Impact of Humidity and Temperature on the Stability of the Optical Properties and Structure of MAPbI3, MA0.7FA0.3PbI3 and (FAPbI3)0.95(MAPbBr3)0.05 Perovskite Thin Films
Abstract
:1. Introduction
2. Experimental Details
2.1. Perovskite Film Preparation
2.2. Real Time Spectroscopic Ellipsometry Data Collection and Analysis
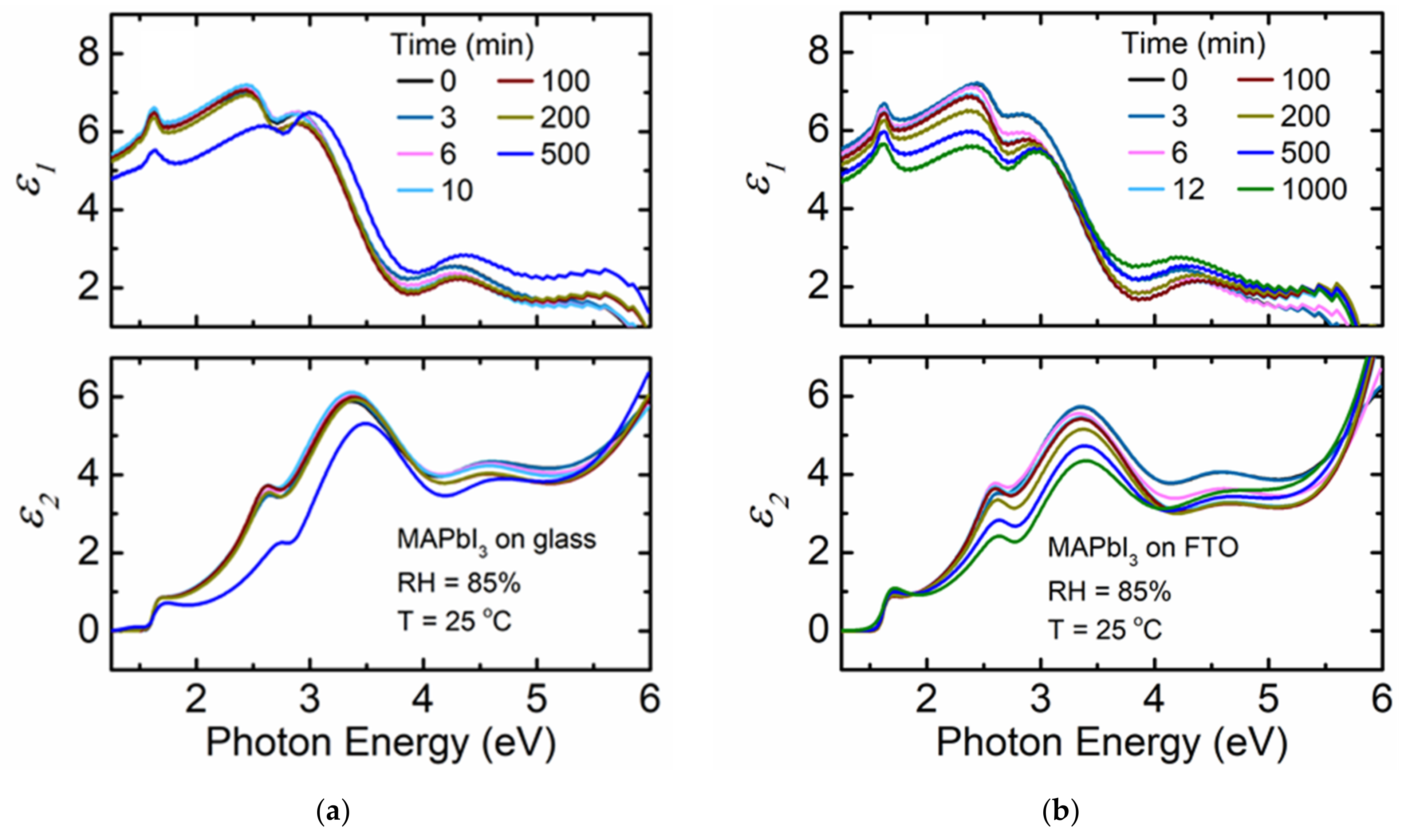
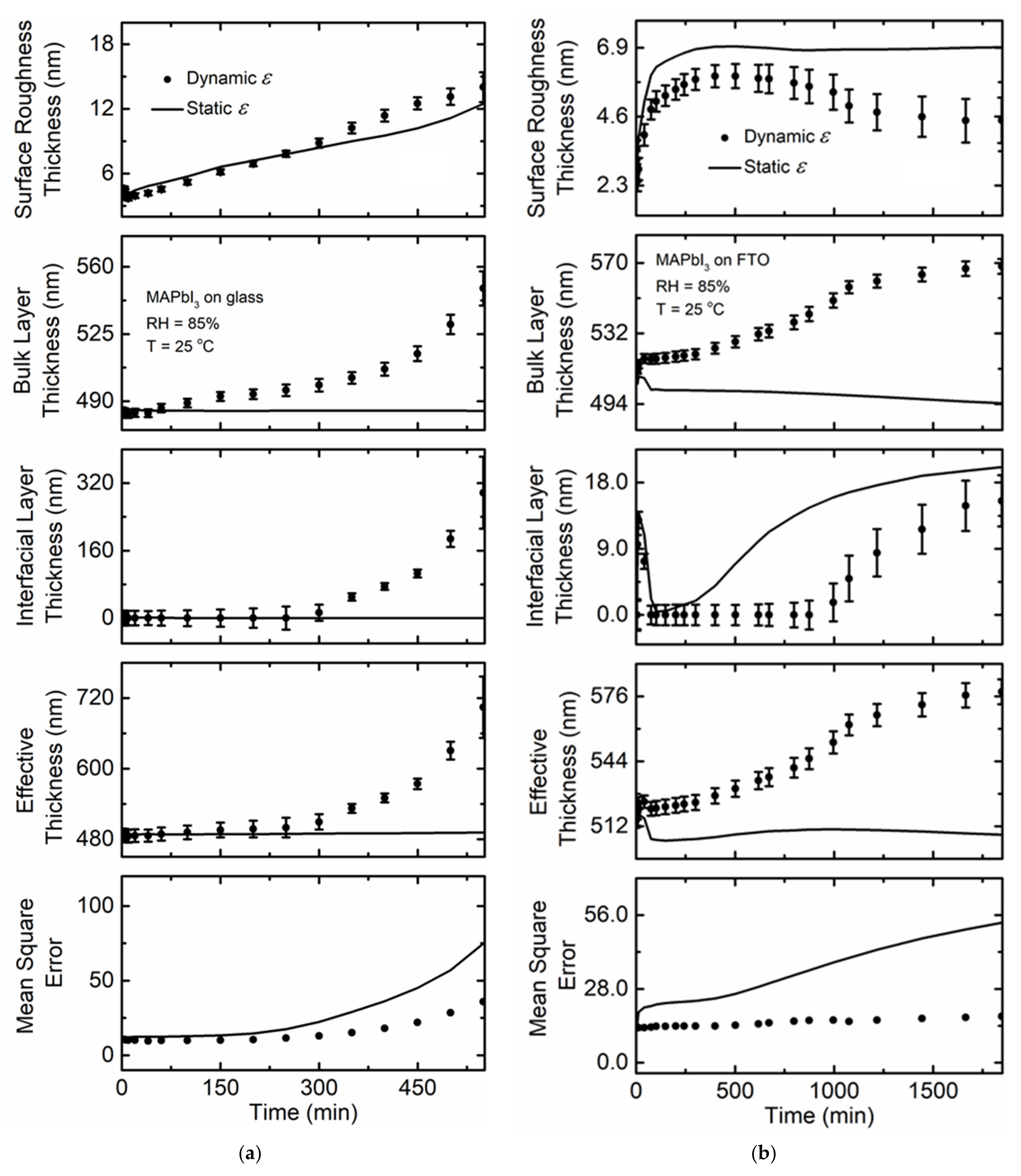
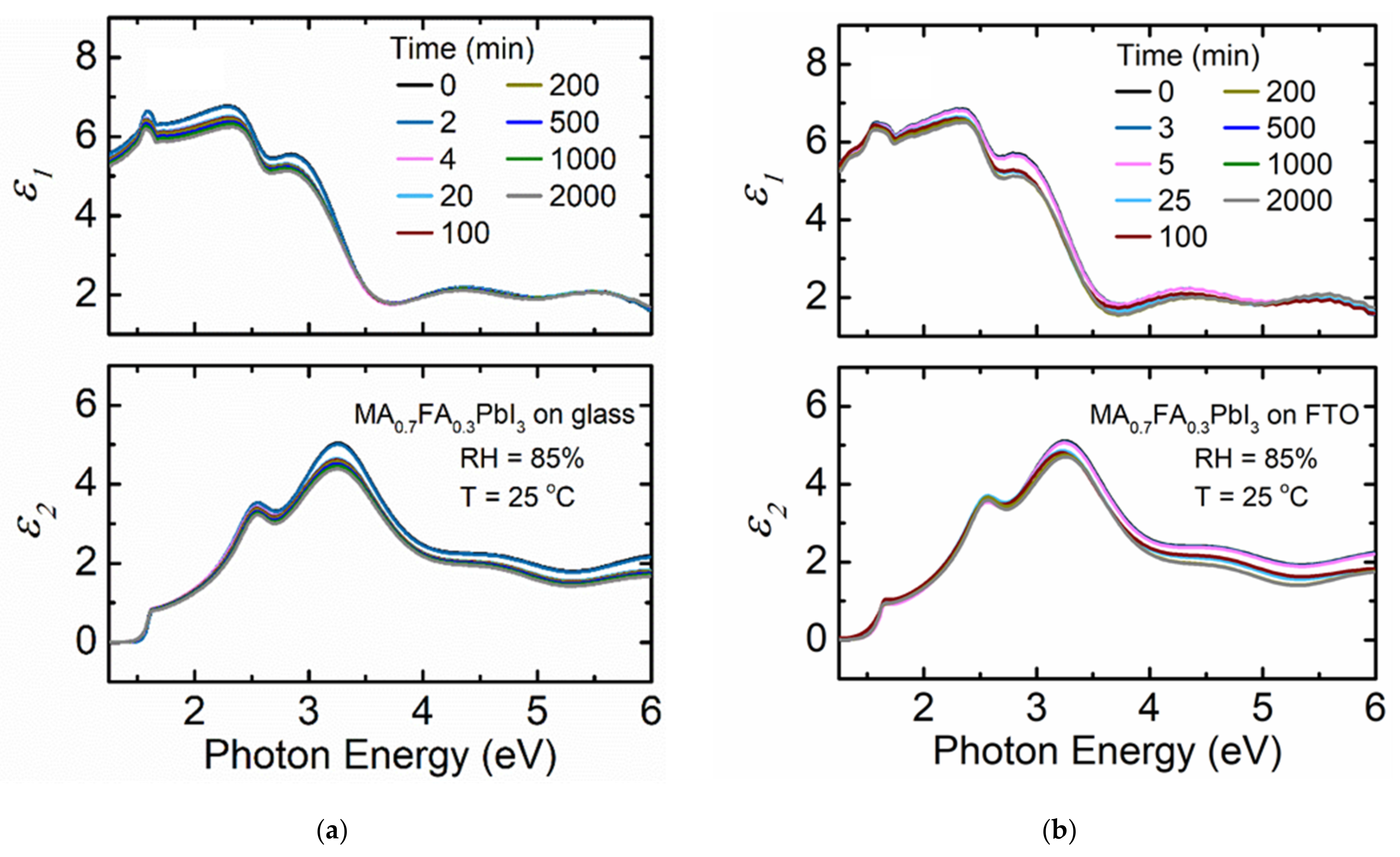
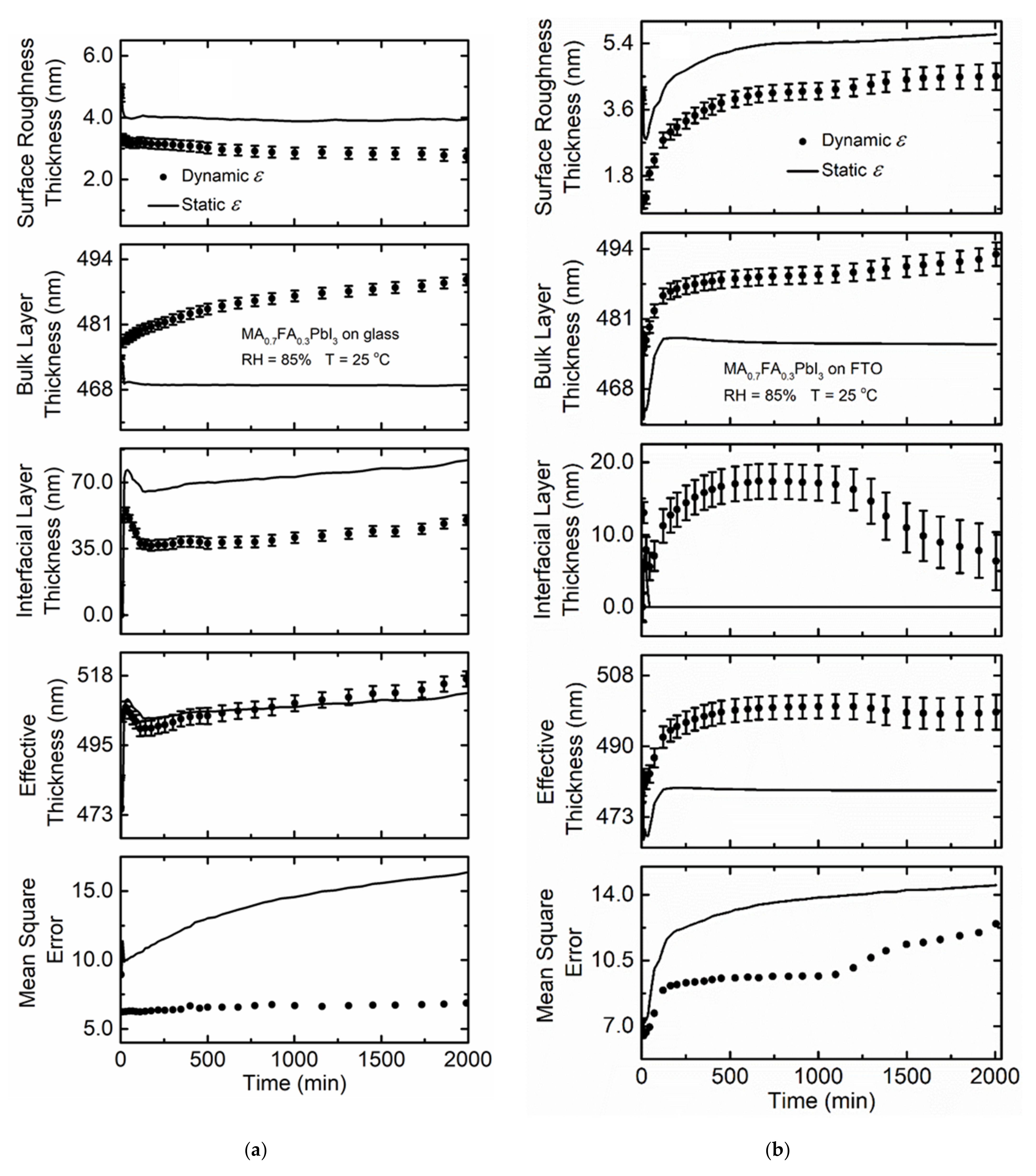
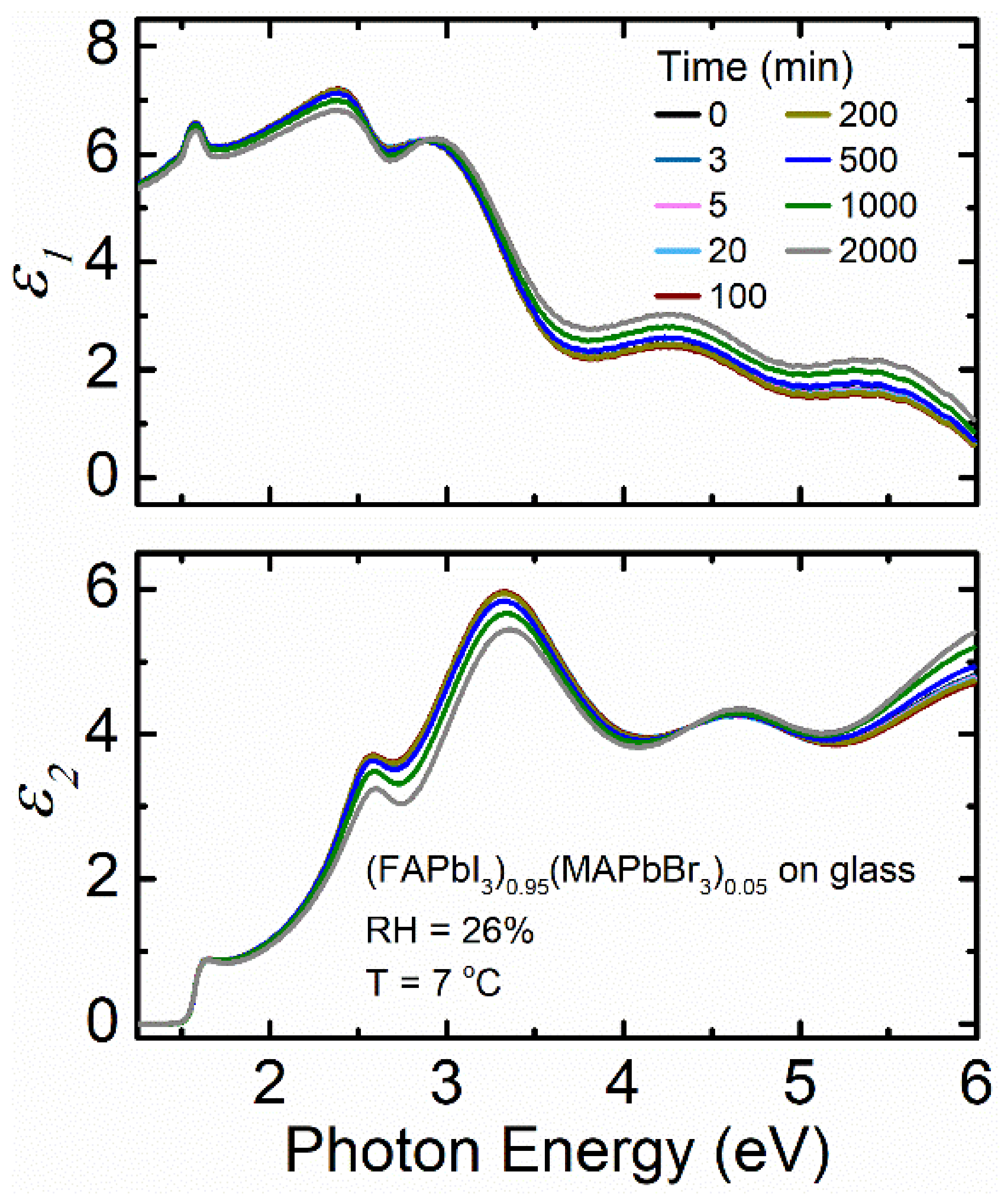
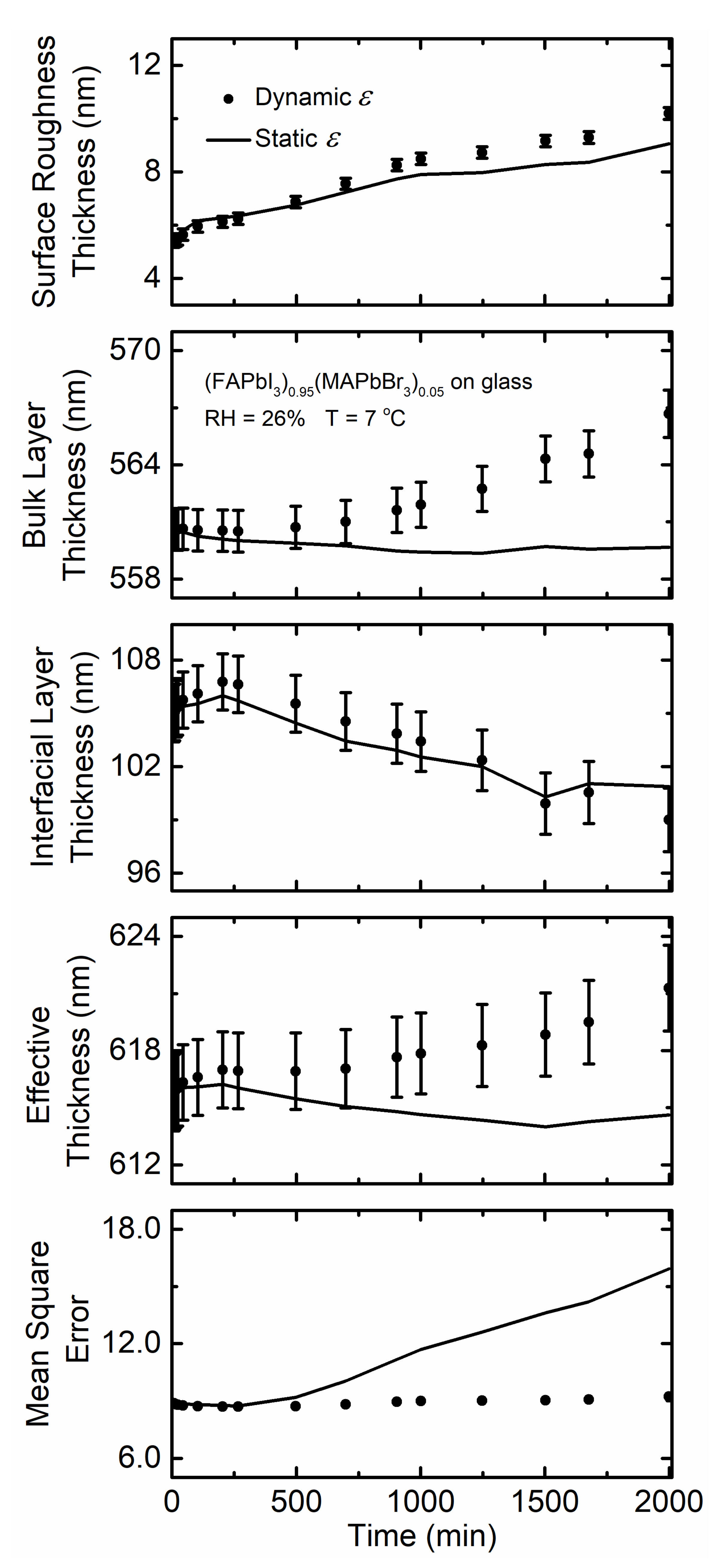
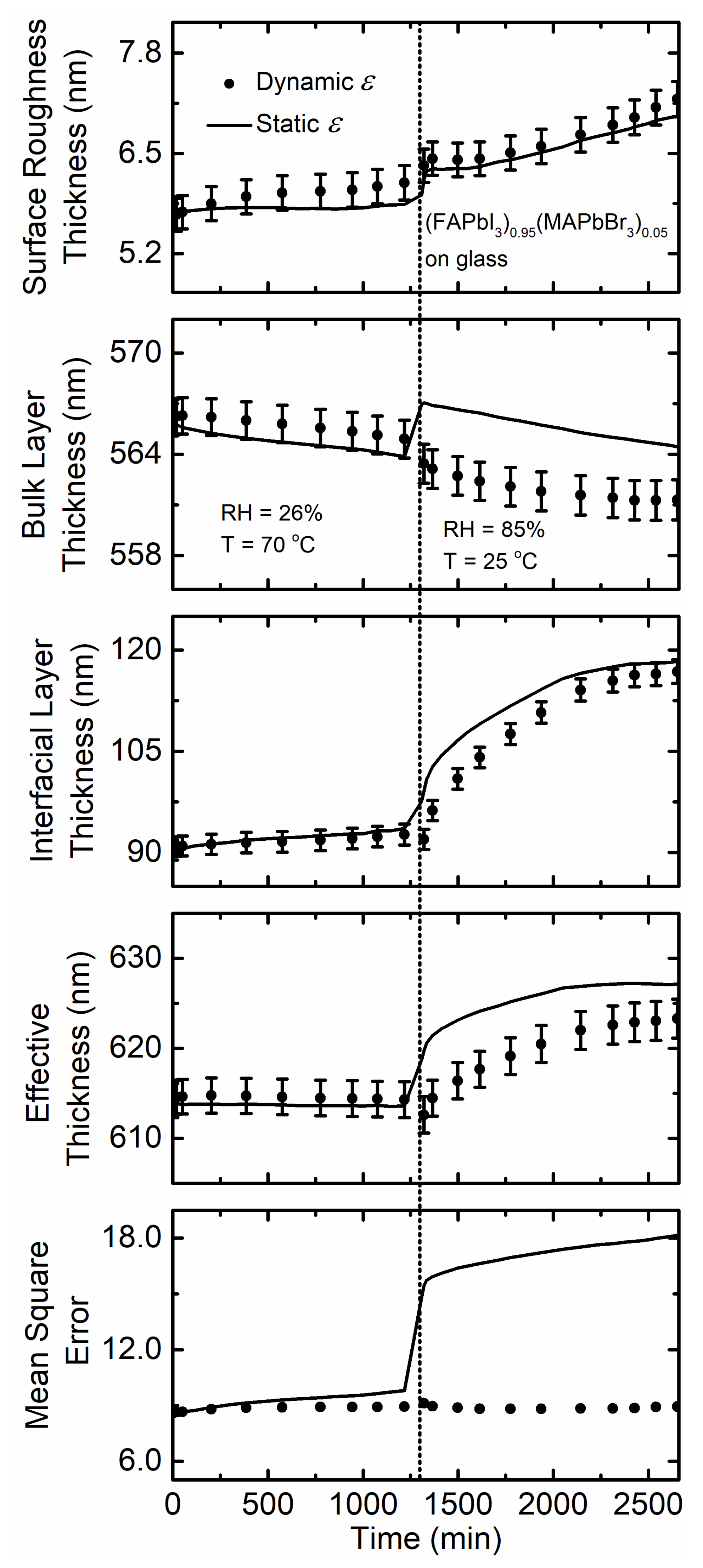
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Werner, J.; Sahli, F.; Kamino, B.; Sacchetto, D.; Brauninger, M.; Walter, A.; Ballif, C.; Despeisse, M.; Nicolay, S.; Niesen, B.; et al. Perovskite/Silicon tandem Solar Cells: Challenges Towards High-Efficiency in 4-Terminal and Monolithic Devices. In Proceedings of the IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017; pp. 3256–3259. [Google Scholar]
- NREL Solar Cell Efficiency Chart. Available online: http://www.nrel.ov/pv/cell-efficiency.html (accessed on 13 February 2021).
- Rehman, W.; McMeekin, D.P.; Patel, J.B.; Milot, R.L.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Photovoltaic mixed-cation lead mixed-halide perovskites: Links between crystallinity, photo-stability, and electronic properties. Energy Environ. Sci. 2017, 10, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Charles, B.; Dillon, J.; Weber, O.J.; Islam, M.S.; Weller, M.T. Understanding the stability of mixed A-cation lead iodide perovskites. J. Mater. Chem. A 2017, 5, 22495–22499. [Google Scholar] [CrossRef] [Green Version]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Baena, J.P.; Saliba, M.; Buonassisi, T.; Gratzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and challenges of perovskite solar cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozny, S.; Yang, M.; Nardes, A.M.; Mercado, C.C.; Ferrere, S.; Reese, M.O.; Zhou, W.; Zhu, K. Controlled Humidity Study on the Formation of Higher Efficiency Formamidinium Lead Triiodide-Based Solar Cells. Chem. Mater. 2015, 27, 4814–4820. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of perovskite photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Senocrate, A.; Acarturk, T.; Kim, G.Y.; Merkle, R.; Starke, U.; Gratzel, M.; Maier, J. Interaction of oxygen with halide perovskites. J. Mater. Chem. A 2018, 6, 10847–10855. [Google Scholar] [CrossRef] [Green Version]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cina, L.; Carlo, A.D.; Ducati, C. In situ observation of heat-induced degradation of perovskite solar cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, K.; Zhao, D.; Cimaroli, A.; Ke, W.; Yan, Y.; Podraza, N.J. Optical monitoring of CH3NH3PbI3 thin films upon atmospheric exposure. J. Phys. D Appl. Phys. 2016, 49, 405102. [Google Scholar] [CrossRef] [Green Version]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the Excited state and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.; Wei, S.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance factor: Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Johnston, A.; Walters, G.; Saidaminoz, M.I.; Huang, Z.; Bertens, K.; Jalarvo, N.; Sargent, E.H. Bromine Incorporation and Suppressed Cation Rotation in Mixed-Halide Perovskites. ACS Nano 2020, 14, 15107–15118. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, V.M. The laws of crystal chemistry. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Subedi, B.; Guan, L.; Ghimire, K.; Uprety, P.; Yan, Y.; Podraza, N.J. Formamidinium + cesium triiodide perovskites: Discrepancies between thin film optical absorption and solar cell efficiency. Sol. Energy Mater. Sol. Cells 2018, 188, 228–233. [Google Scholar] [CrossRef]
- Lv, Y.; Shi, Y.; Song, X.; Liu, J.; Wang, M.; Wang, S.; Feng, Y.; Jin, S.; Hao, C. Bromine Doping as an Efficiency Strategy to Reduce the Interfacial Defects in Hybrid Two-Dimensional/Three-Dimensional Stacking Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 31755–31764. [Google Scholar] [CrossRef]
- Lin, C.; Li, S.; Zhang, W.; Shao, C.; Yang, Z. Effect of Bromine Substitution on the Ion Migration and Optical Absorption in MAPbI3 Perovskite Solar Cells: The First-Principles Study. ACS Appl. Energy Mater. 2018, 1, 1374–1380. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry Principles and Applications; John Wiley & Sons, Ltd.: New York, NY, USA, 2007; pp. 20–32. [Google Scholar]
- Johs, B.; Herzinger, C.M. Quantifying the accuracy of ellipsometer systems. Phys. Status Solidi C 2008, 5, 1031–1035. [Google Scholar] [CrossRef]
- Richter, A.; Guico, R.; Wang, J. Calibrating an ellipsometer using x-ray reflectivity. Rev. Sci. Instrum. 2001, 72, 3004. [Google Scholar] [CrossRef] [Green Version]
- Logothetidis, S.; Stergioudis, G. Studies of density and surface roughness of ultrathin amorphous carbon films with regards to thickness with x-ray reflectometry and spectroscopic ellipsometry. Appl. Phys. Lett. 1997, 71, 2463. [Google Scholar] [CrossRef]
- Kaminska, K.; Amassian, A.; Martinu, L.; Robbie, K. Growth of vacuum evaporated ultraporous silicon studied with spectroscopic ellipsometry and scanning electron microscopy. J. Appl. Phys. 2005, 97, 013511. [Google Scholar] [CrossRef]
- Koirala, P.; Li, J.; Yoon, H.P.; Aryal, P.; Marsillac, S.; Rockett, A.A.; Podraza, N.J.; Collins, R.W. Through-the-glass spectroscopic ellipsometry for analysis of CdTe thin-film solar cells in the superstrate configuration. Prog. Photovolt. Res. Appl. 2016, 24, 1055–1067. [Google Scholar] [CrossRef]
- Leik, N.; Weber, J.W.; Mackus, A.J.M.; Van de Sanden, M.C.M.; Kessels, W.M.M. In situ spectroscopic ellipsometry during atomic layer deposition of Pt, Ru and Pd. J. Phys. D Appl. Phys. 2016, 49, 115504. [Google Scholar] [CrossRef]
- Revol, P.; Perret, D.; Bertin, F.; Fusalba, F.; Rouessac, V.; Chabli, A.; Passemard, G.; Ayral, A. Porosimetry Measurements on Low Dielectric Constant-Thin Layers by Coupling Spectroscopic Ellipsometry and Solvent Adsorption-Desorption. J. Porous Mater. 2005, 12, 113–121. [Google Scholar] [CrossRef]
- Fujiwara, H.; Koh, J.; Rovira, P.I.; Collins, R.W. Assessment of effective-medium theories in the analysis of nucleation and microscopic surface roughness evolution for semiconductor thin films. Phys. Rev. B 2000, 61, 10832. [Google Scholar] [CrossRef]
- Losurdo, M.; Barreca, D.; Capezzuto, P.; Bruno, G.; Tondello, E. Interrelation between nanostructure and optical properties of oxide thin films by spectroscopic ellipsometry. Surf. Coat. Technol. 2002, 151–152, 2–8. [Google Scholar] [CrossRef]
- Wang, C.; Guan, L.; Zhao, D.; Yu, Y.; Grice, C.R.; Song, Z.; Awni, R.A.; Chen, J.; Wang, J.; Zhao, X.; et al. Water Vapor Treatment of Low-Temperature Deposited SnO2 Electron Selective layers for Efficient Flexible Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2118–2124. [Google Scholar] [CrossRef]
- Ke, W.; Xiao, C.; Wang, C.; Saparov, B.; Duan, H.; Zhao, D.; Xiao, Z.; Schulz, P.; Harvey, S.P.; Liao, W.; et al. Employing Lead Thiocyanate Additive to Reduce the Hysteresis and Boost the Fill Factor of Planar Perovskite Solar Cells. Adv. Mater. 2016, 28, 5214–5221. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, C.; Chen, L.; Bista, S.S.; Liu, X.; Li, Y.; Awni, R.A.; Song, Z.; Yan, Y. Correlating Hysteresis and Stability with Organic Cation Composition in the Two-Step Solution-Processed Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 10588–10596. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Rovira, P.; An, I.; Collins, R.W. Rotating-compansator multichannel ellipsometry: Applications for real time Stokes vector spectroscopy of thin film growth. Rev. Sci. Instrum. 1988, 69, 1800. [Google Scholar] [CrossRef]
- Jiang, Y.; Soufiani, A.M.; Gentle, A.; Huang, F.; Ho-Baillie, A.; Green, M.A. Temperature dependent optical properties of CH3NH3PbI3 perovskites perovskite by spectroscopic ellipsometry. Appl. Phys. Lett. 2016, 108, 061905. [Google Scholar] [CrossRef]
- Subedi, B.; Li, C.; Junda, M.M.; Song, Z.; Yan, Y.; Podraza, N.J. Effects of intrinsic and atmospherically induced defects in narrow bandgap (FASnI3)X(MAPbI3)1−X perovskite thin films and solar cells. J. Chem. Phys. 2020, 152, 064705. [Google Scholar] [CrossRef]
- Junda, M.M.; Podraza, N.J. Optical properties of soda lime float glass from 3 mm to 148 nm (0.41 meV to 8.38 eV) by spectroscopic ellipsometry. Surf. Sci. Spectra 2018, 25, 016001. [Google Scholar] [CrossRef]
- Karki Gautam, L. Spectroscopic Ellipsometry Studies of Thin Film Si:H Materials in Photovoltaic Applications from Infrared to Ultraviolet. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, May 2016. [Google Scholar]
- Junda, M.M.; Grice, C.R.; Yan, Y.; Podraza, N.J. Parametric Optical Property Database for CdSe1−XSX Alloys. Electron. Mater. Lett. 2019, 15, 500–504. [Google Scholar] [CrossRef]
- Subedi, B.; Song, Z.; Chen, C.; Li, C.; Ghimire, K.; Junda, M.M.; Subedi, I.; Yan, Y.; Podraza, N.J. Optical and Electronic Losses Arising from Physically Mixed Interfacial Layers in Perovskites Solar Cells. Appl. Mater. Interfaces 2021, 13, 4923–4934. [Google Scholar] [CrossRef]
- Aspnes, D.E. Modulation spectroscopy/electric field effects on the dielectric function of semiconductors. In Handbook of Semiconductors; Balkanski, M., Ed.; North Holland Publishing Company: Amsterdam, The Netherlands, 1980; Volume 2, pp. 109–154. [Google Scholar]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Azarhoosh, P.; Alonso, M.I.; Campoy-Quiles, M.; Weber, O.J.; Yao, J.; Bryant, D.; Weller, M.T.; Nelson, J.; Walsh, A.; et al. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317–6327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 and the effect of post-modification by aluminium oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Song, Z.; Abate, A.; Watthage, S.C.; Liyanage, G.K.; Phillips, A.B.; Steiner, U.; Graetzel, M.; Heben, M.J. Perovskite Solar Cell Stability in Humid Air: Partially Reversible Phase Transitions in the PbI2-CH3NH3I-H2O System. Adv. Energy Mater. 2016, 6, 1600846. [Google Scholar] [CrossRef]
- Jong, U.; Yu, C.; Ri, G.; McMahon, A.P.; Harrison, N.M.; Barnes, P.R.F.; Walsh, A. Influence of water intercalation and hydration on chemical decomposition and ion transport in methylammonium lead halide perovskites. J. Mater. Chem. A 2018, 6, 1067–1074. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Fang, X.; Lv, M.; Lin, B.; Zhang, S.; Ding, J.; Yuan, N. Improvement of the humidity stability of organic-inorganic perovskite solar cells using ultrathin Al2O3 layers prepared by atomic layer deposition. J. Mater. Chem. A 2015, 3, 5360–5367. [Google Scholar] [CrossRef]
- Zhang, L.; Sit, P.H. Ab Initio Study of Interaction of Water, Hydroxyl Radicals, and Hydroxide Ions with CH3NH3PbI3 and CH3NH3PbBr3 Surfaces. J. Phys. Chem. C 2015, 119, 22370–22378. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campony-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Mosconi, E.; Aspiroz, J.M.; De Angelis, F. Ab Initio Molecular Dynamics Simulations of Methylammonium Lead Iodide Perovskite Degradation by Water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Climent-Pascual, E.; Hames, B.C.; Moreno-Ramirez, J.S.; Alvarez, A.L.; Juarez-Perez, E.J.; Mas-Marza, E.; Mora-Sero, I.; DeAndres, A.; Coya, C. Influence of the substrate on the bulk properties of hybrid lead halide perovskite films. J. Mater. Chem. A 2016, 4, 18153–18163. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Rao, H.; Chen, B.; Wang, X.; Kuang, D. A formamidinium-methylammonium lead iodide perovskite single crystal exhibiting exceptional optoelectronic properties and long-term stability. J. Mater. Chem. A 2017, 5, 19431–19438. [Google Scholar] [CrossRef]
- Han, F.; Yang, W.; Zhu, L. Stable High-Efficiency Two-Dimensional Perovskite Solar Cells via Bromine Incorporation. Nanoscale Res. Lett. 2020, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.D.; Verdi, C.; Milot, R.L.; Eperon, G.E.; Perez-Osorio, M.A.; Snaith, H.J.; Giustino, F.; Johnston, M.B.; Herz, L.M. Electron-phonon coupling in hybrid lead halide perovskites. Nat. Commun. 2016, 7, 11755. [Google Scholar] [CrossRef]
- Monserrat, B.; Park, J.; Kim, S.; Walsh, A. Role of electron-phonon coupling and thermal expansion on bandgaps, carrier mobility, and interfacial offsets in kesterite thin-film solar cells. Appl. Phys. Lett. 2018, 112, 193903. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.P.; Chen, X. Temperature dependence of semiconductor bandgaps. Appl. Phys. Lett. 1991, 58, 2924. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Chemical Management for Colorful, Efficient and Stable Inorganic-Organic Hybrid nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Roberts, J.C.; Bedair, S.M.; Reed, M.J.; Liu, S.X.; El-Masry, N.A.; Robins, L.H. Optical band gap dependence on composition and thickness of InXGa1−XN (0 < X < 0.25) grown on GaN. Appl. Phys. Lett. 1999, 75, 2566. [Google Scholar]


| Perovskite Films | MSE Range | Initial Effective Thickness (nm) | Effective Thickness Change (nm) | Minimum Bandgap (eV) | Maximum Bandgap (eV) | Average Bandgap (eV) |
|---|---|---|---|---|---|---|
| MAPbI3 on glass | 10.6–35.5 | 486.8 | 218 | 1.596 | 1.605 | 1.604 |
| MAPbI3 on FTO | 13.5–14.5 | 516.9 | 23 | 1.600 | 1.613 | 1.605 |
| MA0.7FA0.3PbI3 on glass | 6.2–6.5 | 474.3 | 16 | 1.590 | 1.593 | 1.590 |
| MA0.7FA0.3PbI3 on FTO | 6.7–9.6 | 478.1 | 12 | 1.606 | 1.616 | 1.613 |
| (FAPbI3)0.95(MAPbBr3)0.05 on glass at 7 °C with 26% RH | 8.7–8.8 | 615.8 | 1 | 1.560 | 1.565 | 1.562 |
| (FAPbI3)0.95(MAPbBr3)0.05 on glass at 70 °C with 26% RH | 8.6–8.9 | 614.2 | 0 | 1.590 | 1.595 | 1.593 |
| (FAPbI3)0.95(MAPbBr3)0.05 on glass at 25 °C with 85% RH | 8.9–8.8 | 614.5 | 7 | 1.559 | 1.571 | 1.563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumusange, M.S.; Subedi, B.; Chen, C.; Junda, M.M.; Song, Z.; Yan, Y.; Podraza, N.J. Impact of Humidity and Temperature on the Stability of the Optical Properties and Structure of MAPbI3, MA0.7FA0.3PbI3 and (FAPbI3)0.95(MAPbBr3)0.05 Perovskite Thin Films. Materials 2021, 14, 4054. https://doi.org/10.3390/ma14144054
Tumusange MS, Subedi B, Chen C, Junda MM, Song Z, Yan Y, Podraza NJ. Impact of Humidity and Temperature on the Stability of the Optical Properties and Structure of MAPbI3, MA0.7FA0.3PbI3 and (FAPbI3)0.95(MAPbBr3)0.05 Perovskite Thin Films. Materials. 2021; 14(14):4054. https://doi.org/10.3390/ma14144054
Chicago/Turabian StyleTumusange, Marie Solange, Biwas Subedi, Cong Chen, Maxwell M. Junda, Zhaoning Song, Yanfa Yan, and Nikolas J. Podraza. 2021. "Impact of Humidity and Temperature on the Stability of the Optical Properties and Structure of MAPbI3, MA0.7FA0.3PbI3 and (FAPbI3)0.95(MAPbBr3)0.05 Perovskite Thin Films" Materials 14, no. 14: 4054. https://doi.org/10.3390/ma14144054







