Influence of Graphite Layer on Electronic Properties of MgO/6H-SiC(0001) Interface
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
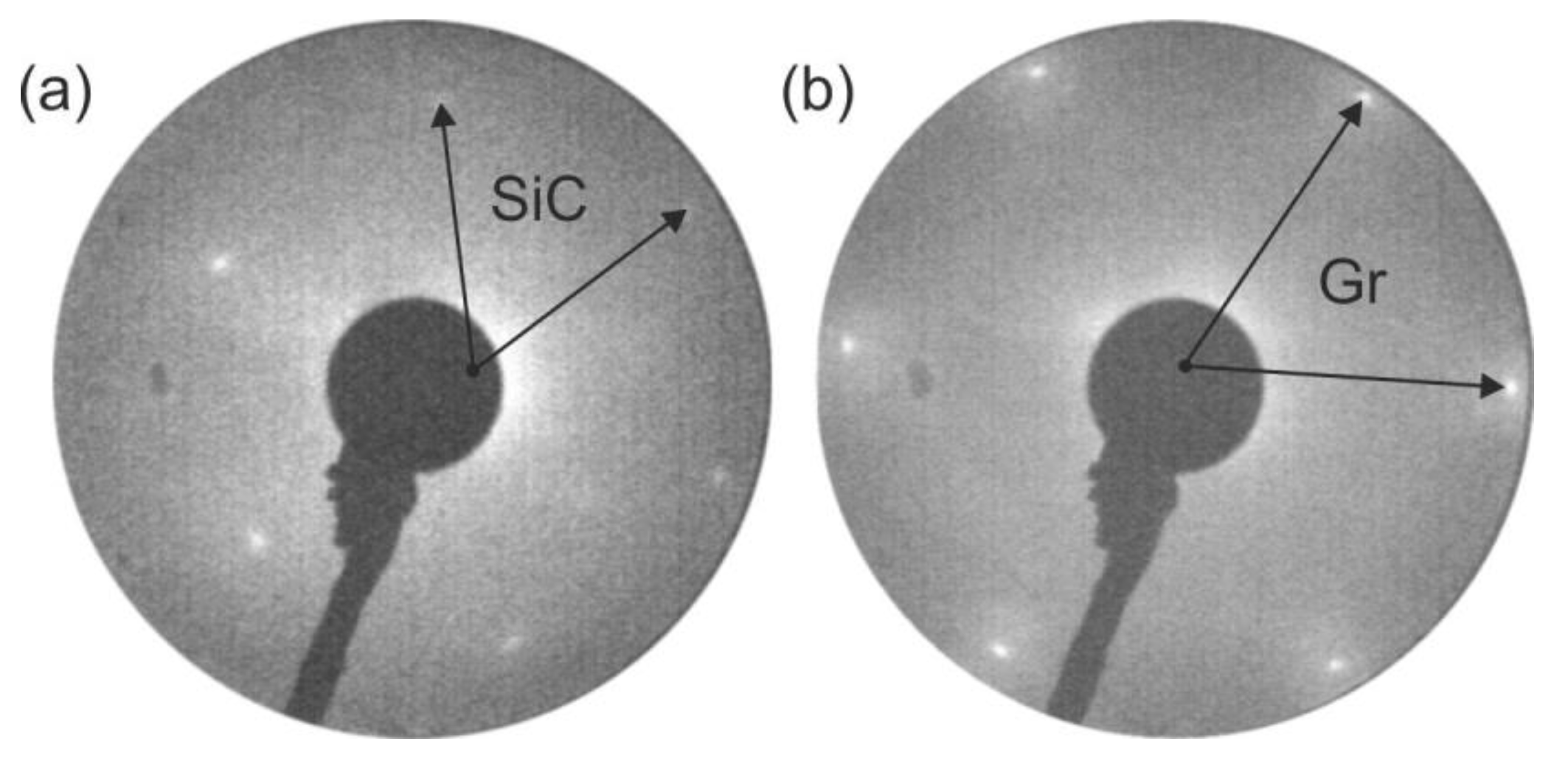
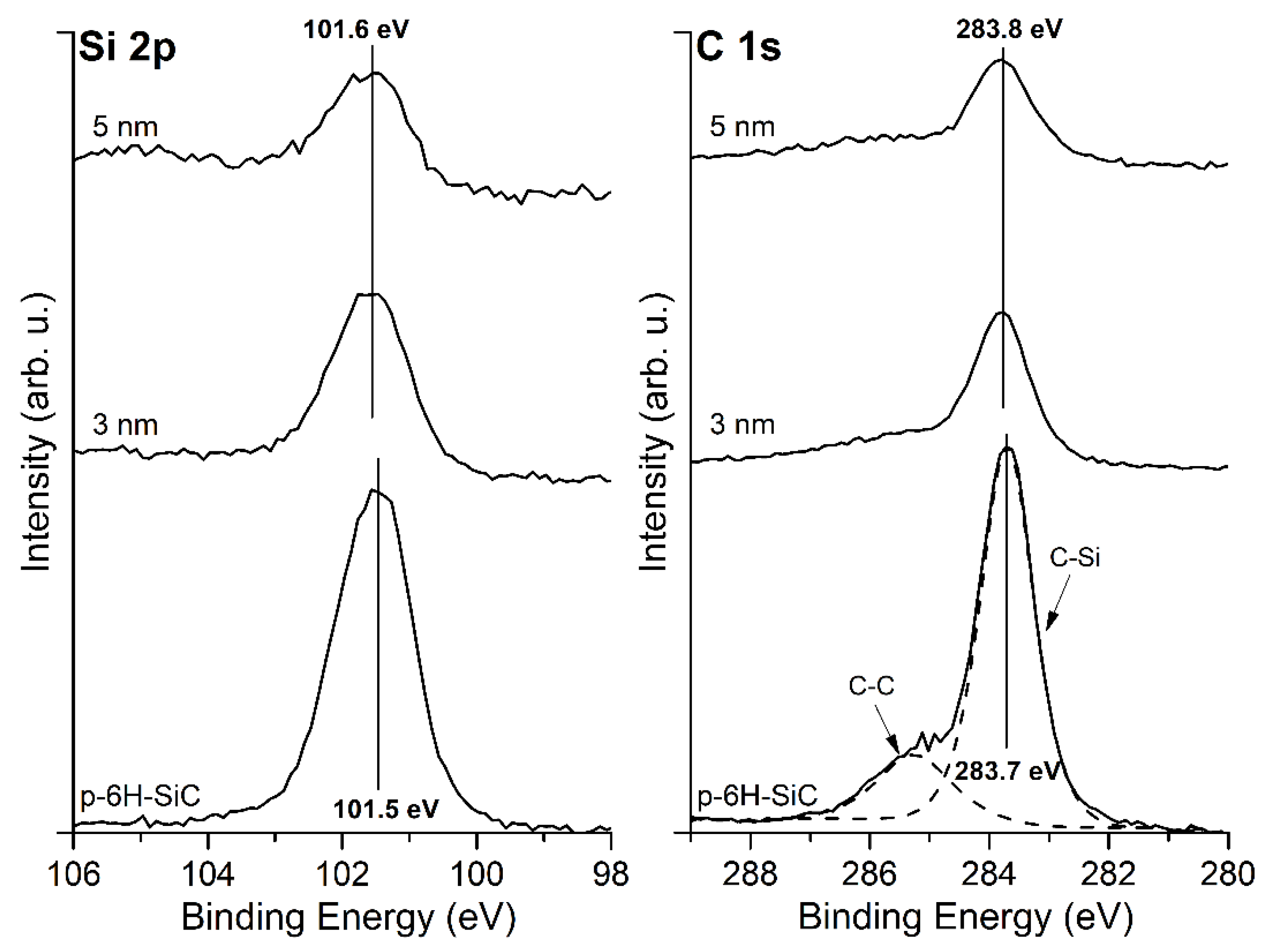
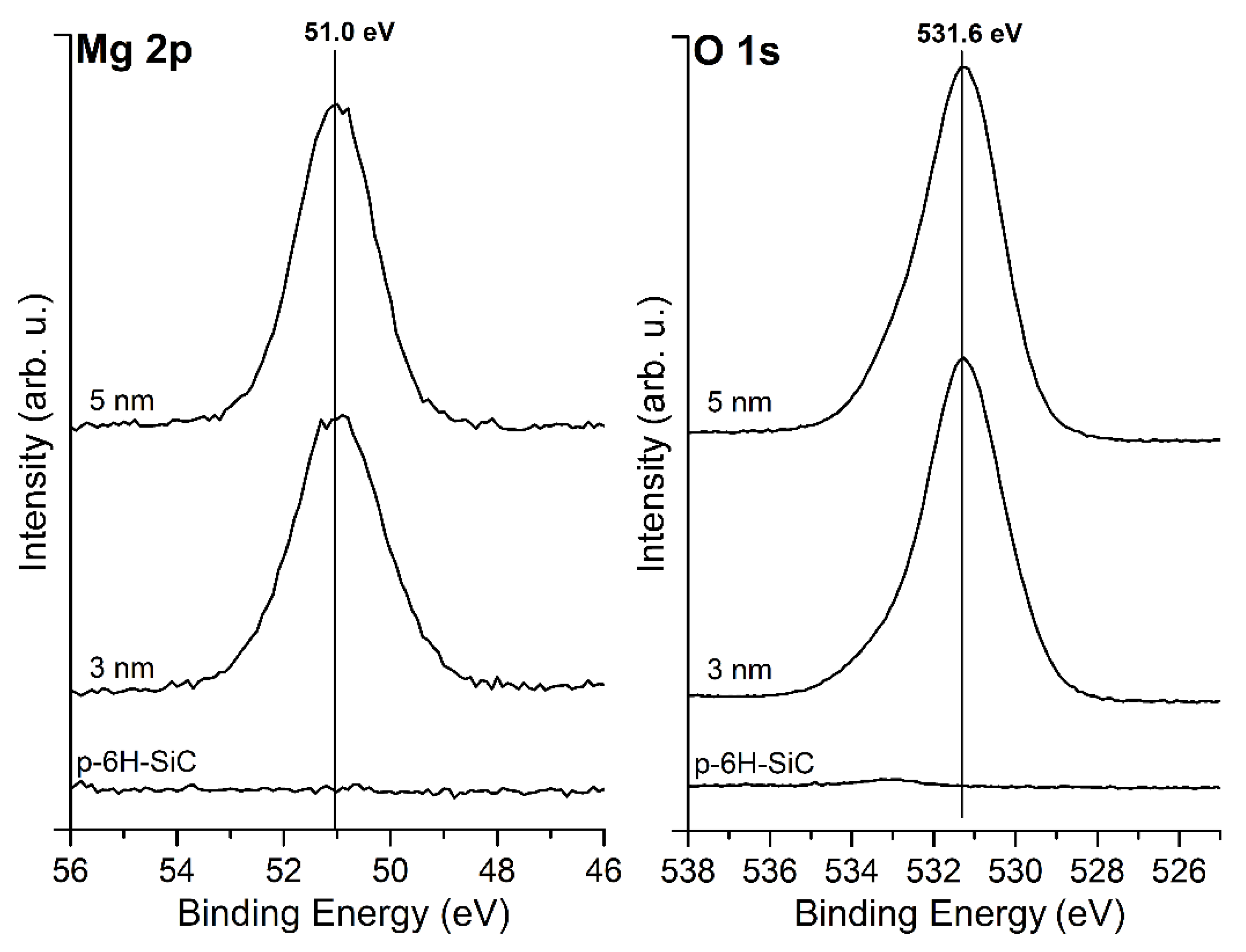
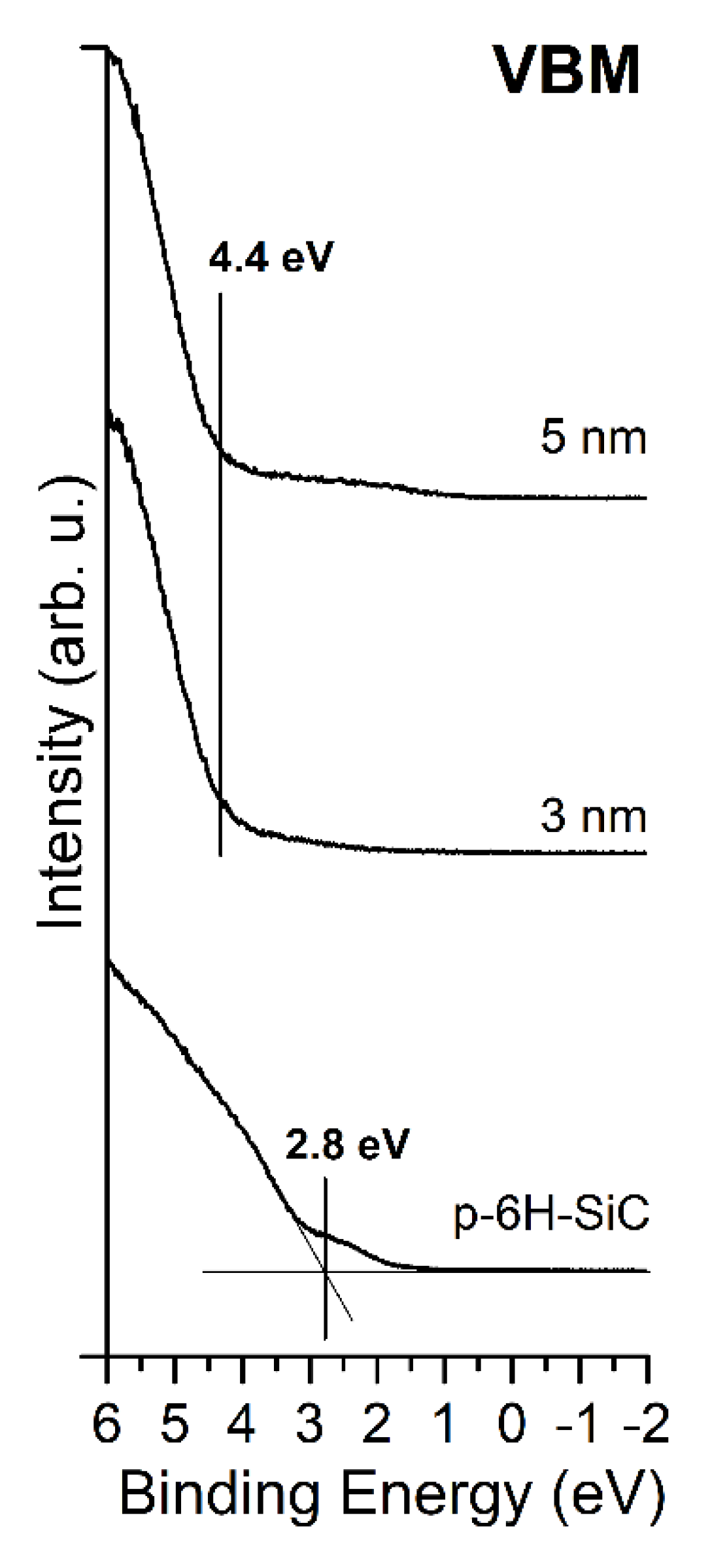
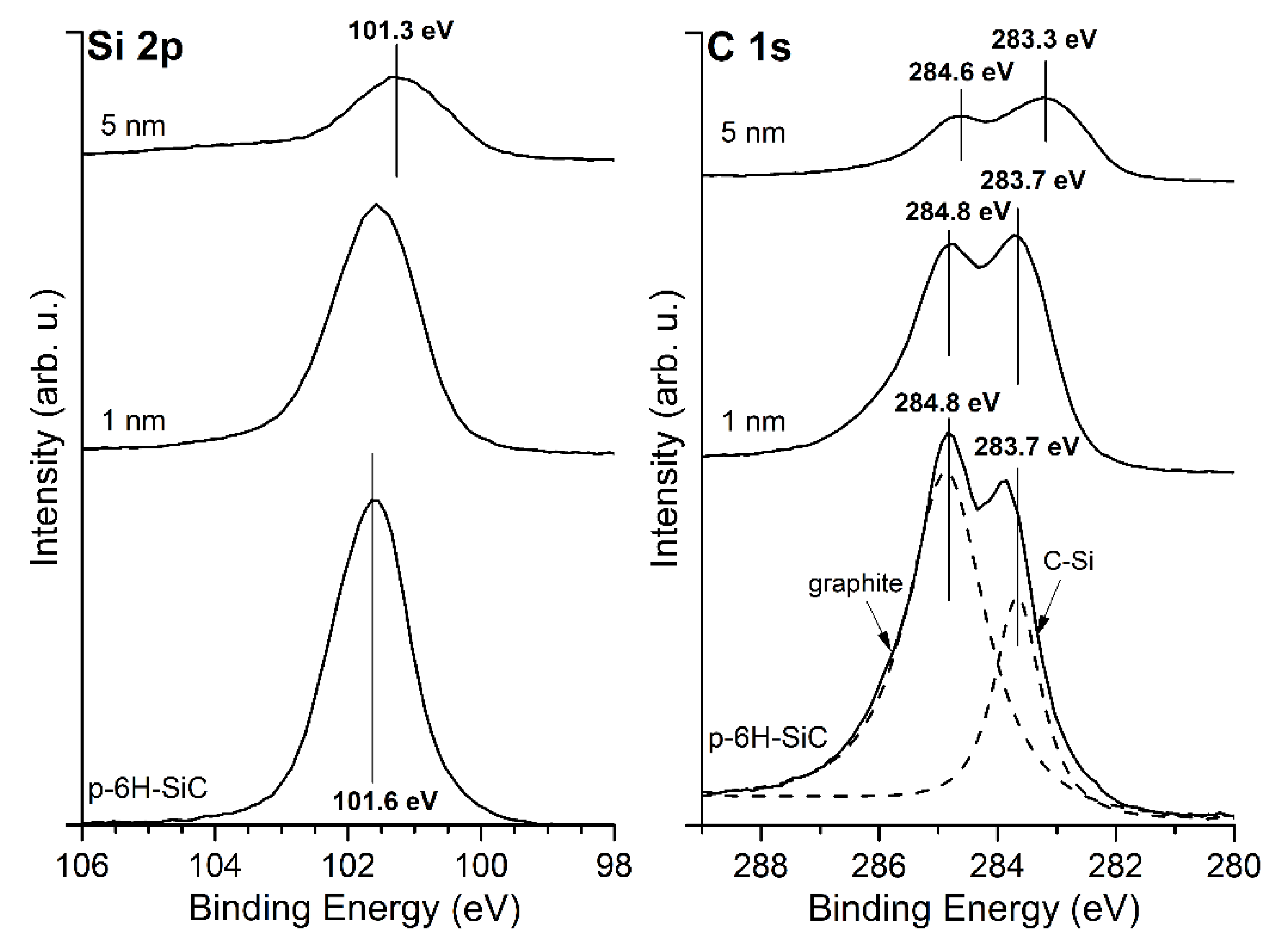
3.1. MgO/SiC(0001) System
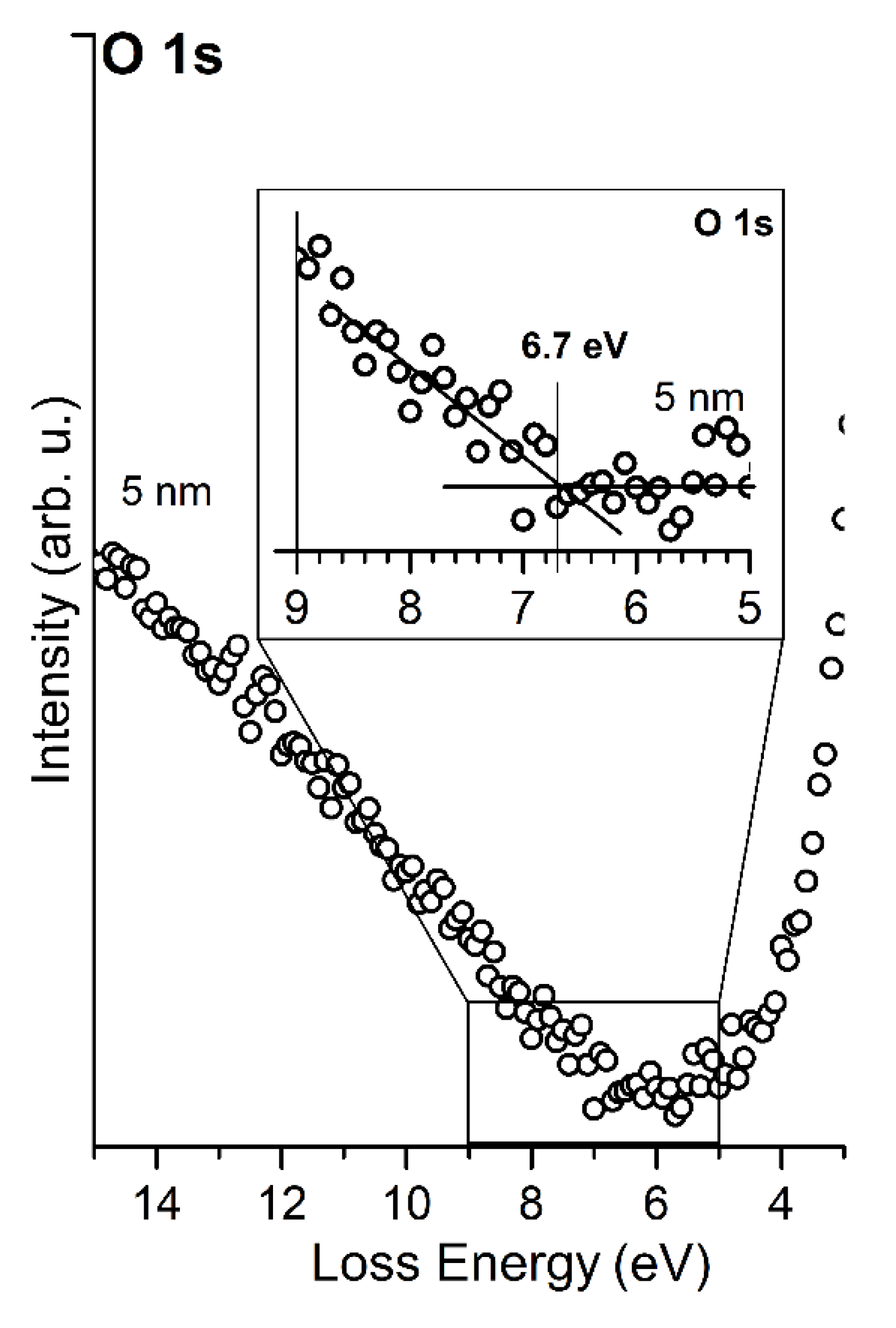
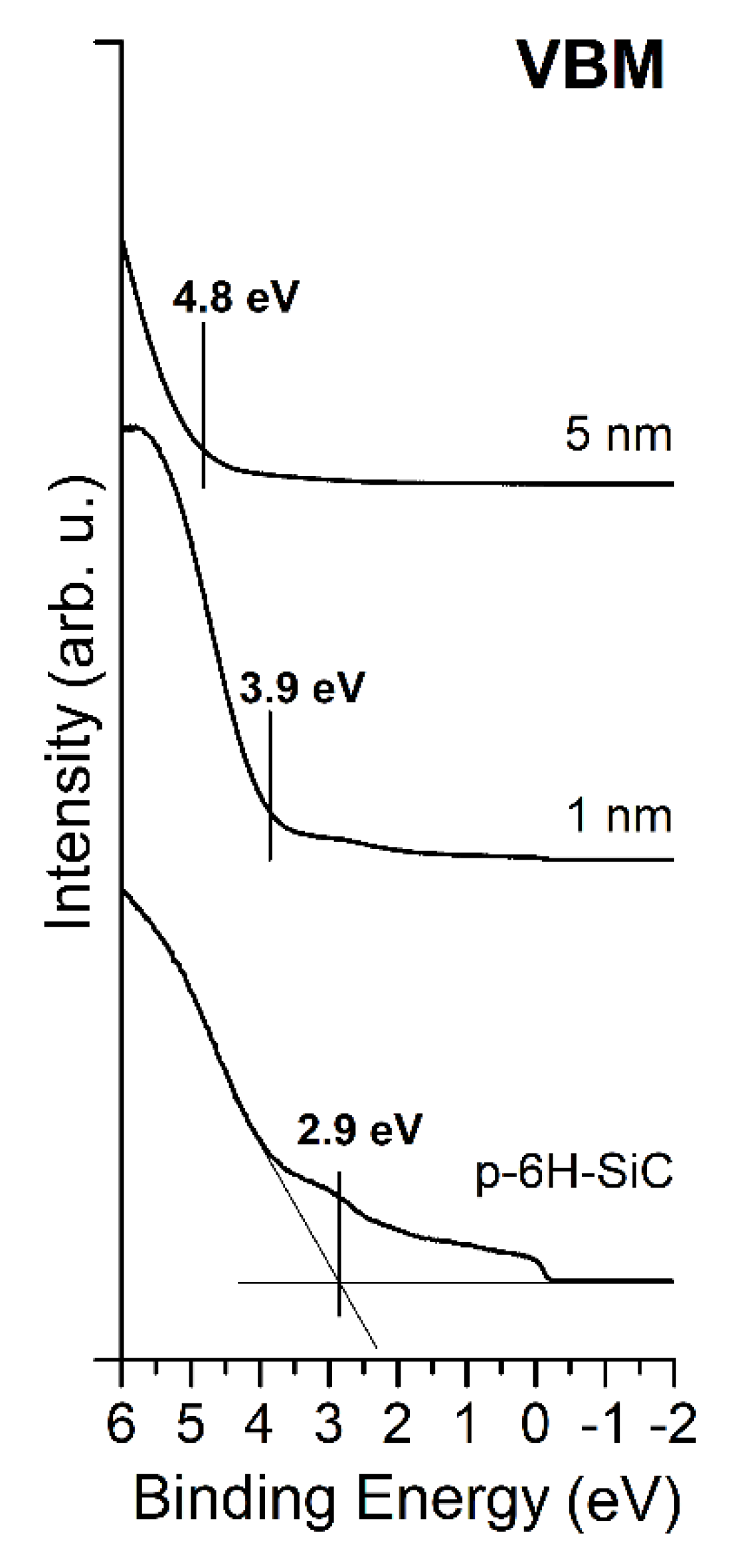
3.2. MgO/Graphite/SiC(0001) System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramanian, M.A.; Shannon, R.D.; Chai, B.H.T.; Abraham, M.M.; Wintersgill, M.C. Dielectric Constants of BeO, MgO, and CaO Using the Two-Terminal Method. Phys. Chem. Miner. 1989, 16, 741–746. [Google Scholar] [CrossRef]
- Dietl, T.; da Silva, E.; Gutowski, J.; Hönerlage, B.; Matsukura, F.; Meyer, B.K.; Strauch, D. New Data and Updates for I-VII, III-V and II-VI Compounds: Volume 44, Subvolume D; Springer Science & Business Media: Berlin, Germany, 2011; ISBN 978-3-642-14147-8. [Google Scholar]
- Chen, J.-Y.; Hsu, W.-H.; Huang, C.-L. Dielectric Properties of Magnesium Oxide at Microwave Frequency. J. Alloys Compd. 2010, 504, 284–287. [Google Scholar] [CrossRef]
- Posadas, A.; Walker, F.J.; Ahn, C.H.; Goodrich, T.L.; Cai, Z.; Ziemer, K.S. Epitaxial MgO as an Alternative Gate Dielectric for SiC Transistor Applications. Appl. Phys. Lett. 2008, 92, 233511. [Google Scholar] [CrossRef]
- Ogidi-Ekoko, O.N.; Goodrich, J.C.; Howzen, A.J.; Peart, M.R.; Strandwitz, N.C.; Wierer, J.J.; Tansu, N. Electrical Properties of MgO/GaN Metal-Oxide-Semiconductor Structures. Solid-State Electron. 2020, 172, 107881. [Google Scholar] [CrossRef]
- Kaneko, Y.; Mikoshiba, N.; Yamashita, T.Y.T. Preparation of MgO Thin Films by RF Magnetron Sputtering. Jpn. J. Appl. Phys. 1991, 30, 1091. [Google Scholar] [CrossRef]
- Cáceres, D.; Colera, I.; Vergara, I.; González, R.; Román, E. Characterization of MgO Thin Films Grown by Rf-Sputtering. Vacuum 2002, 67, 577–581. [Google Scholar] [CrossRef]
- Seyller, T.; Emtsev, K.V.; Speck, F.; Gao, K.-Y.; Ley, L. Schottky Barrier between 6H-SiC and Graphite: Implications for Metal/SiC Contact Formation. Appl. Phys. Lett. 2006, 88, 242103. [Google Scholar] [CrossRef]
- Grodzicki, M.; Mazur, P.; Wasielewski, R.; Ciszewski, A. Formation of Cr Ohmic Contact on Graphitized 6H-SiC(0001) Surface. Opt. Appl. 2013, 43. [Google Scholar] [CrossRef]
- Grodzicki, M.I.Ł.O.S.Z.; Wasielewski, R.A.D.O.S.Ł.A.W.; Mazur, P.; Zuber, S.; Ciszewski, A. TiO2 Thin Films Grown on SiO2–Si(111) by the Reactive Evaporation Method. Opt. Appl. 2013, 43, 99–107. [Google Scholar] [CrossRef]
- Grodzicki, M.; Mazur, P.; Krupski, A.; Ciszewski, A. Studies of Early Stages of Mn/GaN(0001) Interface Formation Using Surface-Sensitive Techniques. Vacuum 2018, 153, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Forbeaux, I.; Themlin, J.-M.; Debever, J.-M. Heteroepitaxial Graphite on 6H-SiC(0001) Interface Formation through Conduction-Band Electronic Structure. Phys. Rev. B 1998, 58, 16396–16406. [Google Scholar] [CrossRef]
- Forbeaux, I.; Themlin, J.-M.; Debever, J.-M. High-Temperature Graphitization of the 6H-SiC (0001) Face. Surf. Sci. 1999, 442, 9–18. [Google Scholar] [CrossRef]
- Shuman, V.B.; Savkina, N.S. Graphitization of Porous 6H-SiC Layers during Thermal Treatment in Vacuum. Tech. Phys. Lett. 2004, 30, 572–574. [Google Scholar] [CrossRef]
- Idczak, K.; Wachowicz, E.; Próchnicka, A.; Markowski, L.; Tringides, M.C. An Investigation of Thin Zn Films on 4H-SiC(0001)Graphene. Appl. Surf. Sci. 2019, 487, 1348–1355. [Google Scholar] [CrossRef]
- Aït-Mansour, K.; Dentel, D.; Kubler, L.; Diani, M.; Derivaz, M.; Bischoff, J.L. Original Ge-Induced Phenomena on Various SiC(0001) Reconstructions. J. Phys. D Appl. Phys. 2007, 40, 6225–6241. [Google Scholar] [CrossRef]
- Charrier, A.; Coati, A.; Argunova, T.; Thibaudau, F.; Garreau, Y.; Pinchaux, R.; Forbeaux, I.; Debever, J.-M.; Sauvage-Simkin, M.; Themlin, J.-M. Solid-State Decomposition of Silicon Carbide for Growing Ultra-Thin Heteroepitaxial Graphite Films. J. Appl. Phys. 2002, 92, 2479–2484. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. C 1s Peak of Adventitious Carbon Aligns to the Vacuum Level: Dire Consequences for Material’s Bonding Assignment by Photoelectron Spectroscopy. ChemPhysChem 2017, 18, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G.; Hultman, L. Reliable Determination of Chemical State in X-Ray Photoelectron Spectroscopy Based on Sample-Work-Function Referencing to Adventitious Carbon: Resolving the Myth of Apparent Constant Binding Energy of the C 1s Peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-Ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Lewandków, R.; Grodzicki, M.; Mazur, P.; Ciszewski, A. Interface Formation of Al2O3 on Carbon Enriched 6H-SiC(0001): Photoelectron Spectroscopy Studies. Vacuum 2020, 177, 109345. [Google Scholar] [CrossRef]
- Wu, P.Y.; Jiang, Y.P.; Zhang, Q.Y.; Jia, Y.; Peng, D.Y.; Xu, W. Comparative Study on Arsenate Removal Mechanism of MgO and MgO/TiO2 Composites: FTIR and XPS Analysis. New J. Chem. 2016, 40, 2878–2885. [Google Scholar] [CrossRef]
- Corneille, J.S.; He, J.; Goodman, D.W. XPS characterization of ultra-thin MgO films on a Mo(100) surface. Surf. Sci. 1994, 306, 269–278. [Google Scholar] [CrossRef]
- Crist, B.V. Handbooks of Monochromatic XPS Spectra: The Elements and Native Oxides; Wiley: Ames, IA, USA, 2000. [Google Scholar]
- Alexander, V.; Naumkin, A.K.-V.; Gaarenstroom, S.W.; Powell, C.J. NIST X-Ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/ (accessed on 27 July 2021).
- Gao, K.Y.; Seyller, T.; Ley, L.; Ciobanu, F.; Pensl, G.; Tadich, A.; Riley, J.D.; Leckey, R.G.C. Al2O3 Prepared by Atomic Layer Deposition as Gate Dielectric on 6H-SiC(0001). Appl. Phys. Lett. 2003, 83, 1830–1832. [Google Scholar] [CrossRef]
- Bell, F.G.; Ley, L. Photoemission Study of SiOx (0x2) Alloys. Phys. Rev. B 1988, 37, 8383–8393. [Google Scholar] [CrossRef]
- Nichols, M.T.; Li, W.; Pei, D.; Antonelli, G.A.; Lin, Q.; Banna, S.; Nishi, Y.; Shohet, J.L. Measurement of Bandgap Energies in Low-k Organosilicates. J. Appl. Phys. 2014, 115, 094105. [Google Scholar] [CrossRef] [Green Version]
- Mather, P.G.; Read, J.C.; Buhrman, R.A. Disorder, Defects, and Band Gaps in Ultrathin (001) MgO Tunnel Barrier Layers. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 73, 205412. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.; Cho, E.; Lee, H.I.; Park, G.S.; Kang, H.J.; Nagatomi, T.; Choi, P.; Choi, B.D. Band Gap and Defect States of MgO Thin Films Investigated Using Reflection Electron Energy Loss Spectroscopy. AIP Adv. 2015, 5, 077167. [Google Scholar] [CrossRef]
- Waldrop, J.R.; Grant, R.W.; Wang, Y.C.; Davis, R.F. Metal Schottky Barrier Contacts to Alpha 6H-SiC. J. Appl. Phys. 1992, 72, 4757–4760. [Google Scholar] [CrossRef]
- Waldrop, J.R. Schottky Barrier Height of Metal Contacts to P-Type Alpha 6H-SiC. J. Appl. Phys. 1994, 75, 4548–4550. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandków, R.; Mazur, P.; Trembułowicz, A.; Sabik, A.; Wasielewski, R.; Grodzicki, M. Influence of Graphite Layer on Electronic Properties of MgO/6H-SiC(0001) Interface. Materials 2021, 14, 4189. https://doi.org/10.3390/ma14154189
Lewandków R, Mazur P, Trembułowicz A, Sabik A, Wasielewski R, Grodzicki M. Influence of Graphite Layer on Electronic Properties of MgO/6H-SiC(0001) Interface. Materials. 2021; 14(15):4189. https://doi.org/10.3390/ma14154189
Chicago/Turabian StyleLewandków, Rafał, Piotr Mazur, Artur Trembułowicz, Agata Sabik, Radosław Wasielewski, and Miłosz Grodzicki. 2021. "Influence of Graphite Layer on Electronic Properties of MgO/6H-SiC(0001) Interface" Materials 14, no. 15: 4189. https://doi.org/10.3390/ma14154189
APA StyleLewandków, R., Mazur, P., Trembułowicz, A., Sabik, A., Wasielewski, R., & Grodzicki, M. (2021). Influence of Graphite Layer on Electronic Properties of MgO/6H-SiC(0001) Interface. Materials, 14(15), 4189. https://doi.org/10.3390/ma14154189






