One Step In-Situ Synthesis of Zinc Oxide Nanoparticles for Multifunctional Cotton Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Activation of Cotton
2.3. In-Situ Synthesis of Zinc Oxide on Cotton
2.4. Characterization and Functional Properties
- Eλ = solar spectra irradiance
- Sλ = relative erythemal spectral response
- Δλ = measured wavelength region in nm
- Tλ = average spectral transmittance (%)
3. Results
3.1. Induced Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) Analysis
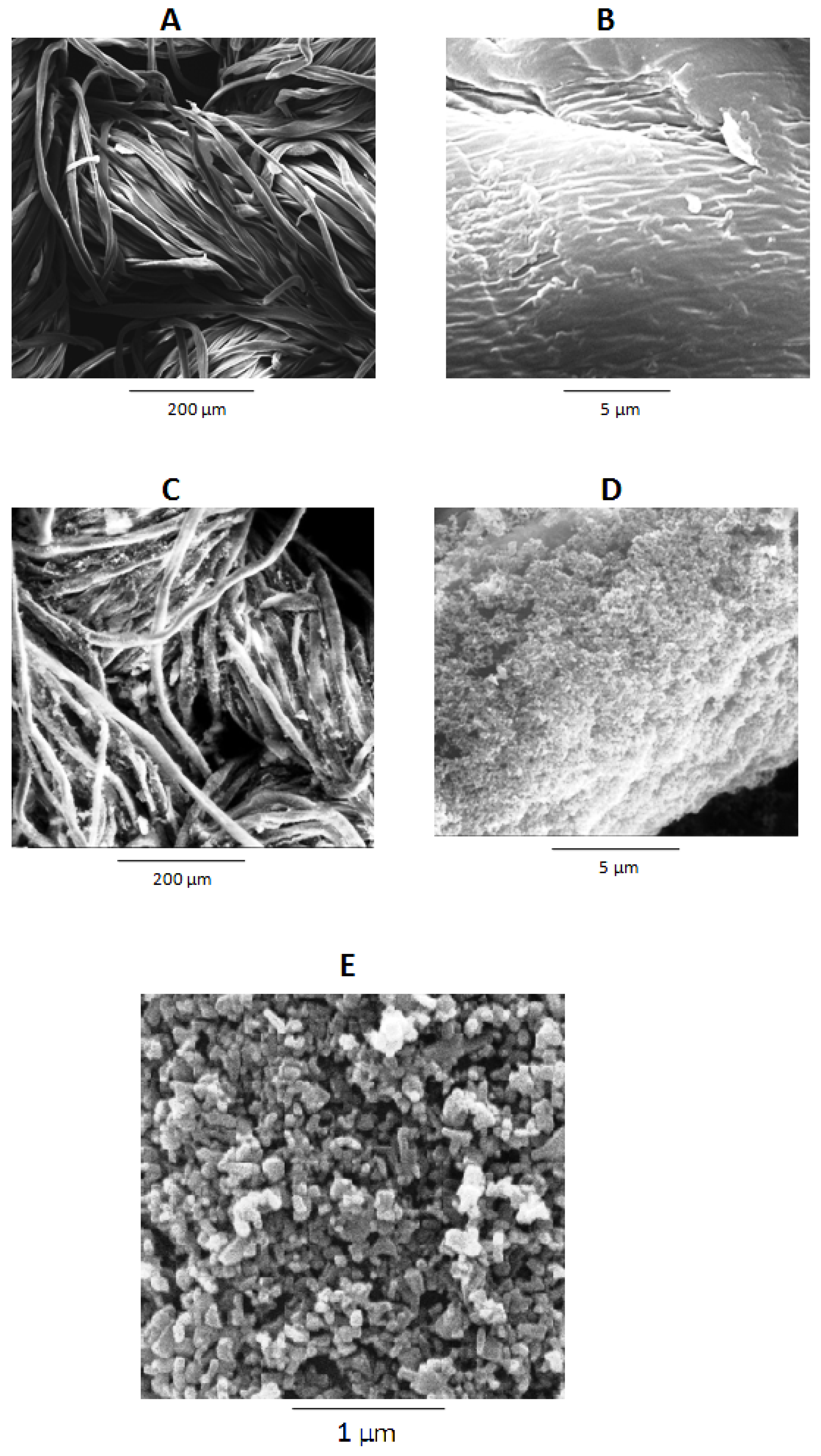
3.2. Surface Morphology
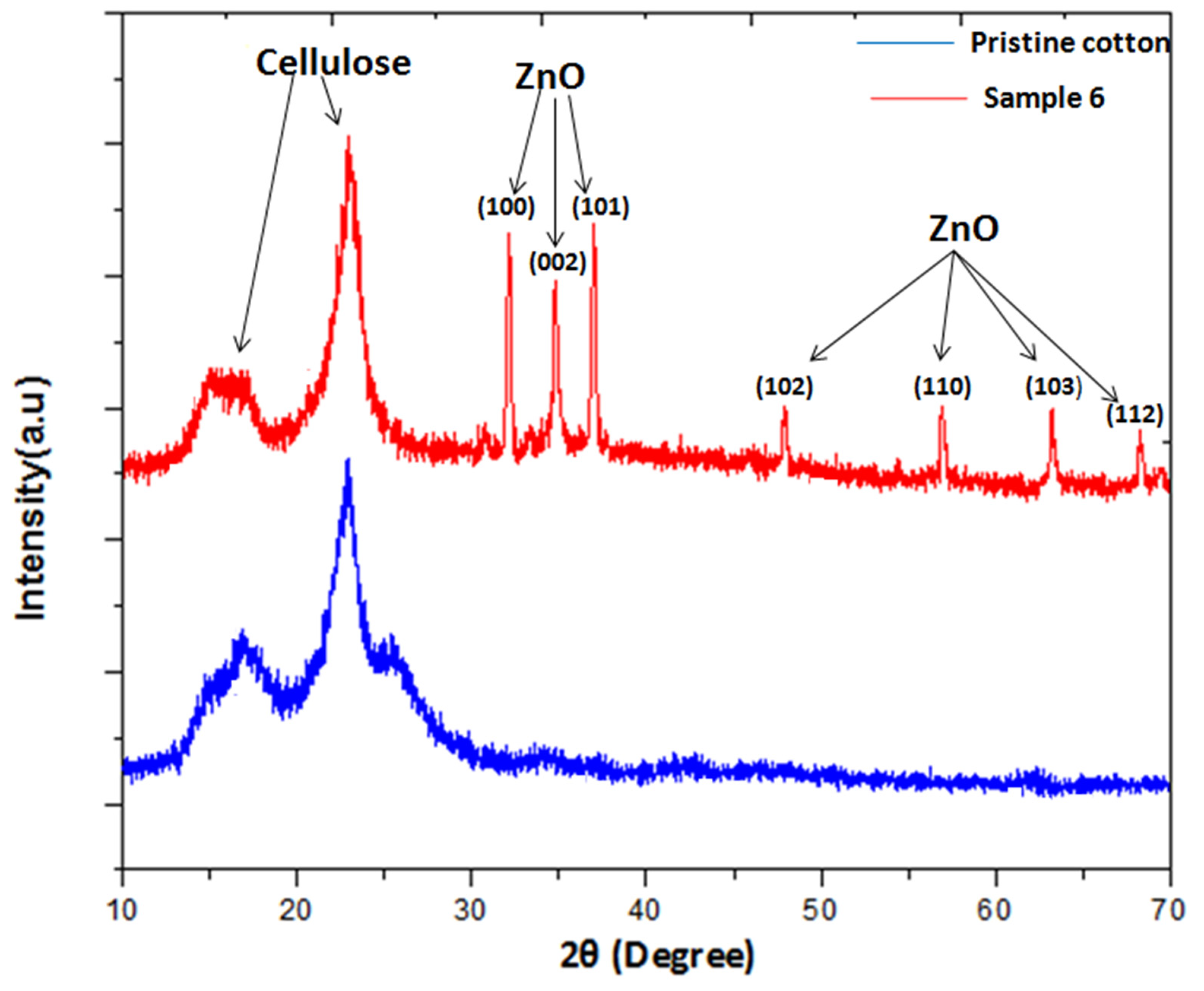
3.3. XRD Analysis
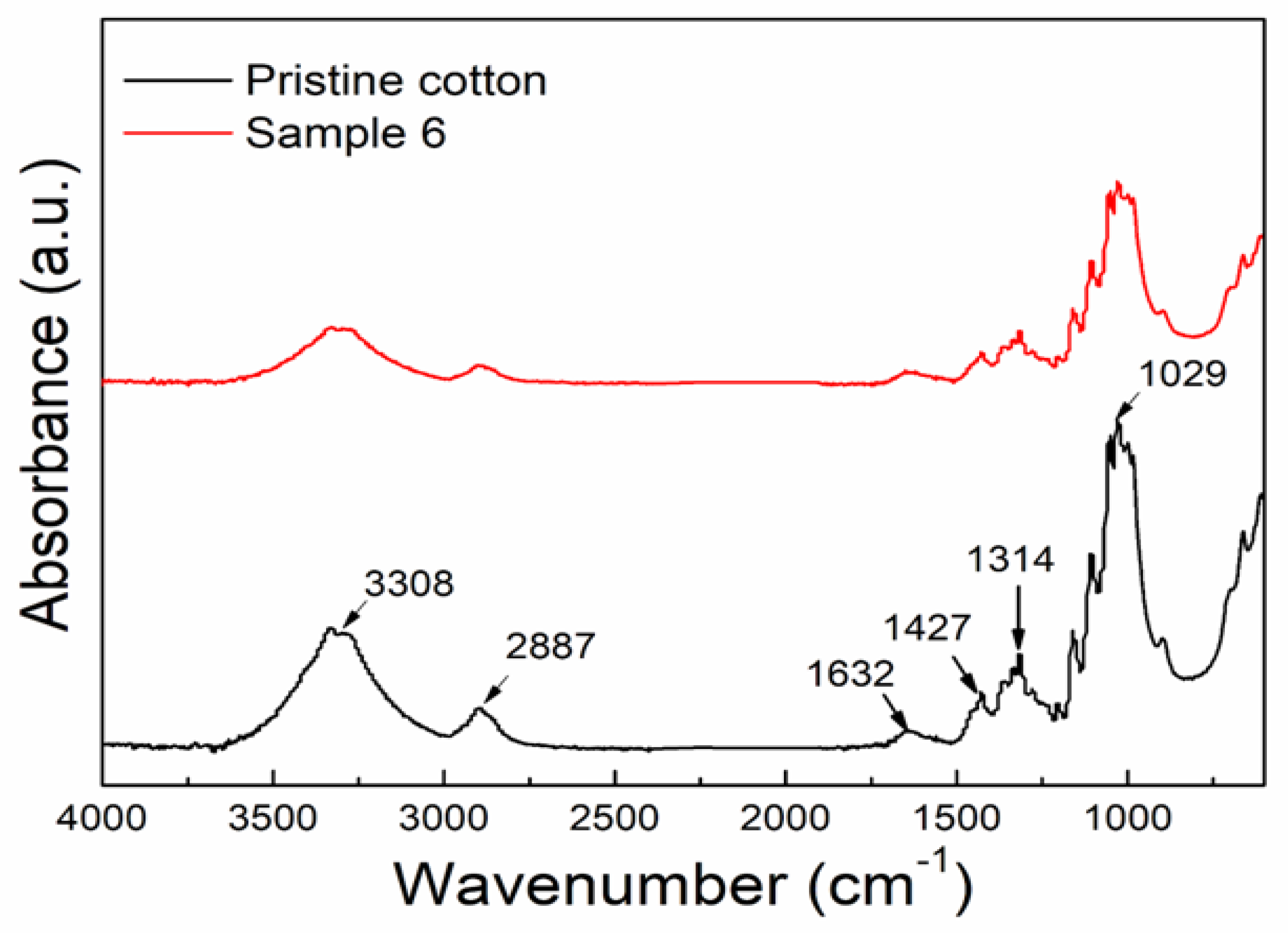
3.4. FTIR Analysis
3.5. UV Protection Factor
3.6. Antibacterial Performance
3.7. Photo Catalytic Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Kadir, A.A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.; Bhattacharyya, A. Nanotechnology—A New Route to High-Performance Functional Textiles. Text. Prog. 2011, 43, 155–233. [Google Scholar] [CrossRef]
- Sawhney, A.P.S.; Condon, B.; Singh, K.V.; Pang, S.S.; Li, G.; Hui, D. Modern Applications of Nanotechnology in Textiles. Text. Res. J. 2008, 78, 731–739. [Google Scholar] [CrossRef]
- Mandai, P.K.; Choi, K.; Min, S.G.; Lee, C.H. Application of Nanotechnology in Food Packaging: An Overview. Korean J. Food Sci. Anim. Resour. 2009, 29, 403–408. [Google Scholar]
- Raut, S.B.; Vasavada, D.A.; Chaudhari, S.B. Nano Particles—Application in Textile Finishing. Man-Made Text. India 2010, 38, 31–36. [Google Scholar]
- Lee, D.; Seo, Y.; Khan, M.S.; Hwang, J.; Jo, Y.; Son, J.; Lee, K.; Park, C.; Chavan, S.; Gilad, A.A.; et al. Use of Nanoscale Materials for the Effective Prevention and Extermination of Bacterial Biofilms. Biotechnol. Bioprocess Eng. 2018, 23, 1–10. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and Multi Metal Oxide Nanoparticles: Synthesis, Antibacterial and Cytotoxic Properties. J. Nanobiotechnol. 2016, 14, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Budama, L.; Çakir, B.A.; Topel, Ö.; Hoda, N. A New Strategy for Producing Antibacterial Textile Surfaces Using Silver Nanoparticles. Chem. Eng. J. 2013, 228, 489–495. [Google Scholar] [CrossRef]
- Ramanujam, K.; Sundrarajan, M. Antibacterial Effects of Biosynthesized MgO Nanoparticles Using Ethanolic Fruit Extract of Emblica Officinalis. J. Photochem. Photobiol. B Biol. 2014, 141, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Radetić, M. Functionalization of Textile Materials with TiO2 Nanoparticles. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Çakir, B.A.; Budama, L.; Topel, Ö.; Hoda, N. Synthesis of ZnO Nanoparticles Using PS-b-PAA Reverse Micelle Cores for UV Protective, Self-Cleaning and Antibacterial Textile Applications. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 414, 132–139. [Google Scholar] [CrossRef]
- Li, S.; Zhu, T.; Huang, J.; Guo, Q.; Chen, G.; Lai, Y. Durable Antibacterial and UV-Protective Ag/TiO2@fabrics for Sustainable Biomedical Application. Int. J. Nanomed. 2017, 12, 2593–2606. [Google Scholar] [CrossRef] [Green Version]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Verbič, A.; Šala, M.; Gorjanc, M. The Influence of in Situ Synthesis Parameters on the Formation of ZnO Nanoparticles and the UPF Value of Cotton Fabric. Tekstilec 2018, 61, 280–288. [Google Scholar] [CrossRef]
- Nguyen, H.T.P.; Nguyen, T.M.T.; Hoang, C.N.; Le, T.K.; Lund, T.; Nguyen, H.K.H.; Huynh, T.K.X. Characterization and Photocatalytic Activity of New Photocatalysts Based on Ag, F-Modified ZnO Nanoparticles Prepared by Thermal Shock Method. Arab. J. Chem. 2020, 13, 1837–1847. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc Oxide Particles: Synthesis, Properties and Applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Zhou, G.C.; Sun, L.Z.; Zhong, X.L.; Chen, X.; Wei, L.; Wang, J.B. First-Principle Study on Bonding Mechanism of ZnO by LDA + U Method. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2007, 368, 112–116. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-from Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, Defects, and Devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Ramamurthi, K.; Ramesh Babu, R.; Sethuraman, K. Visible Light Driven Photocatalytic Activity of ZnO/CuO Nanocomposites Coupled with RGO Heterostructures Synthesized by Solid-State Method for RhB Dye Degradation. Arab. J. Chem. 2020, 13, 3910–3928. [Google Scholar] [CrossRef]
- Le, T.K.; Nguyen, T.M.T.; Nguyen, H.T.P.; Nguyen, T.K.L.; Lund, T.; Nguyen, H.K.H.; Huynh, T.K.X. Enhanced Photocatalytic Activity of ZnO Nanoparticles by Surface Modification with KF Using Thermal Shock Method. Arab. J. Chem. 2020, 13, 1032–1039. [Google Scholar] [CrossRef]
- Barrak, H.; Saied, T.; Chevallier, P.; Laroche, G.; M’nif, A.; Hamzaoui, A.H. Synthesis, Characterization, and Functionalization of ZnO Nanoparticles by N-(Trimethoxysilylpropyl) Ethylenediamine Triacetic Acid (TMSEDTA): Investigation of the Interactions between Phloroglucinol and ZnO@TMSEDTA. Arab. J. Chem. 2019, 12, 4340–4347. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, B.; Endo, T.; Kaneko, S.; Murali, K.R.; John, R. Properties of Sol Gel Synthesized ZnO Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 9474–9485. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Parihar, V.; Raja, M.; Paulose, R. A Brief Review of Structural, Electrical and Electrochemical Properties of Zinc Oxide Nanoparticles. Rev. Adv. Mater. Sci. 2018, 53, 119–130. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Ali, A.; Phull, A.R.; Zia, M. Elemental Zinc to Zinc Nanoparticles: Is ZnO NPs Crucial for Life? Synthesis, Toxicological, and Environmental Concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc Oxide Nanoparticles: A Review of Their Biological Synthesis, Antimicrobial Activity, Uptake, Translocation and Biotransformation in Plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 3527623957. [Google Scholar]
- Gorjanc, M.; Jazbec, K.; Zaplotnik, R.; Vesel, A.; Mozetic, M. Creating Cellulose Fibres with Excellent UV Protective Properties Using Moist CF 4 Plasma and ZnO Nanoparticles. Cellulose 2014, 21, 3007–3021. [Google Scholar] [CrossRef]
- Jazbec, K.; Šala, M.; Mozetič, M.; Vesel, A.; Gorjanc, M. Functionalization of Cellulose Fibres with Oxygen Plasma and ZnO Nanoparticles for Achieving UV Protective Properties. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Primc, G.; Tom, B.; Vesel, A.; Mozeti, M. Biodegradability of Oxygen-Plasma Treated Cellulose Textile Functionalized with ZnO Nanoparticles as Antibacterial Treatment. J. Phys. D Appl. Phys. 2016, 49, 324002. [Google Scholar] [CrossRef]
- Hong, G.; Kim, J.; Lee, J.; Shin, K.; Jung, D.; Kim, J. A Flexible Tactile Sensor Using Seedless Hydrothermal Growth of Zno Nanorods on Fabrics. J. Phys. Commun. 2020, 4, 045002. [Google Scholar] [CrossRef]
- Baruah, B.; Downer, L.; Agyeman, D. Fabric-Based Composite Materials Containing ZnO-NRs and ZnO-NRs-AuNPs and Their Application in Photocatalysis. Mater. Chem. Phys. 2019, 231, 252–259. [Google Scholar] [CrossRef]
- Nurelissa, I.; Ramzuz, M.; Zakaria, N.; Zain, Z.M. Synthesis and Characterization of Zinc Oxide Nanostructures in Biosensor Application. Int. J. Biosens. Bioelectron. 2020, 6, 48–54. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, T.I.; El-Naggar, M.E.; Abdelgawad, A.M.; Hebeish, A. Durable Antibacterial and UV Protections of in Situ Synthesized Zinc Oxide Nanoparticles onto Cotton Fabrics. Int. J. Biol. Macromol. 2016, 83, 426–432. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shaarawy, S.; Hebeish, A.A. Multifunctional Properties of Cotton Fabrics Coated with in Situ Synthesis of Zinc Oxide Nanoparticles Capped with Date Seed Extract. Carbohydr. Polym. 2018, 181, 307–316. [Google Scholar] [CrossRef]
- Zhang, G.; Morikawa, H.; Chen, Y.; Miura, M. In-Situ Synthesis of ZnO Nanoparticles on Bamboo Pulp Fabric. Mater. Lett. 2013, 97, 184–186. [Google Scholar] [CrossRef]
- Ali, A.; Baheti, V.; Vik, M.; Militky, J. Copper Electroless Plating of Cotton Fabrics after Surface Activation with Deposition of Silver and Copper Nanoparticles. J. Phys. Chem. Solids 2020, 137, 109181. [Google Scholar] [CrossRef]
- Scherrer, P. Göttinger Nachrichten Math. Phys 1918, 2, 98–100. [Google Scholar]
- Mansoor, T.; Hes, L.; Skenderi, Z.; Siddique, H.F.; Hussain, S.; Javed, A. Effect of Preheat Setting Process on Heat, Mass and Air Transfer in Plain Socks. J. Text. Inst. 2019, 110, 159–170. [Google Scholar] [CrossRef]
- Ran, J.; He, M.; Li, W.; Cheng, D.; Wang, X. Growing ZnO Nanoparticles on Polydopamine-Templated Cotton Fabrics for Durable Antimicrobial Activity and UV Protection. Polymers 2018, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Jiao, L.; Ma, J.; Dai, H. Preparation and Characterization of Self-Reinforced Antibacterial and Oil-Resistant Paper Using a NaOH/Urea/ZnO Solution. PLoS ONE 2015, 10, e0140603. [Google Scholar] [CrossRef]
- Sun, X.Z.; Bremner, D.H.; Wan, N.; Wang, X. Development of Antibacterial ZnO-Loaded Cotton Fabric Based on in Situ Fabrication. Appl. Phys. A Mater. Sci. Process. 2016, 122, 940. [Google Scholar] [CrossRef]
- Becker, J.; Raghupathi, K.R.; Pierre, J.S.; Zhao, D.; Koodali, R.T. Tuning of the Crystallite and Particle Sizes of ZnO Nanocrystalline Materials in Solvothermal Synthesis and Their Photocatalytic Activity for Dye Degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Trenque, I.; Mornet, S.; Duguet, E.; Gaudon, M. New Insights into Crystallite Size and Cell Parameters Correlation for ZnO Nanoparticles Obtained from Polyol-Mediated Synthesis. Inorg. Chem. 2013, 52, 12811–12817. [Google Scholar] [CrossRef] [PubMed]
- Khorsand Zak, A.; Razali, R.; Abd Majid, W.H.; Darroudi, M. Synthesis and Characterization of a Narrow Size Distribution of Zinc Oxide Nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, P.M.; Balaji, S.; Prabhawathi, V.; Neelakandan, R.; Manoharan, P.T.; Doble, M. Effective Antibacterial Adhesive Coating on Cotton Fabric Using ZnO Nanorods and Chalcone. Carbohydr. Polym. 2010, 79, 717–723. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of Cotton Fabric Scouring by FT-IR ATR Spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Javed, A.; Azeem, M.; Wiener, J.; Thukkaram, M.; Saskova, J.; Mansoor, T. Ultrasonically Assisted In Situ Deposition of ZnO Nano Particles on Cotton Fabrics for Multifunctional Textiles. Fibers Polym. 2021. [Google Scholar] [CrossRef]
- Shao, D.; Gao, Y.; Cao, K.; Wei, Q. Rapid Surface Functionalization of Cotton Fabrics by Modified Hydrothermal Synthesis of ZnO. J. Text. Inst. 2017, 108, 1391–1397. [Google Scholar] [CrossRef]
- Wu, D.; Long, M.; Zhou, J.; Cai, W.; Zhu, X.; Chen, C.; Wu, Y. Synthesis and Characterization of Self-Cleaning Cotton Fabrics Modified by TiO2 through a Facile Approach. Surf. Coatings Technol. 2009, 203, 3728–3733. [Google Scholar] [CrossRef]
- Javed, A.; Azeem, M.; Saskova, J. P024_0663_ UV Protective Fabrics by Application of Ball Milled Neem Tree Leaves. In Proceedings of the 19th World Textile Conference-Autex 2019, Ghent, Belgium, 11–15 June 2019; p. 3. [Google Scholar]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic Effects of Ultraviolet Radiation on the Skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Young, A.R. Acute Effects of UVR on Human Eyes and Skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Piacentini, R.D. The Importance of the Use of Clothes with Solar UV Protection. Curr. Trends Fash. Technol. Text. Eng. 2018, 3, 96–100. [Google Scholar] [CrossRef]
- Gies, P.; Slevin, T.; Harrison, S.; Plowman, P.; Dain, S.; Moller, L.; Mawley, F.; Swift, N. Australian/New Zealand Standard, AS/NZS 4399: 2017: Sun Protective Clothing–Evaluation and Classification; Standards Australia: Sydney, Australia, 2017; ISBN 1760358843. [Google Scholar]
- Alebeid, O.K.; Zhao, T. Review on: Developing UV Protection for Cotton Fabric. J. Text. Inst. 2017, 108, 2027–2039. [Google Scholar] [CrossRef]
- Khan, M.Z.; Militky, J.; Baheti, V.; Fijalkowski, M.; Wiener, J.; Voleský, L.; Adach, K. Growth of ZnO Nanorods on Cotton Fabrics via Microwave Hydrothermal Method: Effect of Size and Shape of Nanorods on Superhydrophobic and UV-Blocking Properties. Cellulose 2020, 27, 10519–10539. [Google Scholar] [CrossRef]
- Anita, S.; Ramachandran, T.; Rajendran, R.; Koushik, C.V.; Mahalakshmi, M. Preparation and Characterization of Zinc Oxide Nanoparticles and a Study of the Anti-Microbial Property of Cotton Fabric Treated with the Particles. J. Text. Appar. Technol. Manag. 2010, 6. Available online: https://ojs.cnr.ncsu.edu/index.php/JTATM/article/view/899/723 (accessed on 15 July 2021).
- Souza, D.A.R.; Gusatti, M.; Ternus, R.Z.; Fiori, M.A.; Riella, H.G. In Situ Growth of ZnO Nanostructures on Cotton Fabric by Solochemical Process for Antibacterial Purposes. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and Antimicrobial Activity of ZnO-NPs Coated Cotton/Starch and Their Functionalized ZnO-Ag/Cotton and Zn(II) Curcumin/Cotton Materials. Sci. Rep. 2020, 10, 5410. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.H.; Shim, K.D.; Kim, D.J.; Jhee, K.H.; Lee, H.W.; Heo, C.H.; Kim, H.M.; Jang, E.S. Antibacterial Mechanism of ZnO Nanoparticles under Dark Conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc Oxide Nanostructure-Modified Textile and Its Application to Biosensing, Photocatalysis, and as Antibacterial Material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef] [PubMed]
- Sudrajat, H. Superior Photocatalytic Activity of Polyester Fabrics Coated with Zinc Oxide from Waste Hot Dipping Zinc. J. Clean. Prod. 2018, 172, 1722–1729. [Google Scholar] [CrossRef]
- Shaban, M.; Abdallah, S.; Khalek, A.A. Characterization and Photocatalytic Properties of Cotton Fibers Modified with ZnO Nanoparticles Using Sol–Gel Spin Coating Technique. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 277–283. [Google Scholar] [CrossRef] [Green Version]

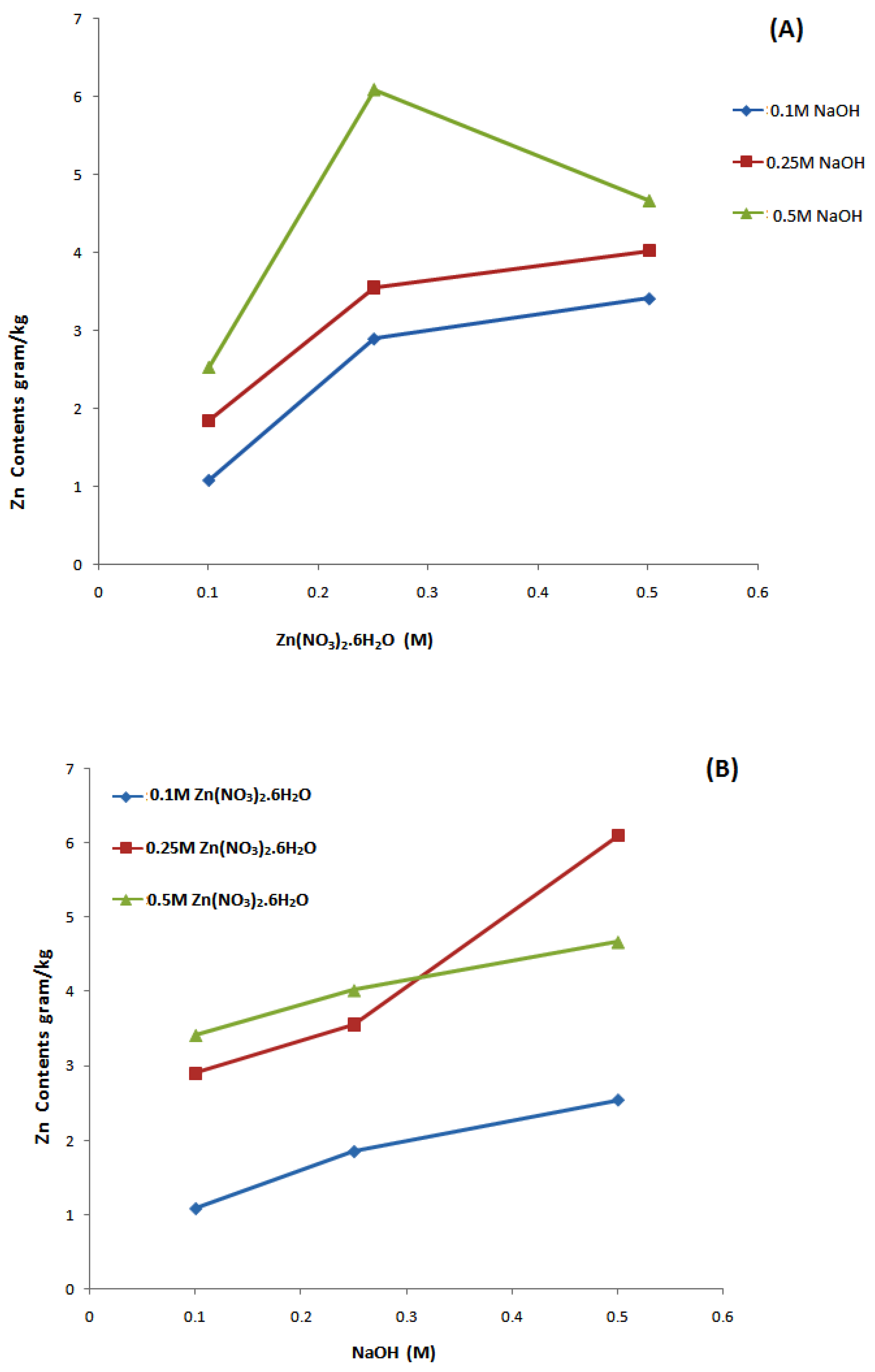
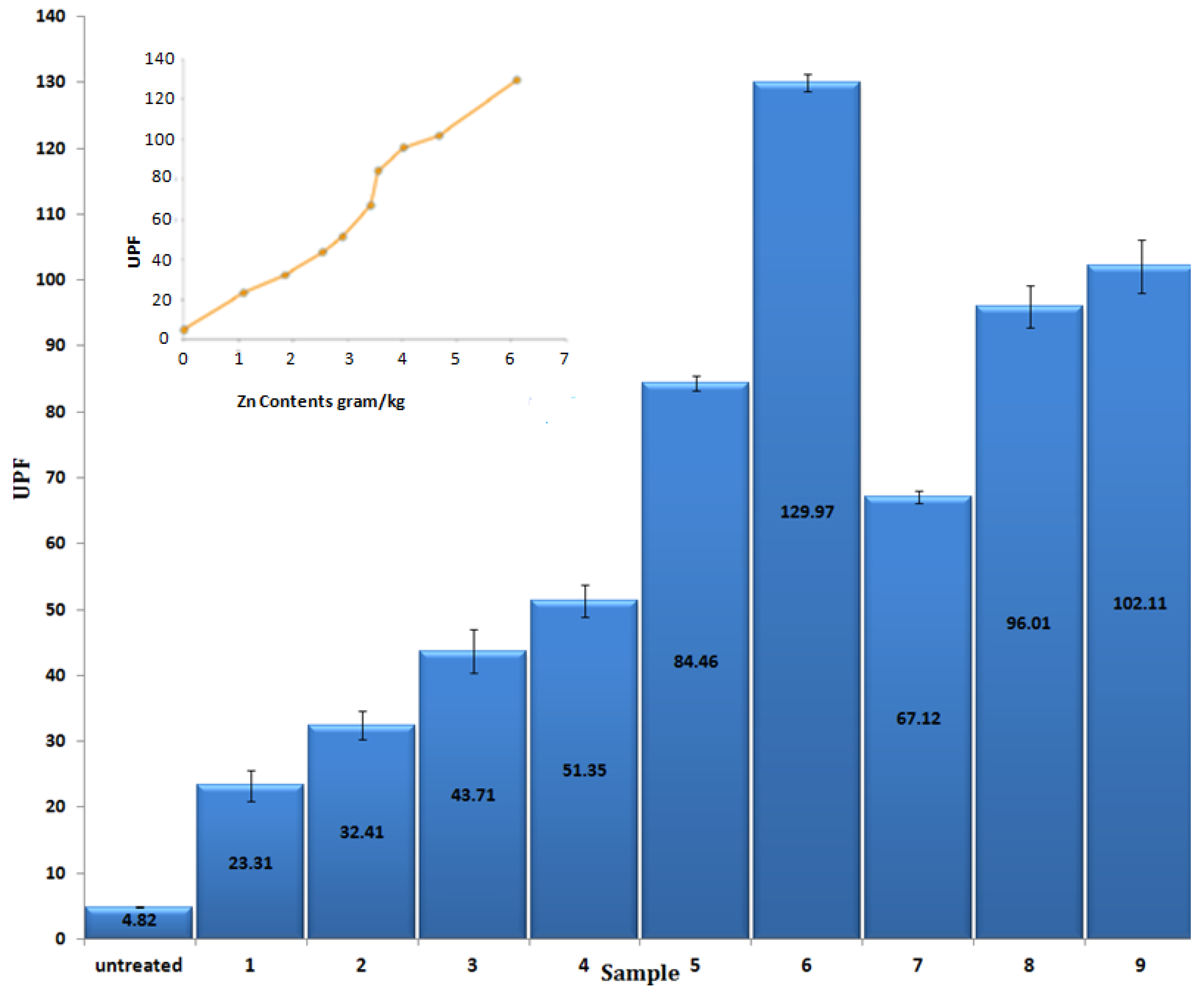
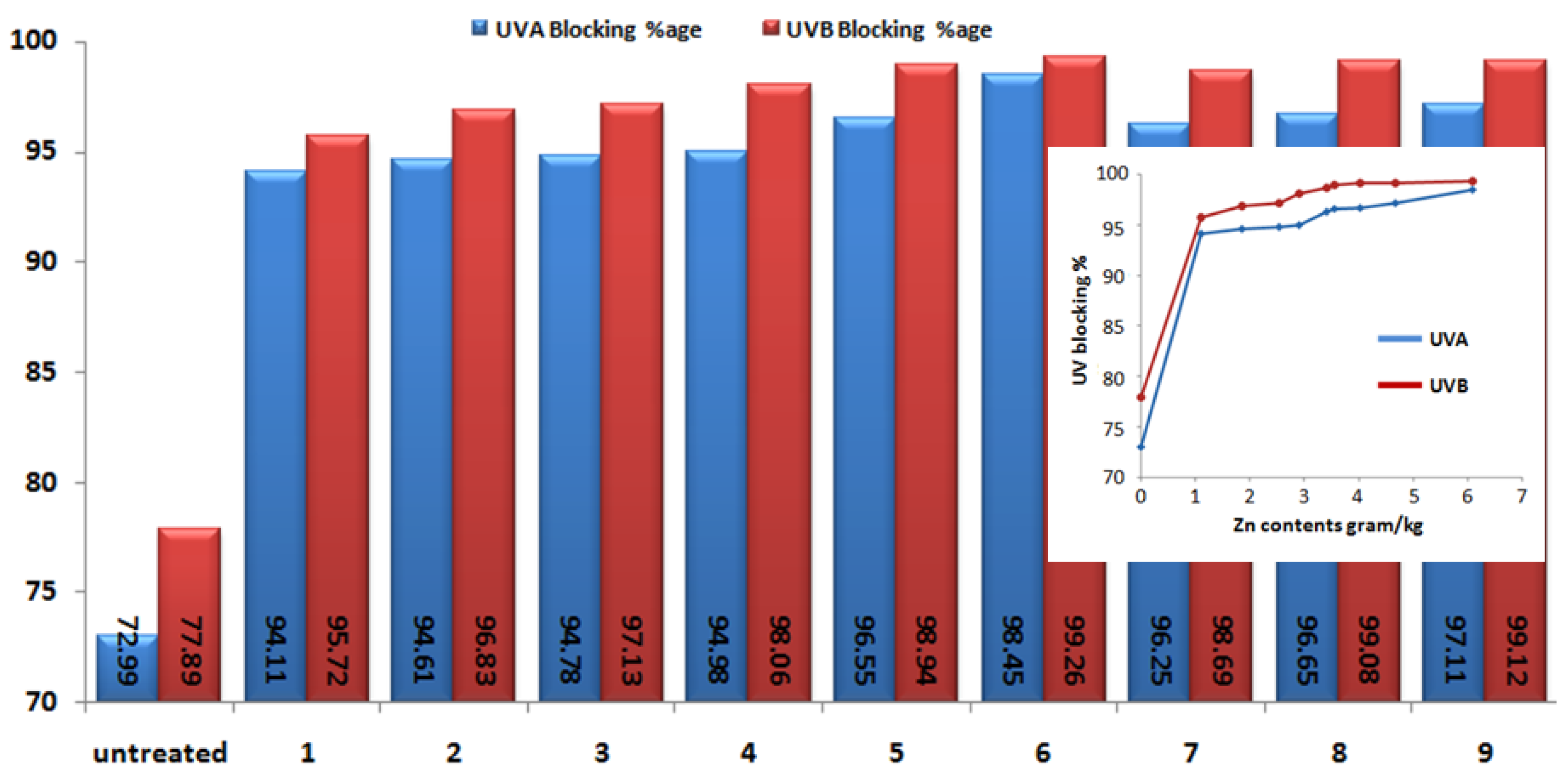
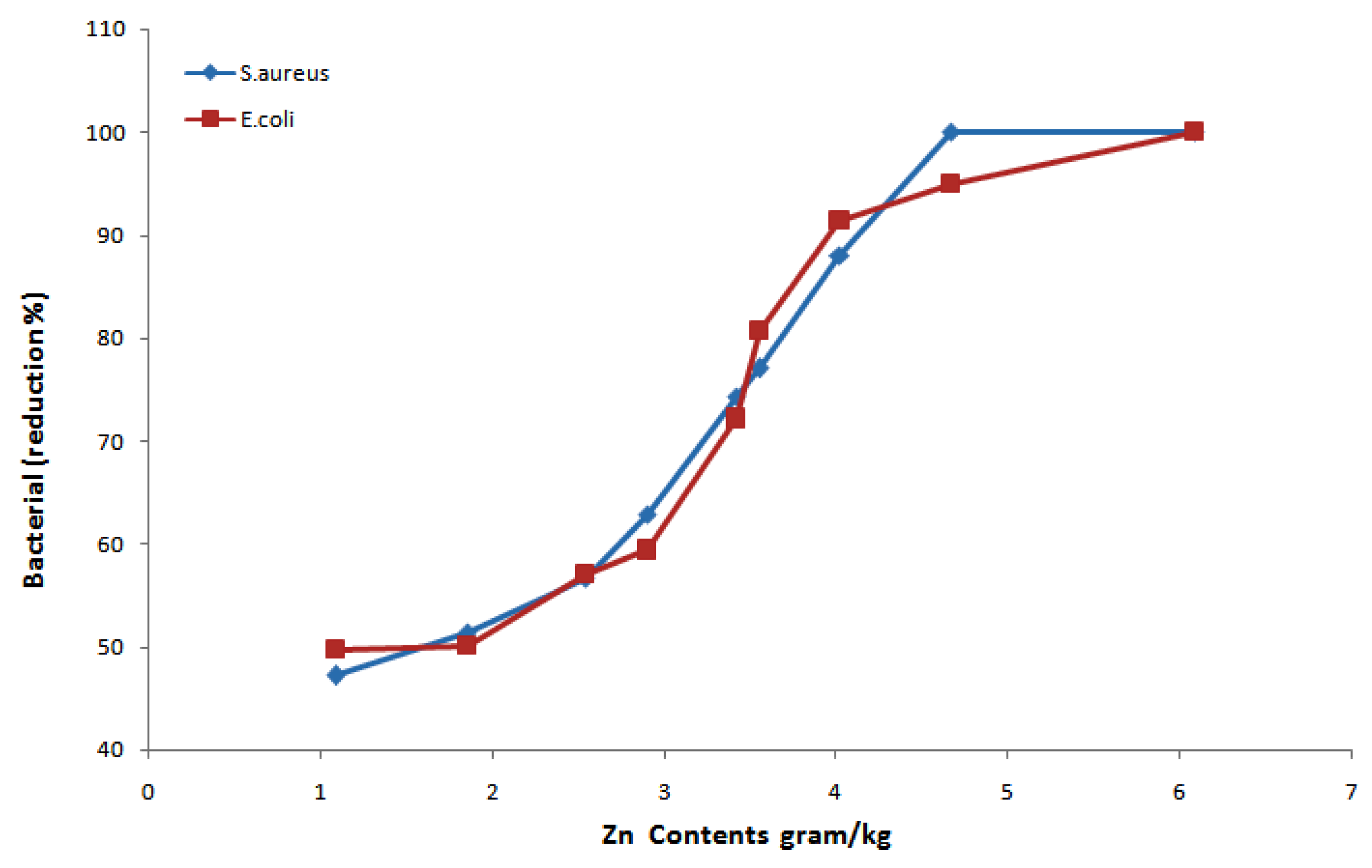


| Sample No. | Zn(NO3)2·6H2O (M) | NaOH (M) | Deposited Amount of Zn Contents onto Fabric (gram/kg) | Standard Deviation |
|---|---|---|---|---|
| Untreated | - | - | - | |
| 1 | 0.1 | 0.1 | 1.089 | 0.041 |
| 2 | 0.1 | 0.25 | 1.851 | 0.034 |
| 3 | 0.1 | 0.5 | 2.541 | 0.061 |
| 4 | 0.25 | 0.1 | 2.902 | 0.052 |
| 5 | 0.25 | 0.25 | 3.553 | 0.071 |
| 6 | 0.25 | 0.5 | 6.091 | 0.095 |
| 7 | 0.5 | 0.1 | 3.418 | 0.064 |
| 8 | 0.5 | 0.25 | 4.021 | 0.066 |
| 9 | 0.5 | 0.5 | 4.671 | 0.083 |
| Method | Precursors | Crystallite Size in nm (XRD) | Ref. |
|---|---|---|---|
| One Step Hydrothermal | Zn(NO3)2·6H2O, NaOH | 20.3–22.4 | This study |
| Solvothermal | Zn(NO3)2·6H2O, tetramethylammonium hydroxide | 4.1 | [51] |
| Precipitation Method | Zinc acetate, Diethylene glycol | 5–35 | [52] |
| Solvothermal | Zinc acetate, Triethanolamine | 33±2 | [53] |
| Precursors | Size of ZnO NPs | Method | UPF | UVA Blocking (%) | UVB Blocking (%) | Mass Load Zn (g/kg) | Ref. |
|---|---|---|---|---|---|---|---|
| Zn(NO3)2·6H2O, NaOH | 20.3–22.4 (by XRD) | One step hydrothermal | 129.97 | 97.11 | 99.12 | 6.091 | This work |
| ZnCl2,NaOH | - | One step hydrothermal | 80.2 | 92.6 | 99.1 | 0.71 | [18] |
| Zn(NO3)2·6H2O, C6H12N4 | 1760 ± 120 (by SEM) | Two step hydrothermal | 157.8 | 99.54 | 99.69 | - | [48] |
| Zn(NO3)2·6H2O, Hexamethyleneteraamine | 55 ± 6.1 (by SEM) | Two step hydrothermal | 114 | 95.8 | 99.3 | - | [66] |
| Sample No. | Zn Contents (g/kg) | S. aureus (Reduction %) | E. coli (Reduction %) | Ref. |
|---|---|---|---|---|
| 1 | 1.089 | 47.23 | 49.76 | |
| 2 | 1.851 | 51.41 | 50.12 | |
| 3 | 2.541 | 56.67 | 57.03 | |
| 4 | 2.902 | 62.85 | 59.43 | This study |
| 5 | 3.553 | 77.06 | 80.65 | |
| 6 | 6.091 | 100.00 | 100.00 | |
| 7 | 3.418 | 74.31 | 72.17 | |
| 8 | 4.021 | 88.09 | 91.47 | |
| 9 | 4.671 | 100.00 | 94.96 | |
| Wet chemical method | - | >99.99 | 80 | [67] |
| Solochemical process | - | 100 | - | [68] |
| Ultrasonic Irradiation Process | - | 100 | 100 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, A.; Wiener, J.; Tamulevičienė, A.; Tamulevičius, T.; Lazauskas, A.; Saskova, J.; Račkauskas, S. One Step In-Situ Synthesis of Zinc Oxide Nanoparticles for Multifunctional Cotton Fabrics. Materials 2021, 14, 3956. https://doi.org/10.3390/ma14143956
Javed A, Wiener J, Tamulevičienė A, Tamulevičius T, Lazauskas A, Saskova J, Račkauskas S. One Step In-Situ Synthesis of Zinc Oxide Nanoparticles for Multifunctional Cotton Fabrics. Materials. 2021; 14(14):3956. https://doi.org/10.3390/ma14143956
Chicago/Turabian StyleJaved, Asif, Jakub Wiener, Asta Tamulevičienė, Tomas Tamulevičius, Algirdas Lazauskas, Jana Saskova, and Simas Račkauskas. 2021. "One Step In-Situ Synthesis of Zinc Oxide Nanoparticles for Multifunctional Cotton Fabrics" Materials 14, no. 14: 3956. https://doi.org/10.3390/ma14143956






