the Sardinian epiclastic rock), the circulation of hydrothermal fluids through the riodacitic and ryolithic volcanic glass precursor resulted in the crystallization of clinoptilolite and smectite, together with a small amount of crystobalite and, sometimes, opal CT [39].
- In the case of rock 7 (the Gray Campanian Ignimbrite), the high emplacement temperatures determined the welding of the glass shards and transformation of the original alcaly trachytic glass into the feldspars that represent its main constituent (more than 90%) [41].
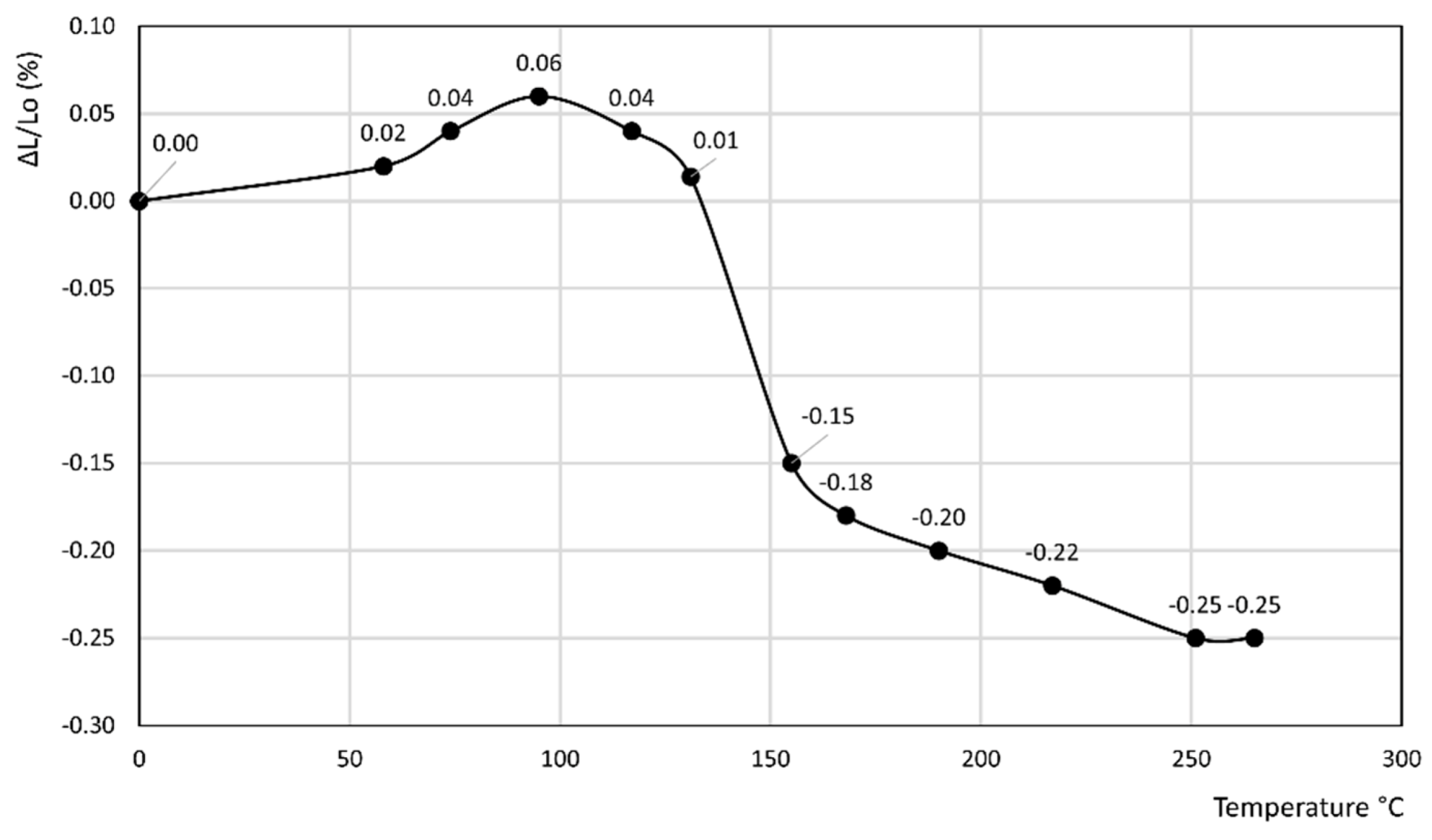
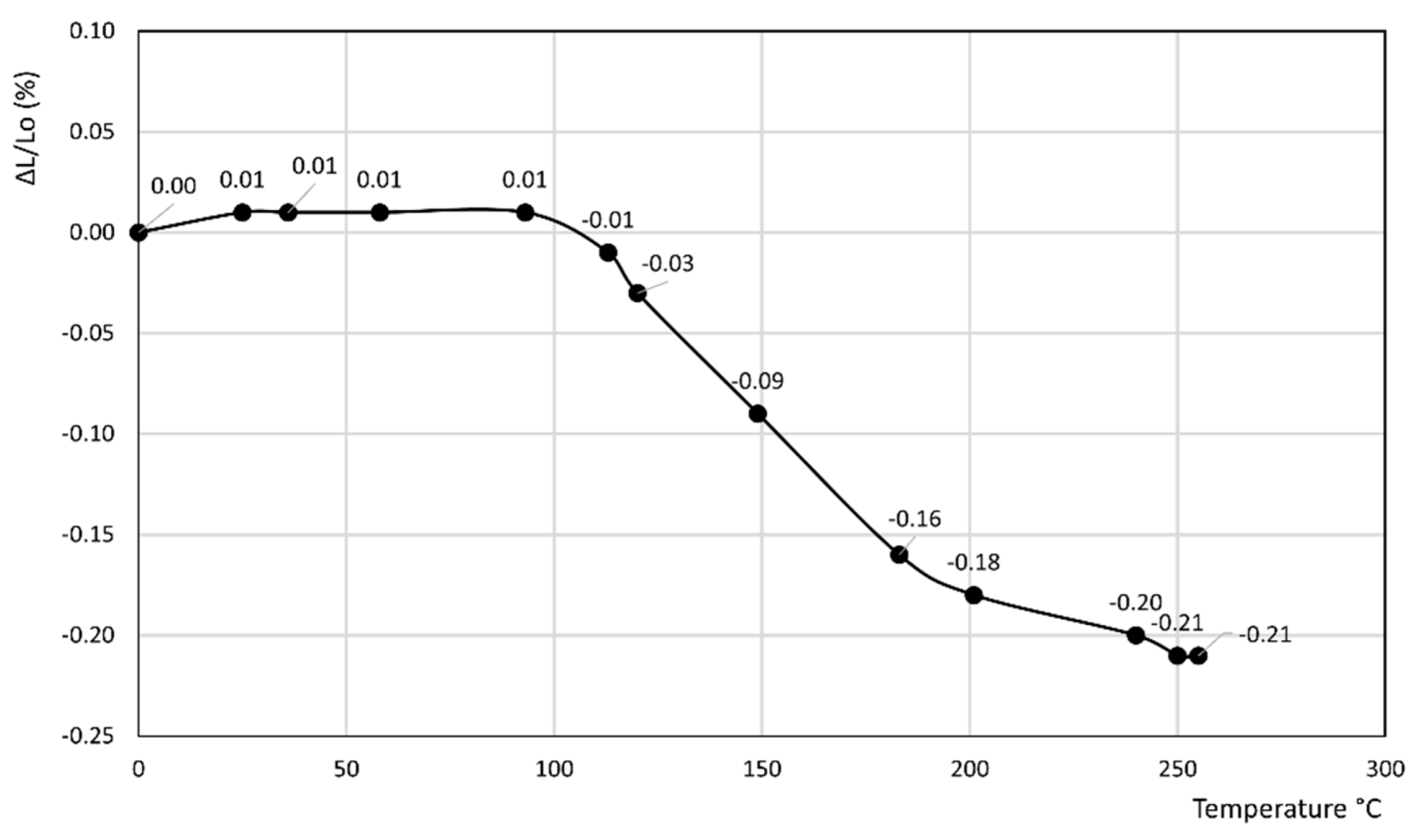
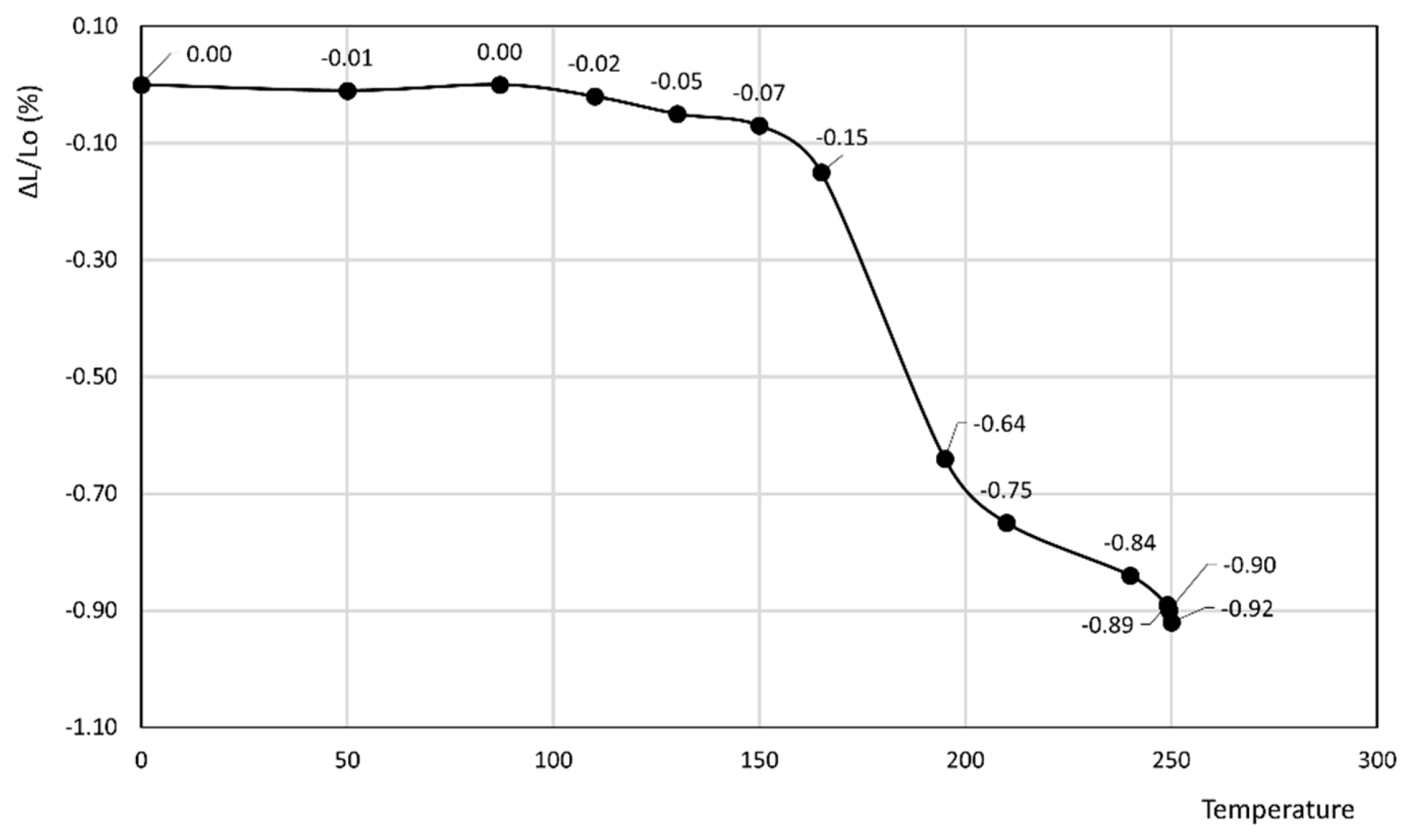
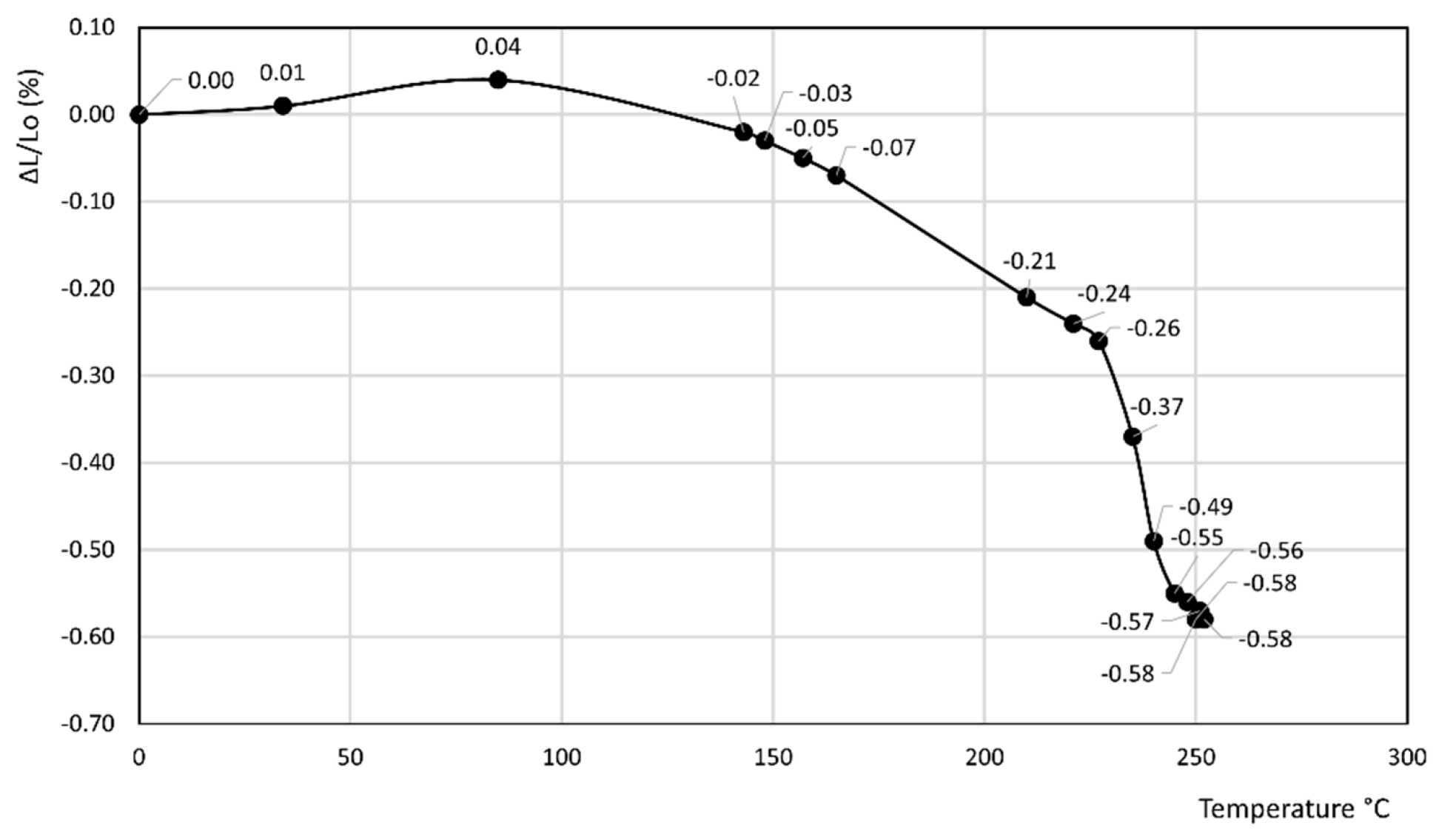
3. Results
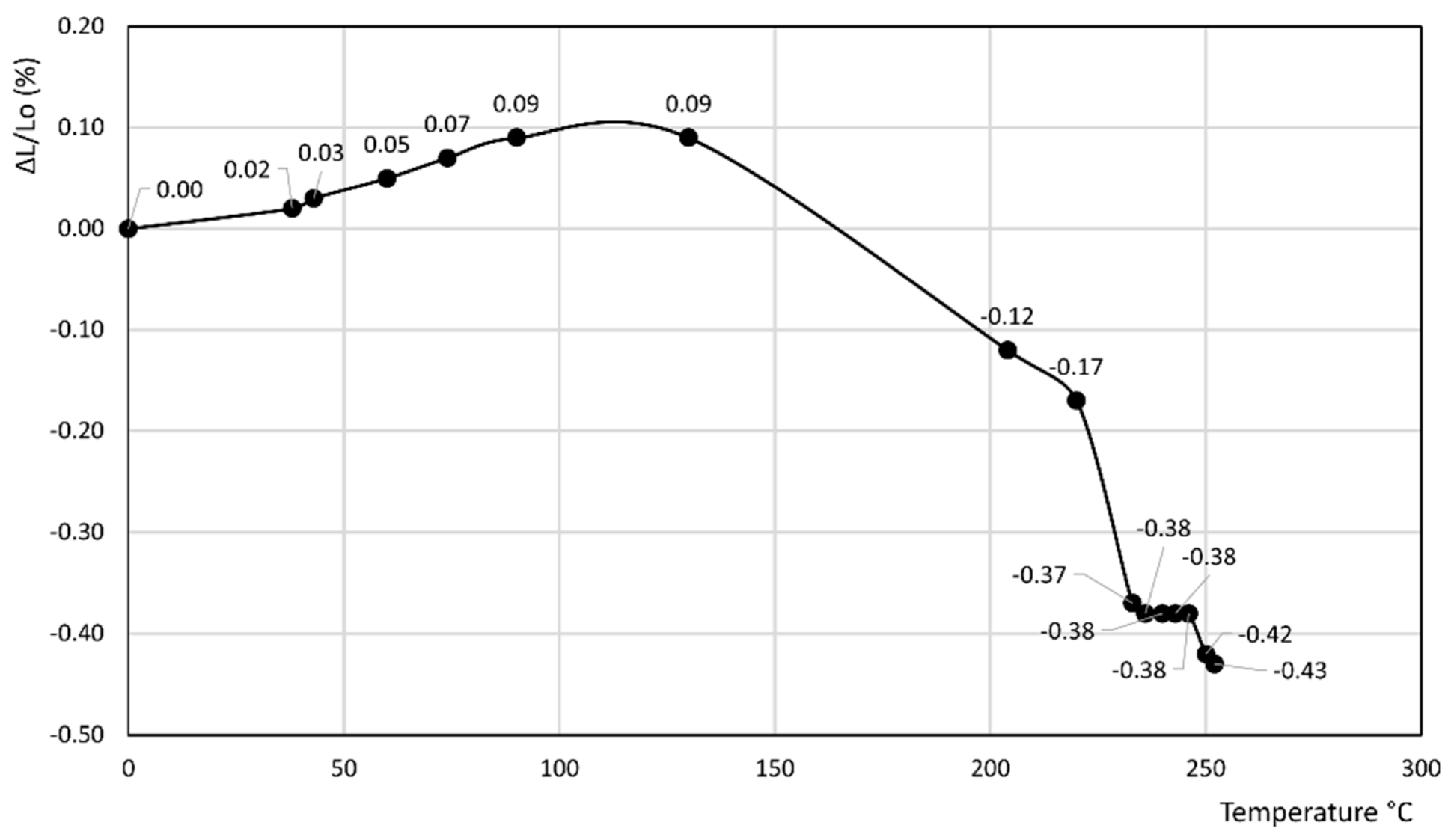
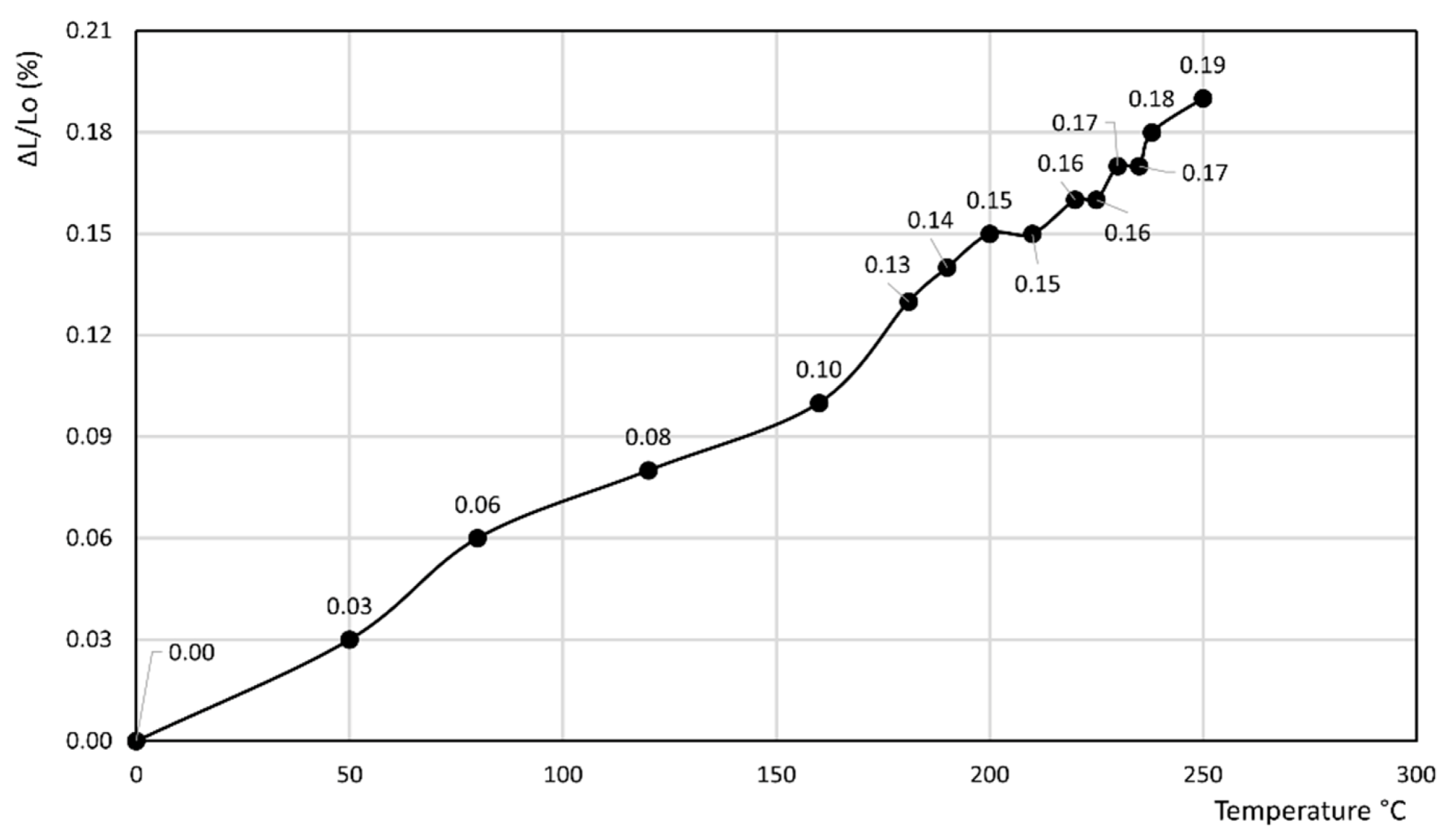
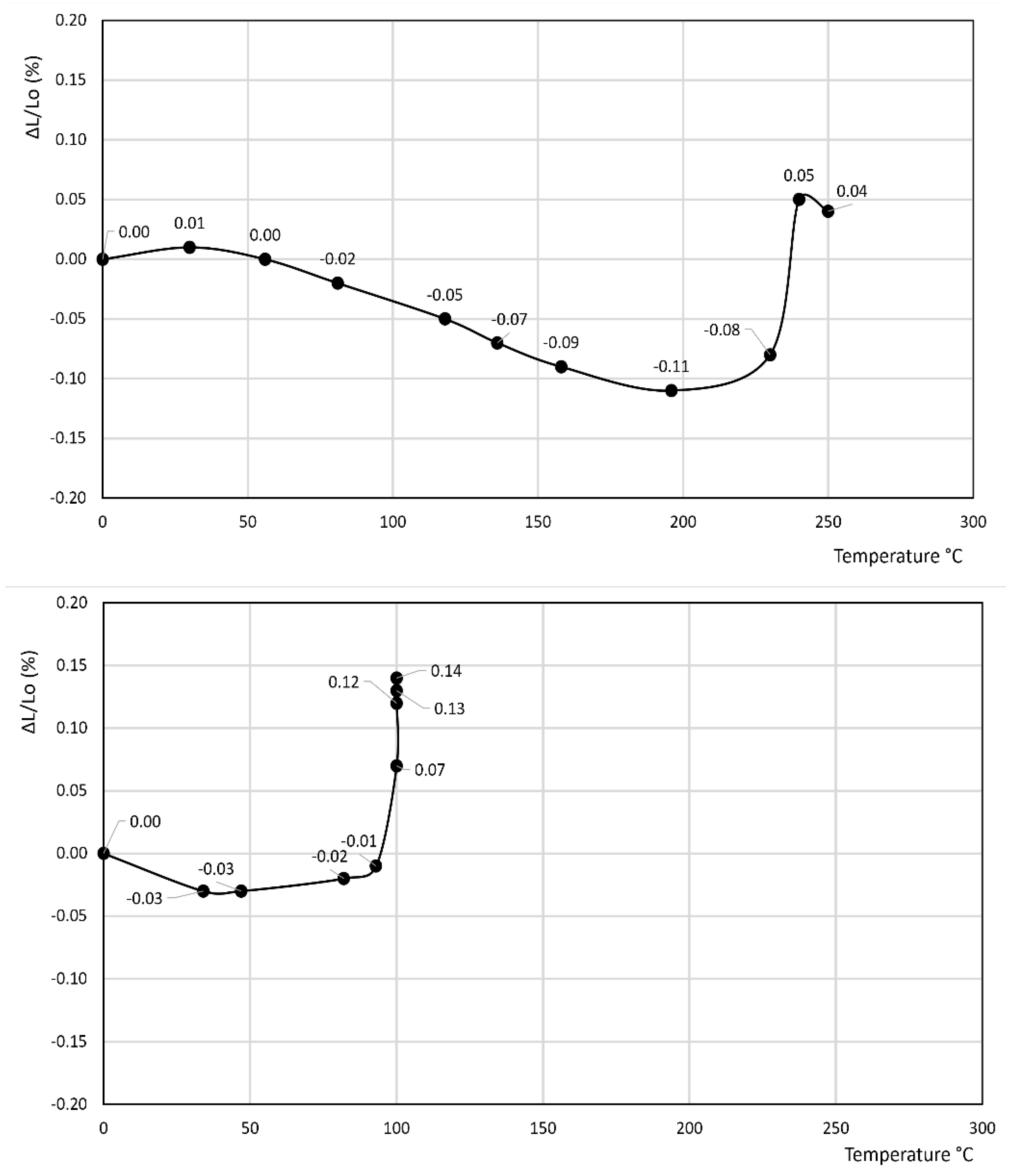
- In the former curve, ΔL/Lo (%) decreases up to 200 °C, thus attaining a minimum of about −0.11%, then rapidly increases up to about 240 °C, thus attaining a maximum of about 0.05%, and finally decreases again to a final value of 0.04% at 250 °C.
- In the latter curve, ΔL/Lo (%) slightly decreases up to 40 °C, attaining a value of −0.03%, thus attaining a plateau at this value in the 40–80 °C temperature range before finally increasing to a final value of 0.13% at 100 °C.
4. Discussion
- Thermal behavior of phillipsite is described in [58]. In this work, the various cation forms of phillipsite were found to undergo thermal collapse at temperatures ranging from 200 to 600 °C.
- Shrinkage resulting from dehydration in chabazite and phillipsite appears likely to be similar on account of their similar Si/Al ratio and cation populations (these zeolites originated from the alteration of the same volcanic glass by weathering) and on account of their similar framework densities (1.58 and 1.45 g/cm3 for phillipsite and chabazite, respectively [59]).
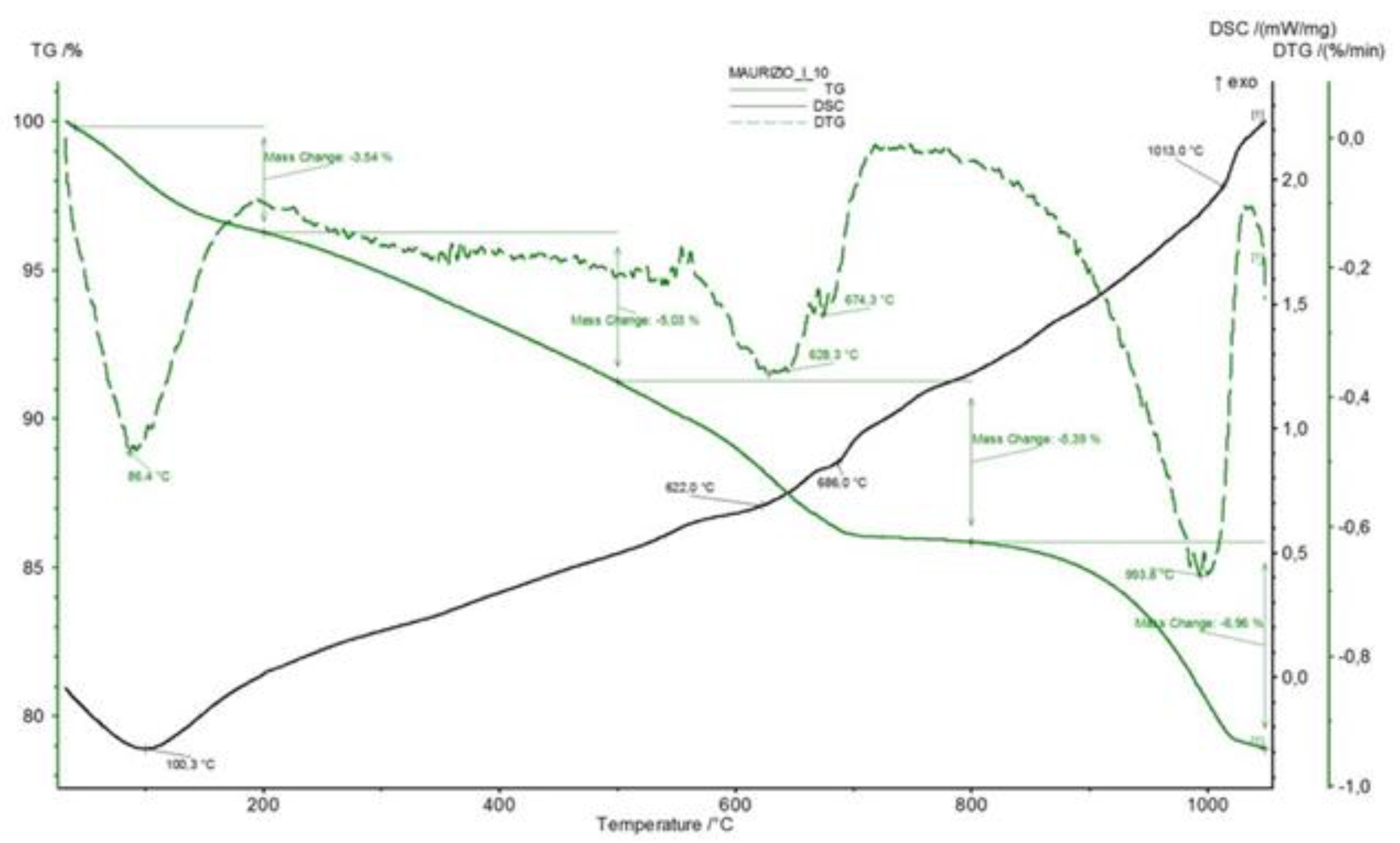
- The amount of water released by zeolites at the various temperatures is strongly related to cation population, as demonstrated confidently in [60]. It is true that the cation populations of the various zeolitic phases present in the rock were not explicitly determined in that study, but the oxide analysis of the rocks (Table 2) gives us this datum, at least to a loose approximation.
- a small shrinkage, which almost exactly counterbalances the plain thermal expansion, thus resulting almost in the absence of appreciable length variations among specimens (rocks 4 and 5);
- a small shrinkage of entity slightly lower than the plain thermal expansion, thus giving rise to slightly positive length variations among specimens (rocks 1, 2, 5 and 6).
- the limited extent to which the variation of the heating rate affects the thermodilametric behavior of the zeolite-bearing materials;
- the fact that the variation of the heating rate does not change the nature of the phenomena occurring [29];
- the fact that the issue most largely affecting the weathering of zeolite-bearing building stones is the difference of thermodilatometric behavior between the plaster layer and the underlying building stones (vide infra).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bish, D.L.; Ming, D.W. Natural Zeolites: Occurrence, Properties, Application. Reviews in Mineralogy & Geochemistry; Mineralogical Society of America: Blacksburg, VA, USA, 2001; Volume 45, p. 654. [Google Scholar]
- Colella, C.; de’ Gennaro, M.; Langella, A.; Pansini, M. Evaluation of Italian natural zeolites in wastewater treatment: Cu and Zn exchange reaction on phillipsite and chabazite. Sep. Sci. Technol. 1998, 33, 467–481. [Google Scholar] [CrossRef]
- Pansini, M.; Colella, C. Dynamic data on lead uptake from water by chabazite. Desalination 1990, 78, 287–295. [Google Scholar] [CrossRef]
- Albino, V.; Cioffi, R.; Pansini, M.; Colella, C. Disposal of lead-containing zeolite sludges in cement matrix. Environ. Technol. 1995, 16, 147–165. [Google Scholar] [CrossRef]
- Albino, V.; Cioffi, R.; Pansini, M.; Colella, C. Evaluation of cement-based solidified materials encapsulating Cd-exchanged natural zeolites. Environ. Technol. 1996, 17, 1215–1224. [Google Scholar]
- Colella, C.; Pansini, M. Lead removal from wastewaters using chabazite tuff. ACS Symp. Ser. 1988, 368, 500–510. [Google Scholar]
- Pansini, M.; de Gennaro, R.; Parlato, L.; de Gennaro, M.; Langella, A.; Marocco, A.; Cappelletti, P.; Mercurio, M. Use of sawing waste from zeolitic tuffs in the manufacture of ceramics. Adv. Mater. Sci. Eng. 2010, 2010, 820541. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, R.; Cappelletti, P.; Cerri, G.; de Gennaro, M.; Dondi, M.; Guarini, G.; Langella, A.; Naimo, D. Influence of zeolites on the sintering and technological properties of porcelain stoneware tiles. J. Eur. Ceram. Soc. 2003, 23, 2237–2245. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, R.; Dondi, M.; Cappelletti, P.; Cerri, G.; de Gennaro, M.; Guarini, G.; Langella, A.; Parlato, L.; Zanelli, C. Zeolite-feldspar epiclastic rocks as flux in ceramic tile manufacturing. Microporous Mesoporous Mater. 2007, 105, 273–278. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, R.; Cappelletti, P.; Cerri, G.; de Gennaro, M.; Dondi, M.; Graziano, S.F.; Langella, A. Campanian Ignimbrite as raw material for lightweight aggregates. Appl. Clay Sci. 2007, 37, 115–126. [Google Scholar] [CrossRef]
- De Gennaro, R.; Langella, A.; D’Amore, M.; Dondi, M.; Colella, A.; Cappelletti, P.; de Gennaro, M. Use of zeolite-rich rocks and waste materials for the production of structural lightweight concretes. Appl. Clay Sci. 2008, 41, 61–72. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, R.; Cappelletti, P.; Cerri, G.; de Gennaro, M.; Dondi, M.; Langella, A. Zeolitic tuffs as raw materials for lightweight aggregates. Appl. Clay Sci. 2004, 25, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Mercurio, M.; Bish, D.L.; Cappelletti, P.; de Gennaro, B.; de Gennaro, M.; Grifa, C.; Izzo, F.; Mercurio, V.; Morra, V.; Langella, A. The combined use of steam-treated bentonites and natural zeolites in the oenological refining process. Mineral. Mag. 2016, 80, 347–362. [Google Scholar] [CrossRef]
- Cappelletti, P.; Rapisardo, G.; de Gennaro, B.; Colella, A.; Langella, A.; Graziano, S.F.; Bish, D.L.; de Gennaro, M. Immobilization of Cs and Sr in aluminosilicate matrices derived from natural zeolites. J. Nucl. Mater. 2011, 414, 451–457. [Google Scholar] [CrossRef]
- Mercurio, M.; Langella, A.; Cappelletti, P.; de Gennaro, B.; Monetti, V.; de Gennaro, M. May the use of Italian volcanic zeolite-rich tuffs as additives in animal diet represent a risk for the human health? Period. Mineral. 2012, 81, 393–407. [Google Scholar]
- Cappelletti, P.; Colella, A.; Langella, A.; Mercurio, M.; Catalanotti, L.; Monetti, V.; de Gennaro, B. Use of surface modified natural zeolite (SMNZ) in pharmaceutical preparations Part 1. Mineralogical and technological characterization of some industrial zeolite-rich rocks. Microporous Mesoporous Mater. 2017, 250, 232–244. [Google Scholar] [CrossRef]
- Basile, A.; Cacciola, G.; Colella, C.; Mercadante, L.; Pansini, M. Thermal conductivity of natural zeolite-PTFE composites. Heat Recovery Syst. CHP 1992, 12, 497–503. [Google Scholar] [CrossRef]
- Colella, C.; de Gennaro, M.; Aiello, R. Use of Zeolitic Tuff in the Building Industry. Rev. Mineral. Geochem. 2001, 45, 551–587. [Google Scholar] [CrossRef]
- Cardone, V. Il Tufo Nudo Nell’architettura Napoletana; CUEN: Napoli, Italy, 1990; pp. 63–72. [Google Scholar]
- Steindlberger, E. Volcanic tuff from Hesse (Germany) and their weathering behavior. Special issue: Stone decay hazards. Environ. Geol. 2004, 46, 378–390. [Google Scholar] [CrossRef]
- Granato, A.; Apicella, A. Utilization of fluorinated polymers in surface protection of zeolite-bearing tuff. Mater. Eng. 1994, 5, 329–342. [Google Scholar]
- De Gennaro, M.; Calcaterra, D.; Cappelletti, P.; Langella, A.; Morra, V. Building stone and related weathering in the architecture of the ancient city of Naples. J. Cult. Herit. 2000, 1, 399–414. [Google Scholar] [CrossRef]
- Dyer, A.; Judanne, W.M. Thermal analysis of synthetic zeolite A series. Thermochim. Acta 1974, 10, 299–304. [Google Scholar] [CrossRef]
- Dyer, A. Thermomechanical analysis of zeolites. Anal. Proc. 1981, 18, 447–450. [Google Scholar]
- Siegel, H.; Schmitz, W.; Schollner, R.; Dyer, A. Thermal analysis of NaMgA zeolites. Thermochim. Acta 1983, 61, 329–340. [Google Scholar] [CrossRef]
- Siegel, H.; Schmitz, W.; Schollner, R.; Dyer, A. Thermomechanical analysis and X-ray heating studies of (Ca, Na)-a zeolites. Thermochim. Acta 1985, 93, 561–564. [Google Scholar] [CrossRef]
- Dyer, A. Thermal analysis of zeolites. Thermochim. Acta 1987, 110, 521–526. [Google Scholar] [CrossRef]
- Dyer, A.; Saghal, M.A. Standardization of TMA (dilatometry) for application of the study of synthetic zeolites. Thermochim. Acta 1988, 132, 127–131. [Google Scholar] [CrossRef]
- Colantuono, A.; Dal Vecchio, S.; Mascolo, G.; Pansini, M. Thermal shrinkage of various cation forms of zeolite A. Thermochim. Acta 1997, 296, 59–66. [Google Scholar] [CrossRef]
- Ullrich, B.; Adolphi, P.; Zwahr, H.; Schomburg, J. Derivative dilatometric study of natural zeolites. Zeolites 1989, 9, 412–417. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Ferone, C.; Mascolo, G.; Pansini, M. Dilatometry of Na-, K-, Ca- and NH4-clinoptilolite. Thermochim. Acta 1999, 336, 105–110. [Google Scholar] [CrossRef]
- Colantuono, A.; Dal Vecchio, S.; Mascolo, G.; Pansini, M. Dilatometric behaviour of chabazite. J. Therm. Anal. 1996, 47, 281–289. [Google Scholar] [CrossRef]
- Colantuono, A.; Dal Vecchio, S.; Ferone, C.; Mascolo, G.; Pansini, M. Dilatometric characterization of natural phillipsite and chabazite-bearing materials. In Natural Zeolites for the Third Millennium; Colella, C., Mumpton, F.A., Eds.; De Frede: Naples, Italy, 2000; pp. 299–304. [Google Scholar]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, F.; Lvov, Y. Halloysite/keratin nanocomposite for human hairs photoprotection coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Stoporev, A.; Mendgaziev, R.; Artemova, M.; Semenov, A.; Novikov, A.; Kiiamov, A.; Emelianov, A.; Rodionova, T.; Fakhrullin, R.; Shchukin, D. Ionic chlathrate hydrates loaded into a cryogel-halloysite clay composite for cold storage. Appl. Clay Sci. 2021, 191, 105618. [Google Scholar] [CrossRef]
- Cappelletti, P.; Langella, A.; Cruciani, G. Crystal-chemistry and synchrotron Rietveld refinement of two different clinoptilolites from volcanoclastites of North-western Sardinia. Eur. J. Mineral. 1999, 11, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Cerri, G.; Langella, A.; Pansini, M.; Cappelletti, P. Methods of Determining Cation Exchange Capacities for Clinoptilolite-Rich Rocks of the Logudoro Region in Northern Sardinia, Italy. Clays Clay Miner. 2002, 50, 127–135. [Google Scholar] [CrossRef]
- Langella, A.; Pansini, M.; Cerri, G.; Cappelletti, P.; de’ Gennaro, M. Thermal behaviour of natural and cation-exchanged clinoptilolite from Sardinia (Italy). Clays Clay Miner. 2003, 51, 625–633. [Google Scholar] [CrossRef]
- Cerri, G.; Cappelletti, P.; Langella, A.; de’ Gennaro, M. Zeolitization of Oligo-Miocene volcaniclastic rocks from Logudoro (northern Sardinia, Italy). Contrib. Mineral. Petrol. 2001, 140, 404–421. [Google Scholar] [CrossRef]
- Cappelletti, P.; Cerri, G.; Colella, A.; de Gennaro, M.; Langella, A.; Perrotta, A.; Scarpati, C. Post-eruptive processes in the Campanian Ignimbrite. Mineral. Petrol. 2003, 79, 79–97. [Google Scholar] [CrossRef]
- Langella, A.; Bish, D.L.; Cappelletti, P.; Cerri, G.; Colella, A.; de Gennaro, R.; Graziano, S.F.; Perrotta, A.; Scarpati, C.; de Gennaro, M. New insights into the mineralogical facies distribution of Campanian Ignimbrite, a relevant Italian industrial material. Appl. Clay Sci. 2013, 72, 55–73. [Google Scholar] [CrossRef]
- Scarpati, C.; Sparice, D.; Perrotta, A. Dynamics of large pyroclastic currents inferred by the interna architecture of the Campanian Ignimbrite. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- De Gennaro, M.; Cappelletti, P.; Langella, A.; Perrotta, A.; Scarpati, C. Genesis of zeolites in the Neapolitan Yellow Tuff: Geological, volcanological and mineralogical evidence. Contrib. Mineral. Petrol. 2000, 139, 17–35. [Google Scholar] [CrossRef]
- Italiana Zeolite (CBC Group Company) Record: Report of analysis performed by the Department of Earth Sciences, Environment and Resources Federico II University of Naples. Head of the Laboratory P. Cappelletti. Code PG/2020//0096686 of18/11/2020.
- De Gennaro, R. Caratterizzazione di Tufi Zeolitizzati Italiani per un Possibile Impiego in Campo Ceramico. Ph.D. Thesis, University of Sassari, Sassari, Italy, 2000. [Google Scholar]
- Italiana Zeolite (CBC Group Company) Record: Report of analysis performed by the Department of Earth Sciences Department of Earth Sciences of the Federico II University of Naples. Head of the laboratory M. de Gennaro. Code 974/2004.
- Italiana Zeolite (CBC Group Company) Record: Report of analysis performed by the Department of Earth Sciences of the Federico II University of Naples. Head of the laboratory M. de Gennaro. Code PG/2008/0073407.
- Italiana Zeolite (CBC Group Company) Record: Report of analysis performed by the Department of Earth Sciences Environment and Resources Federico II University of Naples. Head of the laboratory P. Cappelletti. Code PG/2020/0064054.
- Marocco, A.; Liguori, B.; Dell’Agli, G.; Pansini, M. Sintering behaviour of celsian based ceramics obtained from the thermal conversion of (Ba, Sr)-exchanged zeolite A. J. Eur. Ceram. Soc. 2011, 31, 1965–1973. [Google Scholar] [CrossRef]
- Clayden, N.; Esposito, E.; Ferone, C.; Pansini, M. 27Al and 28Si NMR study of the thermal transformation of Ba-exchanged zeolite A into monoclinic celsian. J. Mater. Chem. 2003, 13, 1681–1685. [Google Scholar] [CrossRef]
- Italiana Zeolite Company Record (2020): Certificate of analysis performed by the Department of Earth Sciences, Environment and Resources Federico II University of Naples. Head of the laboratory P. Cappelletti. Code PG/20200055438.
- D’Auria, S. Impiego di Materiali Zeolitizzati per la Produzione di Prodotti Ceramici. Experimental Thesis in Applied Mineralogy, Universiti of Naples “Federico II”, Naples, Italy, 2006. [Google Scholar]
- De Gennaro, M.; Franco, E.; Langella, A.; Mirra, P.; Morra, V. Le phillipsiti dei tufi gialli del Napoletano. Period. Mineral. 1982, 51, 287–310. [Google Scholar]
- Italiana Zeolite Company Record: Certificate of analysis performed by the Department of Earth Sciences, Environment and Resources Federico II University of Naples. Head of the laboratory V. Morra. Code PG 2021/0012839.
- CLINOSA s.r.l. Report Tecnico per la Concessione Mineraria per la Coltivazione di Zeoliti; 2016; p. 59. [Google Scholar]
- Italiana Zeolite Company Record: Certificate of analysis performed by the Department of Earth Sciences, Environment and Resources Federico II University of Naples. Head of the Laboratory A. Colella. Code PG 2021/0012843.
- Italiana Zeolite Company Record: Certificate of analysis performed by the Department of Earth Sciences, Environment and Resources Federico II University of Naples. Head of the Laboratory A. Colella. Code PG 2021/0022739.
- Langella, A.; de Gennaro, M.; Pansini, M.; Colella, C.; Cantalini, C. Thermal Stability of Some Italian Natural Phillipsite; Proc. J. Med. C.A.T. 93; Balbi, N., Ed.; Université de Corse: Corte (Corse), France, 1993; pp. 229–232. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieve: Structure, Chemistry and Use; Wiley: New York, NY, USA, 1974; p. 771. [Google Scholar]
- Esposito, S.; Marocco, A.; Dell’Agli, G.; de Gennaro, B.; Pansini, M. Relationships between the Water Content of Zeolites and Their Cation Population. Microporous Mesoporous Mater. 2015, 202, 36–43. [Google Scholar] [CrossRef]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin, Germany, 1985; p. 410. [Google Scholar]
- Heap, M.J.; Farquharson, J.I.; Kushnar, A.R.L.; Lavallèe, Y.; Baud, P.; Gilg, H.A.; Reuschlé, T. The influence of water on the strength of Neapolitan Yellow Tuff, the most widely used building stones in Naples (Italy). Bull. Volcanol. 2018, 80, 1–15. [Google Scholar] [CrossRef]
- De Gennaro, M.; Incoronato, A.; Mastrolorenzo, G.; Adabbo, M.; Spina, G. Depositional mechanisms and alteration processes in different types of pyroclatic deposits from Campi Flegrei volcanic field (Souther Italy). J. Volcanol. Geotherm. Res. 1999, 91, 303–320. [Google Scholar] [CrossRef]




| Sample | Biotite | Feldspars | Clino-Pyroxene | Cristobalite | Clinoptilolite | Chabazite | Phillipsite | Analcime | Quarz | Amorphous and Clays | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 IC Dugenta | 3 (±1) | 21 (±3) | 4 (±1) | - | - | 34 (±2) | 16 (±2) | tr | - | 22 (±4) | [44] $ |
| 2 TF Piandirena | 2 (±2) | 16 (±3) | 5 (±1) | - | - | 52 (±4) | 4 (±1) | 3 (±1) | - | 18 (±2) | [45] $ |
| 3 IC Comiziano | 1 (±1) | 30 (±5) | 5 (±1) | - | - | 27 (±2) | 21 (±1) | 1 (±1) | - | 15 (±2) | [45] $ |
| 4 TG Chiaiano | 0, 2 | 23 (±4) | - | - | - | 8 (±2) | 55 (±2) | 3 (±1) | - | 12 (±2) | [46] $ |
| 5 TG Toledo | tr | 13 (±2) | - | - | - | 30 (±1) | 37 (±2) | 3 (±1) | - | 17 (±2) | [47] $ |
| 6 CL Anela | 2 (±1) | 10 (±2) | 6 (±2) | 58 (±2) | - | - | - | 7 (±1) | 17 (±4) | [48] $ | |
| 7 IC Grigia Faicchio | 1 (±2) | 93 (±3) | - | - | - | - | 6 (±2) | [41] |
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI § | Total | TG §§ | Ref. Bib. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 IC Dugenta | 54.80 | - | 15.90 | 3.65 | 0.12 | 1.18 | 4.57 | 1.25 | 5.24 | 0.13 | 12.58 | 99.42 | 14.20 | [51] |
| 2 TF Piandirena | 50.89 | 0.61 | 17.43 | 5.21 | - | 2.29 | 6.39 | 0.96 | 6.04 | - | 10.70 | 100.52 | 11.61 | [45] $ |
| 3 IC Comiziano | 54.52 | 0.45 | 15.19 | 4.00 | 0.17 | 0.88 | 4.14 | 1.04 | 6.98 | 0.10 | 12.53 | 99.88 | 10.21 | [52] $ |
| 4 TG Chiaiano | 52.08 | - | 16.52 | 3.50 | - | 1.02 | 2.71 | 2.06 | 8.67 | - | 13.72 | 100.28 | 13.47 | [53] $ |
| 5 TG Toledo | 50.70 | - | 16.97 | 3.47 | 0.09 | 1.23 | 3.63 | 2.60 | 7.21 | 0.12 | 14.17 | 100.50 | 14.32 | [54] |
| 6 CL Anela | 65.62 | - | 12.88 | 0.04 | - | 1.08 | 3.22 | 1.32 | 1.94 | - | 13.09 | 100.00 | 12.09 | [55] $ |
| 7 IC Grigia Faicchio | 60.23 | 0.45 | 18.65 | 4.05 | 0.22 | 0.54 | 1.87 | 4.56 | 7.54 | 0.11 | 1.85 | 100.08 | 2.40 | [41] |
| Sample | Open Porosity (%) | Reference |
|---|---|---|
| 1 IC Dugenta | 48.02 | [56] $ |
| 2 TF Piandirena | 48.54 | [56] $ |
| 3 IC Comiziano | 45.69 | [56] $ |
| 4 TG Chiaiano | 52.25 | [56] $ |
| 5 TG Toledo | 50.01 | [56] $ |
| 6 CL Anela | 35.87 | [56] $ |
| 7 IC Grigia Faicchio | 48.82 | [56] $ |
| Plaster | 44.63 | [57] $ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pansini, M.; Cappi, A.; Monetti, V.; Di Clemente, E.; de Gennaro, M.; D’Amore, M.; Buccino, R.; Santimone Nuzzi, P.; de Gennaro, B. Thermodilatometric Study of the Decay of Zeolite-Bearing Building Materials. Materials 2021, 14, 3551. https://doi.org/10.3390/ma14133551
Pansini M, Cappi A, Monetti V, Di Clemente E, de Gennaro M, D’Amore M, Buccino R, Santimone Nuzzi P, de Gennaro B. Thermodilatometric Study of the Decay of Zeolite-Bearing Building Materials. Materials. 2021; 14(13):3551. https://doi.org/10.3390/ma14133551
Chicago/Turabian StylePansini, Michele, Angelo Cappi, Vincenzo Monetti, Enrico Di Clemente, Maurizio de Gennaro, Marco D’Amore, Rosa Buccino, Pierpaolo Santimone Nuzzi, and Bruno de Gennaro. 2021. "Thermodilatometric Study of the Decay of Zeolite-Bearing Building Materials" Materials 14, no. 13: 3551. https://doi.org/10.3390/ma14133551









