ZnO Films Incorporation Study on Macroporous Silicon Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Macroporous Silicon Substrates
2.2. Deposition of ZnO Thin Films by the Sol–Gel Spin Coating Method
2.3. Deposition of ZnO Thin Films by the USP Method
2.4. Characterization of ZnO Thin Films
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526. [Google Scholar] [CrossRef]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% Efficient Perovskite Solar Cell Based on ZnO Nanorods: An Effective Charge Collection System. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Kong, X.; Gao, Z.; Han, L.; Li, X. Enhanced electrical properties of ZnO transparent conducting films prepared by electron beam annealing. Appl. Surf. Sci. 2018, 428, 191–198. [Google Scholar] [CrossRef]
- Liu, X.; Pan, K.; Li, W.; Hu, D.; Liu, S.; Wang, Y. Optical and gas sensing properties of Al-doped ZnO transparent conducting films prepared by sol–gel method under different heat treatments. Ceram. Int. 2014, 40, 9931–9939. [Google Scholar] [CrossRef]
- Pan, M.-C.; Wu, T.-H.; Bui, T.-A.; Shih, W.-C. Fabrication of highly c-axis textured ZnO thin films piezoelectric transducers by RF sputtering. J. Mater. Sci. Mater. Electron. 2012, 23, 418–424. [Google Scholar] [CrossRef]
- Panda, D.; Simanjuntak, F.M.; Chandrasekaran, S.; Pattanayak, B.; Singh, P.; Tseng, T.-Y. Barrier Layer Induced Switching Stability in Ga:ZnO Nanorods Based Electrochemical Metallization Memory. IEEE Trans. Nanotechnol. 2020, 19, 764–768. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, X.; Luo, J.; Li, Y.; Jin, H.; Dong, S.; Luo, J.; Duan, H.; Fu, Y. Nebulization using ZnO/Si surface acoustic wave devices with focused interdigitated transducers. Surf. Coat. Technol. 2019, 367, 127–134. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Aydemir, G.; Utlu, G.; Çetinel, A. Growth and characterization of ZnO nanostructures on porous silicon substrates: Effect of solution temperature. Chem. Phys. Lett. 2019, 737, 136827. [Google Scholar] [CrossRef]
- Ali, G.G.; Sulaiman, A.K.A.; Karomi, I.B. The effect of the ZnO thickness layer on the porous silicon properties deposited by chemical vapor deposition. AIP Conf. Proc. 2018, 2034, 020009. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Eswar, K.A.; Rouhi, J.; Husairi, F.; Dalvand, R.; Alrokayan, S.A.; Khan, H.A.; Mahmood, M.R.; Abdullah, S. Hydrothermal growth of flower-like ZnO nanostructures on porous silicon substrate. J. Mol. Struct. 2014, 1074, 140–143. [Google Scholar] [CrossRef]
- Martínez, L.; Holguín-Momaca, J.; Karthik, T.; Olive-Méndez, S.; Campos-Alvarez, J.; Agarwal, V. Sputtering temperature dependent growth kinetics and CO2 sensing properties of ZnO deposited over porous silicon. Superlattices Microstruct. 2016, 98, 8–17. [Google Scholar] [CrossRef]
- Znaidi, L.; Chauveau, T.; Tallaire, A.; Liu, F.; Rahmani, M.; Bockelee, V.; Vrel, D.; Doppelt, P. Textured ZnO thin films by sol–gel process: Synthesis and characterizations. Thin Solid Films 2016, 617, 156–160. [Google Scholar] [CrossRef]
- Flores, G.; Carrillo, J.; Luna, J.; Martinez, R.M.; Sierra-Fernandez, A.; Milosevic, O.; Rabanal, M. Synthesis, characterization and photocatalytic properties of nanostructured ZnO particles obtained by low temperature air-assisted-USP. Adv. Powder Technol. 2014, 25, 1435–1441. [Google Scholar] [CrossRef]
- Nayef, U.M.; Muayad, M.W.; Khalaf, H.A. Effect of ZnO layers on Porous silicon properties. Int. J. Electrochem. Sci. 2014, 9, 2278–2284. [Google Scholar]
- Karthik, T.; Martinez, L.; Agarwal, V. Porous silicon ZnO/SnO2 structures for CO2 detection. J. Alloy. Compd. 2018, 731, 853–863. [Google Scholar] [CrossRef]
- Shabannia, R. Vertically aligned ZnO nanorods on porous silicon substrates: Effect of growth time. Prog. Nat. Sci. 2015, 25, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.; Aswal, D.; Gupta, S. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Li, C.-F.; Hsu, C.-Y.; Li, Y.-Y. NH3 sensing properties of ZnO thin films prepared via sol–gel method. J. Alloy. Compd. 2014, 606, 27–31. [Google Scholar] [CrossRef]
- Colmenares, N.; Correr, W.; Mastelaro, V.R. Deposition Rate Influence in O3 Sensing Response of Sputtered ZnO Thin Films. Proceedings 2017, 1, 429. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.D.; Patil, G.; Kajale, D.; Gaikwad, V.; Jain, G. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloy. Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Kaur, M.; Sharma, P.K.; Datta, N.; Kailasaganapathi, S.; Bhattacharya, S.; Debnath, A.; Aswal, D.; Gupta, S. Ethanol sensing properties of pure and Au modified ZnO nanowires. Sens. Actuators B Chem. 2013, 187, 313–318. [Google Scholar] [CrossRef]
- Cheng, I.-K.; Lin, C.-Y.; Pan, F.-M. Gas sensing behavior of ZnO toward H2 at temperatures below 300 °C and its dependence on humidity and Pt-decoration. Appl. Surf. Sci. 2021, 541, 148551. [Google Scholar] [CrossRef]
- Kumar, Y.; Herrera-Zaldivar, M.; Singh, F.; Olive-Méndez, S.F.; Kanjilal, D.; Kumar, S.; Agarwal, V. Cathodoluminescence and photoluminescence of swift ion irradiation modified zinc oxide-porous silicon nanocomposite. Mater. Sci. Eng. B 2012, 177, 1476–1481. [Google Scholar] [CrossRef]
- Mizsei, J. Gas sensor applications of porous Si layers. Thin Solid Films 2007, 515, 8310–8315. [Google Scholar] [CrossRef]
- Singh, P.; Simanjuntak, F.M.; Wu, Y.-C.; Kumar, A.; Zan, H.-W.; Tseng, T.-Y. Sensing performance of gas sensors fabricated from controllably grown ZnO-based nanorods on seed layers. J. Mater. Sci. 2020, 55, 8850–8860. [Google Scholar] [CrossRef]
- Leonardi, S.G. Two-Dimensional Zinc Oxide Nanostructures for Gas Sensor Applications. Chemosensors 2017, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Ureña, F.; Olsen, S.H.; Escobedo-Cousin, E.; Minamisawa, R.A.; Raskin, J.-P. Roughness analysis in strained silicon-on-insulator wires and films. J. Appl. Phys. 2014, 116, 124503. [Google Scholar] [CrossRef] [Green Version]
- Rosli, N.; Halim, M.M.; Chahrour, K.M.; Hashim, R. Incorporation of Zinc Oxide on Macroporous Silicon Enhanced the Sensitivity of Macroporous Silicon MSM Photodetector. ECS J. Solid State Sci. Technol. 2020, 9, 105005. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Hamid, M.A.A.; Shamsudin, R.; Al-Khalqi, E.M.; Keng, L.K.; Ahmed, N.M. Electrochemical Hydrogen Peroxide Sensor Based on Macroporous Silicon. Sensors 2018, 18, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, L.; Li, X.; Shen, X.; Wang, A. Effect of aging time of ZnO sol on the structural and optical properties of ZnO thin films prepared by sol–gel method. Appl. Surf. Sci. 2010, 256, 4543–4547. [Google Scholar] [CrossRef]
- Tangirala, V.K.; Vargas, I.R.; Zanabria, A.G.H.; Martínez-Ayala, L.; Ruíz, H.D.M. Diseño y fabricación del equipo de rocío químico asistido por ultra sonicación. TEPEXI Boletín Científico de la Escuela Superior Tepeji del Río 2019, 6, 14–19. [Google Scholar] [CrossRef]
- Bedia, A.; Bedia, F.Z.; Aillerie, M.; Maloufi, N.; Benyoucef, B. Influence of the Thickness on Optical Properties of Sprayed ZnO Hole-blocking Layers Dedicated to Inverted Organic Solar Cells. Energy Procedia 2014, 50, 603–609. [Google Scholar] [CrossRef]
- Gadallah, A.-S.; El-Nahass, M.M. Structural, Optical Constants and Photoluminescence of ZnO Thin Films Grown by Sol-Gel Spin Coating. Adv. Condens. Matter Phys. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.J.; Ravichandran, K. Effect of source material on the transparent conducting properties of sprayed ZnO:Al thin films for solar cell applications. J. Phys. Chem. Solids 2013, 74, 841–848. [Google Scholar] [CrossRef]
- Suchea, M.; Christoulakis, S.; Moschovis, K.; Katsarakis, N.; Kiriakidis, G. ZnO transparent thin films for gas sensor applications. Thin Solid Films 2006, 515, 551–554. [Google Scholar] [CrossRef]
- Khan, Z.R.; Khan, M.S.; Zulfequar, M. Optical and Structural Properties of ZnO Thin Films Fabricated by Sol-Gel Method. Mater. Sci. Appl. 2011, 2, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Shakti, N.; Gupta, P. Structural and Optical Properties of Sol-gel Prepared ZnO Thin Film. Appl. Phys. Res. 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Sorescu, D.C.; Deng, X. Tunable Lattice Constant and Band Gap of Single- and Few-Layer ZnO. J. Phys. Chem. Lett. 2016, 7, 1335–1340. [Google Scholar] [CrossRef]
- Singh, S.; Thiyagarajan, P.; Mohan Kant, D.; Anita, D.; Thirupathiah, S.; Tiwari, B.; Kottaisamy, M.; Ramachandra Rao, M.S. Structure, microstructure and physical properties of ZnO based materials in various forms: Bulk, thin film and nano. Phys. D App. Phys. 2007, 40, 6312–6327. [Google Scholar] [CrossRef]
- Chen, J.; Sun, K. Growth of vertically aligned ZnO nanorod arrays as antireflection layer on silicon solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 930–934. [Google Scholar] [CrossRef]
- Nesheva, D.; Dzhurkov, V.; Stambolova, I.; Blaskov, V.; Bineva, I.; Moreno, J.M.C.; Preda, S.; Gartner, M.; Hristova-Vasileva, T.; Shipochka, M. Surface modification and chemical sensitivity of sol gel deposited nanocrystalline ZnO films. Mater. Chem. Phys. 2018, 209, 165–171. [Google Scholar] [CrossRef]
- Taabouche, A.; Bouabellou, A.; Kermiche, F.; Hanini, F.; Sedrati, C.; Bouachiba, Y.; Benazzouz, C. Preparation and characterization of Al-doped ZnO piezoelectric thin films grown by pulsed laser deposition. Ceram. Int. 2016, 42, 6701–6706. [Google Scholar] [CrossRef]
- Velázquez-Nevárez, G.A.; Vargas-García, J.R.; Aguilar-Hernández, J.; Vega-Becerra, O.E.; Chen, F.; Shen, Q.; Zhang, L. Optical and Electrical Properties of (002)-Oriented ZnO Films Prepared on Amorphous Substrates by Sol-Gel Spin-Coating. Mater. Res. 2016, 19, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Fitriana, F.; Septiani, N.L.W.; Irzaman, I.; Ferdiansjah, F.; Fahmi, M.Z.; Adhika, D.R.; Suyatman, S.; Nugraha, N.; Yuliarto, B. Preparation of (002)-oriented ZnO for CO gas sensor. Mater. Res. Express 2019, 6, 064003. [Google Scholar] [CrossRef]
- Miranda, M.A.R.; Sasaki, J.M. The limit of application of the Scherrer equation. Acta Crystallogr. Acta Crystallogr. Sect. A Found. Crystallogr. 2018, A74, 54–65. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction: A Practical Approach; Plenum Press Publishing: New York, NY, USA, 2013; pp. 3–19. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Shen, H.; Yin, Y.; Lu, L.; Shen, J.; Tang, Z. The effects of porous silicon on the crystalline properties of ZnO thin films. J. Phys. Chem. Solids 2009, 70, 967–971. [Google Scholar] [CrossRef]
- Hur, T.-B.; Hwang, Y.-H.; Kim, H.-K.; Lee, I.J. Strain effects in ZnO thin films and nanoparticles. J. Appl. Phys. 2006, 99, 064308. [Google Scholar] [CrossRef]
- Ghosh, R.; Basak, D.; Fujihara, S. Effect of substrate-induced strain on the structural, electrical, and optical properties of polycrystalline ZnO thin films. J. Appl. Phys. 2004, 96, 2689–2692. [Google Scholar] [CrossRef]
- Bueno, C.; Pacio, M.; Osorio, E.; Perez, R.; Juarez, H. Effect of annealing atmosphere on optic-electric properties of ZnO thin films. Rev. Mex. Fis. 2017, 63, 569–574. [Google Scholar]
- Xu, J.; Xue, Z.; Qin, N.; Cheng, Z.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuators B Chem. 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; De Leeuw, N.H. Structural and Optical Properties of ZnO Thin Films Prepared by Molecular Precursor and Sol–Gel Methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, M.; Nagaraja, H. Effect of current density on morphological, structural and optical properties of porous silicon. Mater. Today Chem. 2017, 3, 10–14. [Google Scholar] [CrossRef]
- Rahmani, A.; Remache, L.; Guendouz, M.; Aida, M.S.; Hebboul, Z. Impact of the meso-PSi substrate on ZnO thin films deposited by spray pyrolysis technique for UV photodetectors. Appl. Phys. A 2021, 127, 1–10. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, B.; Yadav, P.; Bhatt, V.; Kumar, M.; Singh, K.; Abhyankar, A.; Kumar, A.; Yun, J.-H. Effect of structural defects, surface roughness on sensing properties of Al doped ZnO thin films deposited by chemical spray pyrolysis technique. Ceram. Int. 2016, 43, 3562–3568. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Fan, K.-S.; Chen, S.-H.; Tsai, C.-H. Preparation and characterization of ZnO transparent semiconductor thin films by sol–gel method. J. Alloy. Compd. 2010, 495, 126–130. [Google Scholar] [CrossRef]
- Salazar-Kuri, U.; Antúnez, E.; Estevez, J.; Olive-Méndez, S.F.; Silva-González, N.; Agarwal, V. Formation of different micro-morphologies from VO 2 and ZnO crystallization using macro-porous silicon substrates. J. Phys. Chem. Solids 2016, 104, 21–31. [Google Scholar] [CrossRef]
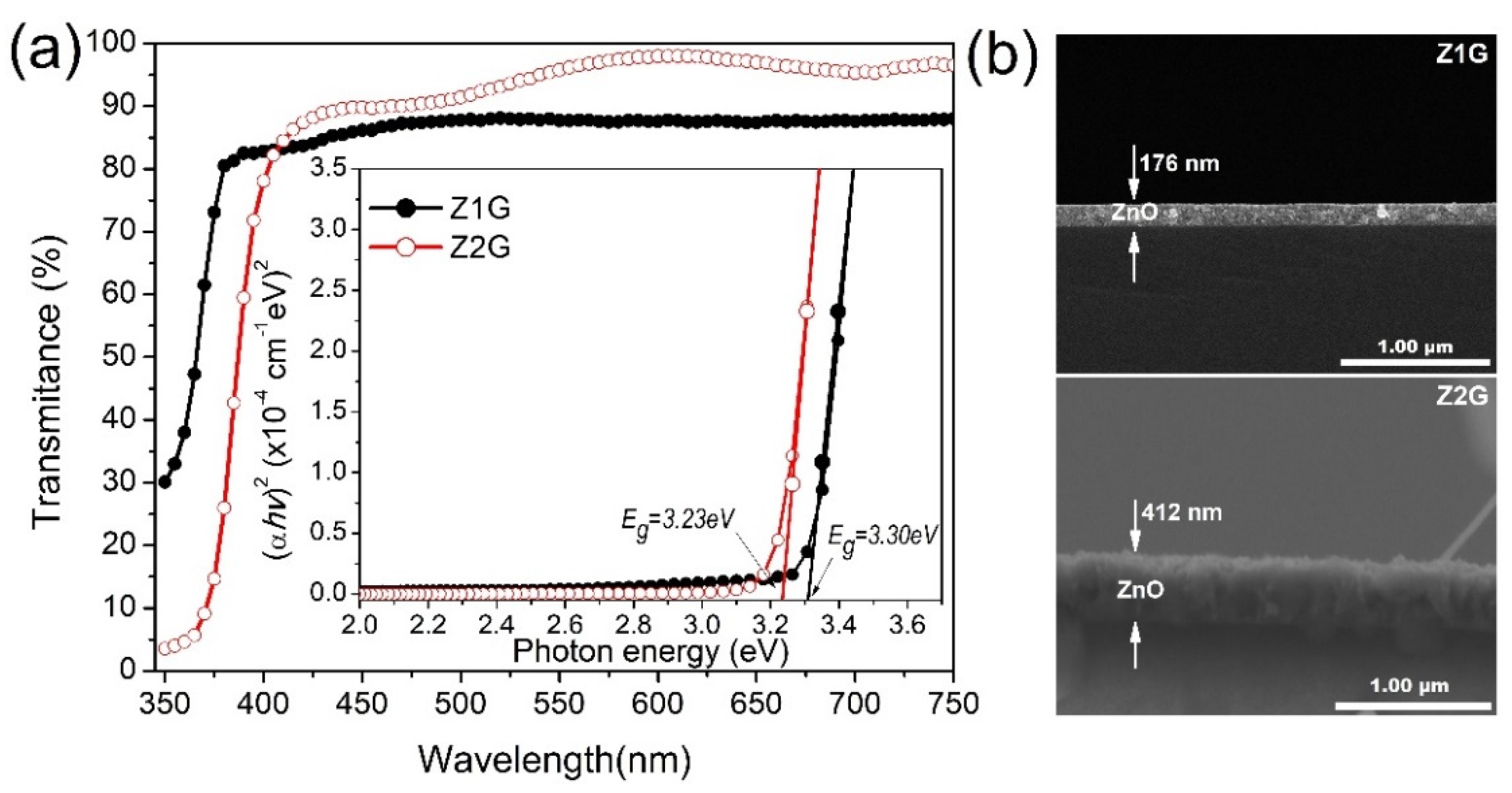
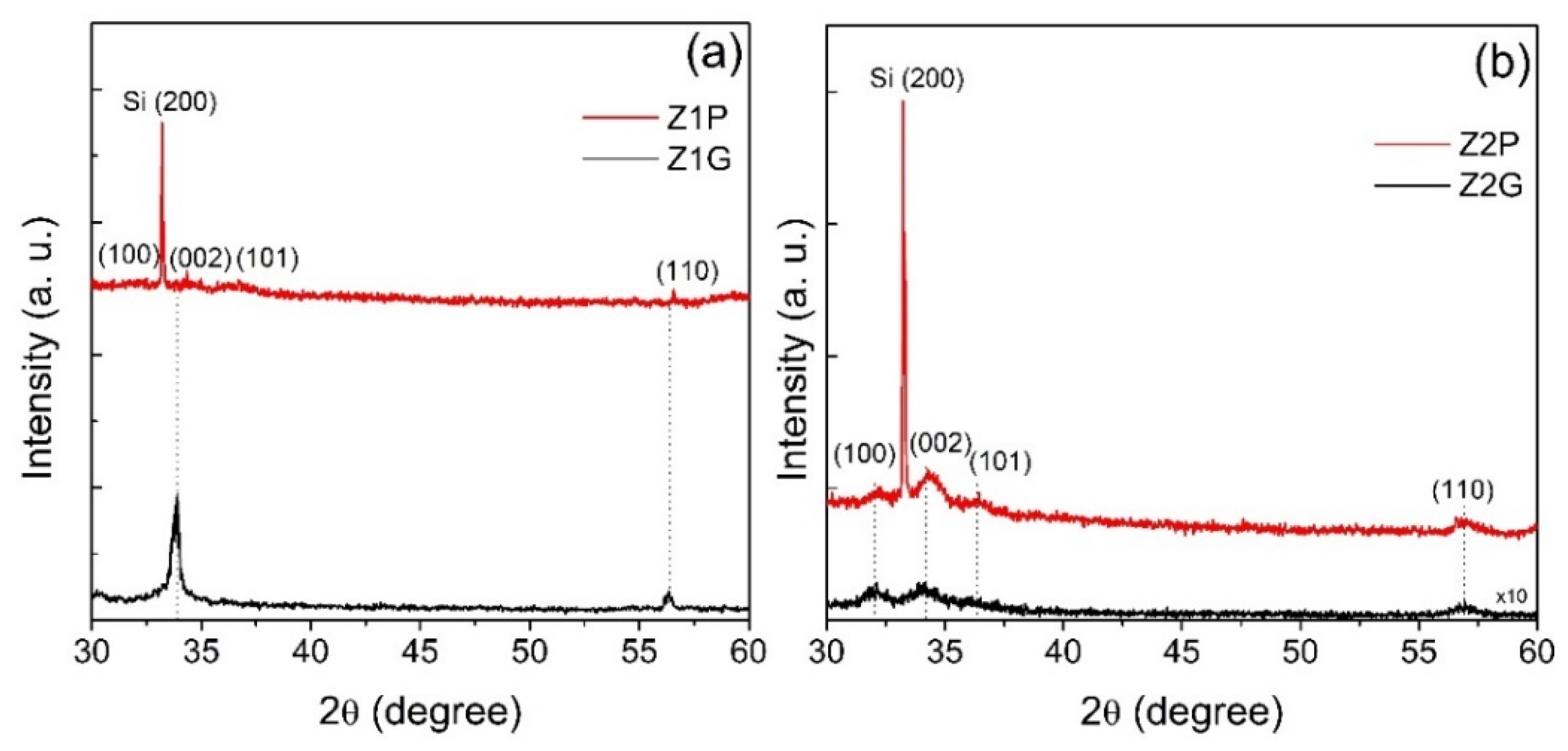
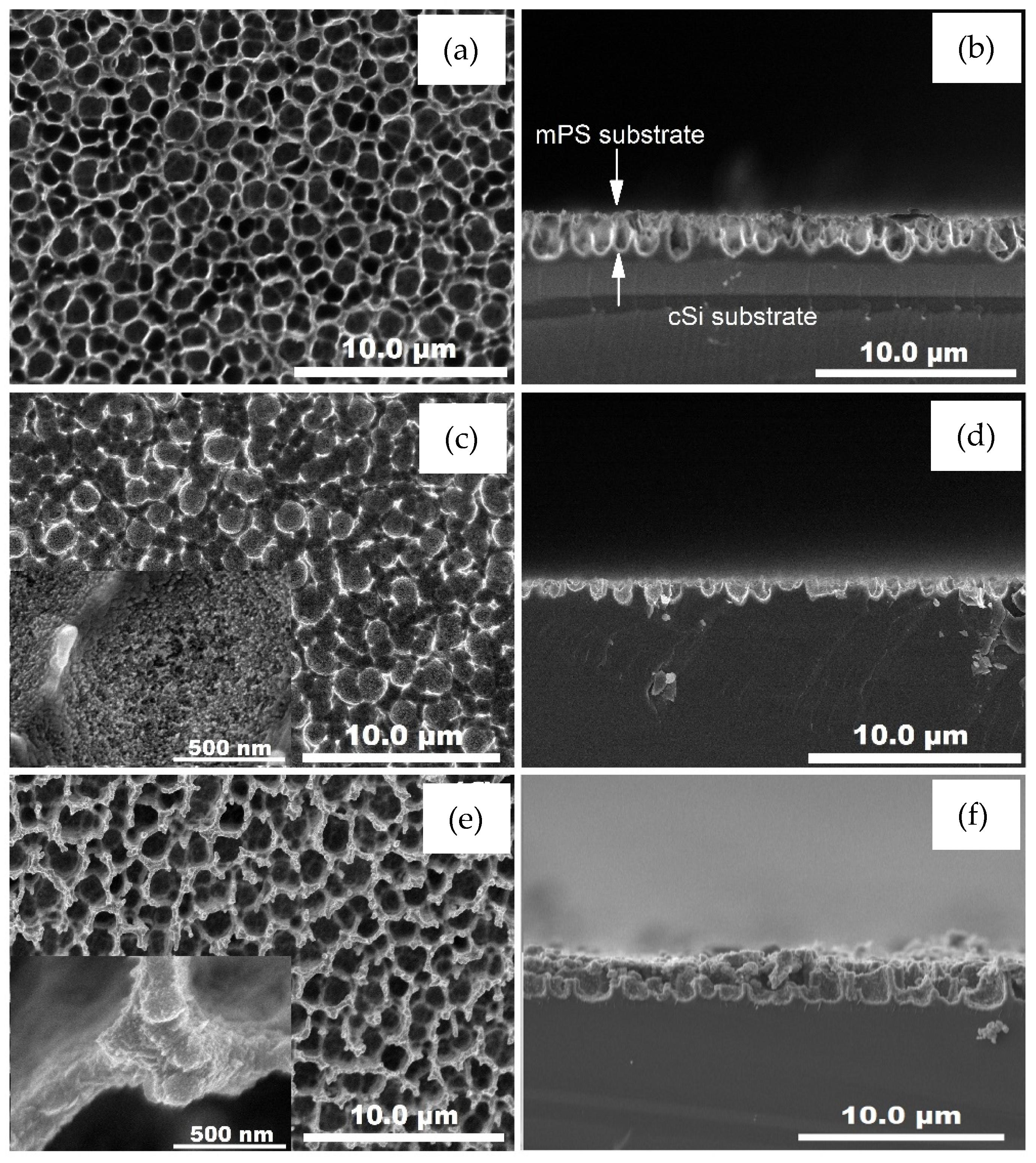
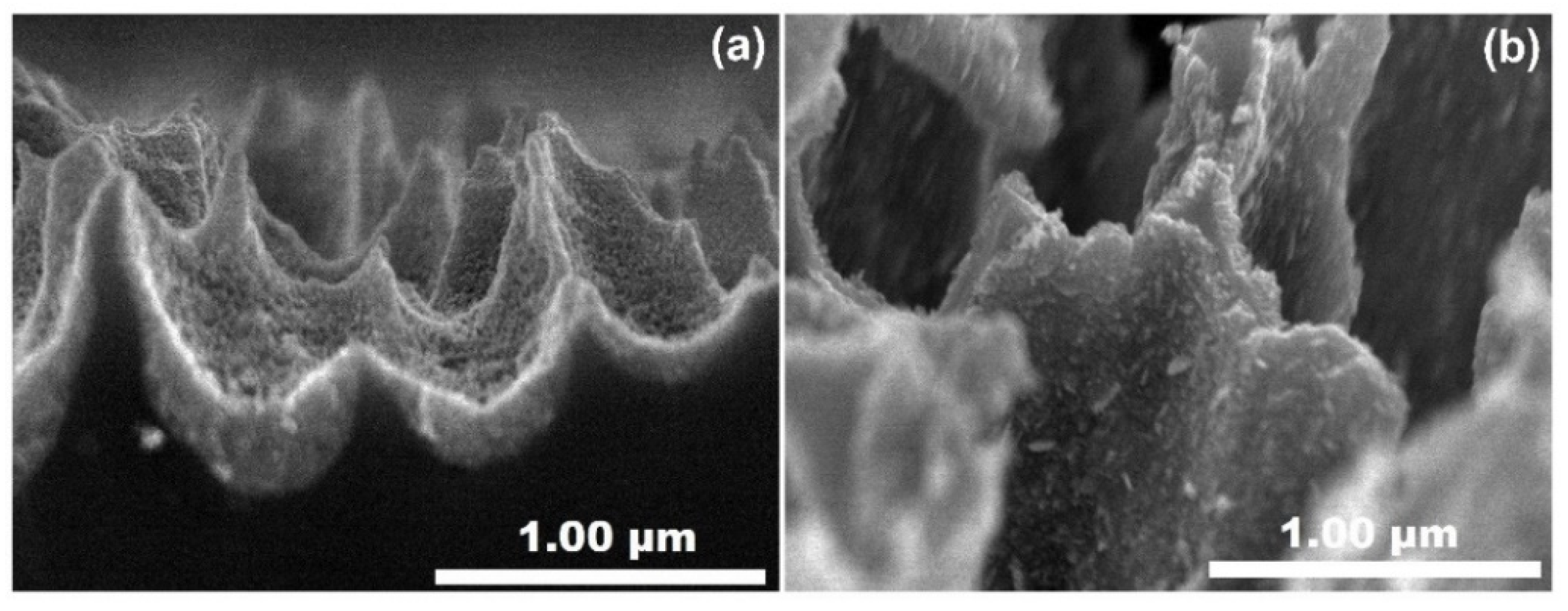
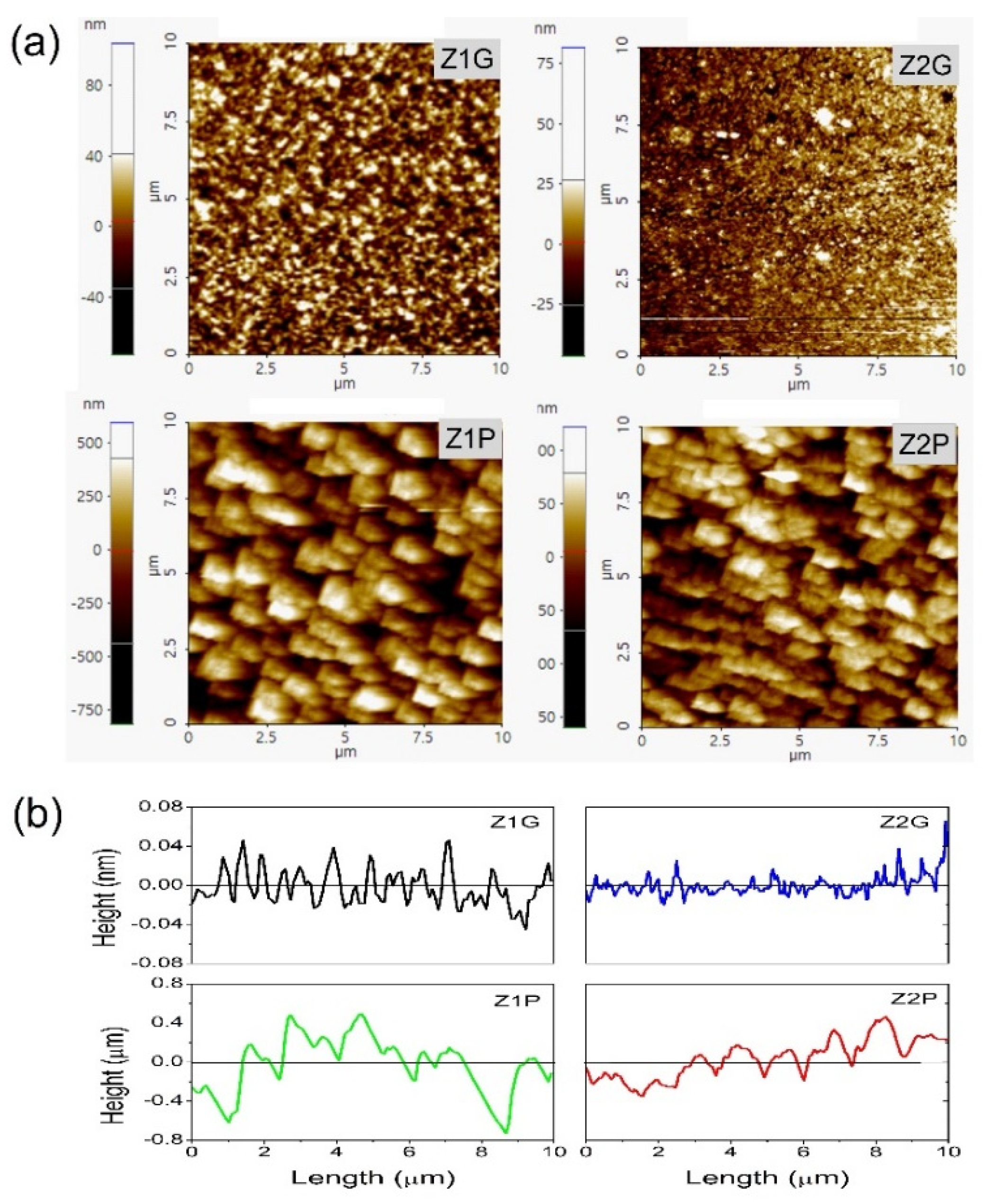
| Sample | Characteristics |
|---|---|
| Z1G | ZnO on glass substrate by sol–gel spin coating method |
| Z1P | ZnO on mPS substrate by sol–gel spin coating method |
| Z2G | ZnO on glass substrate by USP method |
| Z2P | ZnO on mPS substrate by USP method |
| Sample | FWHM (002) | 2 θ (°) | Crystallite Size (nm) | ε |
|---|---|---|---|---|
| Z1G | 0.4213 | 33.8145 | 20 | 0.100826 |
| Z1P | 0.5312 | 34.4291 | 15 | 0.126851 |
| Z2G | 1.3099 | 34.0601 | 6 | 0.313116 |
| Z2P | 0.9811 | 34.4287 | 8 | 0.234288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.; García-Salgado, G.; Morales-Morales, F.; Campillo, B.; Hernández, A.G.; Karthik, T.V.K.; Jiménez-Vivanco, M.R.; Campos-Álvarez, J. ZnO Films Incorporation Study on Macroporous Silicon Structure. Materials 2021, 14, 3697. https://doi.org/10.3390/ma14133697
Martínez L, García-Salgado G, Morales-Morales F, Campillo B, Hernández AG, Karthik TVK, Jiménez-Vivanco MR, Campos-Álvarez J. ZnO Films Incorporation Study on Macroporous Silicon Structure. Materials. 2021; 14(13):3697. https://doi.org/10.3390/ma14133697
Chicago/Turabian StyleMartínez, Lizeth, Godofredo García-Salgado, Francisco Morales-Morales, Bernardo Campillo, Angélica G. Hernández, Tangirala V. K. Karthik, María R. Jiménez-Vivanco, and José Campos-Álvarez. 2021. "ZnO Films Incorporation Study on Macroporous Silicon Structure" Materials 14, no. 13: 3697. https://doi.org/10.3390/ma14133697






