Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Mixed Double Flow Focusing Nozzle
2.2. Computational Model
2.2.1. Governing Equations
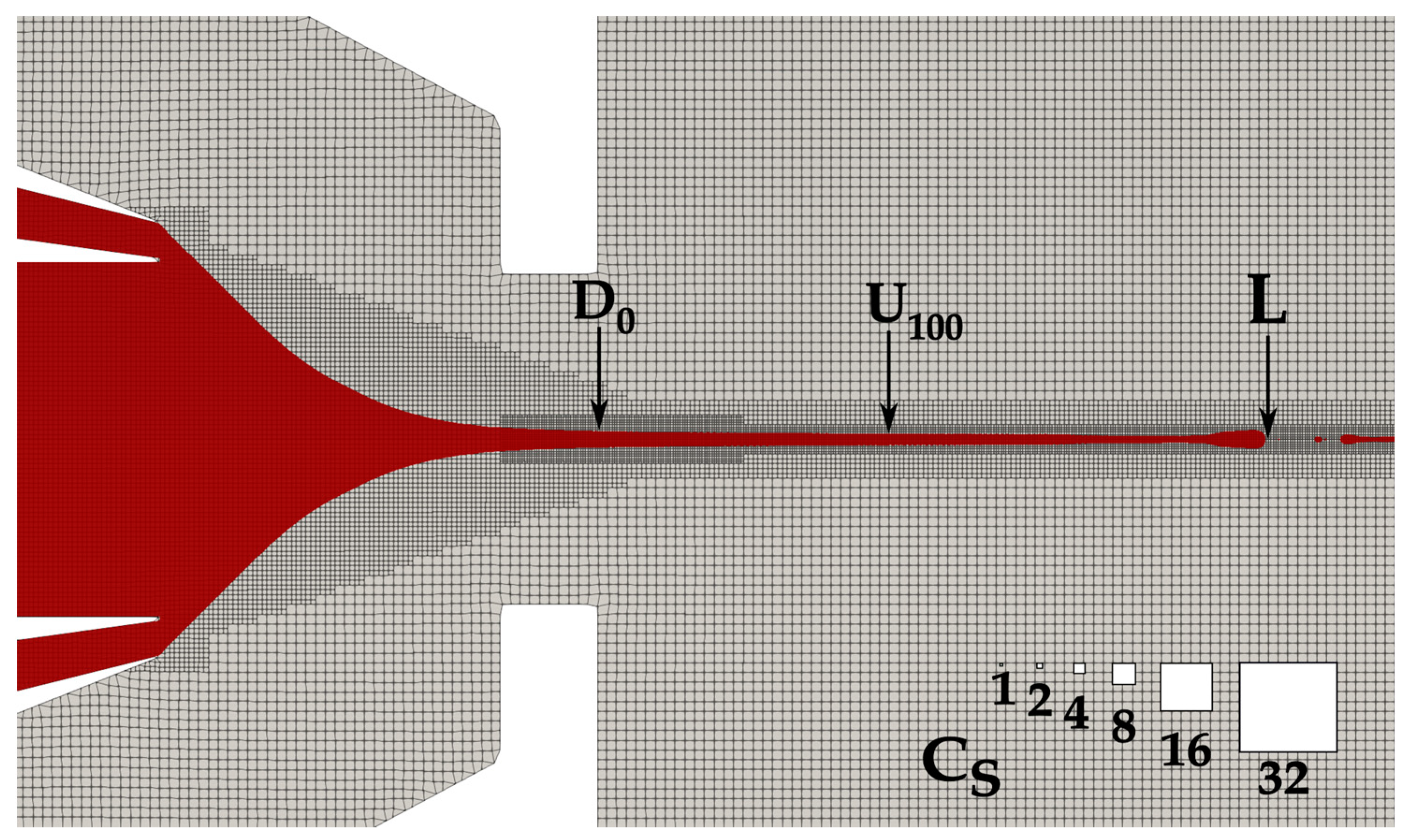
2.2.2. Computational Domain and Boundary Conditions
2.2.3. Solution Procedure and Computer Hardware
2.2.4. Liquid Properties
3. Results
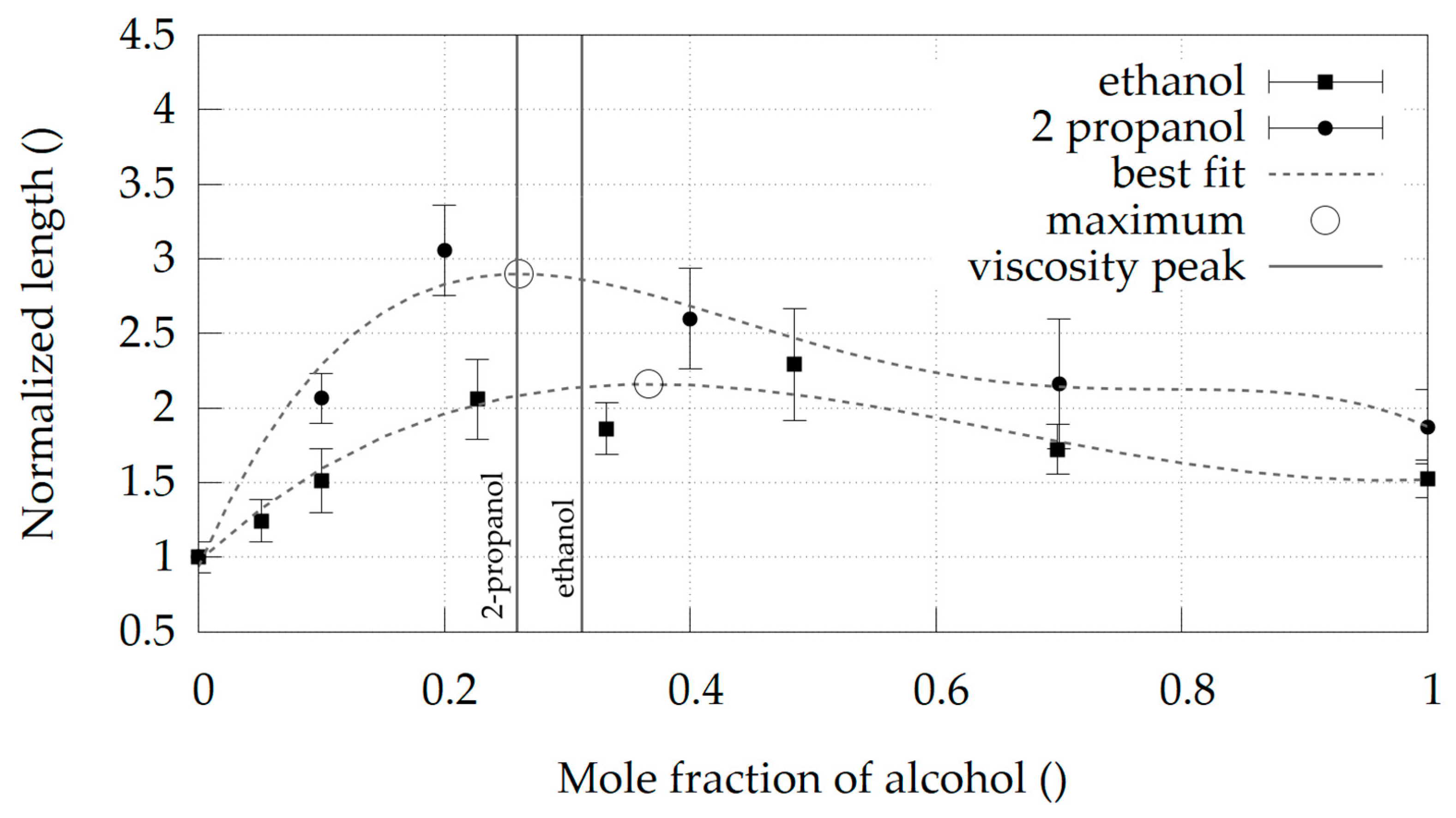
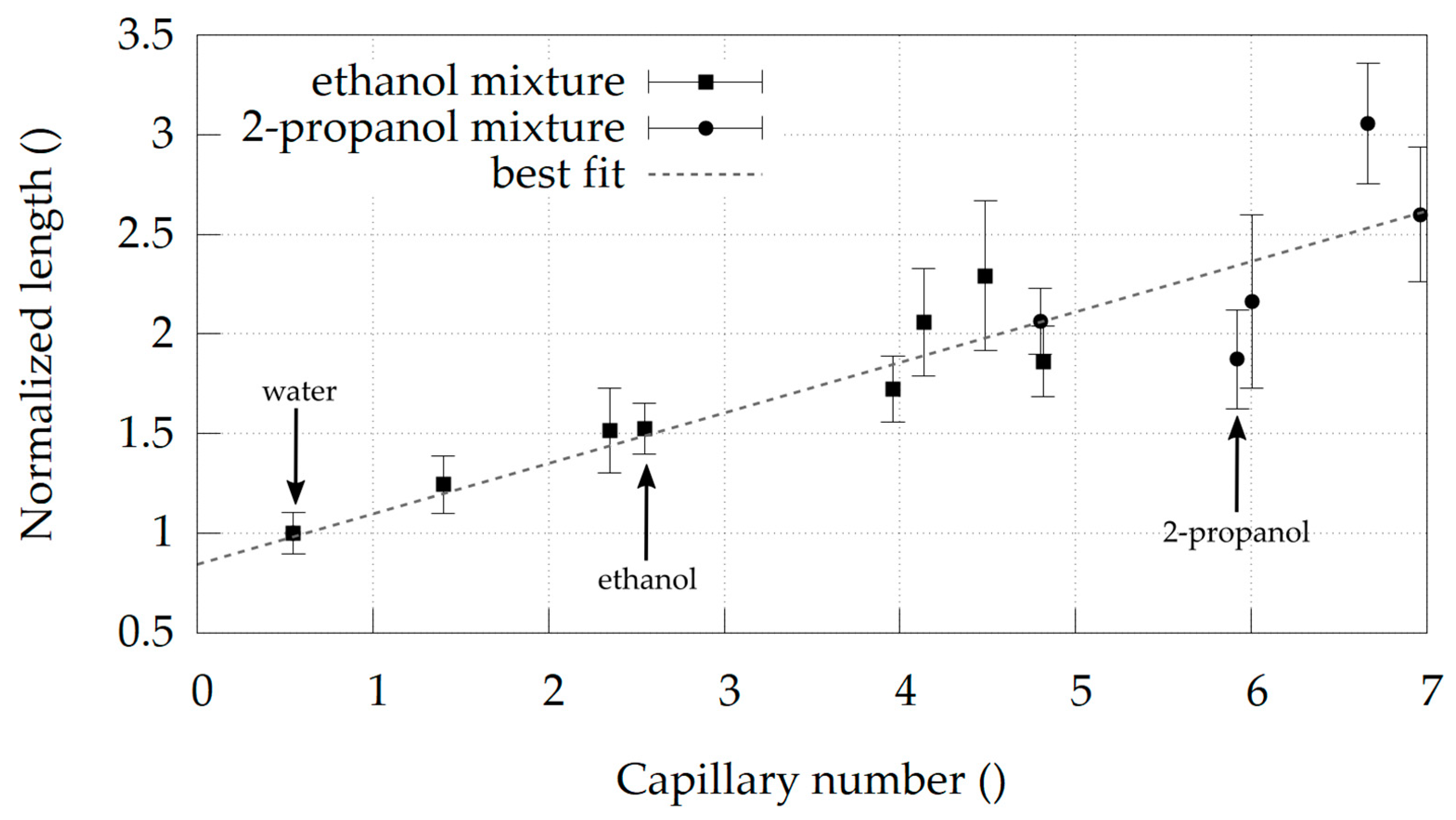
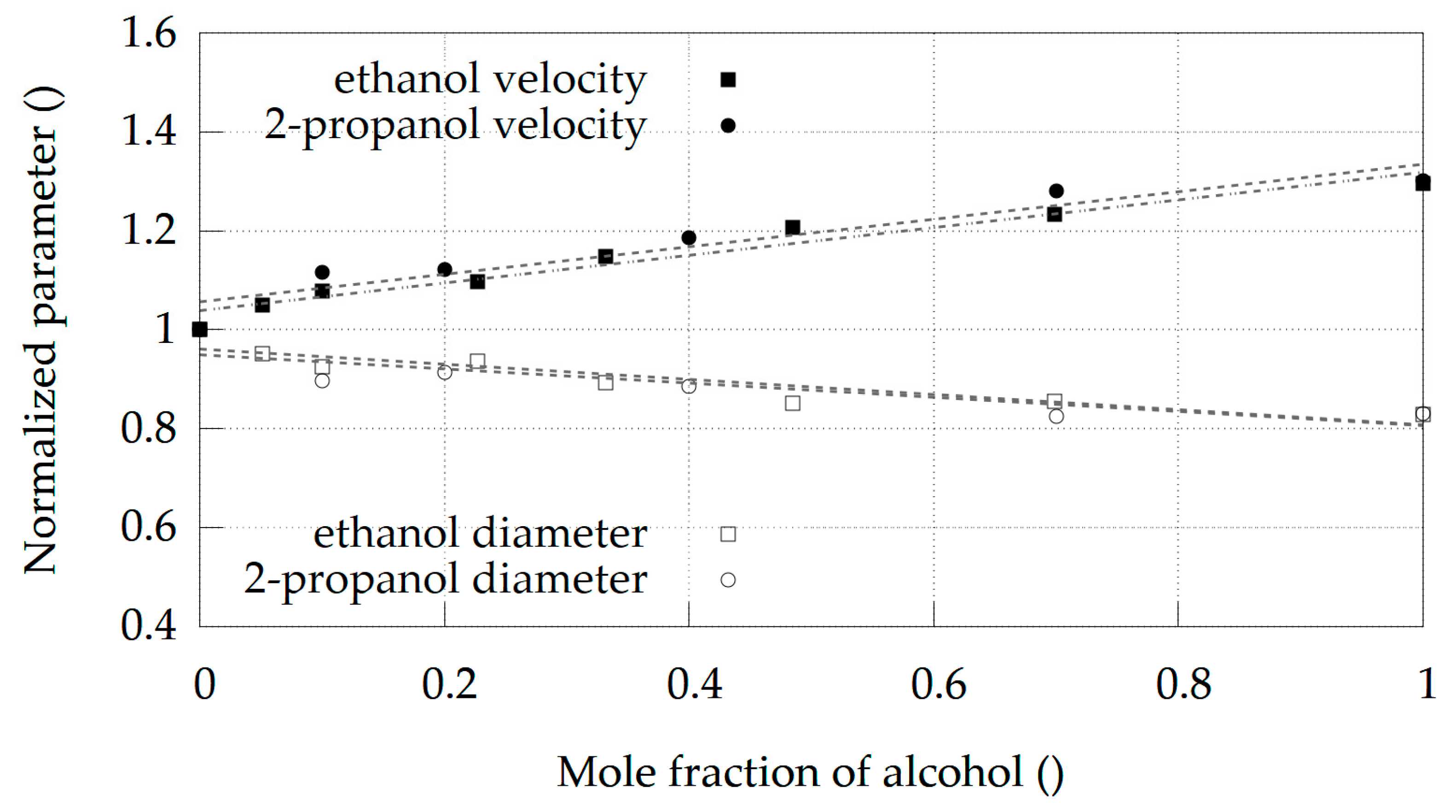
Water + Ethanol and Water + 2-Propanol Mixtures
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S. Microfluidics and Microfabrication; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9781441915429. [Google Scholar]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Boutet, S.; Fromme, P.; Mark, S.H. X-ray Free Electron Lasers; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783030005504. [Google Scholar]
- Oberthuer, D.; Knoska, J.; Wiedorn, M.O.; Beyerlein, K.R.; Bushnell, D.A.; Kovaleva, E.G.; Heymann, M.; Gumprecht, L.; Kirian, R.A.; Barty, A.; et al. Double-flow focused liquid injector for efficient serial femtosecond crystallography. Sci. Rep. 2017, 7, 44628. [Google Scholar] [CrossRef]
- Herrada, M.A.; Ganan-Calvo, A.M.; Ojeda-Monge, A.; Bluth, B.; Riesco-Chueca, P. Liquid flow focused by a gas: Jetting, dripping, and recirculation. Phys. Rev. E 2008, 78, 036323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wu, L.; Zhao, Y.; Chen, Y. Study of compound drop formation in axisymmetric microfluidic devices with different geometries. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 87–98. [Google Scholar] [CrossRef]
- Dupin, M.M.; Halliday, I.; Care, C.M. Simulation of a microfluidic flow-focusing device. Phys. Rev. E 2006, 73, 055701. [Google Scholar] [CrossRef]
- Ganan-Calvo, A.M. Generation of Steady Liquid Microthreads and Micron-Sized Monodisperse Sprays in Gas Streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Zahoor, R.; Belšak, G.; Bajt, S.; Šarler, B. Simulation of liquid micro-jet in free expanding high-speed co-flowing gas streams. Microfluid. Nanofluid. 2018, 22, 87. [Google Scholar] [CrossRef]
- Zahoor, R.; Regvar, R.; Bajt, S.; Šarler, B. A numerical study on the influence of liquid properties on gas-focused micro-jets. Prog. Comput. Fluid Dyn. Int. J. 2019, 1, 1. [Google Scholar] [CrossRef]
- Zahoor, R.; Bajt, S.; Šarler, B. Numerical investigation on influence of focusing gas type on liquid micro-jet characteristics. Int. J. Hydromechatron. 2018, 1, 222. [Google Scholar] [CrossRef]
- Zahoor, R.; Bajt, S.; Šarler, B. Influence of Gas Dynamic Virtual Nozzle Geometry on Micro-Jet Characteristics. Int. J. Multiph. Flow 2018, 104, 152–165. [Google Scholar] [CrossRef]
- Šarler, B.; Zahoor, R.; Bajt, S. Alternative Geometric Arrangements of the Nozzle Outlet Orifice for Liquid Micro-Jet Focusing in Gas Dynamic Virtual Nozzles. Materials 2021, 14, 1572. [Google Scholar] [CrossRef]
- Van Vu, T.; Homma, S.; Wells, J.C.; Takakura, H.; Tryggvason, G. Numerical Simulation of Formation and Breakup of a Three-Fluid Compound Jet. J. Fluid Sci. Technol. 2011, 6, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Knoška, J.; Adriano, L.; Awel, S.; Beyerlein, K.R.; Yefanov, O.; Oberthuer, D.; Murillo, G.E.P.; Roth, N.; Sarrou, I.; Villanueva-Perez, P.; et al. Ultracompact 3D microfluidics for time-resolved structural biology. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Kirian, R.A.; Weierstall, U.; Zatsepin, N.A.; Faragó, T.; Baumbach, T.; Wilde, F.; Niesler, F.B.P.; Zimmer, B.; Ishigami, I.; et al. Three-dimensional-printed gas dynamic virtual nozzles for x-ray laser sample delivery. Opt. Express 2016, 24, 11515–11530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-J. Numerical Heat Transfer and Fluid Flow. Nucl. Sci. Eng. 1981, 78, 196–197. [Google Scholar] [CrossRef]
- Hirt, C.W.; Nichols, B.D. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Roenby, J.; Bredmose, H.; Jasak, H. A computational method for sharp interface advection. R. Soc. Open Sci. 2016, 3, 160405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A tensorial approach to computational continuum mechanics using object-oriented techniques. Comput. Phys. 1998, 12, 620–631. [Google Scholar] [CrossRef]
- Brackbill, J.U.; Kothe, D.B.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Courant, R.; Friedrichs, K.; Lewy, H. On the Partial Difference Equations of Mathematical Physics. IBM J. Res. Dev. 1967, 11, 215–234. [Google Scholar] [CrossRef]
- Datta, B.N. Numerical Linear Algebra and Applications; SIAM: Philadelphia, PA, USA, 2010. [Google Scholar]
- Issa, R.I. Solution of the implicitly discretized fluid flow equations by operator-splitting. J. Comput. Phys. 1986, 62, 40–65. [Google Scholar] [CrossRef]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar] [CrossRef]
- Matvienko, A.; Mandelis, A. Ultrahigh-resolution pyroelectric thermal-wave technique for the measurement of thermal diffusivity of low-concentration water-alcohol mixtures. Rev. Sci. Instrum. 2005, 76, 104901. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol+ Water from 20 to 50 °C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Pang, F.M.; Seng, C.E.; Teng, T.T.; Ibrahim, M.H. Densities and viscosities of aqueous solutions of 1-propanol and 2-propanol at temperatures from 293.15 K to 333.15 K. J. Mol. Liq. 2007, 136, 71–78. [Google Scholar] [CrossRef]
- Arnaud, R.; Avedikian, L.; Morel, J.P. Chaleurs molaires de quelques mélanges hydroorganiques riches en eau. J. Chim. Phys. 1972, 69, 45–49. [Google Scholar] [CrossRef]

| Quantity | Reg | Rel | We | Q | µR | ρR |
|---|---|---|---|---|---|---|
| Minimum | 125.96 | 12.17 | 102.21 | 0.46 | 52.63 | 4741.4 |
| Maximum | 127.2 | 79.67 | 843.50 | 0.59 | 157.63 | 6009.6 |
| Xi | Surface Tension (N/m) ×10−3 | Density (kg/m3) | Dynamic Viscosity (kg/ms) ×10−3 | Molar Weight (kg/mol) | Specific Heat (W/mK) | Prandtl Number |
|---|---|---|---|---|---|---|
| 0 | 72.800 | 1000.000 | 1.000 | 18.000 | 4184 | 6.080 |
| 0.051 | 47.961 | 981.445 | 1.634 | 19.412 | 3966 | 13.083 |
| 0.100 | 39.443 | 967.026 | 2.181 | 20.792 | 3748 | 19.821 |
| 0.154 | 33.521 | 951.516 | 2.593 | 22.311 | 3530 | 26.447 |
| 0.227 | 31.743 | 931.372 | 2.903 | 24.366 | 3312 | 32.938 |
| 0.332 | 28.896 | 904.432 | 2.987 | 27.340 | 3094 | 37.440 |
| 0.485 | 26.410 | 871.692 | 2.671 | 31.606 | 2876 | 36.705 |
| 0.699 | 24.862 | 837.380 | 1.997 | 37.603 | 2658 | 29.722 |
| 1.000 | 22.300 | 789.000 | 1.120 | 46.000 | 2440 | 19.860 |
| Xi | Surface Tension (N/m) ×10−3 | Density (kg/m3) | Dynamic Viscosity (kg/ms) ×10−3 | Molar Weight (kg/mol) | Specific Heat (W/mK) | Prandtl Number |
|---|---|---|---|---|---|---|
| 0 | 72.800 | 1000.000 | 1.000 | 18.000 | 4184 | 6.080 |
| 0.100 | 27.369 | 956.672 | 2.947 | 22.223 | 4433 | 18.680 |
| 0.200 | 24.776 | 922.029 | 3.692 | 26.431 | 3941 | 24.226 |
| 0.400 | 23.869 | 864.935 | 3.514 | 34.847 | 3426 | 24.622 |
| 0.700 | 22.507 | 809.778 | 2.643 | 47.471 | 2989 | 19.778 |
| 1.000 | 21.146 | 791.200 | 2.411 | 60.095 | 2552 | 18.468 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belšak, G.; Bajt, S.; Šarler, B. Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions. Materials 2021, 14, 3614. https://doi.org/10.3390/ma14133614
Belšak G, Bajt S, Šarler B. Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions. Materials. 2021; 14(13):3614. https://doi.org/10.3390/ma14133614
Chicago/Turabian StyleBelšak, Grega, Saša Bajt, and Božidar Šarler. 2021. "Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions" Materials 14, no. 13: 3614. https://doi.org/10.3390/ma14133614
APA StyleBelšak, G., Bajt, S., & Šarler, B. (2021). Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions. Materials, 14(13), 3614. https://doi.org/10.3390/ma14133614







