Morphology, Thermo-Mechanical Properties and Biodegradibility of PCL/PLA Blends Reactively Compatibilized by Different Organic Peroxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methodology
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Dynamic Mechanical Analysis (DMA)
2.3.4. Mechanical Tests
2.3.5. Rheological Properties
2.3.6. Atomic Forace Microscopy (AFM)
2.3.7. Biodegradability Tests
3. Results and Discussion
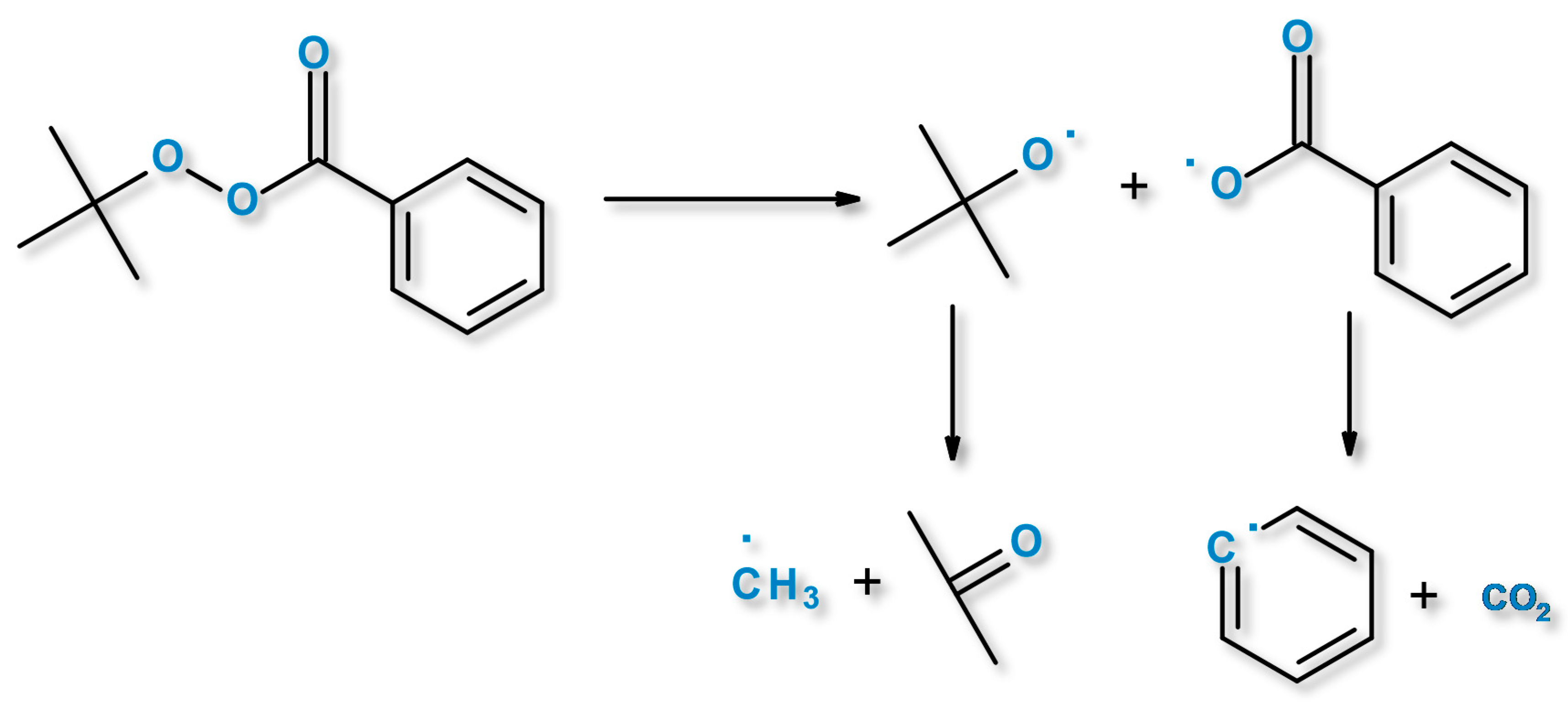
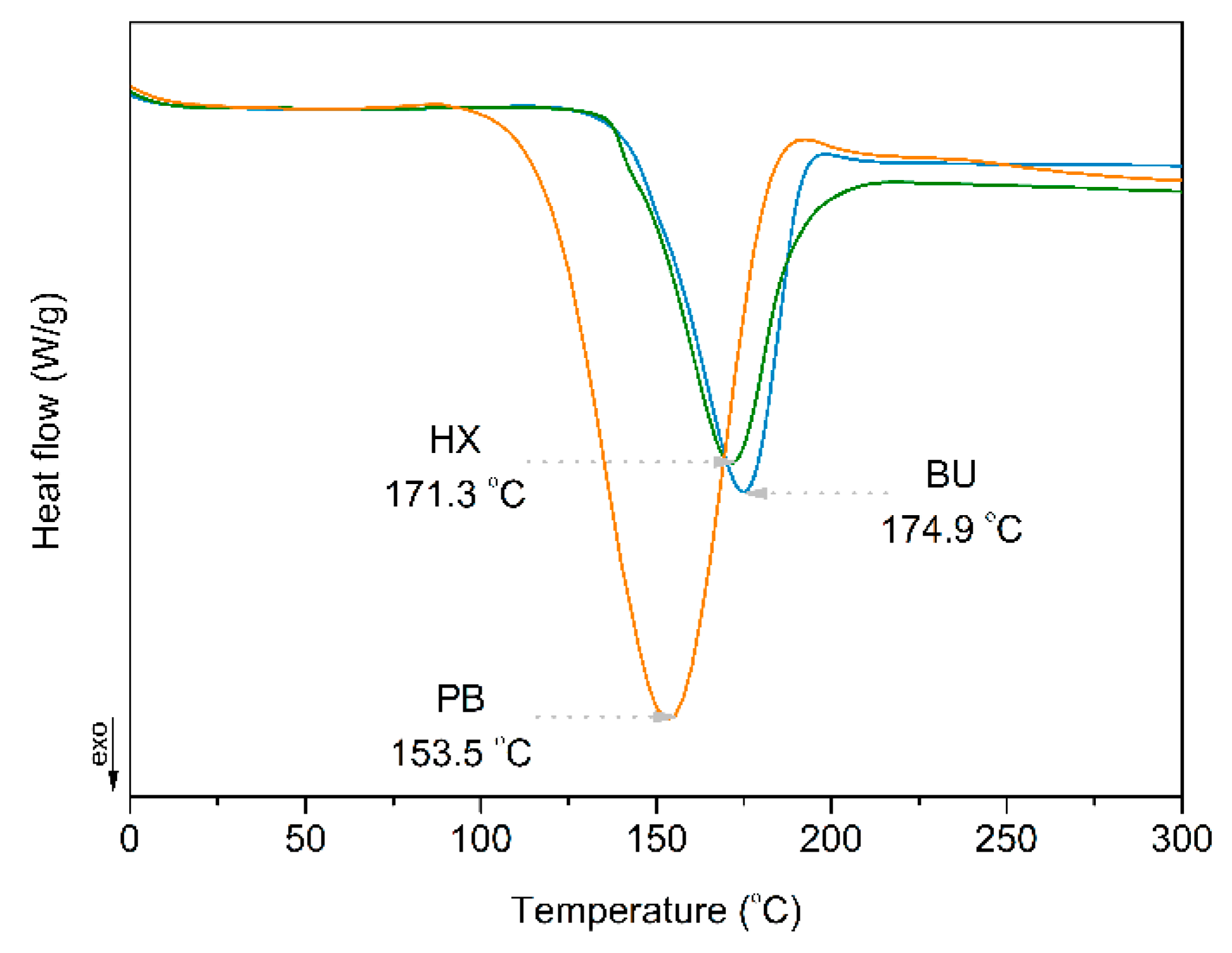
3.1. DSC of Organic Peroxides and Their Influence on PCL/PLA Blends Thermal Properties
3.2. Thermal Stability of PCL/PLA Blends
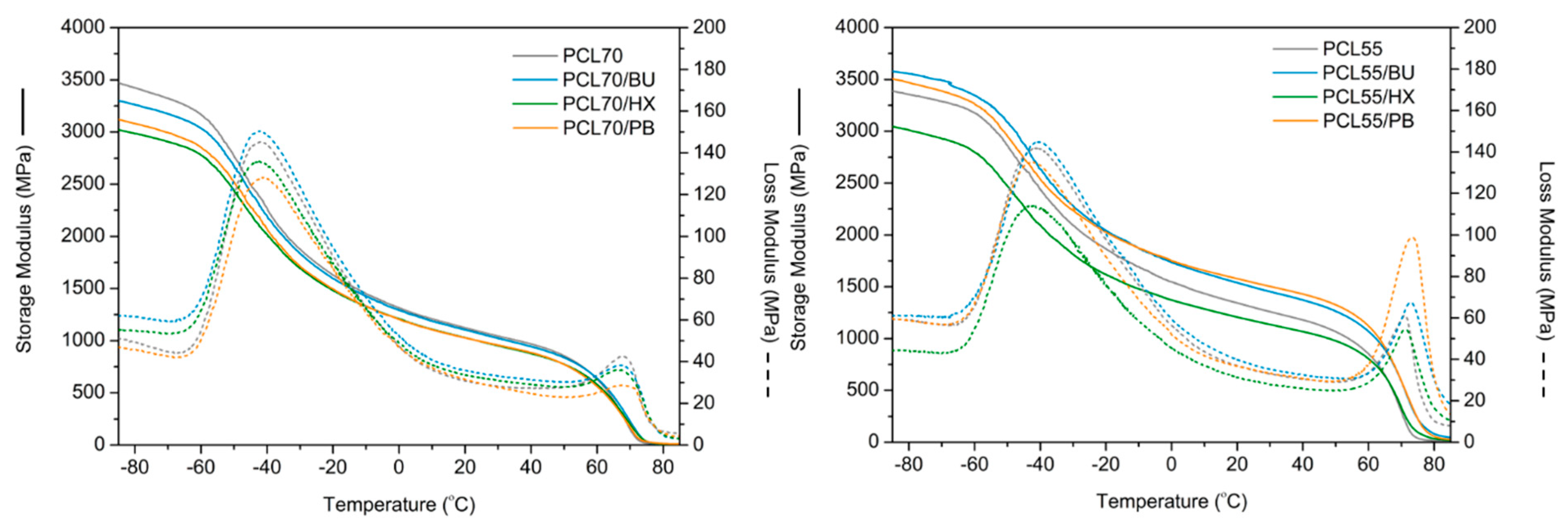
3.3. Dynamic Mechanical Analysis
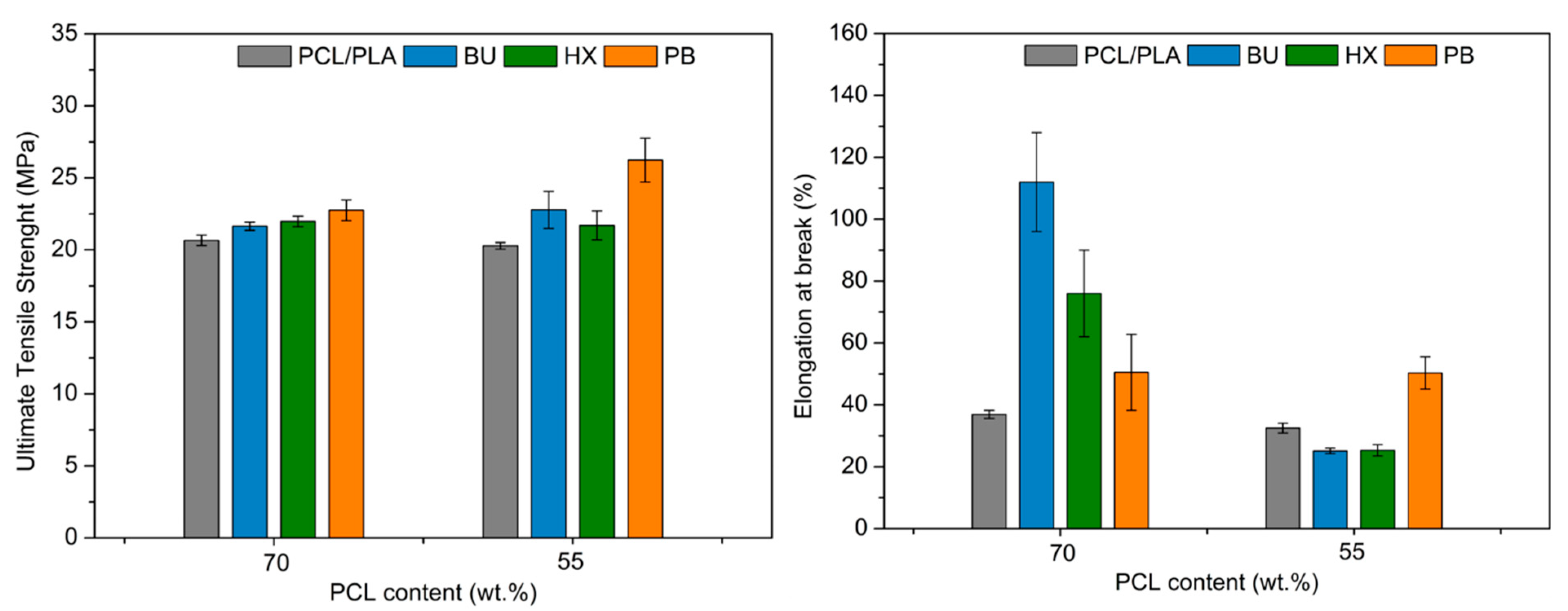
3.4. Mechanical Tests
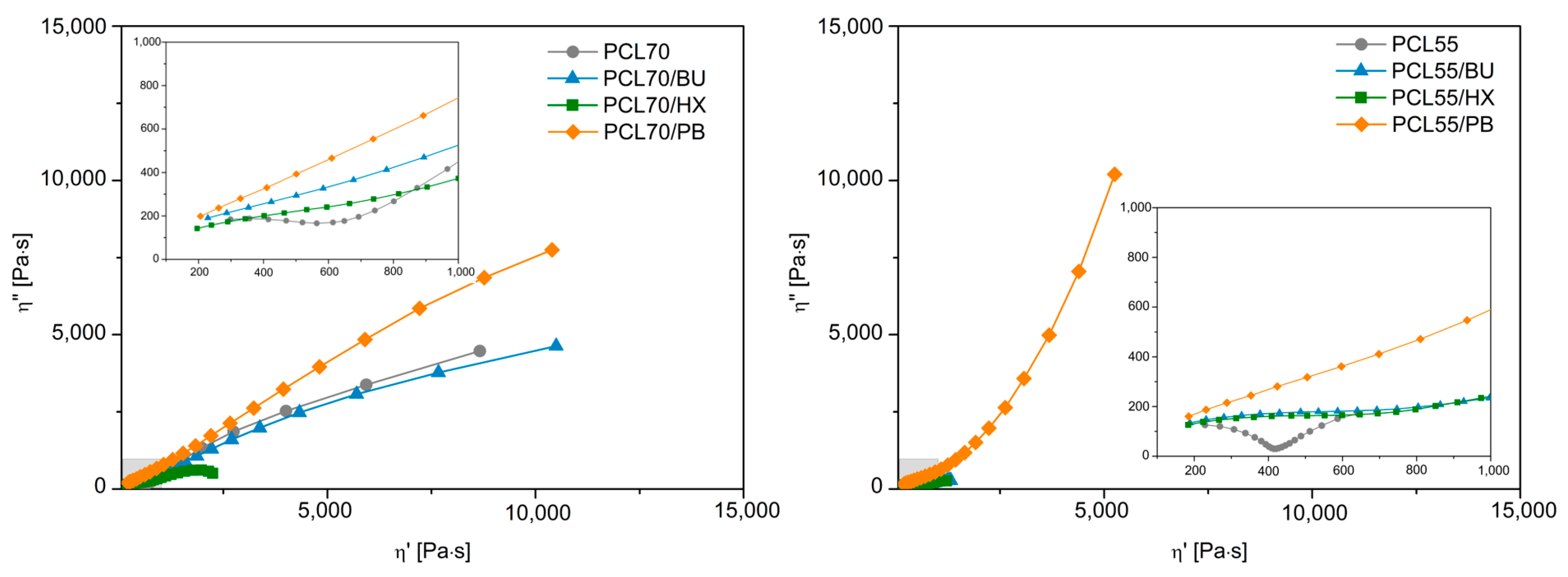
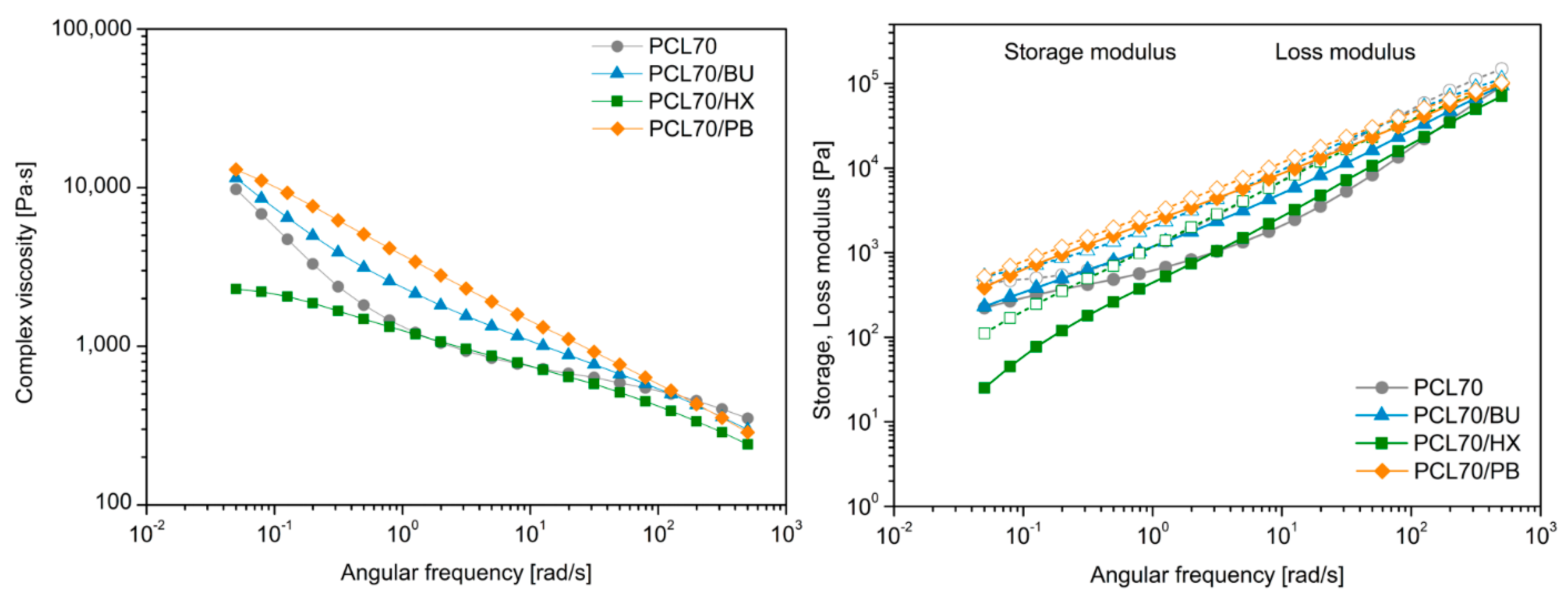
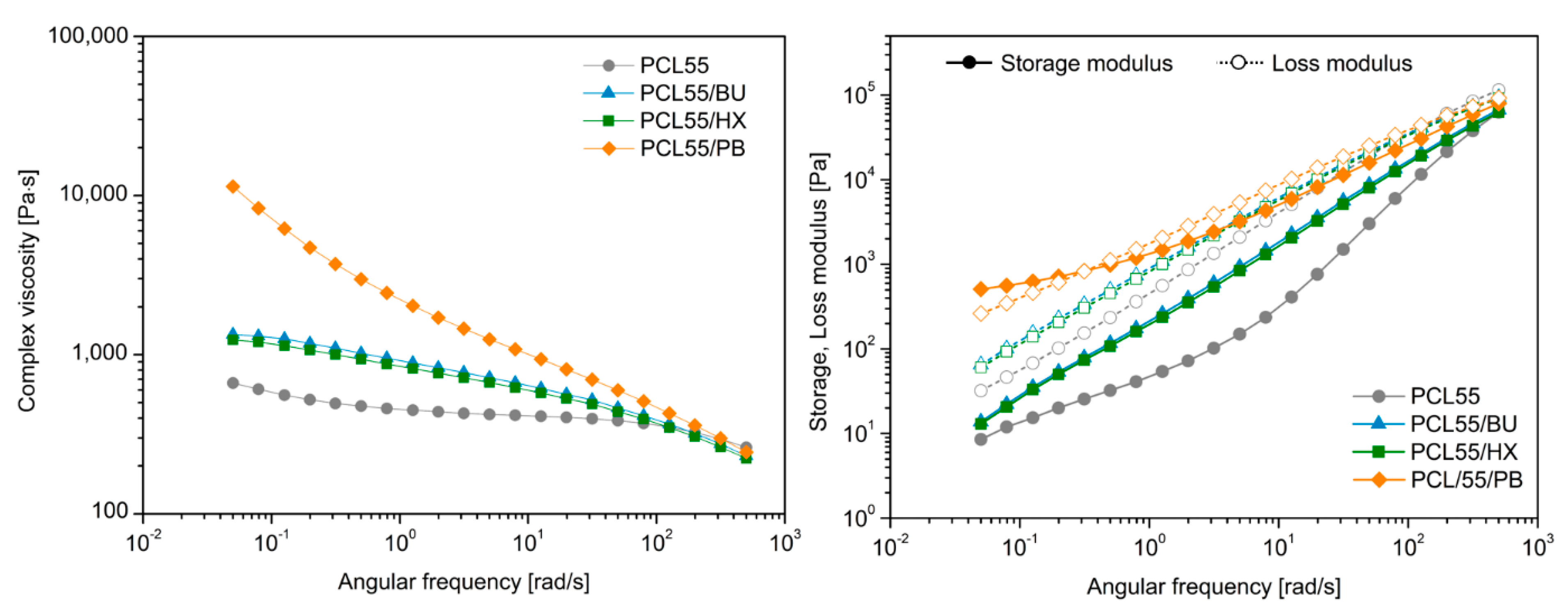
3.5. Rheological Study
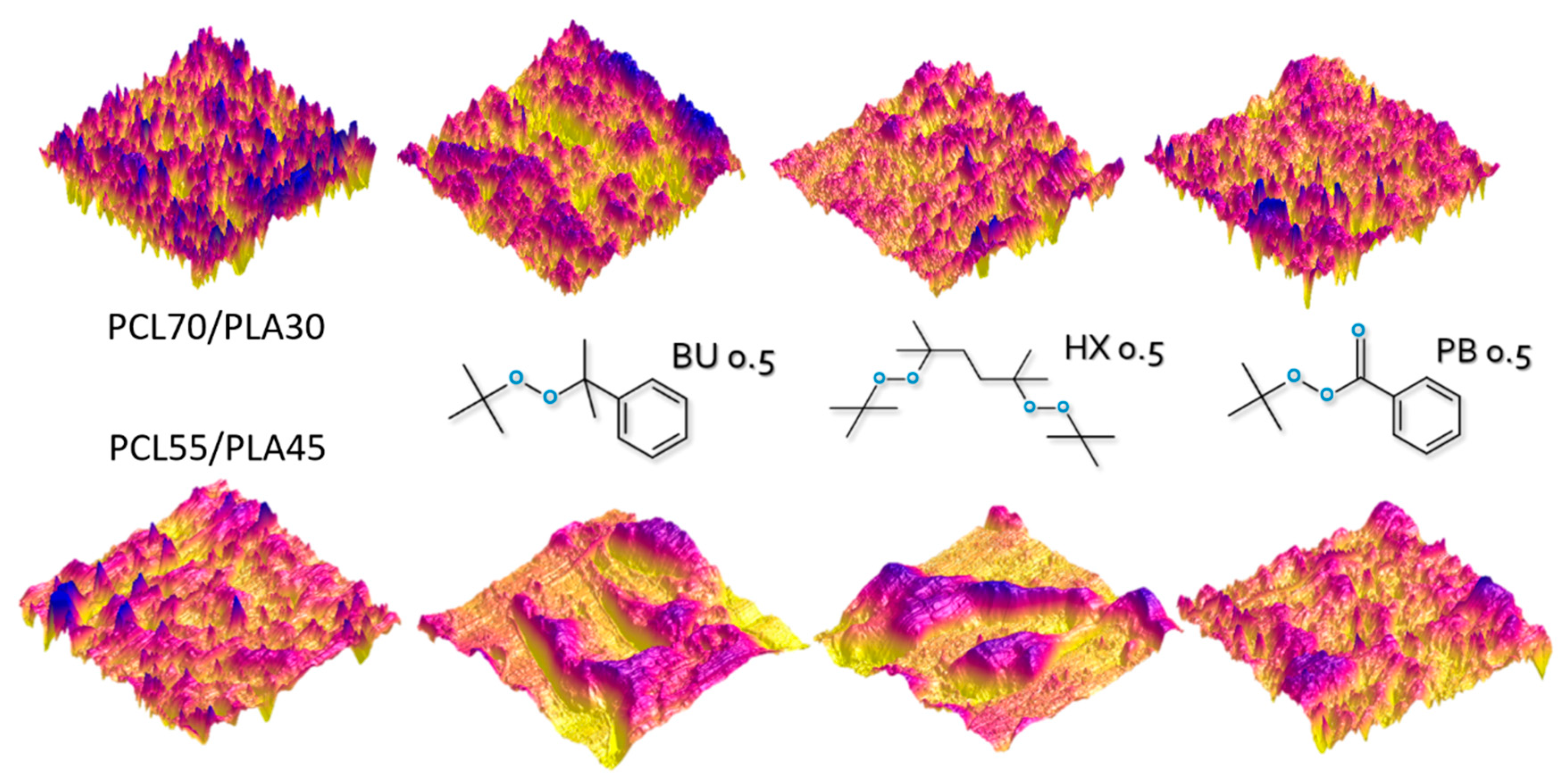
3.6. Morphology
3.7. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based(bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef]
- Nicolais, L.; Di Maio, E.; Iannace, S. Biodegradable composites. In Wiley Encyclopedia of Composites; Nicolais, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly(ε-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2014. [Google Scholar] [CrossRef]
- Das, M.; Mandal, B.; Katiyar, V. Sustainable routes for synthesis of poly(ε-caprolactone): Prospects in chemical industries. In Advances in Sustainable Polymers; Katiyar, V., Kumar, A., Mulchandani, N., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Prem, N.; Schale, F.; Zimmermann, K.; Gowda, D.K.; Odenbach, S. Synthesis and characterization of the properties of thermosensitive elastomers with thermoplastic and magnetic particles for application in soft robotics. J. Appl. Polym. Sci. 2021, e51296. [Google Scholar] [CrossRef]
- Oyama, T.; Kobayashi, S.; Okura, T.; Sato, S.; Tajima, K.; Isono, T.; Satoh, T. Biodegradable compatibilizers for poly(hydroxyalkanoate)/poly(ε-caprolactone) blends through click reactions with end-functionalized microbial poly(hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2019, 7, 7969–7978. [Google Scholar] [CrossRef]
- Gassner, F.; Owen, A. Physical properties of poly(β-hydroxybutyrate)-poly(ε-caprolactone) blends. Polymer 1994, 35, 2233–2236. [Google Scholar] [CrossRef]
- Vinci, G.; Ruggieri, R.; Billi, A.; Pagnozzi, C.; Di Loreto, M.V.; Ruggeri, M. Sustainable management of organic waste and recycling for bioplastics: A LCA approach for the Italian case study. Sustainability 2021, 13, 6385. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of bio-based products on waste management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef] [Green Version]
- Funabashi, M.; Ninomiya, F.; Kunioka, M. Biodegradation of polycaprolactone powders proposed as reference test materials for international standard of biodegradation evaluation method. J. Polym. Environ. 2007, 15, 7–17. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers. A review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2019, 3, 27–35. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly(lactic acid) and poly(glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Achmad, F.; Yamane, K.; Quan, S.; Kokugan, T. Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators. Chem. Eng. J. 2009, 151, 342–350. [Google Scholar] [CrossRef]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic acid): A versatile biobased polymer for the future with multifunctional properties–from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards controlled degradation of poly(lactic) acid in technical applications. C J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and biodegradation of poly(lactic acid). In Synthesis, Structure and Properties of Poly(Lactic Acid); Di Lorenzo, M., Androsch, R., Eds.; Advances in Polymer Science Book Series; Springer: Cham, Switzerland, 2017; Volume 279. [Google Scholar] [CrossRef]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Solati-Hashjin, M.; Abu Osman, N.A. Polycaprolactone/starch composite: Fabrication, structure, properties, and applications. J. Biomed. Mater. Res. Part. A 2015, 103A, 2482–2498. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.J.; Zhang, X.; He, C. Fully biodegradable poly(lactic acid)/starch blends: A review of toughening strategies. Int. J. Biol. Macromol. 2018, 109, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly(lactic) acid and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef]
- Adeodato Vieira, M.G.; Altenhofen da Silva, M.; Oliveira dos Santos, L.; Masumi Beppu, M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the toughness and thermal resistance of polyoxymethylene/poly(lactic acid) blends: Evaluation of structure–properties correlation for reactive processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened renewable PLA reactive multiphase blends system: Phase morphology and performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xiao, A.; Yu, B.; Bhat, G.; Zhu, F. Effect of PCL and compatibilizer on the tensile and barrier properties of PLA/PCL films. Polym. Korea. 2017, 41, 181–188. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarria, G.; Eguizabal, J.L. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Cohn, D.; Salomon, A.H. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials. 2005, 26, 2297. [Google Scholar] [CrossRef]
- Dandan Doganci, M.; Aynali, F.; Doganci, E.; Ozkoc, G. Mechanical, thermal and morphological properties of poly(lactic acid) by using star-shaped poly(ε-caprolactone) with POSS core. Eur. Polym. J. 2019, 121, 109316. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites-Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Lan, X.; Wu, F.; Xie, B.; Yang, M. Morphology, rheology, crystallization behavior, and mechanical properties of poly(lactic acid)/poly(butylene succinate)/dicumyl peroxide reactive blends. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of partial crosslinking on morphology and properties of the poly(β-hydroxybutyrate)/poly(d,l-lactic acid) blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Poly(ε-caprolactone)/poly(lactic acid) blends compatibilized by peroxide initiators: Comparison of two strategies. Polymers 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Przybysz-Romatowska, M.; Marć, M.; Klein, M.; Saeb, M.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(lactic acid) (PLA)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test. 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Simon, G.; Kukaleva, N. Rheological and viscoelastic behavior of HDPE/cctamethyl-POSS nanocomposites. Macromolecules 2006, 39, 1839–1849. [Google Scholar] [CrossRef]
- Tesfaye, M.; Patwa, R.; Dhar, P.; Katiyar, V. Nanosilk-grafted poly(lactic acid) films: Influence of cross-linking on rheology and thermal stability. ACS Omega 2017, 2, 7071–7084. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Wang, G.; Zhao, D. Study on rheological behavior of polypropylene/clay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 3609–3617. [Google Scholar] [CrossRef]
- Walczak, M.; Swiontek Brzezinska, M.; Sionkowska, A.; Michalska, M.; Jankiewicz, U.; Deja-Sikora, E. Biofilm formation on the surface of polylactide during its biodegradation in different environments. Colloids Surfaces B Biointerfaces 2015, 136, 340–345. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Management 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Folino, A.; Karageorgiou, A.; Calabrò, C.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]


| Parameter | PCL 6800 | PLA 3251D |
|---|---|---|
| Density, g/cm3 | 1.15 | 1.24 |
| Melting point, °C | 58–60 | 155–170 |
| Glass transition (Tg), °C | −60 | 55–60 |
| Average molecular weight (Mw), g/mol | 80,000 | 55,400 |
| Melt flow index (MFI)190 °C/2.16kg, g/10 min | 4.1 | 35.0 |
| Sample Name | Polymers (wt. %) | Organic Peroxides (wt. %) | |||
|---|---|---|---|---|---|
| PCL | PLA | BU | HX | BP | |
| PCL70 | 70 | 30 | - | - | - |
| PCL70/BU | 70 | 30 | 0.5 | - | - |
| PCL70/HX | 70 | 30 | - | 0.5 | - |
| PCL70/PB | 70 | 30 | - | - | 0.5 |
| PCL55 | 55 | 45 | - | - | - |
| PCL55/BU | 55 | 45 | 0.5 | - | - |
| PCL55/HX | 55 | 45 | - | 0.5 | - |
| PCL55/PB | 55 | 45 | - | - | 0.5 |
| Sample Code | Tm PCL (°C) | ΔHm PCL (J/g) | Tc PCL (°C) | ΔHc PCL (J/g) | Tm PLA (°C) | ΔHm PLA (J/g) | Tcc PLA (°C) | ΔHcc PLA (J/g) | Xc PCL (%) | Xc PLA (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL70 | 57.8 | 38.7 | 32.7 | 42.9 | 169.5 | 15.3 | 105.6 | 9.6 | 40.7 | 20.3 |
| PCL70/BU | 57.6 | 36.4 | 32.8 | 42.9 | 168.3 | 13.9 | 102.0 | 8.6 | 38.2 | 18.9 |
| PCL70/HX | 57.5 | 36.7 | 32.9 | 42.7 | 168.1 | 14.0 | 102.5 | 8.7 | 38.4 | 18.9 |
| PCL70/PB | 58.1 | 37.7 | 34.4 | 40.9 | 168.3 | 14.1 | 98.5 | 7.2 | 39.6 | 24.5 |
| PCL55 | 57.1 | 29.4 | 32.9 | 34.0 | 169.2 | 19.8 | 104.4 | 13.4 | 39.3 | 15.2 |
| PCL55/BU | 57.2 | 29.8 | 32.0 | 32.8 | 168.1 | 22.4 | 101.2 | 13.8 | 39.8 | 20.4 |
| PCL55/HX | 57.0 | 27.6 | 32.7 | 32.9 | 168.2 | 24.6 | 101.2 | 14.9 | 36.9 | 23.0 |
| PCL55/PB | 57.7 | 27.9 | 33.6 | 31.5 | 168.6 | 19.6 | 104.7 | 13.3 | 37.3 | 14.9 |
| Sample Codes | T−2% (°C) | T−5% (°C) | T−10% (°C) | T−50% (°C) | R716.5 °C (%) |
|---|---|---|---|---|---|
| PCL70 | 324.0 | 334.3 | 343.1 | 391.2 | 1.87 |
| PCL70/BU | 324.6 | 333.9 | 342.5 | 391.6 | 3.13 |
| PCL70/HX | 317.6 | 331.2 | 341.0 | 389.9 | 0.44 |
| PCL70/PB | 322.8 | 333.9 | 342.8 | 391.4 | 1.50 |
| PCL55 | 317.5 | 329.5 | 338.4 | 379.5 | 1.89 |
| PCL55/BU | 316.8 | 329.4 | 338.7 | 376.5 | 0.94 |
| PCL55/HX | 318.7 | 329.4 | 338.4 | 378.1 | 1.92 |
| PCL55/PB | 317.9 | 328.1 | 337.3 | 379.4 | 3.50 |
| Sample Codes | Biodegradability [% TOD] After | ||
|---|---|---|---|
| 7 Days | 14 Days | 28 Days | |
| PCL70 | 1.87 ± 0.10 | 7.24 ± 0.26 | 21.04 ± 0.52 |
| PCL70/BU | 0.70 ± 0.04 | 4.18 ± 0.10 | 7.23 ± 0.16 |
| PCL70/HX | 0.23 ± 0.01 | 1.79 ± 0.04 | 4.04 ± 0.12 |
| PCL70/PB | 0.31 ± 0.01 | 2.03 ± 0.05 | 4.04 ± 0.09 |
| PCL55 | 1.22 ± 0.04 | 4.73 ± 0.12 | 15.93 ± 0.16 |
| PCL55/BU | 0.39 ± 0.02 | 2.13 ± 0.09 | 9.37 ± 0.19 |
| PCL55/HX | 0.10 ± 0.01 | 1.05 ± 0.05 | 3.77 ± 0.20 |
| PCL55/PB | 0.16 ± 0.02 | 1.76 ± 0.07 | 6.78 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybysz-Romatowska, M.; Barczewski, M.; Mania, S.; Tercjak, A.; Haponiuk, J.; Formela, K. Morphology, Thermo-Mechanical Properties and Biodegradibility of PCL/PLA Blends Reactively Compatibilized by Different Organic Peroxides. Materials 2021, 14, 4205. https://doi.org/10.3390/ma14154205
Przybysz-Romatowska M, Barczewski M, Mania S, Tercjak A, Haponiuk J, Formela K. Morphology, Thermo-Mechanical Properties and Biodegradibility of PCL/PLA Blends Reactively Compatibilized by Different Organic Peroxides. Materials. 2021; 14(15):4205. https://doi.org/10.3390/ma14154205
Chicago/Turabian StylePrzybysz-Romatowska, Marta, Mateusz Barczewski, Szymon Mania, Agnieszka Tercjak, Józef Haponiuk, and Krzysztof Formela. 2021. "Morphology, Thermo-Mechanical Properties and Biodegradibility of PCL/PLA Blends Reactively Compatibilized by Different Organic Peroxides" Materials 14, no. 15: 4205. https://doi.org/10.3390/ma14154205






