Phosphate Cements Based on Calcined Dolomite: Influence of Calcination Temperature and Silica Addition
Abstract
:1. Introduction
2. Materials and Methods
- Calcined magnesite (M), industrial product (Tremag, Tulcea, Romania), obtained by the calcination of magnesite at 1500 °C; the residue on sieve 90 microns mesh was 7.93%.
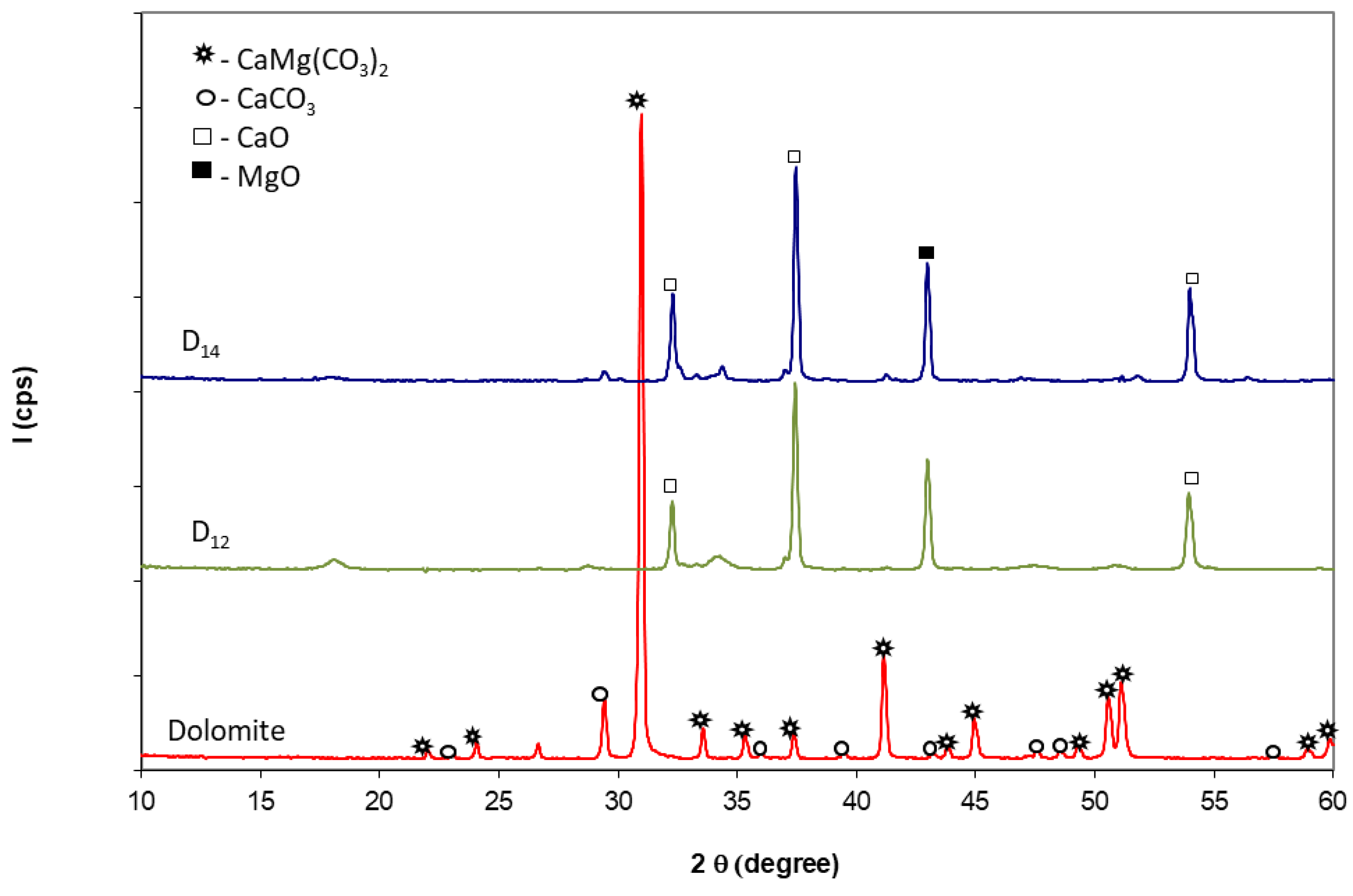
- Calcined dolomites, obtained by thermal treatment of natural dolomite (Rodbungrup, Bucharest, Romania) at 1200 °C (D12) and 1400 °C (D14) for 3 h. The natural dolomite had a content of 47% CaCO3 and 37.5% MgCO3 and a residue on 90 microns mesh of 24.83%. After the thermal treatment, the calcined dolomites were ground up to a fineness corresponding to total passing through a 90 microns sieve.
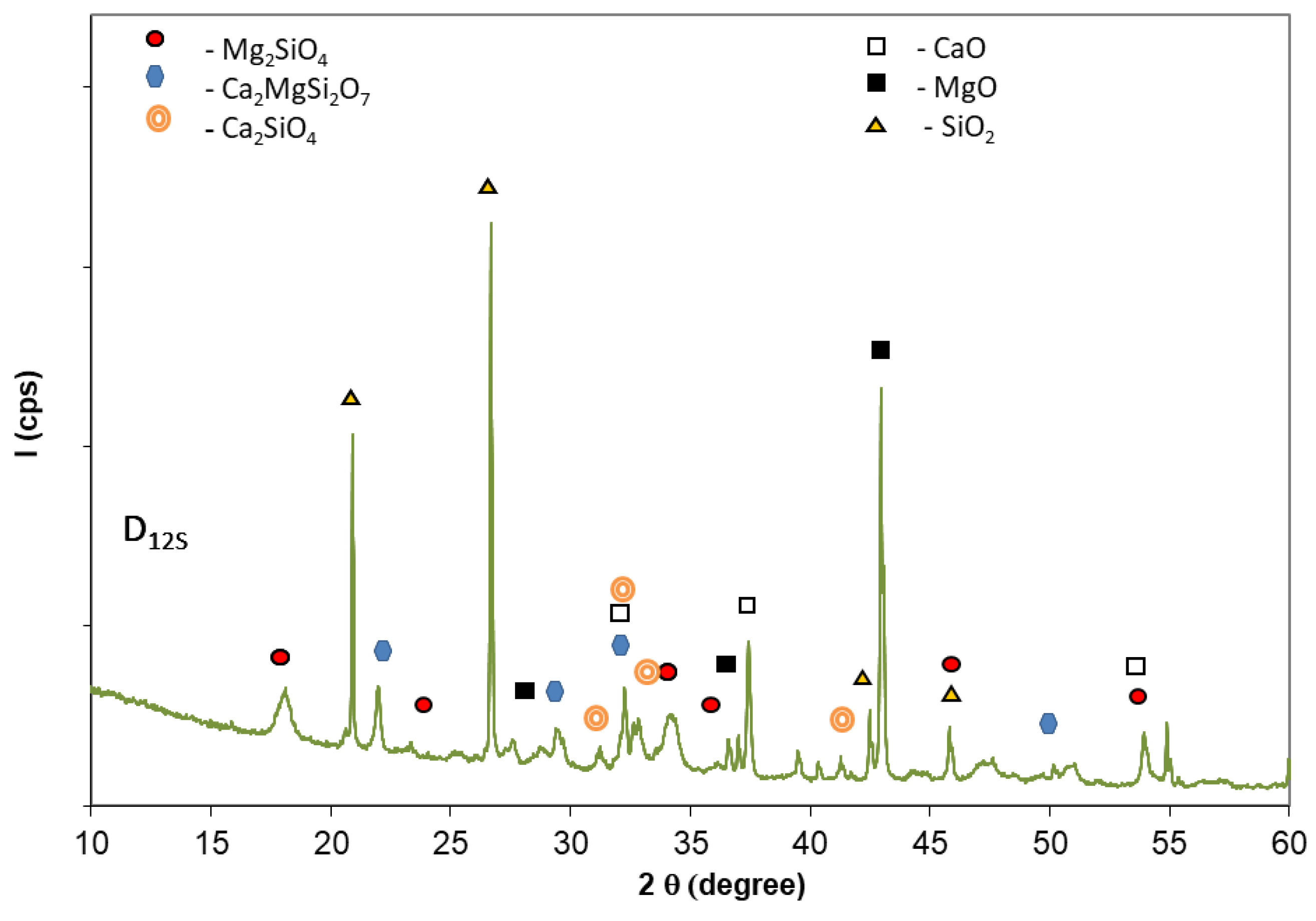
- Calcined mixture of dolomite and quartz sand (D12S); the sand (Societe Nouvelle du Litoral, Leucate, France) had a fineness corresponding to total passing through a 200 microns sieve. The dolomite to quartz sand ratio was 1.5, and the thermal treatment was performed at 1200 °C for 1 h, based on the results reported by Yu et.al. [14]. The rate of heating was 10 °C/minute, and the cooling was performed in the oven.
- Potassium dihydrogen phosphate (KH2PO4—MKP), chemical reagent Sigma-Aldrich (Darmstadt, Germany).
- Setting retarder—borax (B)—chemical reagent Sigma-Aldrich (Darmstadt, Germany).
3. Results
4. Conclusions
- The thermal treatment of dolomite at 1200 °C and 1400 °C leads to the decomposition of calcium magnesium carbonate into CaO and MgO. The increase in calcination temperature, from 1200 to 1400 °C reduces the reactivity of calcium and magnesium oxides vs. water or phosphate (MKP) solution; for the phosphate cements based on dolomite calcined at 1200 °C, an important increase in paste temperature during the setting and paste’s expansion was noticed due to the high reactivity of oxides (CaO and MgO); the increase of thermal treatment temperature at 1400 °C determines a decrease of the oxides’ reactivity, and for a higher KH2PO4 dosage (corresponding to D14/KH2PO4 = 2 weight ratio), the pastes have measurable compressive strength at early ages. Nevertheless, for all specimens based on dolomite calcined at 1400 °C, the compressive strengths dramatically decrease after 7 days of hardening, which is most probably due to a delayed hydration of CaO and MgO.
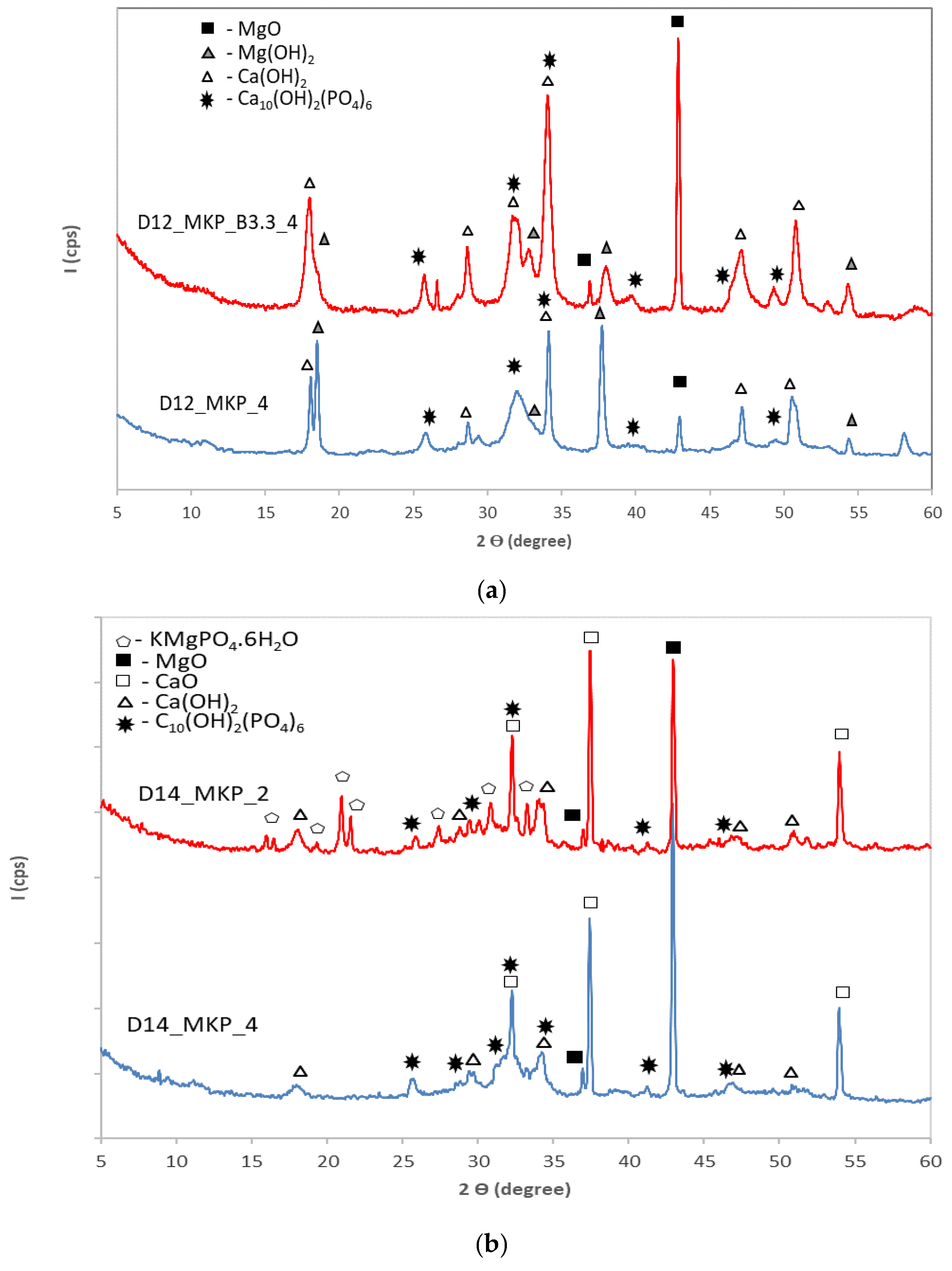
- The main compounds observed in hardened phosphate binders based on calcined dolomite were calcium and magnesium hydroxides; in the case of specimens with a lower dosage of MKP (corresponding to D14/MKP = 4 weight ratio)—along with Ca(OH)2 and Mg(OH)2, a new compound was detected by XRD—hydroxyapatite (HAp); HAp results from the reaction of calcium with phosphate, which is brought into the system by MKP. For a higher dosage of MKP (corresponding to D14/MKP = 2 weight ratio) on the XRD patterns, peaks specific for the K-struvite (KMgPO4.6H2O) compound are also present.
- In order to obtain a solid precursor for CMPC synthesis by the calcination of dolomite at relatively low temperature, a mixture of dolomite and quartz sand was thermally treated at 1200 °C for 1 h. The compressive strengths of resulting CMPC are lower in comparison with those assessed on phosphate cement based on calcined magnesite; however, they steadily increase up to 28 days. The lower values of compressive strengths assessed on these compositions are mainly due to the lower content of MgO available in this type of calcined dolomite (D12S) for the formation of K-struvite.
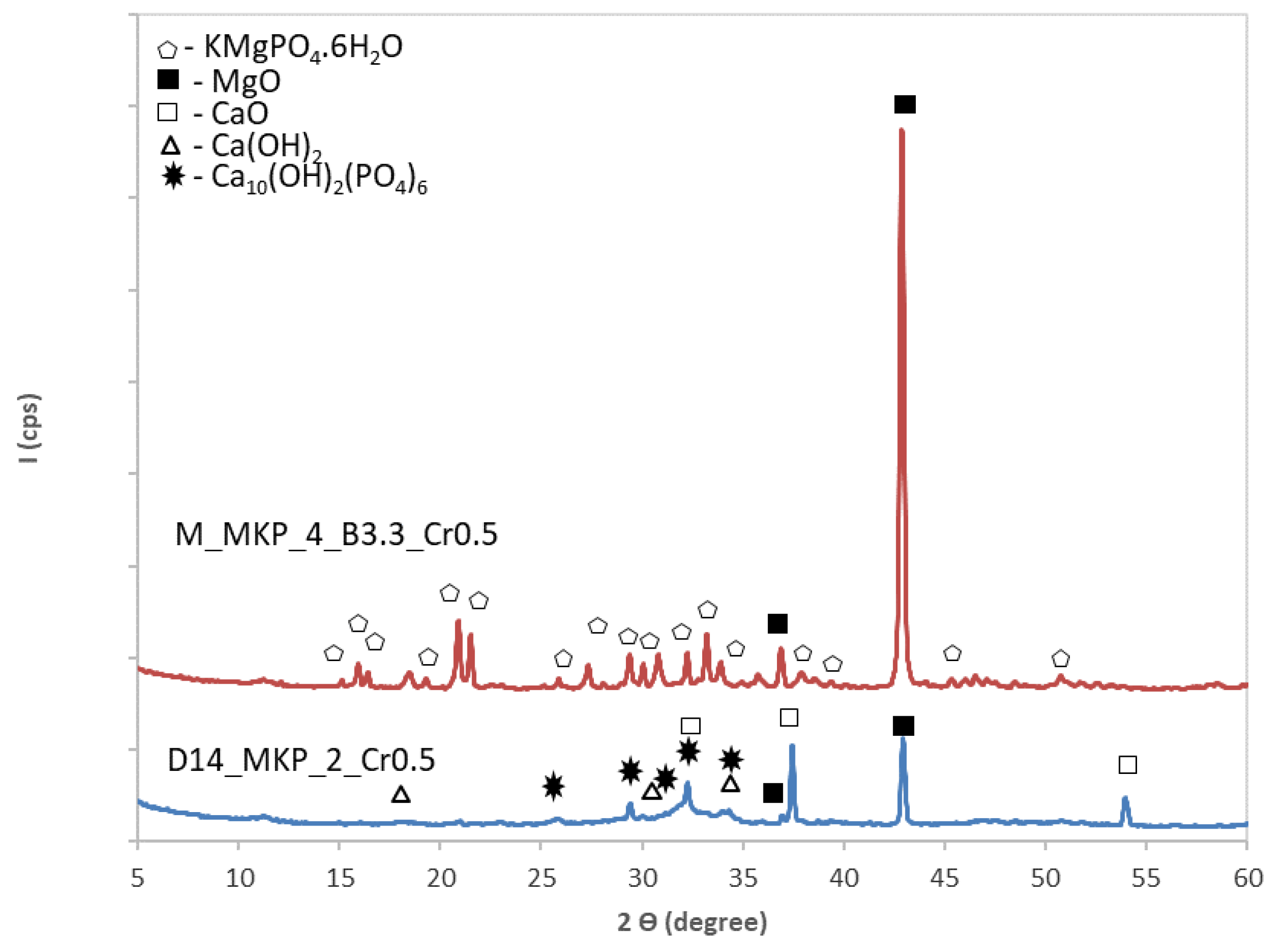
- The partial substitution of calcined magnesite and calcined dolomite with an industrial waste product with chromium content (corresponding to a Cr dosage of 0.5 wt % and 1 wt %) led to a significant decrease of compressive strength. In the case of MPC based on calcined magnesite, this decrease can be explained by the reduction of K-struvite amount due to the replacement of calcined magnesite and KH2PO4 with chromium waste, as well as by the increase of the water-to-solid ratio (necessary to obtain an adequate workability). For the CMPC based on dolomite calcined at 1400 °C (D14), the replacement of active components (D14 and KH2PO4) with chromium waste further inhibits the formation of K-struvite, and the compressive strengths decrease with the increase of the waste content. In the case of CMPC based on D12S, the lower compressive strengths assessed for specimen with chromium waste are also explained by the lower amount of K-struvite formed in this system (due to the decrease of MgO available for the reaction with KH2PO4).
- Phosphate cements based on calcined magnesite with a waste content corresponding to 1 and 0.5 wt % Cr can effectively reduce the Cr leaching for pastes cured for 28 days; in the case of phosphate cements based on calcined dolomite (D14), it was found that for a waste dosage corresponding to 0.5 wt % Cr, after 28 days of curing, the concentration of leached Cr is below the limit value imposed by the legislation currently in force. Nevertheless, considering the evolution of mechanical strength vs. time for this phosphate cement, it seems necessary to extend the evaluation of leached chromium for longer curing times (over 28 days). Although the CMPCs based on D12S developed adequate compressive strengths even at longer hardening times (28 days), the amount of chromium leached exceeded the limit imposed by the current legislation most probably due to the decrease of K-struvite content. The chromium immobilization in this type of CMPC can be improved if the appropriate amount of D12S (or chromium waste) is selected.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, B.; Ma, H.; Li, Z. Influence of magnesia-to-phosphate molar ratio on microstructures, mechanical properties and thermal conductivity of magnesium potassium phosphate cement paste with large water-to-solid ratio. Cem. Concr. Res. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Sun, S. Effect of M/P and Borax on the Hydration Properties of Magnesium Potassium Phosphate Cement Blended with Large Volume of Fly Ash. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 1159–1167. [Google Scholar] [CrossRef]
- Yue, L.; Bing, C. Factors that affect the properties of magnesium phosphate cement. Constr. Build. Mater. 2013, 47, 977–983. [Google Scholar]
- Jiang, Z.; Zhang, L.; Geng, T.; Lai, Y.; Zheng, W.; Huang, M. Study on the Compressive Properties of Magnesium Phosphate Cement Mixing with Eco-Friendly Coir Fiber Considering Fiber Length. Material 2020, 13, 3194. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials for EU. Available online: http://ec.europa.eu/DocsRoom/documents/11911/attachments/1/translations/en/renditions/native (accessed on 23 May 2021).
- Alvarado, E.; Torres-Martinez, L.M.; Fuentes, A.F.; Quintana, P. Preparation and characterization of MgO powders obtained from different magnesium salts and the mineral dolomite. Polyhedron 2000, 19, 2345–2351. [Google Scholar] [CrossRef]
- Subagjo; Wulandari, W.; Adinata, P.M.; Fajrin, A. Thermal decomposition of dolomite under CO2-air atmosphere. In AIP Conference Proceedings, Proceedings of the 1st International Process Metallurgy Conference (IPMC 2016), Bandung, Indonesia, 10–11 November 2016; AIP publishing: Dresden, Germany, 2017; Volume 1805. [Google Scholar] [CrossRef] [Green Version]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Wulandari, W.; Subagjo; Mursito, A.T.; Juanjaya, F.J.; Alwi, M.F. Performance of Dolomite Calcination in a Bench-Scale Rotary Kiln. In MATEC Web of Conferences, Proceedings of the The 24th Regional Symposium on Chemical Engineering (RSCE 2017), Semarang, Indonesia, 15–16 November 2017; EDP Science: Les Ulis, France, 2018; Volume 156, pp. 704–708. [Google Scholar]
- Roques, H.; Nugroho-Jeudy, L.; Lebugle, A. Phosphorus removal from wastewater by half-burned dolomite. Wat. Res. 1991, 25, 959–965. [Google Scholar] [CrossRef]
- Engler, P.; Santana, M.W.; Mittleman, M.L.; Balazs, D. Non isothermal, In situ XRD analysis of dolomite decomposition. Thermochim. Acta 1989, 140, 67–76. [Google Scholar] [CrossRef]
- Mako, E. The effect of quartz content on the mechanical activation of dolomite. J. Eur. Ceram. Soc. 2007, 27, 535–540. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Thermal decomposition of natural dolomite. Bull. Mater. Sci. 2007, 30, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Qian, J.; Wang, F.; Qin, J.; Dai, X.; You, C.; Jia, X. Study of using dolomite ores as raw materials to produce magnesium phosphate cement. Constr. Build. Mater. 2020, 253, 119147. [Google Scholar] [CrossRef]
- Singh, D.; Wagh, A.S.; Cunnane, J.C.; Mayberry, J.L. Chemically bonded phosphate ceramics for low-level mixed-waste stabilization. J. Environ. Sci. Health 1997, 32, 527–541. [Google Scholar] [CrossRef]
- Deng, Q.; Lai, Z.; Yan, T.; Wu, J.; Liu, M.; Lu, Z.; Lv, S. Effect of Cr (III) on hydration, microstructure of magnesium phosphate cement, and leaching toxicity evaluation. Environ. Sci. Pollut. Res. 2021, 28, 15290–15304. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dai, J.G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sua, Y.; Yanga, J.; Liuc, D.; Zhena, S.; Lind, N.; Zhoue, Y. Effects of municipal solid waste incineration fly ash on solidification/ stabilization of Cd and Pb by magnesium potassium phosphate cement. J. Environ. Chem. Eng. 2016, 4, 259–265. [Google Scholar] [CrossRef]
- Vijan, C.A.; Badanoiu, A.; Nicoara, A.I.; Barcan, I. Effect of lead and nickel on the hardening processes and properties of phosphate cements. Rev. Rom. Mater. 2020, 50, 510–520. [Google Scholar]
- ATSDR, Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/csem/chromium/signs_and_symptoms.html (accessed on 29 June 2021).
- Oproiu, C.; Parvan, M.; Voicu, G.; Badanoiu, A.I. Inertization of an industrial waste rich in chromium in composite portland cements. Rev. Rom. Mater. 2018, 48, 458–466. [Google Scholar]
- SR EN 459-2. 2001-Building Lime. Part 2. Test Methods; ASRO: Bucharest, Romania, 2001. [Google Scholar]
- SR EN 12457-4:2003. Characterization of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 10 mm (without or with Size Reduction); ASRO: Bucharest, Romania, 2003. [Google Scholar]
- Teoreanu, I. The Basics of Inorganic Binder Technology (in Romanian); Editura Didactică și Pedagogică: Bucharest, Romania, 1993. [Google Scholar]
- Schorcht, F.; Kourti, I.; Scalet, B.M.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide (Integrated Pollution Prevention and Control). Available online: https://ec.europa.eu/jrc/en/publication/reference-reports/best-available-techniques-bat-reference-document-production-cement-lime-and-magnesium-oxide (accessed on 23 May 2021).
- Stork, M.; Meindetsma, W.; Overgaag, M.; Neelis, M. A Competitive and Efficient Lime Industry. Cornerstone for a Sustainable Europe; Technical Report; Ecofys by order of EuLA: Brussels, Belgium, 2014; pp. 1–60. [Google Scholar]
- Hashimoto, H.; Komaki, E.; Hayashi, F.; Uematsu, T. Partial Decomposition of Dolomite in CO2. J. Solid State Chem. 1980, 33, 181–188. [Google Scholar] [CrossRef]
- Wells, L.S.; Taylor, K. Hydration of magnesia in dolomitic hydrated limes and putties. J. Res. Natl. Bur. Stand. 1937, 19, 215–236. [Google Scholar] [CrossRef]
- Wagn, A.S. Chemically Bonded Phosphate Ceramics; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Vijan, C.A.; Badanoiu, A. The influence of potassium phosphate and fly ash addition on the setting time and mechanical strengths of magnesium phosphate cement. Univ. Politeh. Buchar. Sci. Bull. Ser. B 2020, 82, 21–32. [Google Scholar]
- Romanian Ministerial Order no. 95/2005 on the Establishment of Acceptance Criteria and Preliminary Acceptance Procedures for Landfill Waste and the National List of Waste Accepted in Each Landfill Class (According to 2003/33/EC). Available online: https://www.ecotic.ro/wp-content/uploads/2015/07/1e62400fcaa487767564187df6343e669b6f36ad.pdf (accessed on 23 May 2021).
- Rouff, A.A. The use of TG/DSC–FT-IR to assess the effect of Cr sorption on struvite stability and composition. J. Therm. Anal. Calorim. 2012, 110, 1217–1223. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. Hydroxyapatite as an advanced adsorbent for removal of heavy metal ions from water: Focus on its applications and limitations. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Campisi, S.; Evangelisti, C.; Postole, G.; Gervasini, A. Combination of Interfacial Reduction of Hexavalent Chromium and Trivalent Chromium Immobilization on Tin-Functionalized Hydroxyapatite Material. Appl. Surf. Sci. 2021, 539, 148227. [Google Scholar] [CrossRef]


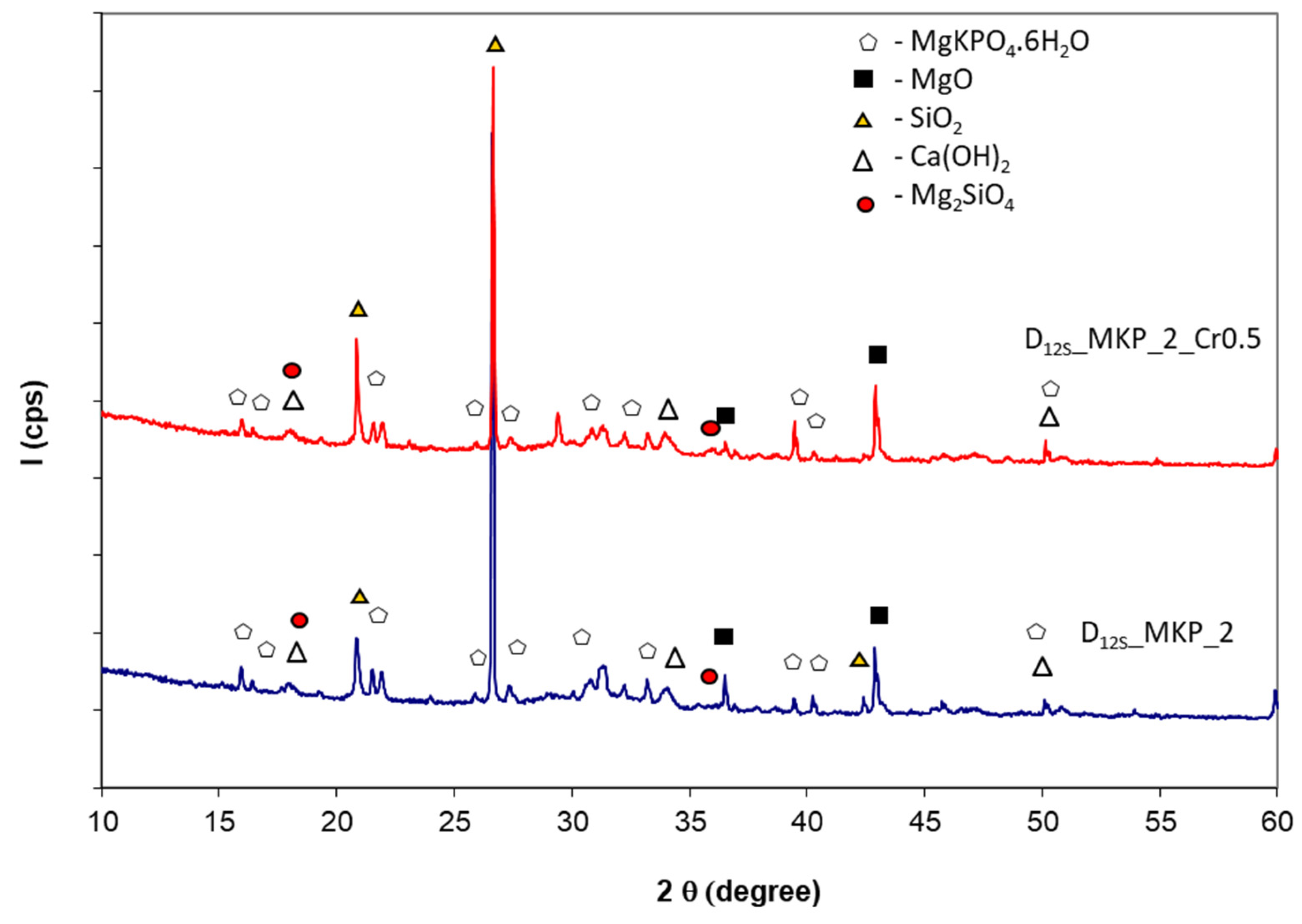
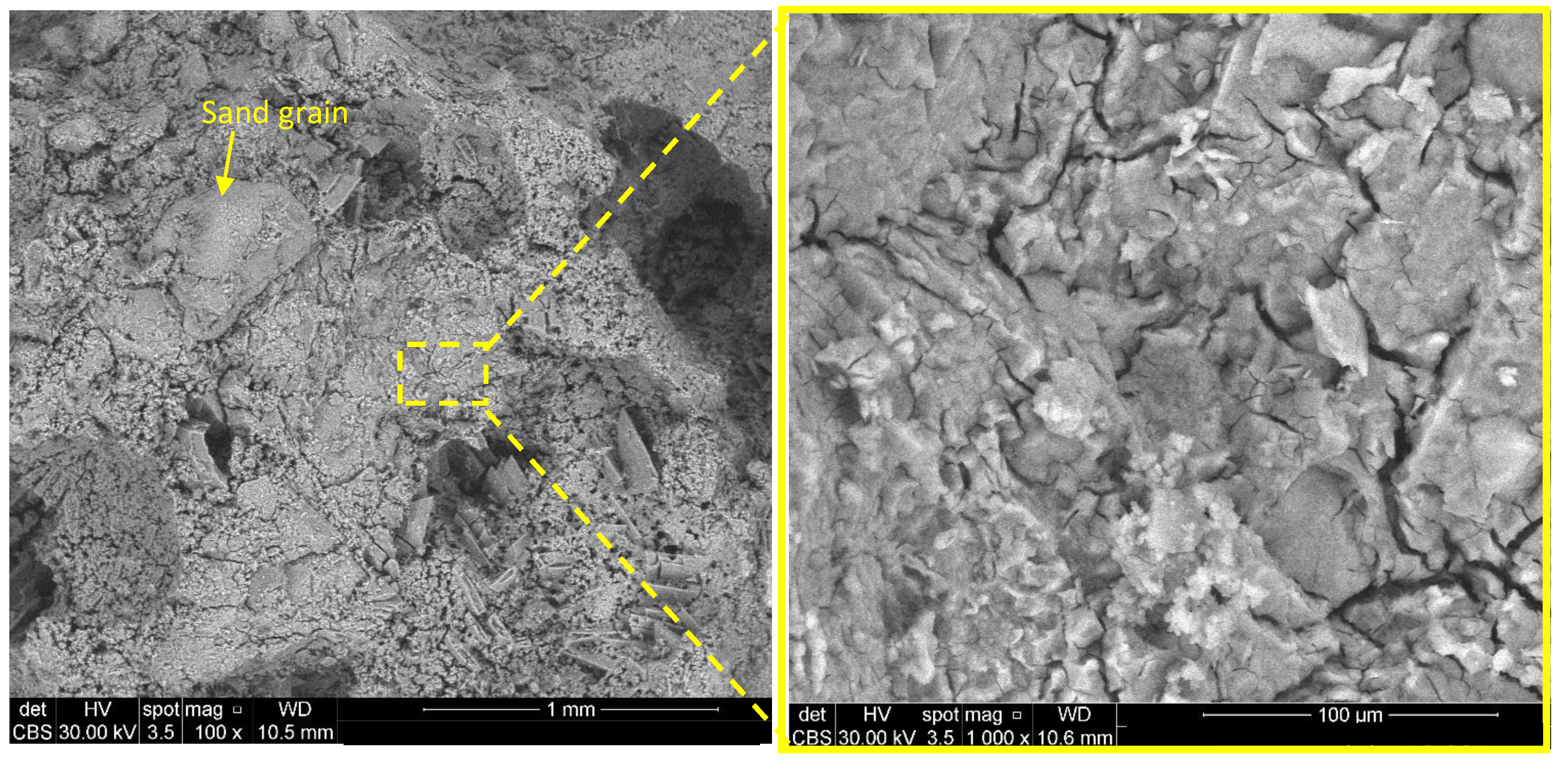
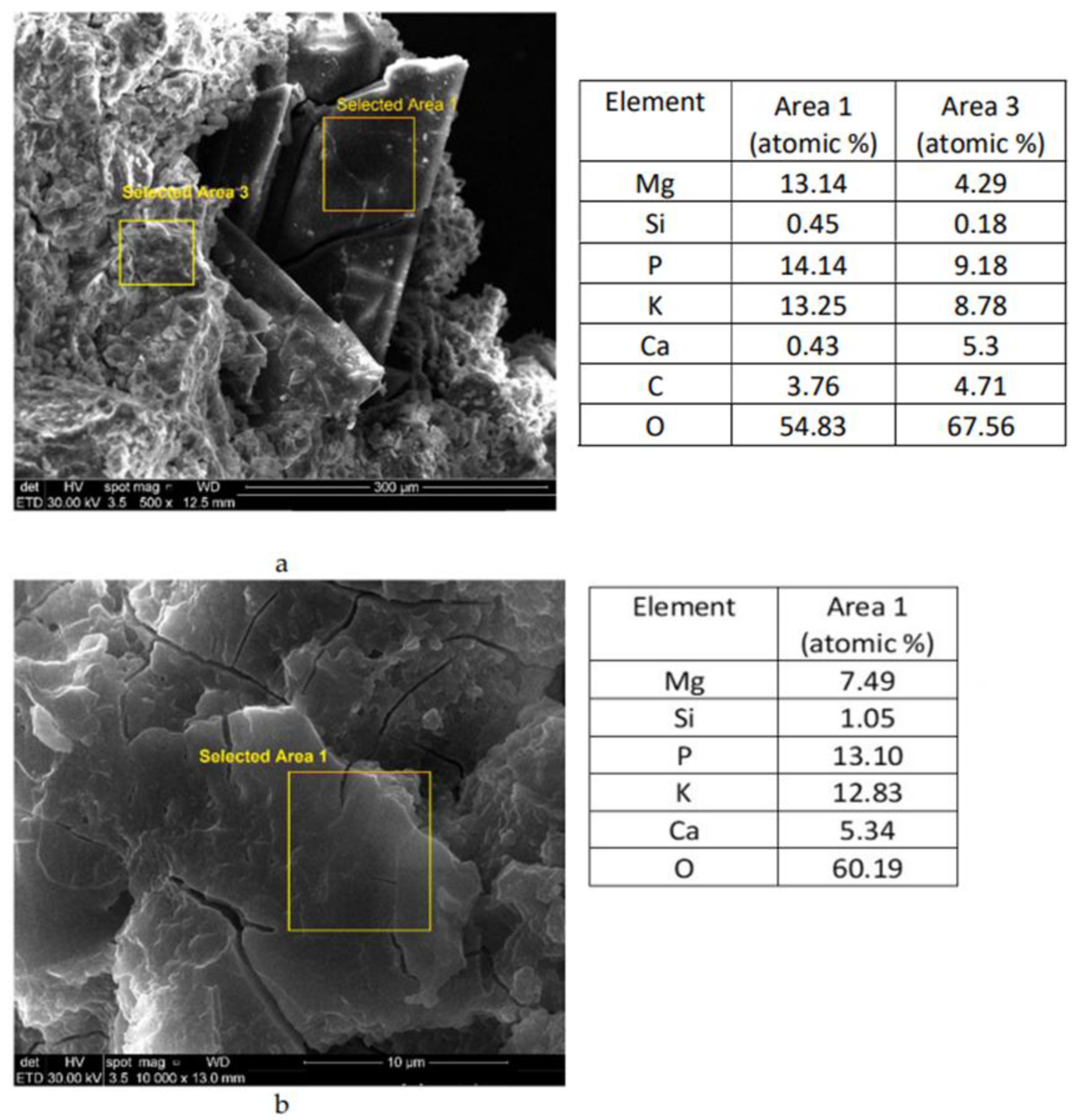

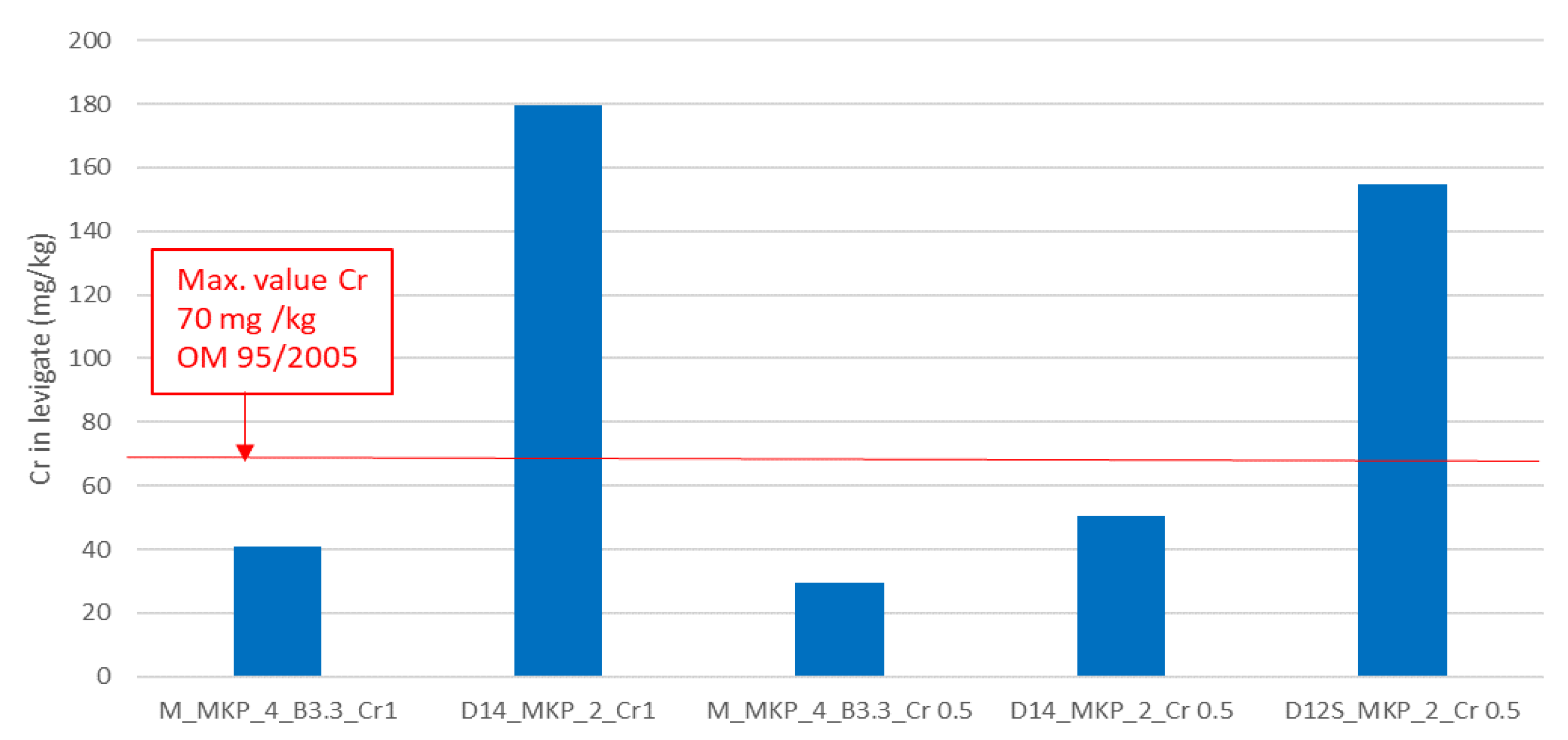
| Sample | Calcined Magnesite (M) wt % | Calcined Dolomite (D) wt % | KH2PO4 (MKP) wt % | Borax * (B) wt % | Sand wt % | Cr Waste ** % wt % | M or D to MKPRatio (wt) | Water to Solid Ratio (wt) | Calcination Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| M_MKP_4_B3.3 | 80 | - | 20 | 3.3 | - | - | 4 | 0.2 | 1500 |
| M_MKP_4_B3.3_Cr 1 | 50.4 | - | 12.6 | 3.3 | - | 37 | 4 | 0.35 | 1500 |
| M_MKP_4_B3.3_Cr_0.5 | 64.8 | - | 16.2 | 3.3 | - | 19 | 4 | 0.35 | 1500 |
| D12_W | - | 100 | - | - | - | - | - | 0.8 | 1200 |
| D12_MKP_4 | - | 80 | 20 | - | - | - | 4 | 0.67 | 1200 |
| D12_MKP_B3.3_4 | - | 80 | 20 | 3.3 | - | - | 4 | 0.67 | 1200 |
| D12_MKP_2.5 | - | 71.43 | 28.57 | - | - | - | 2.5 | 0.55 | 1200 |
| D12S_MKP_2 | - | 40.2 | 33 | - | 26.8 | - | 2 | 0.2 | 1200 |
| D14_MKP_4 | - | 80 | 20 | - | - | - | 4 | 0.3 | 1400 |
| D14_MKP_2 | - | 67 | 33 | - | - | 2 | 0.2 | 1400 | |
| D14_MKP_2_Cr1 | - | 42 | 21 | - | - | 37 | 2 | 0.35 | 1400 |
| D14_MKP_2_Cr0.5 | - | 54 | 27 | - | - | 19 | 2 | 0.35 | 1400 |
| D12S_MKP_2_Cr0.5 | - | 32.4 | 27 | - | 21.6 | 19 | 2 | 0.2 | 1200 |
| Sample | Tmax * (°C) | tmax ** (min) | Obs. |
|---|---|---|---|
| D12_W | 98 | 4 | Expansion |
| D12_MKP_4 | 90 | 26 | Expansion |
| D12_MKP_2.5 | 90 | 10 | Expansion |
| Specimens | Compressive Strength (MPa) | |||
|---|---|---|---|---|
| 1 Day | 3 Days | 7 Days | 28 Days | |
| M_MKP_4_B3.3 | 16.87 | 21.62 | 26.5 | 27.1 |
| M_MKP_4_B3.3_Cr0.5 | 0.6 | 1 | 1.15 | 1.5 |
| M_MKP_4_B3.3_Cr1 | 0 | 0 | 0 | 0 |
| D14_MKP_4 | 0 | 0 | 0 | 0 |
| D14_MKP_2 | 7.1 | 10.8 | 12.2 | 0 |
| D14_MKP_2_Cr0.5 | 1.5 | 1.7 | 2 | 0 |
| D14_MKP_2_Cr1 | 0 | 0 | 0 | 0 |
| D12S_MKP_2 | 2.8 | 6.3 | 7.2 | 9.2 |
| D12S_MKP_2_Cr0.5 | 0 | 3.5 | 5.4 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijan, C.A.; Badanoiu, A.; Voicu, G.; Nicoara, A.I. Phosphate Cements Based on Calcined Dolomite: Influence of Calcination Temperature and Silica Addition. Materials 2021, 14, 3838. https://doi.org/10.3390/ma14143838
Vijan CA, Badanoiu A, Voicu G, Nicoara AI. Phosphate Cements Based on Calcined Dolomite: Influence of Calcination Temperature and Silica Addition. Materials. 2021; 14(14):3838. https://doi.org/10.3390/ma14143838
Chicago/Turabian StyleVijan, Cristina Andreea, Alina Badanoiu, Georgeta Voicu, and Adrian Ionut Nicoara. 2021. "Phosphate Cements Based on Calcined Dolomite: Influence of Calcination Temperature and Silica Addition" Materials 14, no. 14: 3838. https://doi.org/10.3390/ma14143838
APA StyleVijan, C. A., Badanoiu, A., Voicu, G., & Nicoara, A. I. (2021). Phosphate Cements Based on Calcined Dolomite: Influence of Calcination Temperature and Silica Addition. Materials, 14(14), 3838. https://doi.org/10.3390/ma14143838







