Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
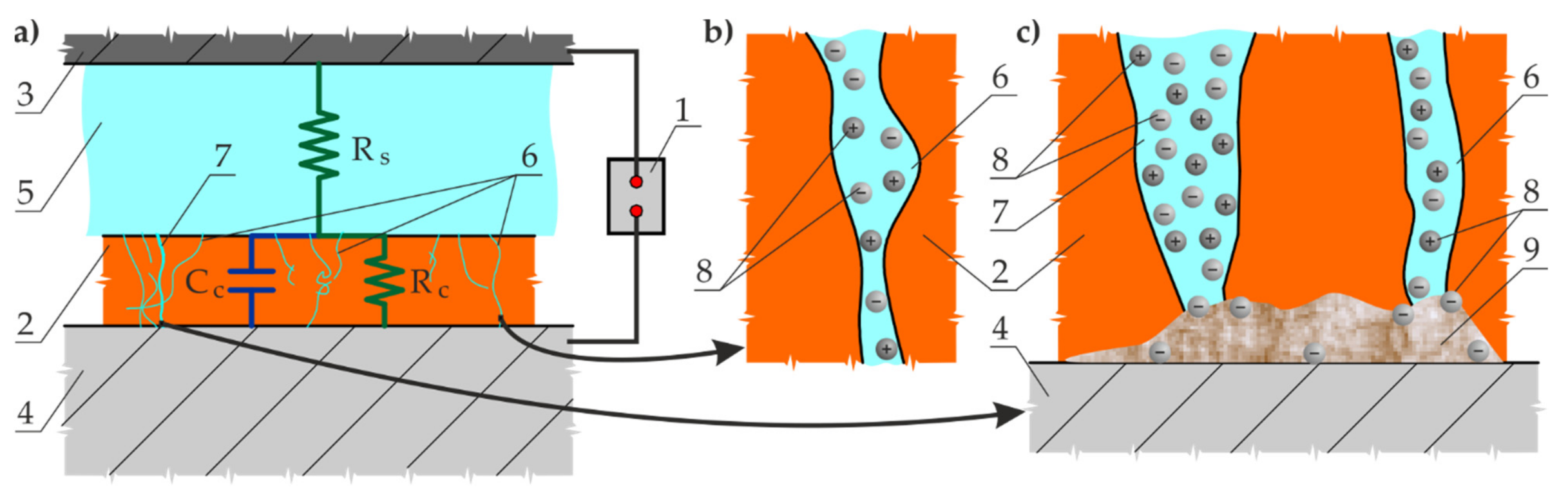
2. Proposed Methods to Diagnose Large Anti-Corrosion Coatings
| Barrier Properties | |
|---|---|
| ≤6 | Low |
| 6–8 | Mean |
| 8–10 | High |
| >10 | Very high |
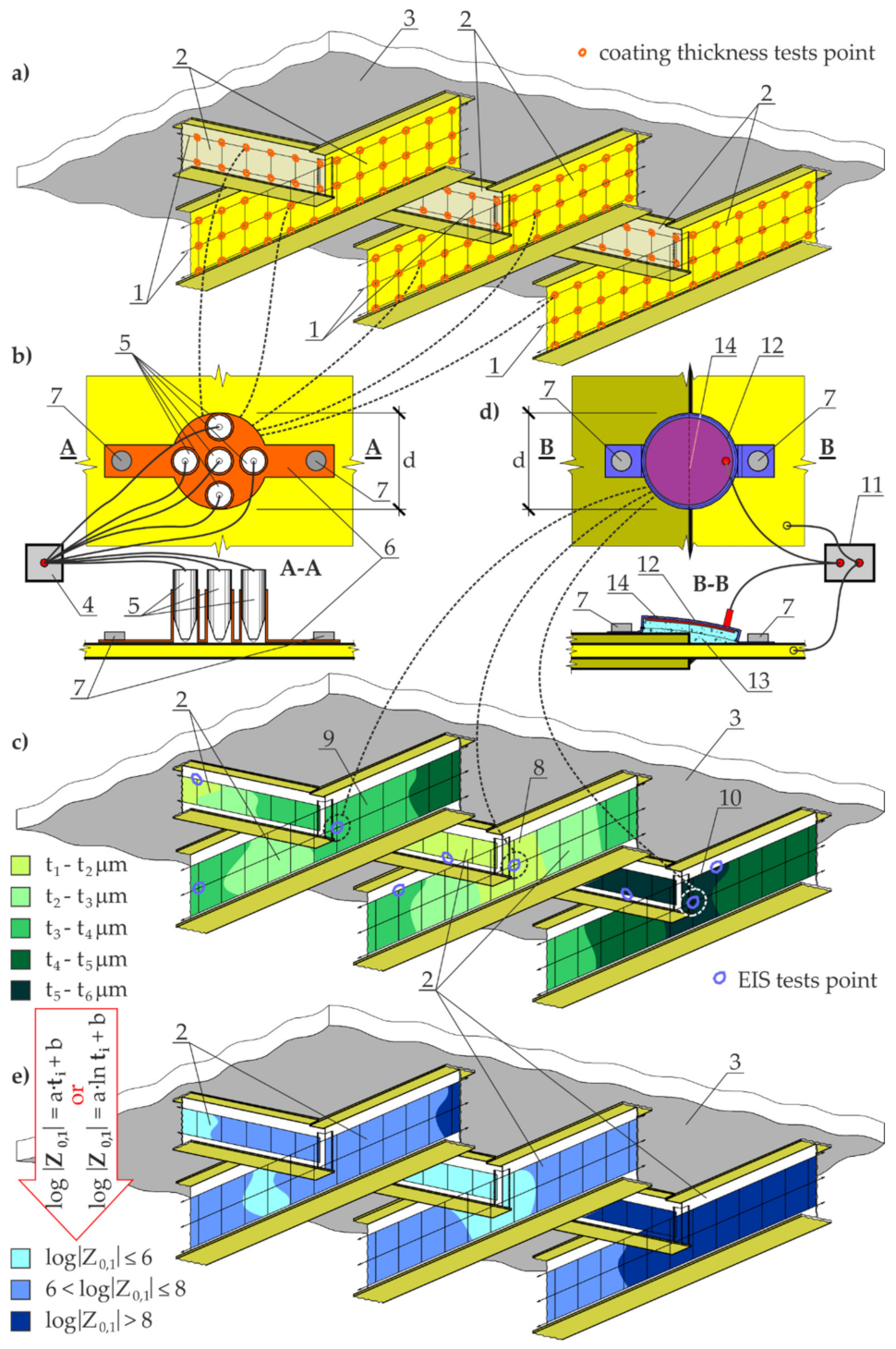
3. Materials
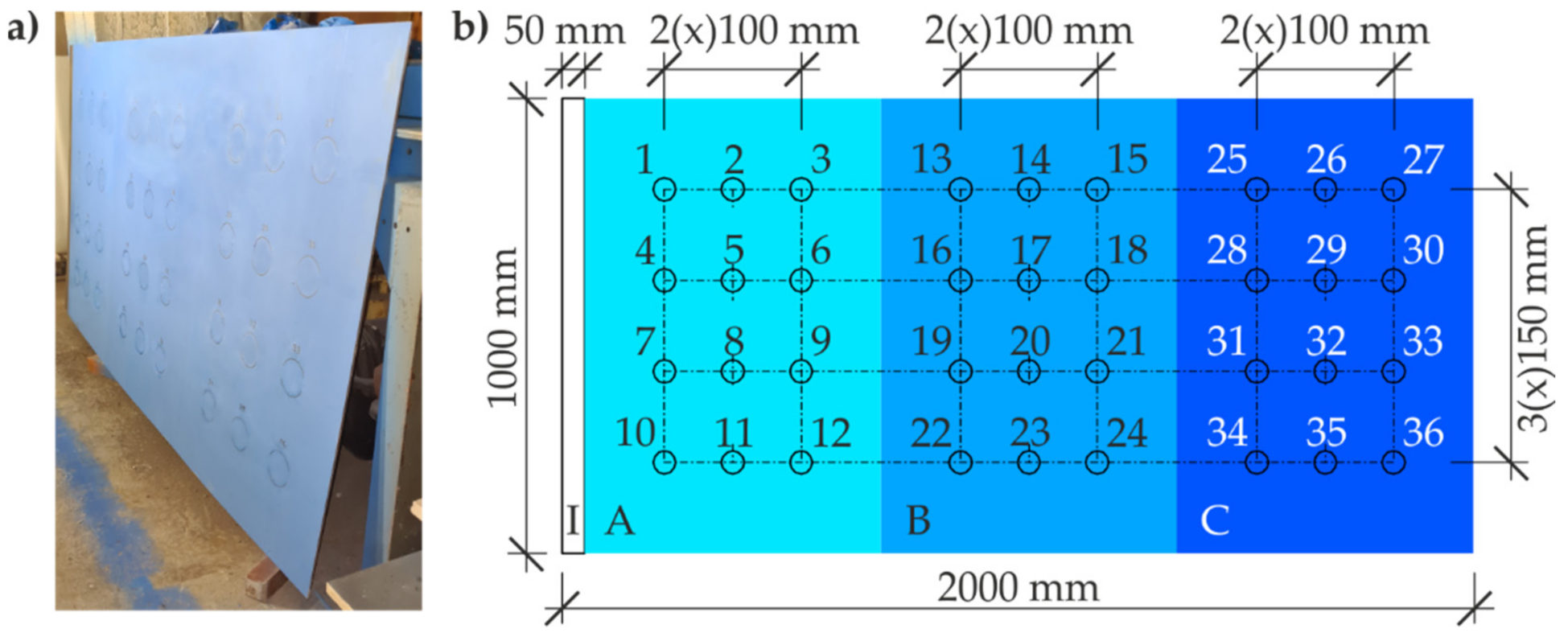
3.1. Test Materials
3.2. Materials Used for Tests
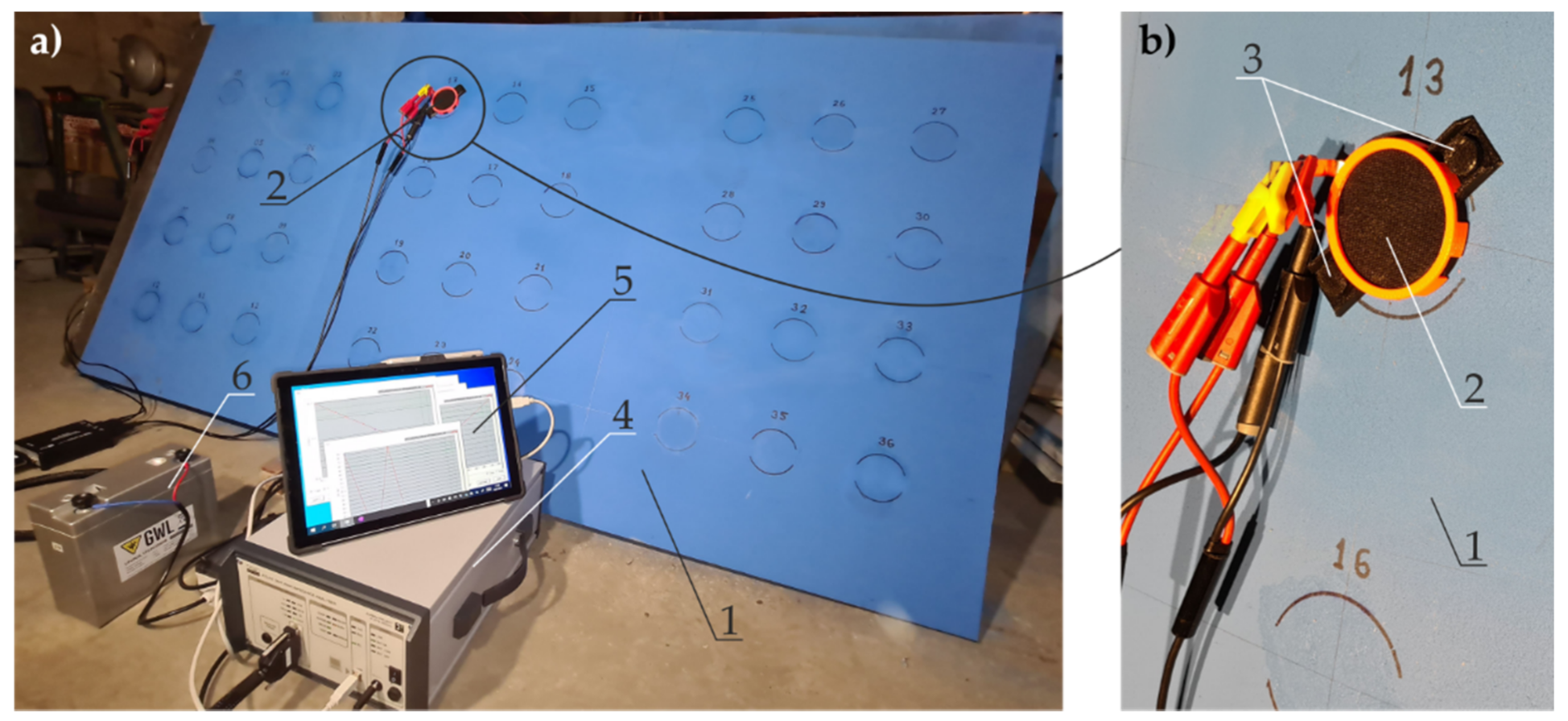
4. Methods
4.1. Selection of an Electrolyte Solution
4.2. Comparative Tests of Coating Impedance Using a Probe Filled with Liquid and Gel Electrolyte

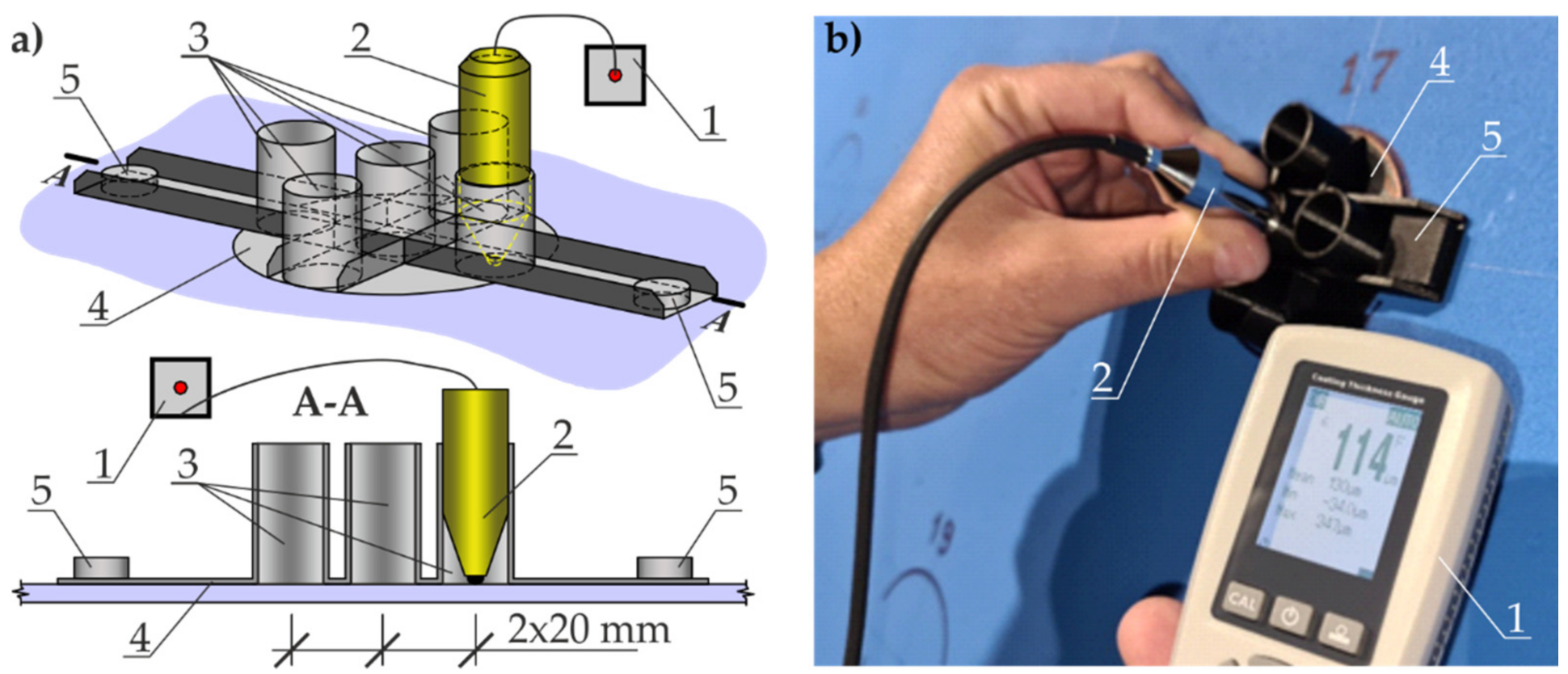
4.3. Measurements of Coating Thickness by Electromagnetic Method with Template
4.4. Measurements of Coating Impedance by the EIS with a Probe Filled with Gel Electrolyte
5. Results
5.1. Comparative Tests of Conductivity of Aqueous and Gel Electrolyte
5.2. Comparative Tests of Coating Impedance Using the Probe Filled with Aqueous and Gel Electrolyte
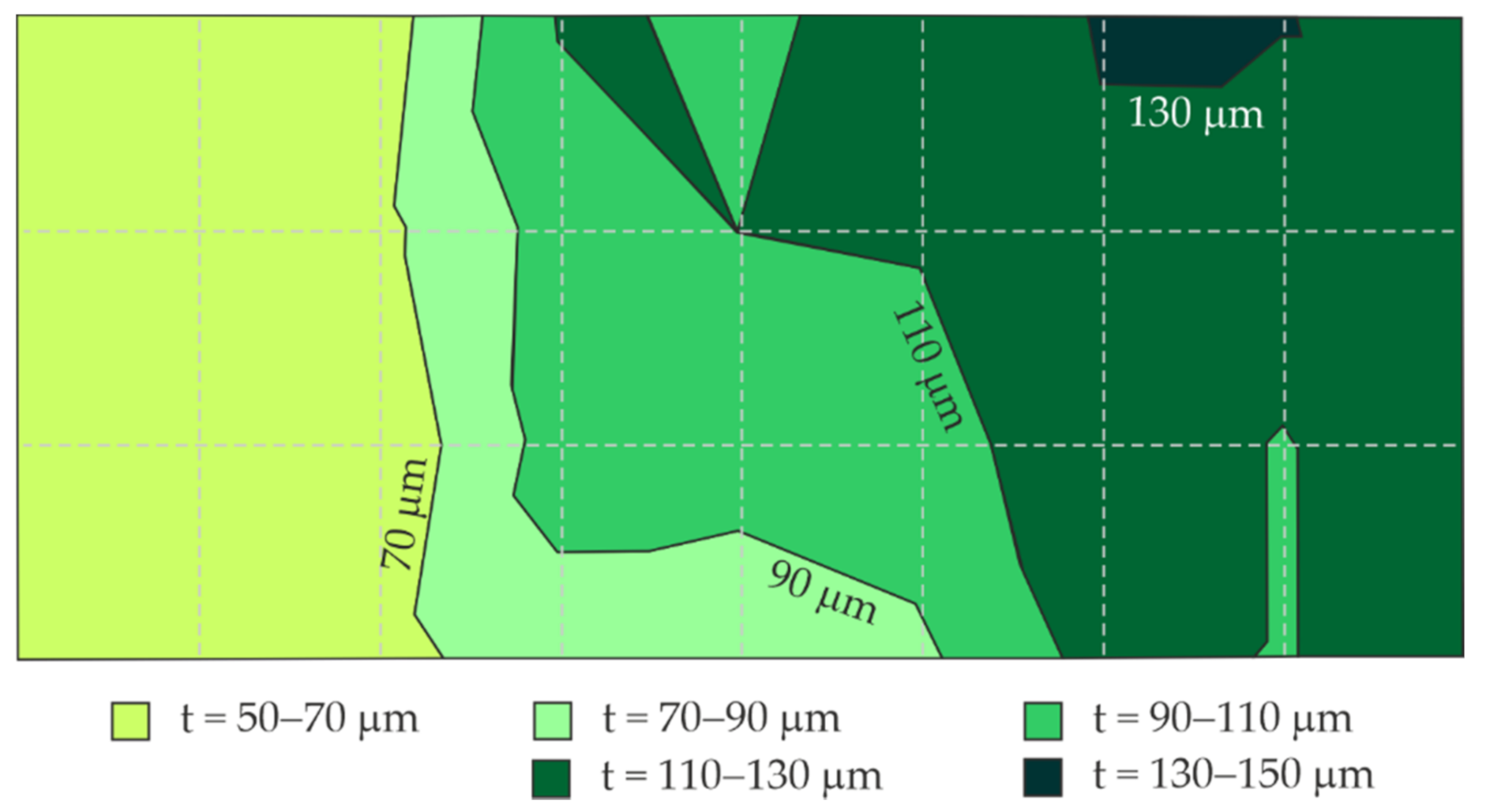
5.3. Measurements of Coating Thickness by Magnetic Induction
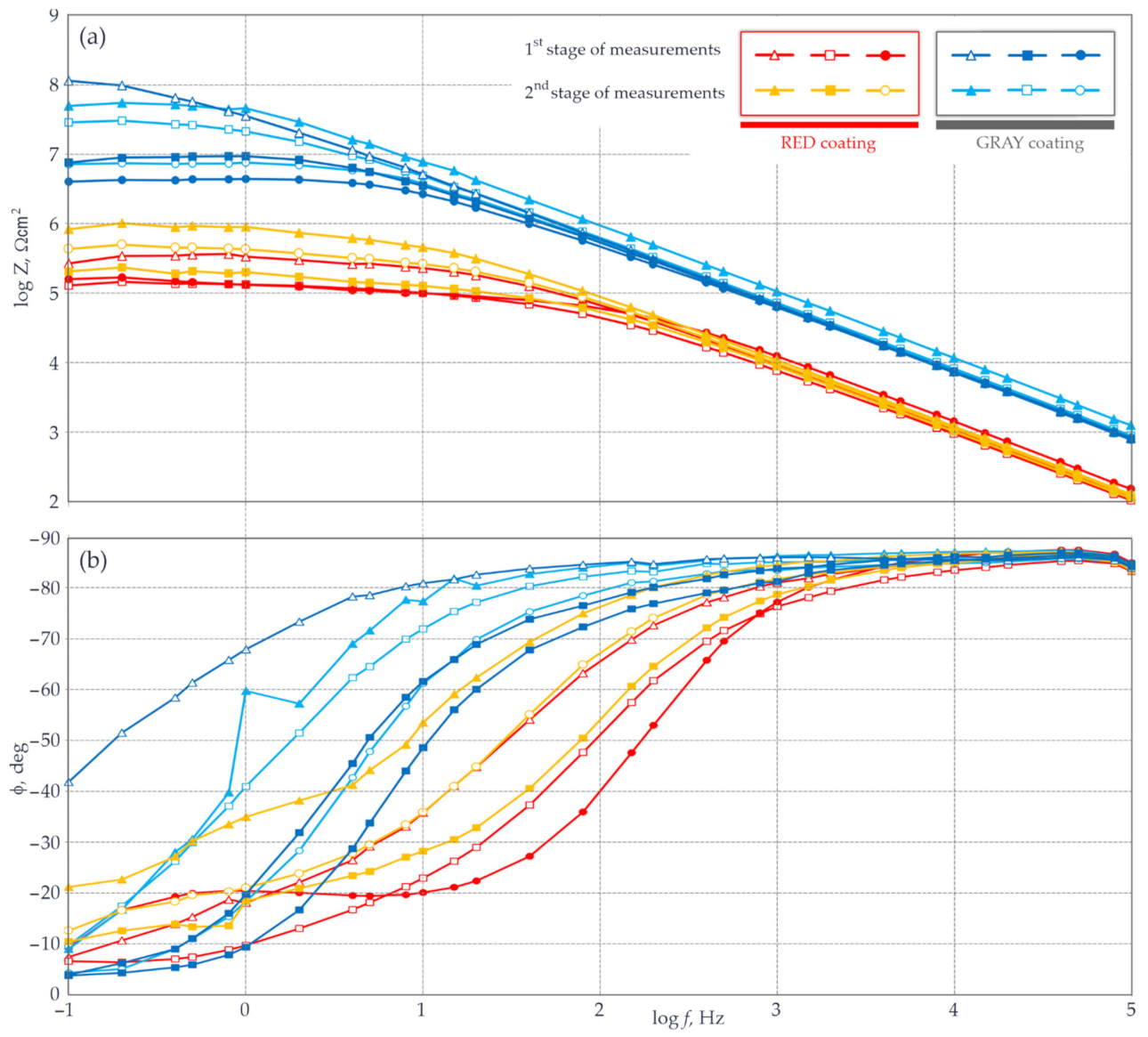
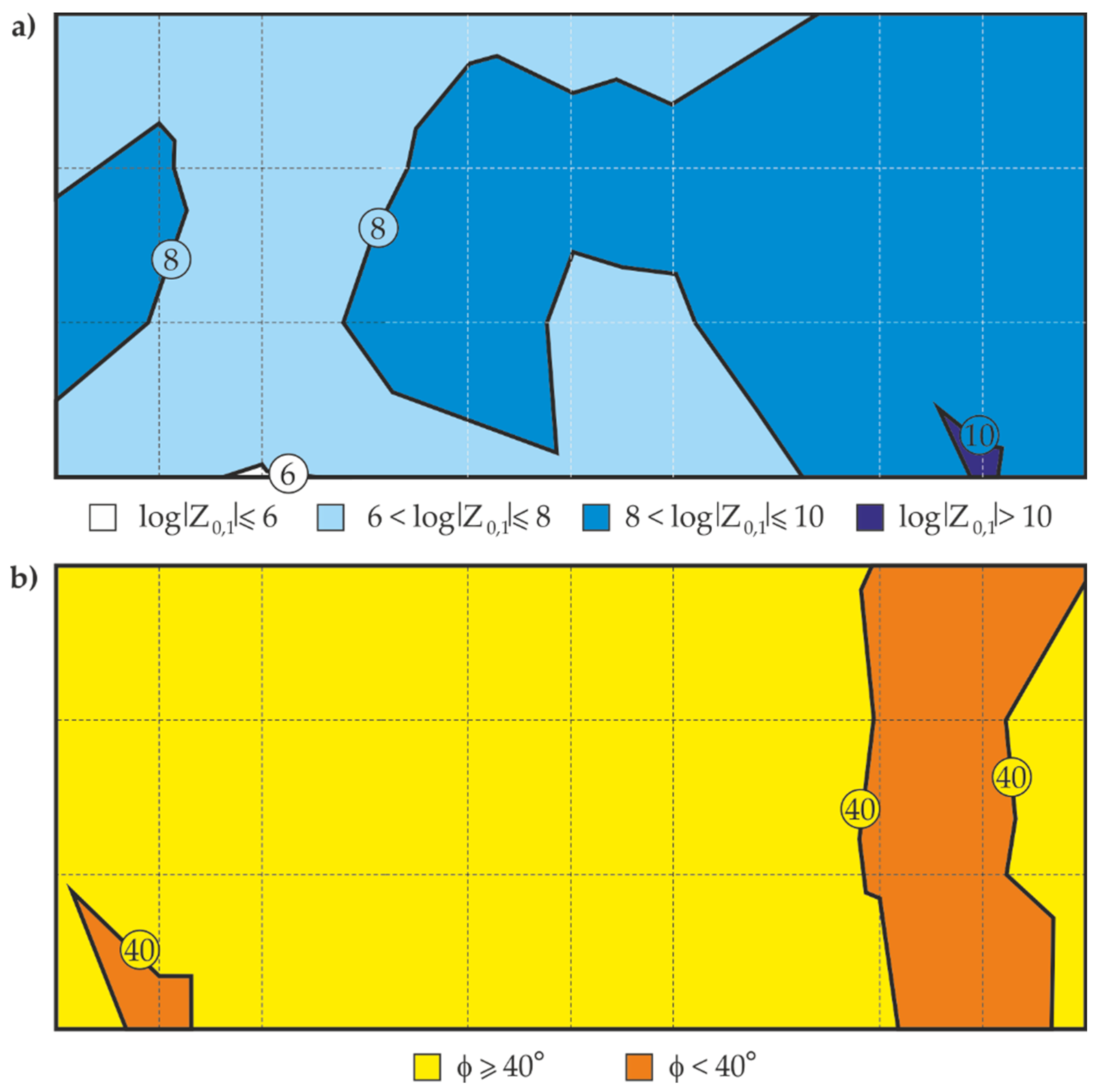
5.4. Measurements of Coating Impedance by the EIS with a Probe Filled with gel Electrolyte
6. Discussion
7. Conclusions
- The method of electrochemical impedance spectroscopy can be used not only for testing non-conductive coatings on metals under laboratory conditions, but also, after the required adaptation, for testing anti-corrosion coatings on large elements of steel structures.
- Adaptation of the EIS to the in situ tests was mainly based on using the flexible housing of the measuring probe with the integrated flexible auxiliary electrode, the shape of which adjusted to the test surface, and using electrolyte gel instead of the traditional aqueous electrolyte.
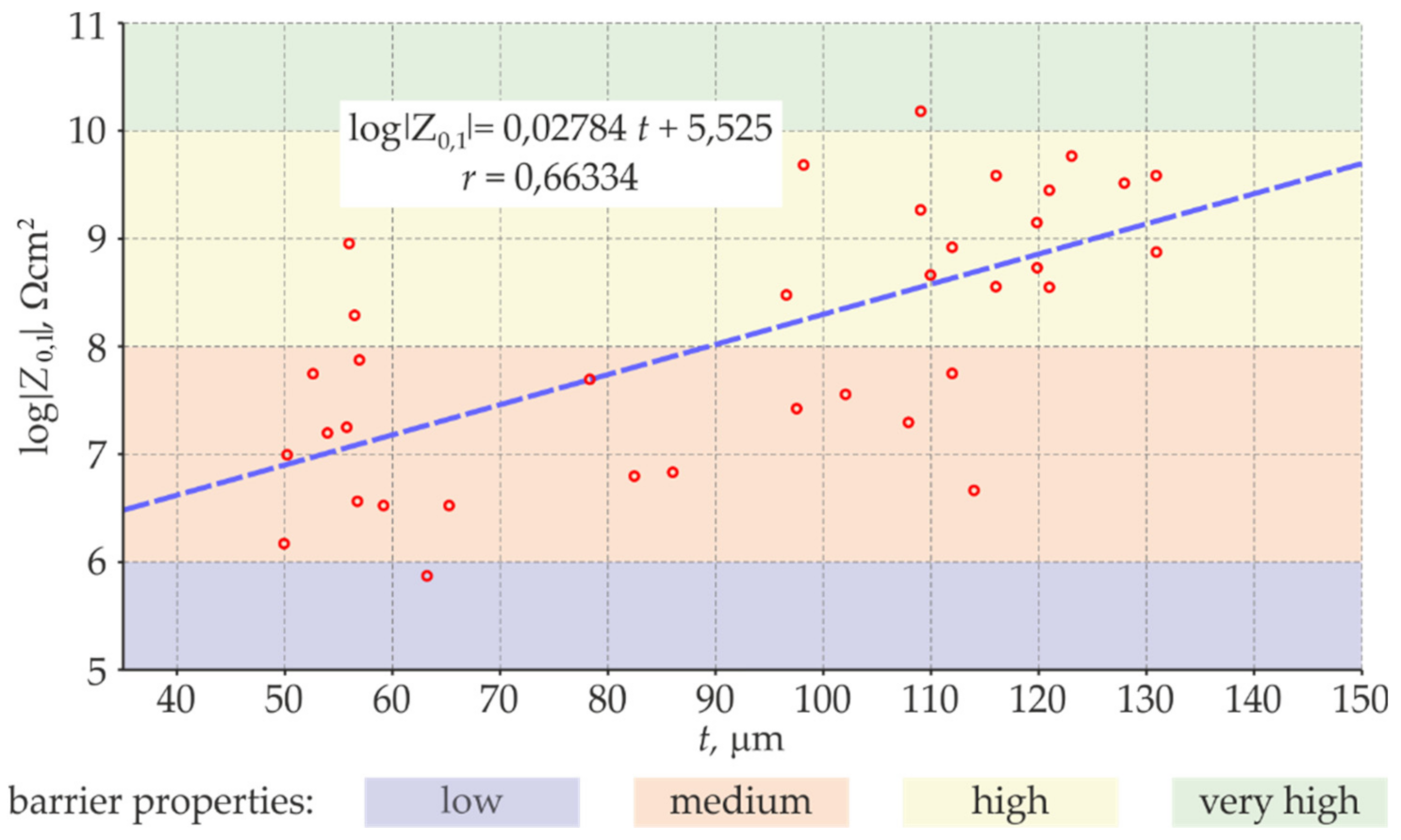
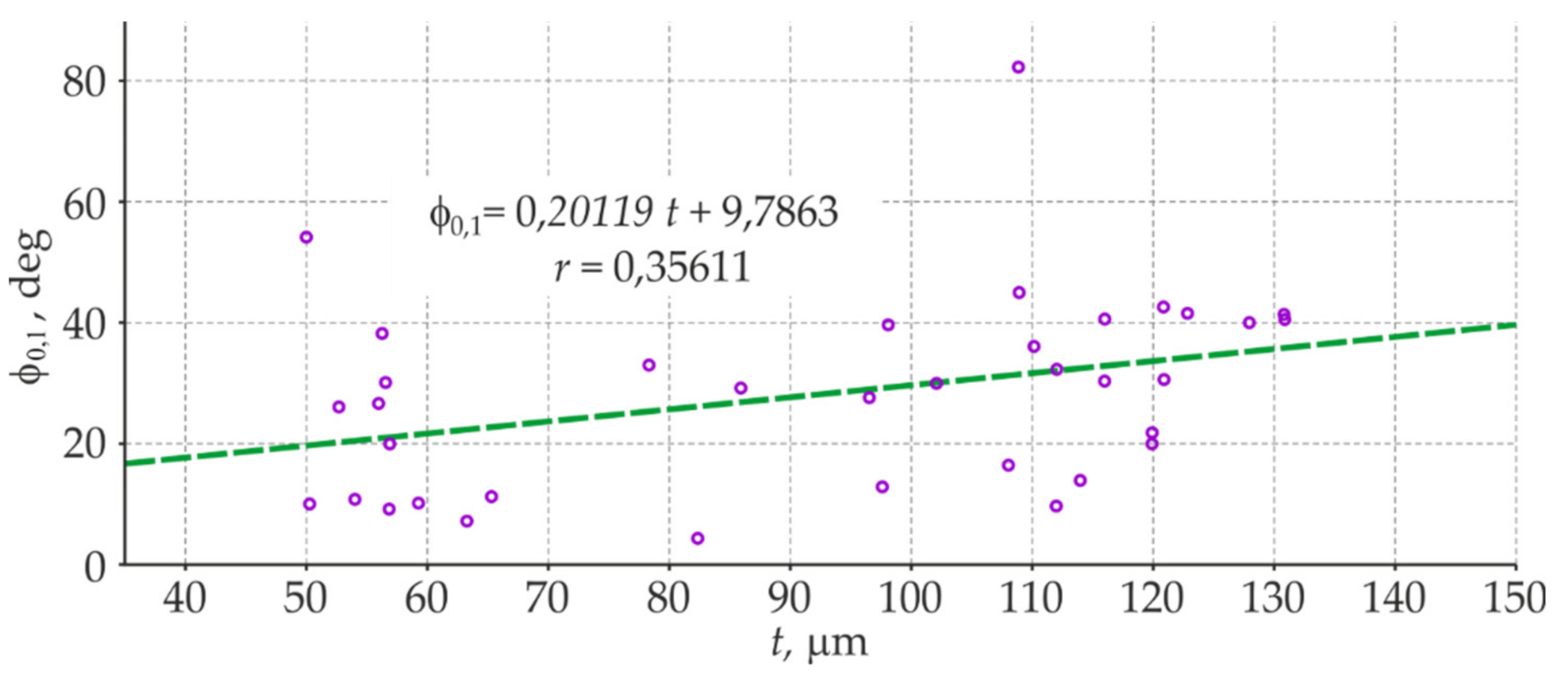
- The coating thickness measured at the same test points with the EIS and electromagnetic gauge (average values of a few measurements) demonstrated a significant relationship and a high correlation between the logarithm of the impedance modulus and the average thickness of the coating. The specified relationship between the phase-shift angle and the mean coating thickness had a low correlation, and thus could not be used as an auxiliary parameter in this methodology.
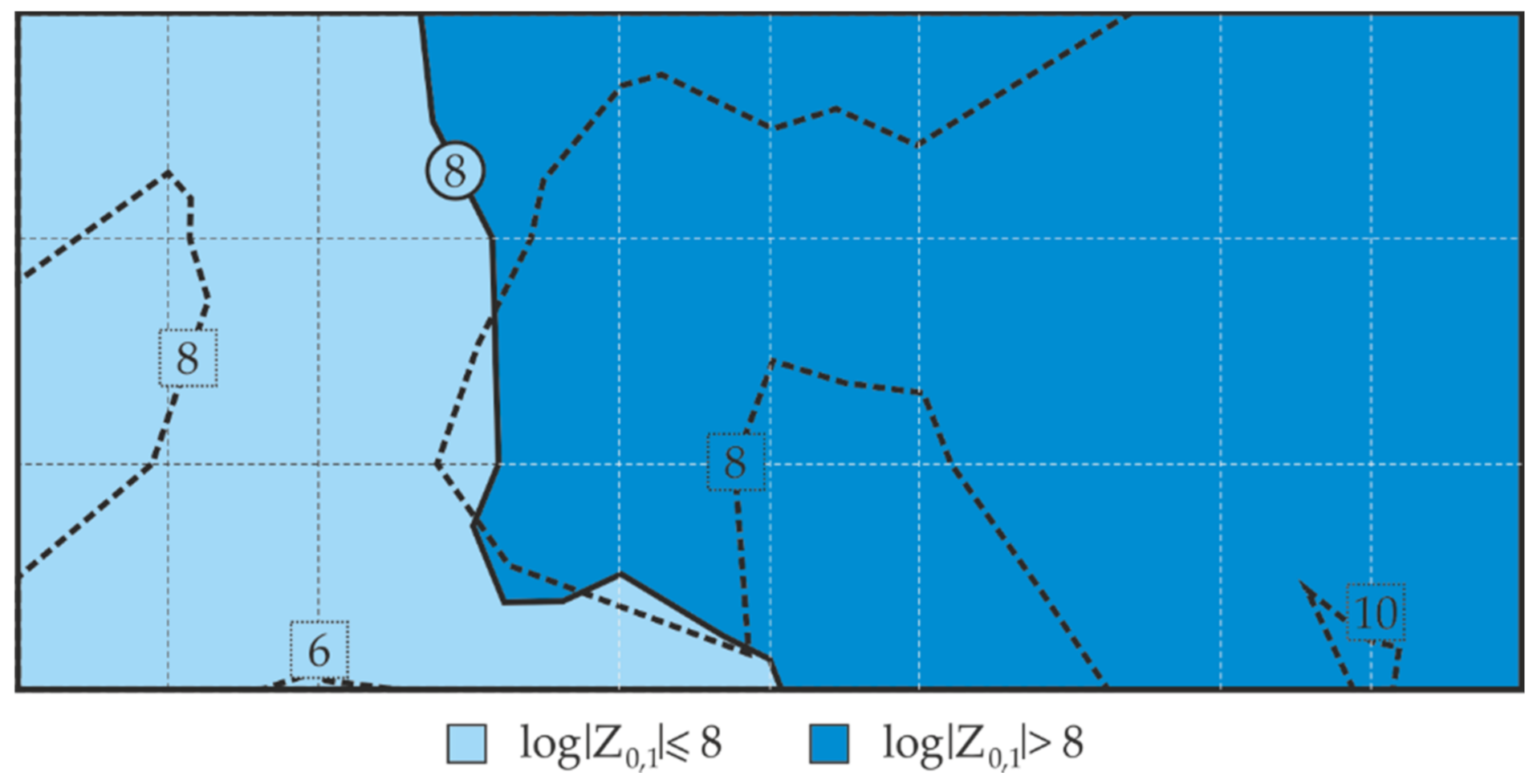
- Following the proposed measurement methodology, a high correlation between the logarithm of the impedance modulus and the mean thickness of the anti-corrosion coating was obtained for parts of the test steel structure and was used to determine the empirical relationships between these parameters. Then, the distribution of the barrier properties of the non-conductive anti-corrosion coating on the whole surface of the test steel structure could be determined on the basis of nothing but quick measurements of the coating thickness.
- The proposed test procedure is currently at the stage of preliminary tests and requires further measurements and analyses. In particular, the tests on the effect of different types of non-conductive coatings and their thickness are required, and on the recommended number of the test points and the number of measurements of the coating thickness at each test point. On the other hand, the presented results and adaptive details of the EIS method for testing large steel structures indicated that this methodology can be recognised as a quantitative method of testing anti-corrosion coatings that is relatively quick compared to currently applied qualitative methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Królikowska, A. Requirements for paint systems for the steel bridges in Poland. Prog. Org. Coat. 2000, 39, 37–39. [Google Scholar] [CrossRef]
- Zubielewicz, M.; Kamińska-bach, G.; Królikowska, A. Metody badań systemów powłokowych do długoletniej ochrony przed korozją. Probl. Kolejnictwa 2016, 170, 103–110. [Google Scholar]
- Ahmed, H.B.; Ramadan, A.M.; Nour, M.A.; El-Malak, S.S.A.; Gomaa, A.E.A.Z. Innovative precursor for manufacturing of superior enhancer of intumescence for paint: Thermal insulative coating for steel structures. Prog. Org. Coat. 2018, 118, 129–140. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X. The Application Research of Coating Thickness Detection Using the Tree Classifier. Adv. Mater. Res. 2011, 230-232, 1034–1038. [Google Scholar] [CrossRef]
- Królikowska, A.; Augustyński, Ł. Evolution of the Requirements for Anticorrosion Protection of Road and Bridge Infrastructure. Transp. Res. Procedia 2016, 14, 4000–4009. [Google Scholar] [CrossRef][Green Version]
- Królikowska, A.; Zubielewicz, M. Wybrane problemy, na które trzeba zwracać uwagę, stosując jako zabezpieczenie antykorozyjne powłokę cynkową zanurzeniową lub system duplex z tą powłoką. Ochrona Przed Korozją 2008, 51, 360–364. [Google Scholar]
- Rani, N.; Singh, A.K.; Alam, S.; Bandyopadhyay, N.; Denys, M.B. Optimization of phosphate coating properties on steel sheet for superior paint performance. J. Coat. Technol. Res. 2012, 9, 629–636. [Google Scholar] [CrossRef]
- Zubielewicz, M.; Kamińska-Tarnawska, E.; Kozłowska, A. Protective properties of organic phosphate-pigmented coatings on phosphated steel substrates. Prog. Org. Coat. 2005, 53, 276–285. [Google Scholar] [CrossRef]
- ISO 4628-1: 2016. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 1: General Introduction and Designation System, 4th ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 4628-2: 2016. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 2: Assessment of Degree of Blistering, 3rd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 4628-3: 2016. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 3: Assessment of Degree of Rusting, 3rd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 4628-4: 2016. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 4: Assessment of Degree of Cracking, 3rd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 4628-5: 2016. Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 5: Assessment of Degree of Flaking, 3rd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 4628-6: 2011. Paints and varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 6: Assessment of Degree of Chalking by Tape Method, 3rd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2011. [Google Scholar]
- ASTM D610-08: 2019. Standard Practice for Evaluation Degree of Rusting on Painted Steel Saces; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM D714-02: 2017. Standard Test Method for Evaluating Degree of Blistering of Paints; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM D5162: 2015. Standard Practice for Discontinuity (Holiday) Testing of Nonconductive Protective Coating on Metallic Substrates; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- McIntyre, J.M.; Pham, H.Q. Electrochemical impedance spectroscopy; a tool for organic coatings optimizations. Prog. Org. Coat. 1996, 27, 201–207. [Google Scholar] [CrossRef]
- Kendig, M.; Scully, J. Basic Aspects of Electrochemical Impedance Application for the Life Prediction of Organic Coatings on Metals. Corrosion 1990, 46, 22–29. [Google Scholar] [CrossRef]
- Mayne, J.E.O. The Mechanism of the Protective Action of Paints, in Corrosion, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 2, pp. 14:22–14:38. [Google Scholar]
- Elsner, C.; Cavalcanti, E.; Ferraz, O.; Di Sarli, A. Evaluation of the surface treatment effect on the anticorrosive performance of paint systems on steel. Prog. Org. Coat. 2003, 48, 50–62. [Google Scholar] [CrossRef]
- Bordziłowski, J. Wykorzystanie elektrochemicznej spektroskopii impedancyjnej w badaniach korozyjnych. Ochrona Przed Korozją 2008, 5s/A, 27–32. [Google Scholar]
- ISO 16773-2: 2016. Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens—Part 2: Collection of Data, 2nd ed.; ISO/TC35/SC9: Geneva, Switzerland, 2016. [Google Scholar]
- Beiro, M.; Collazo, A.; Izquierdo, M.; Nóvoa, X.R.; Pérez, C. Characterisation of barrier properties of organic paints: The zinc phosphate effectiveness. Prog. Org. Coat. 2003, 46, 97–106. [Google Scholar] [CrossRef]
- Mahdavian-Ahadi, M.; Attar, M. Another approach in analysis of paint coatings with EIS measurement: Phase angle at high frequencies. Corros. Sci. 2006, 48, 4152–4157. [Google Scholar] [CrossRef]
- Szociński, M.; Darowicki, K.; Schaefer, K. Application of impedance imaging to evaluation of organic coating degradation at a local scale. J. Coat. Technol. Res. 2012, 10, 65–72. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Multivariate analysis of impedance data obtained for coating systems of varying thickness applied on steel. Prog. Org. Coat. 2014, 77, 2000–2006. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, C.; Song, J.; Deng, C.; Liu, M.; Dai, M. Electrochemical Corrosive Behaviors of Fe-Based Amorphous/Nanocrystalline Coating on Stainless Steel Prepared by HVOF-Sprayed. Coatings 2019, 9, 226. [Google Scholar] [CrossRef]
- Jaśniok, T.; Jaśniok, M.; Zybura, A. Studies on corrosion rate of reinforcement in reinforced concrete water tanks. Ochrona Przed Korozją 2013, 56, 227–234. [Google Scholar]
- Jaśniok, M.; Śliwka, A.; Zybura, A. Zastosowanie pomiarów polaryzacyjnych do oceny stanu zbrojenia żelbetowej podpory wiaduktu. Ochrona Przed Korozją 2010, 53, 220–224. [Google Scholar]
- Jaśniok, T.; Jaśniok, M. Prosta metoda ograniczenia zasięgu polaryzacji w badaniach szybkości korozji zbrojenia w betonie. Ochrona Przed Korozją 2016, 1, 20–23. [Google Scholar] [CrossRef]
- Jaśniok, M.; Kołodziej, J.; Pamuła, M. Corrosion tests of the reinforced concrete crane beam after 60-year operation. Ochrona Przed Korozją 2015, 58, 140–146. [Google Scholar] [CrossRef]
- Jaśniok, T.; Jaśniok, M. Influence of Rapid Changes of Moisture Content in Concrete and Temperature on Corrosion Rate of Reinforcing Steel. Procedia Eng. 2015, 108, 316–323. [Google Scholar] [CrossRef]
- Bordziłowski, J.; Darowicki, K.; Krakowiak, S.; Królikowska, A. Impedance measurements of coating properties on bridge structures. Prog. Org. Coat. 2003, 46, 216–219. [Google Scholar] [CrossRef]
- ISO 2178: 2016. Non-Magnetic Coatings on Magnetic Substrates—Measurement of Coating Thickness—Magnetic Method, 3rd ed.; ISO/TC107: Geneva, Switzerland, 2016. [Google Scholar]
- Faber, M.; Kroon, I.; Sørensen, J. Sensitivities in structural maintenance planning. Reliab. Eng. Syst. Saf. 1996, 51, 317–329. [Google Scholar] [CrossRef]
- Farizhendy, M.M.; Noorzai, E.; Golabchi, M. Implementing the NSGA-II genetic algorithm to select the optimal repair and maintenance method of jack-up drilling rigs in Iranian shipyards. Ocean Eng. 2020, 211, 107548. [Google Scholar] [CrossRef]
- Rackwitz, R. Optimizing systematically renewed structures. Reliab. Eng. Syst. Saf. 2001, 73, 269–279. [Google Scholar] [CrossRef]
- Oetjen, H. Principals and field experience with the 0.1 Hz VLF method regarding the test of medium voltage distribution cables. In Proceedings of the Conference Record of the 2004 IEEE International Symposium on Electrical Insulation, Indianapolis, IN, USA, 19–22 September 2004; pp. 376–379. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Insulation performance evaluation of HV AC/DC XLPE cables by 0.1 Hz tan δ test on circumferentially peeled samples. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3941–3950. [Google Scholar] [CrossRef]
- Binder, A. Considerations of the place of assumptions in correlational analysis. Am. Psychol. 1959, 14, 504–510. [Google Scholar] [CrossRef]
- Vetter, T.R. Fundamentals of Research Data and Variables. Anesthesia Analg. 2017, 125, 1375–1380. [Google Scholar] [CrossRef]
- Kwak, S.K.; Kim, J.H. Statistical data preparation: Management of missing values and outliers. Korean J. Anesthesiol. 2017, 70, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Correlation in restricted ranges of data. BMJ 2011, 342, d556. [Google Scholar] [CrossRef] [PubMed]
- ISO 8501-1: 2007. Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previou, 2nd ed.; ISO/TC 35: Geneva, Switzerland, 2007. [Google Scholar]
- ISO 9223:2012. Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation, 2nd ed.; ISO/TC 156: Geneva, Switzerland, 2012. [Google Scholar]
- Rodgers, J.L.; Nicewander, W.A. Thirteen Ways to Look at the Correlation Coefficient. Am. Stat. 1988, 42, 59. [Google Scholar] [CrossRef]
- Wackerly, D.D.; Mendenhall, W., III; Scheaffer, R.L. Multivariate Probability distributions. In Mathematical Statistics with Applications; Thomson Brooks/Cole: Belmont, CA, USA, 2008; pp. 223–295. [Google Scholar]




| Coating | 1 Layer | 2 Layers | 3 Layers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Row/column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | 57 ± 9 | 56 ± 11 | 59 ± 3 | 112 ± 4 | 108 ± 9 | 114 ± 5 | 131 ± 4 | 131 ± 7 | 116 ± 4 |
| 2 | 53 ± 8 | 57 ± 4 | 65 ± 15 | 97 ± 10 | 110 ± 10 | 112 ± 9 | 128 ± 8 | 121 ± 8 | 121 ± 9 |
| 3 | 56 ± 3 | 57 ± 4 | 54 ± 4 | 98 ± 7 | 98 ± 12 | 102 ± 6 | 123 ± 8 | 109 ± 2 | 120 ± 1 |
| 4 | 50 ± 7 | 50 ± 2 | 63 ± 9 | 82 ± 4 | 78 ± 4 | 86 ± 6 | 116 ± 12 | 109 ± 6 | 120 ± 10 |
| Coating | 1 Layer | 2 Layers | 3 Layers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Row/column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | 6.56 | 7.27 | 6.54 | 7.76 | 7.30 | 6.68 | 8.88 | 9.60 | 9.60 |
| 2 | 7.76 | 8.29 | 6.53 | 8.48 | 8.66 | 8.93 | 9.52 | 9.45 | 8.55 |
| 3 | 8.96 | 7.89 | 7.21 | 9.69 | 7.44 | 7.57 | 9.76 | 9.27 | 9.16 |
| 4 | 7.02 | 6.19 | 5.89 | 6.80 | 7.70 | 6.85 | 8.58 | 10.19 | 8.76 |
| Coating | 1 Layer | 2 Layers | 3 Layers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Row/column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | 19.8 | 26.8 | 10.1 | 9.9 | 16.7 | 14.2 | 41.5 | 40.6 | 40.9 |
| 2 | 26.3 | 30.5 | 11.3 | 27.7 | 36.0 | 32.6 | 40.4 | 42.8 | 30.8 |
| 3 | 38.3 | 9.3 | 10.9 | 39.6 | 13.0 | 30.0 | 41.7 | 45.0 | 22.0 |
| 4 | 10.2 | 54.2 | 7.4 | 4.5 | 33.0 | 29.1 | 30.4 | 82.5 | 20.0 |
| Coating | 1 Layer | 2 Layers | 3 Layers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Row/column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | 6.78 (−3%) | 6.74 (+7%) | 6.86 (−5%) | 8.86 (−14%) | 8.71 (−19%) | 8.94 (−34%) | 9.58 (−8%) | 9.58 (0%) | 9.01 (+6%) |
| 2 | 6.63 (+15%) | 6.78 (+18%) | 7.08 (−8%) | 8.29 (+2%) | 8.79 (−1%) | 8.86 (+1%) | 9.47 (+1%) | 9.20 (+3%) | 9.20 (−8%) |
| 3 | 7.10 (+21%) | 7.13 (+10%) | 7.05 (+2%) | 8.27 (+15%) | 8.27 (−11%) | 8.38 (−11%) | 8.97 (+8%) | 8.58 (+7%) | 8.88 (+3%) |
| 4 | 6.94 (+1%) | 6.94 (−12%) | 7.30 (−24%) | 7.83 (−15%) | 7.72 (0%) | 7.94 (−16%) | 8.77 (−2%) | 8.58 (+16%) | 8.88 (−1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśniok, T.; Jaśniok, M.; Skórkowski, A. Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy. Materials 2021, 14, 3959. https://doi.org/10.3390/ma14143959
Jaśniok T, Jaśniok M, Skórkowski A. Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy. Materials. 2021; 14(14):3959. https://doi.org/10.3390/ma14143959
Chicago/Turabian StyleJaśniok, Tomasz, Mariusz Jaśniok, and Artur Skórkowski. 2021. "Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy" Materials 14, no. 14: 3959. https://doi.org/10.3390/ma14143959
APA StyleJaśniok, T., Jaśniok, M., & Skórkowski, A. (2021). Diagnostics of Large Non-Conductive Anti-Corrosion Coatings on Steel Structures by Means of Electrochemical Impedance Spectroscopy. Materials, 14(14), 3959. https://doi.org/10.3390/ma14143959








