Experimental Investigation of the Apparent Thermal Conductivity of Microencapsulated Phase-Change-Material Slurry at the Phase-Transition Temperature
Abstract
:1. Introduction
1.1. Phase-Change Material and Working Fluids in Heat-Exchange Systems
1.2. Thermal Conductivity of Micro-/Nanoencapsulated PCM Slurries—Previous Research
2. Methodology and Materials
2.1. Preparation of the Slurry
2.2. Experimental Facility
2.3. Procedure of the Experimental Research and the Conditions of the Experiment
3. Results and Discussion
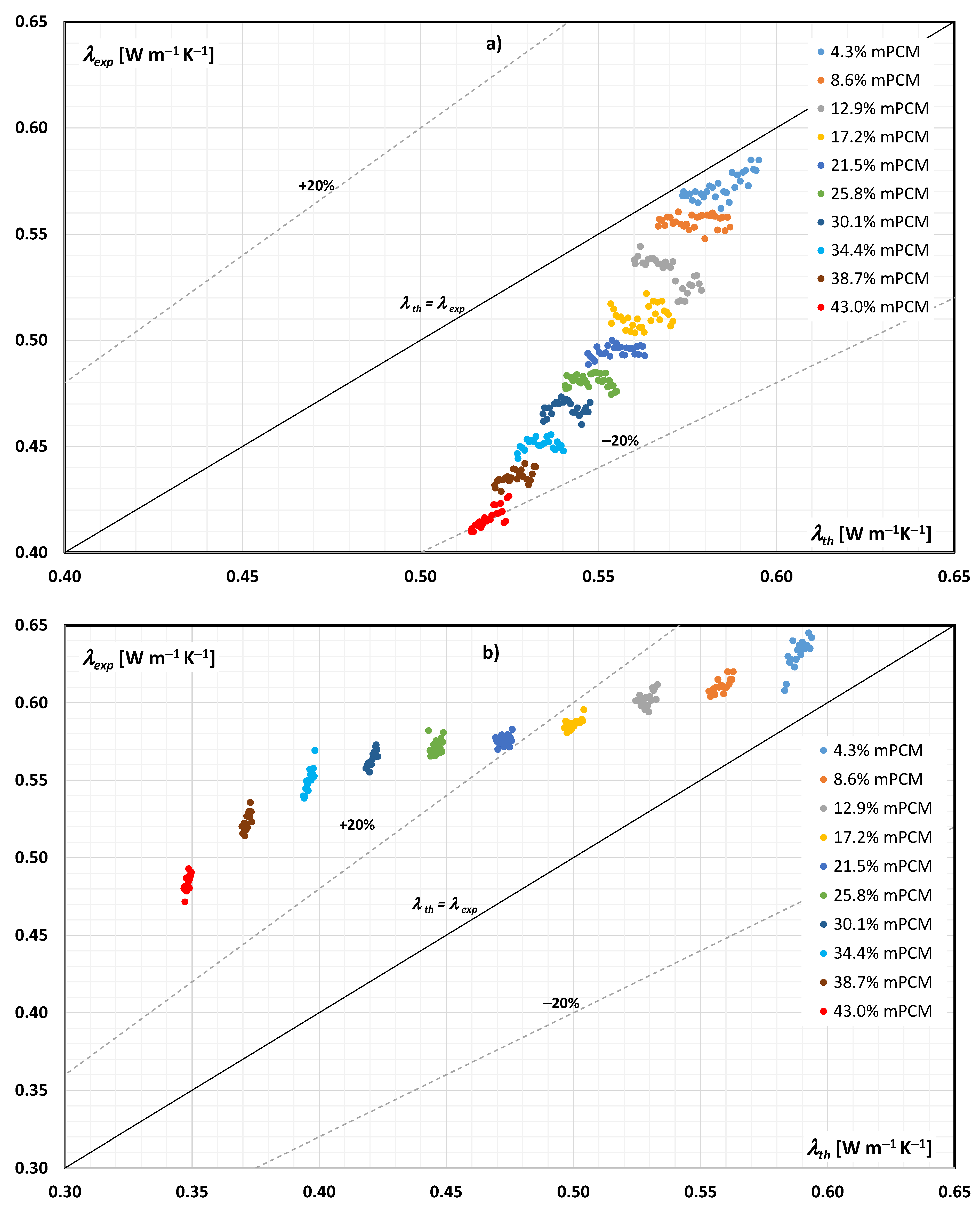
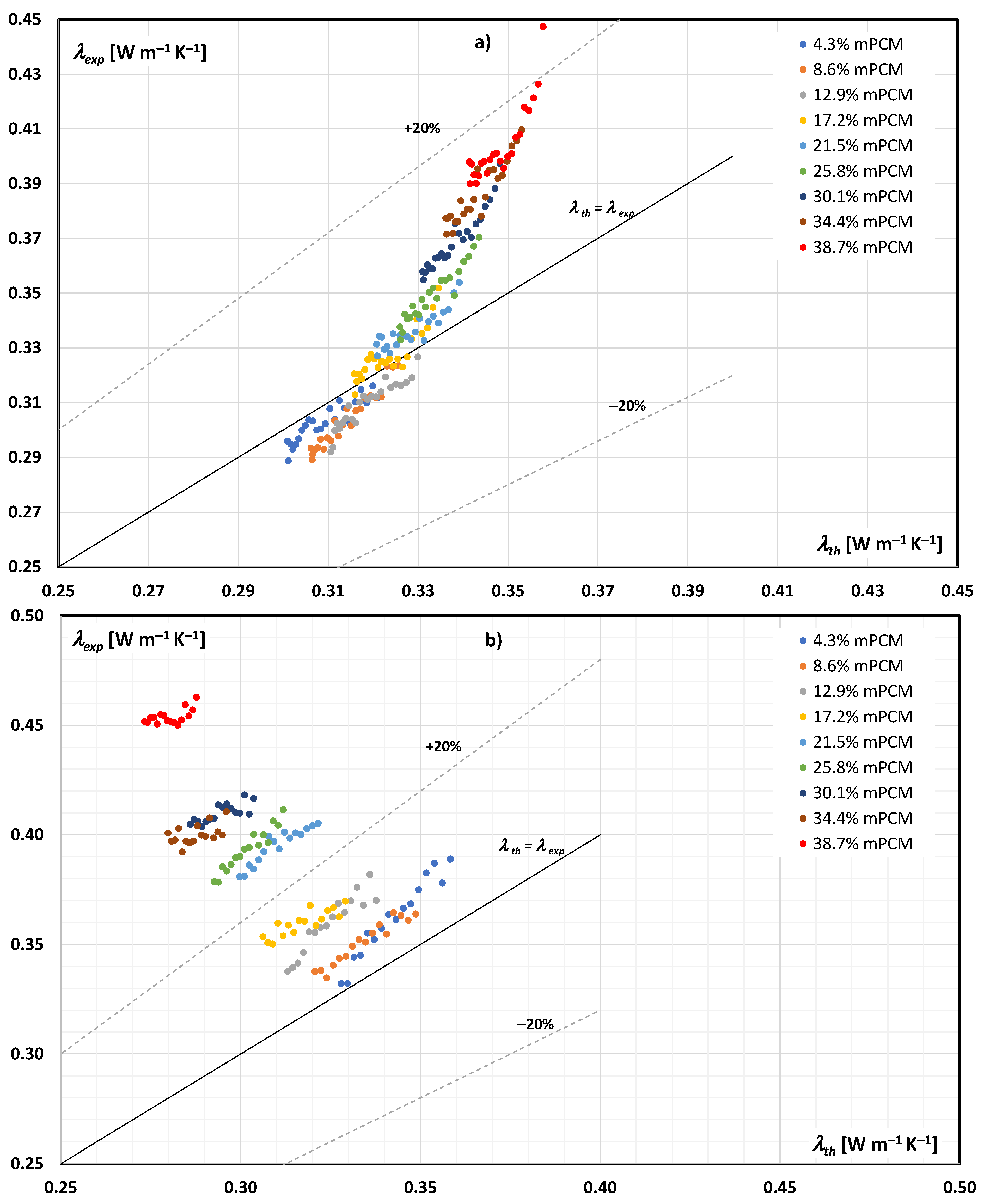
3.1. Experimental vs. Theoretical Thermal Conductivity of the mPCM Slurry
3.2. Discussion of Research Results
4. Summary and Conclusions
- An increase in the temperature of the slurry caused an increase in its thermal conductivity, both when the PCM was in the form of a solid and a liquid;
- The thermal conductivity of the mPCM slurry when the PCM was in liquid form was greater than the thermal conductivity of the slurry when the PCM was solid;
- During the phase transformation, a significant increase in the thermal conductivity of the slurry was observed, and its peak took place when the temperature of the slurry reached the temperature declared by the manufacturer at which the phase-transition peak occurs;
- The thermal conductivity value (apparent thermal conductivity) obtained during the phase transformation did not correspond to the actual value, but results from the imperfection of the measurement method;
- The thermal conductivity of an mPCM slurry can be determined from Maxwell’s dependence with an accuracy of ±20% when the PCM is in the form of a solid in the entire tested range, and to a limited extent (mPCM mass share up to 20%) when the PCM is in liquid form in the slurry;
- Work is required to set standards for measurement methods or standardization of the preparation of mPCM slurry samples in order to measure their thermal conductivity, especially at the temperature at which the PCM undergoes a phase change.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Commission Staff Working Document Energy Storage—The Role of Electricity; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Pandey, A.K.; Hossain, M.S.; Tyagi, V.V.; Rahim, N.A.; Selvaraj, J.A.L.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids. 2020, 5–6. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2020, 101913. [Google Scholar] [CrossRef]
- Palacios, A.; Cong, L.; Navarro, M.E.; Ding, Y.; Barreneche, C. Thermal conductivity measurement techniques for characterizing thermal energy storage materials—A review. Renew. Sustain. Energy Rev. 2019, 108, 32–52. [Google Scholar] [CrossRef]
- Pons, O.; Aguado, A.; Fernández, A.I.; Cabeza, L.F.; Chimenos, J.M. Review of the use of phase change materials (PCMs) in buildings with reinforced concrete structures. Mater. Constr. 2014, 64, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.C.; Nizamuddin, S.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects 2019, 20. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends Food Sci. Technol. 2019, 91, 116–128. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.; Wang, J.; Li, Y.; Rao, Z. Experimental research on flow and heat transfer characteristics of latent functional thermal fluid with microencapsulated phase change materials. Int. J. Heat Mass Transf. 2017, 115, 737–742. [Google Scholar] [CrossRef]
- Karaipekli, A.; Erdoğan, T.; Barlak, S. The stability and thermophysical properties of a thermal fluid containing surface-functionalized nanoencapsulated PCM. Thermochim. Acta 2019, 682. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Zheng, H. A comprehensive review of hydrodynamic mechanisms and heat transfer characteristics for microencapsulated phase change slurry (MPCS) in circular tube. Renew. Sustain. Energy Rev. 2019, 114, 109312. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zeng, R.; Zhang, Y.; Wang, X.; Niu, J.; Li, Y.; Di, H. An experimental study of convective heat transfer with microencapsulated phase change material suspension: Laminar flow in a circular tube under constant heat flux. Exp. Therm. Fluid Sci. 2008, 32, 1638–1646. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ling, X. An experimental study of the latent functionally thermal fluid with micro-encapsulated phase change material particles flowing in microchannels. Appl. Therm. Eng. 2016, 105, 209–216. [Google Scholar] [CrossRef]
- Wang, X.; Niu, J.; Li, Y.; Wang, X.; Chen, B.; Zeng, R.; Song, Q.; Zhang, Y. Flow and heat transfer behaviors of phase change material slurries in a horizontal circular tube. Int. J. Heat Mass Transf. 2007, 50, 2480–2491. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhao, C.Y. Thermal and rheological properties of microencapsulated phase change materials. Renew. Energy 2011, 36, 2959–2966. [Google Scholar] [CrossRef]
- Joseph, M.; Sajith, V. An investigation on heat transfer performance of polystyrene encapsulated n-octadecane based nanofluid in square channel. Appl. Therm. Eng. 2019, 147, 756–769. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Sopian, K.; Chaichan, M.T.; Kazem, H.A.; Ibrahim, A.; Mat, S.; Ruslan, M.H. Evaluation of the nanofluid and nano-PCM based photovoltaic thermal (PVT) system: An experimental study. Energy Convers. Manag. 2017, 151, 693–708. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; Cui, G.; Dou, B.; Lu, W.; Yan, X. Fabrication of a novel nano phase change material emulsion with low supercooling and enhanced thermal conductivity. Renew. Energy 2020, 151, 542–550. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Peñalosa, C.; Zalba, B. Experimental analysis of the influence of microcapsule mass fraction on the thermal and rheological behavior of a PCM slurry. Appl. Therm. Eng. 2014, 63, 11–22. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Liu, L.; Wang, Y.; Chai, L.; Yang, Z.; Chen, H.; Tan, C. Stability and thermophysical properties of binary propanol-water mixtures-based microencapsulated phase change material suspensions. J. Heat Transfer. 2015, 137. [Google Scholar] [CrossRef]
- Wang, F.; Fang, X.; Zhang, Z. Preparation of phase change material emulsions with good stability and little supercooling by using a mixed polymeric emulsifier for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 381–390. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.C.; Yan, W.M.; Amani, M. Microencapsulated n-eicosane PCM suspensions: Thermophysical properties measurement and modeling. Int. J. Heat Mass Transf. 2018, 125, 792–800. [Google Scholar] [CrossRef]
- Xu, B.; Chen, C.; Zhou, J.; Ni, Z.; Ma, X. Preparation of novel microencapsulated phase change material with Cu-Cu2O/CNTs as the shell and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2019, 200, 109980. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Rao, Z.; Xie, J. Experiment on heat storage characteristic of microencapsulated phase change material slurry. Sol. Energy Mater. Sol. Cells 2011, 95, 2726–2733. [Google Scholar] [CrossRef]
- Allouche, Y.; Varga, S.; Bouden, C.; Oliveira, A.C. Experimental determination of the heat transfer and cold storage characteristics of a microencapsulated phase change material in a horizontal tank. Energy Convers. Manag. 2015, 94, 275–285. [Google Scholar] [CrossRef]
- Fischer, L.; Maranda, S.; Stamatiou, A.; von Arx, S.; Worlitschek, J. Experimental investigation on heat transfer with a Phase Change Dispersion. Appl. Therm. Eng. 2019, 147, 61–73. [Google Scholar] [CrossRef]
- Micronal® 5428 X, (n.d.). Available online: https://microteklabs.com/pdfs/MPDS3300-0041%20Rev%201.pdf (accessed on 4 February 2021).
- Thermophysical Properties of Fluid Systems, (n.d.). Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 2 February 2021).
- Karkri, M.; Lachheb, M.; Albouchi, F.; Nasrallah, S.B.; Krupa, I. Thermal properties of smart microencapsulated paraffin/plaster composites for the thermal regulation of buildings. Energy Build. 2015, 88, 183–192. [Google Scholar] [CrossRef]
- Wang, F.; Lin, W.; Ling, Z.; Fang, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218–234. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Barison, S.; Marcos, M.A.; Prado, J.I.; Rossi, S.; Bobbo, S.; Fedele, L. Development of paraffinic phase change material nanoemulsions for thermal energy storage and transport in low-temperature applications. Appl. Therm. Eng. 2019, 159, 113868. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Mossaz, S.; Gruss, J.A.; Ferrouillat, S.; Skrzypski, J.; Getto, D.; Poncelet, O.; Berne, P. Experimental study on the influence of nanoparticle PCM slurry for high temperature on convective heat transfer and energetic performance in a circular tube under imposed heat flux. Appl. Therm. Eng. 2015, 81, 388–398. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Augood, P.C.; Newborough, M.; Highgate, D.J. Thermal behaviour of phase-change slurries incorporating hydrated hydrophilic polymeric particles. Exp. Therm. Fluid Sci. 2001, 25, 457–468. [Google Scholar] [CrossRef]
- Bai, Z.; Miao, Y.; Xu, H.; Gao, Q. Experimental study on thermal storage and heat transfer performance of microencapsulated phase-change material slurry. Therm. Sci. Eng. Prog. 2020, 17, 100362. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145. [Google Scholar] [CrossRef]
- Kruzel, M.; Bohdal, T.; Sikora, M. Heat transfer and pressure drop during refrigerants condensation in compact heat exchangers. Int. J. Heat Mass Transf. 2020, 161, 120283. [Google Scholar] [CrossRef]
- Bohdal, T.; Kruzel, M. International Journal of Heat and Mass Transfer Refrigerant condensation in vertical pipe minichannels under various heat flux density level. Int. J. Heat Mass Transf. 2020, 146, 118849. [Google Scholar] [CrossRef]
- Bohdal, T.; Charun, H.; Kruzel, M.; Sikora, M. An investigation of heat transfer coefficient during refrigerants condensation in vertical pipe minichannels. E3S Web Conf. 2018, 293. [Google Scholar] [CrossRef]
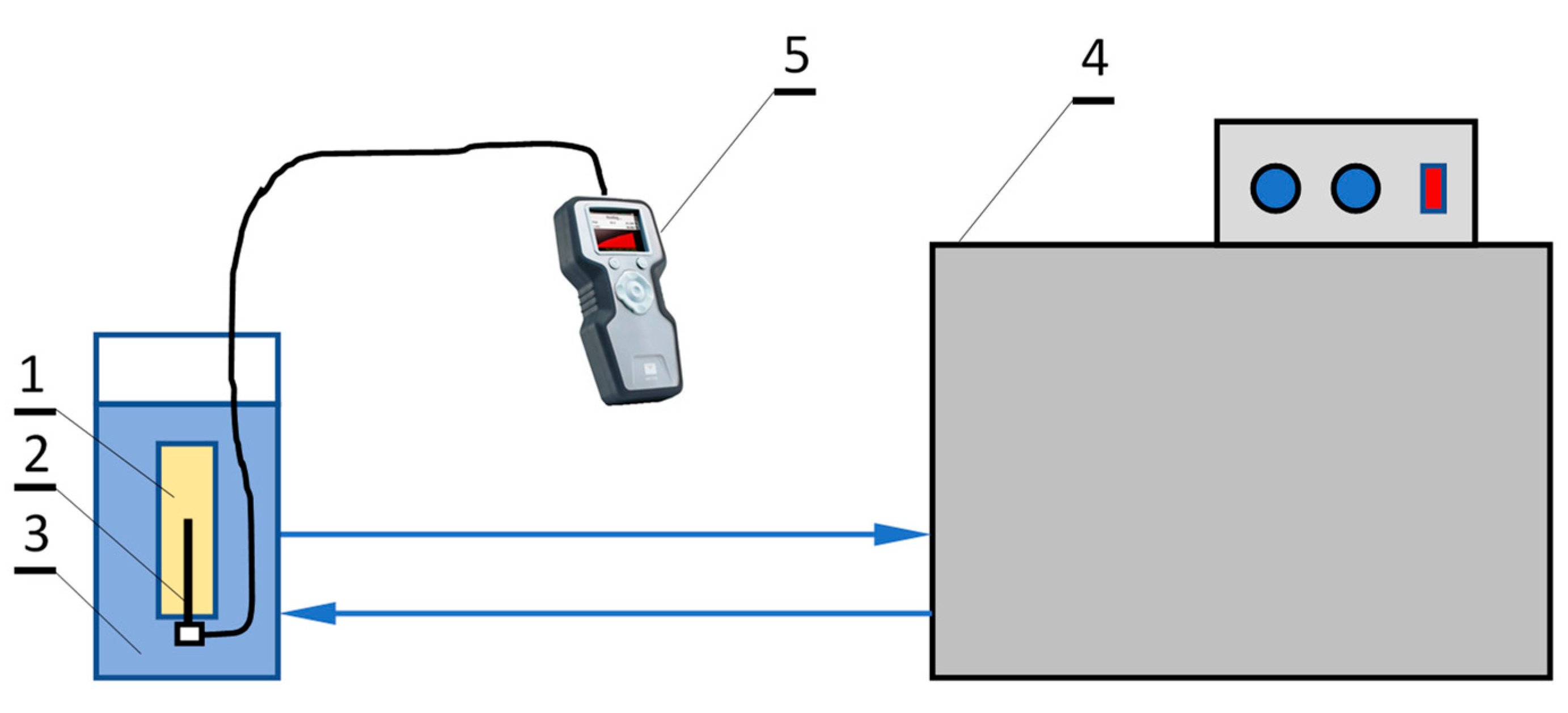


| Mass Fractions of Slurry Components (%) | ||
|---|---|---|
| mPCM | Glycol | Water |
| 43.0 | 0 | 57.0 |
| 38.7 | 3.7 | 57.6 |
| 34.4 | 7.4 | 58.2 |
| 30.1 | 11.1 | 58.8 |
| 25.8 | 14.8 | 59.4 |
| 21.5 | 18.5 | 60.0 |
| 17.2 | 22.2 | 60.6 |
| 12.9 | 25.9 | 61.2 |
| 8.6 | 29.6 | 61.8 |
| 4.3 | 33.3 | 62.4 |
| 0 | 37.0 | 63.0 |
| Temperature (°C) | Thermal Conductivity (W m−1K−1) | Temperature (°C) | Thermal Conductivity (W m−1K−1) | Temperature (°C) | Thermal Conductivity (W m−1K−1) |
|---|---|---|---|---|---|
| 10.0 | 0.58005 | 20.0 | 0.59846 | 30.0 | 0.61550 |
| 10.5 | 0.58099 | 20.5 | 0.59935 | 30.5 | 0.61631 |
| 11.0 | 0.58193 | 21.0 | 0.60024 | 31.0 | 0.61711 |
| 11.5 | 0.58287 | 21.5 | 0.60112 | 31.5 | 0.61790 |
| 12.0 | 0.58380 | 22.0 | 0.6020 | 32.0 | 0.61869 |
| 12.5 | 0.58474 | 22.5 | 0.60288 | 32.5 | 0.61948 |
| 13.0 | 0.58567 | 23.0 | 0.60375 | 33.0 | 0.62026 |
| 13.5 | 0.58660 | 23.5 | 0.60462 | 33.5 | 0.62103 |
| 14.0 | 0.58753 | 24.0 | 0.60548 | 34.0 | 0.62180 |
| 14.5 | 0.58846 | 24.5 | 0.60634 | 34.5 | 0.62257 |
| 15.0 | 0.58938 | 25.0 | 0.60719 | 35.0 | 0.62333 |
| 15.5 | 0.59030 | 25.5 | 0.60805 | 35.5 | 0.62408 |
| 16.0 | 0.59122 | 26.0 | 0.60889 | 36.0 | 0.62483 |
| 16.5 | 0.59214 | 26.5 | 0.60973 | 36.5 | 0.62557 |
| 17.0 | 0.59305 | 27.0 | 0.61057 | 37.0 | 0.62631 |
| 17.5 | 0.59396 | 27.5 | 0.61141 | 37.5 | 0.62704 |
| 18.0 | 0.59487 | 28.0 | 0.61223 | 38.0 | 0.62777 |
| 18.5 | 0.59577 | 28.5 | 0.61306 | 38.5 | 0.62849 |
| 19.0 | 0.59667 | 29.0 | 0.61388 | 39.0 | 0.62921 |
| 19.5 | 0.59757 | 29.5 | 0.61469 | 39.5 | 0.62992 |
| Mass Fraction of Microcapsules | MAPE (%) | |||
|---|---|---|---|---|
| Water | Ergolid | |||
| PCM Solid | PCM Liquid | PCM Solid | PCM Liquid | |
| 4.3% mPCM | 2.1 | 6.8 | 2.1 | 5.1 |
| 8.6% mPCM | 3.7 | 8.6 | 3.4 | 4.8 |
| 12.9% mPCM | 7.1 | 12.2 | 3.1 | 9.7 |
| 17.2% mPCM | 10.0 | 14.7 | 1.4 | 11.9 |
| 21.5% mPCM | 12.1 | 17.9 | 2.4 | 21.5 |
| 25.8% mPCM | 13.9 | 22.0 | 4.6 | 23.2 |
| 30.1% mPCM | 15.6 | 25.4 | 8.4 | 28.2 |
| 34.4% mPCM | 18.4 | 28.0 | 11.1 | 28.1 |
| 38.7% mPCM | 20.9 | 29.0 | 13.7 | 38.3 |
| 43.0% mPCM | 24.7 | 28.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M. Experimental Investigation of the Apparent Thermal Conductivity of Microencapsulated Phase-Change-Material Slurry at the Phase-Transition Temperature. Materials 2021, 14, 4124. https://doi.org/10.3390/ma14154124
Dutkowski K, Kruzel M. Experimental Investigation of the Apparent Thermal Conductivity of Microencapsulated Phase-Change-Material Slurry at the Phase-Transition Temperature. Materials. 2021; 14(15):4124. https://doi.org/10.3390/ma14154124
Chicago/Turabian StyleDutkowski, Krzysztof, and Marcin Kruzel. 2021. "Experimental Investigation of the Apparent Thermal Conductivity of Microencapsulated Phase-Change-Material Slurry at the Phase-Transition Temperature" Materials 14, no. 15: 4124. https://doi.org/10.3390/ma14154124
APA StyleDutkowski, K., & Kruzel, M. (2021). Experimental Investigation of the Apparent Thermal Conductivity of Microencapsulated Phase-Change-Material Slurry at the Phase-Transition Temperature. Materials, 14(15), 4124. https://doi.org/10.3390/ma14154124







