Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Testing Material
2.2. Oil Extraction from MangoSseed
2.3. Characterization of Mango Seed Oil
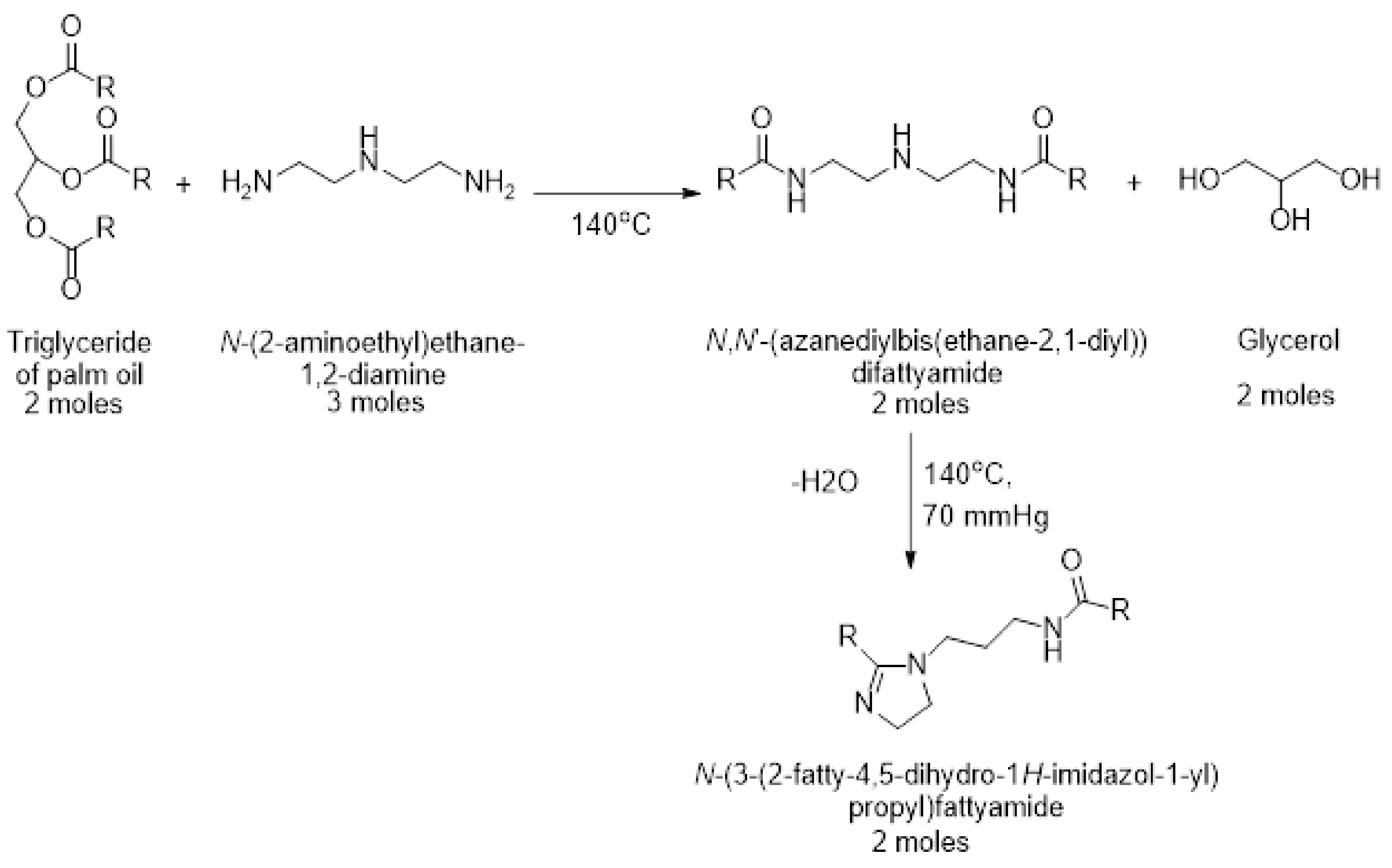
2.4. Synthesis of N-(3-(2-fatty-4, 5-dihydro-1H-imidazol-1-yl) propyl) Fatty Amide
2.5. Electrochemical Tests
3. Results and Discussion
3.1. Extraction of Mango Seed Oil and Optimization Parameters
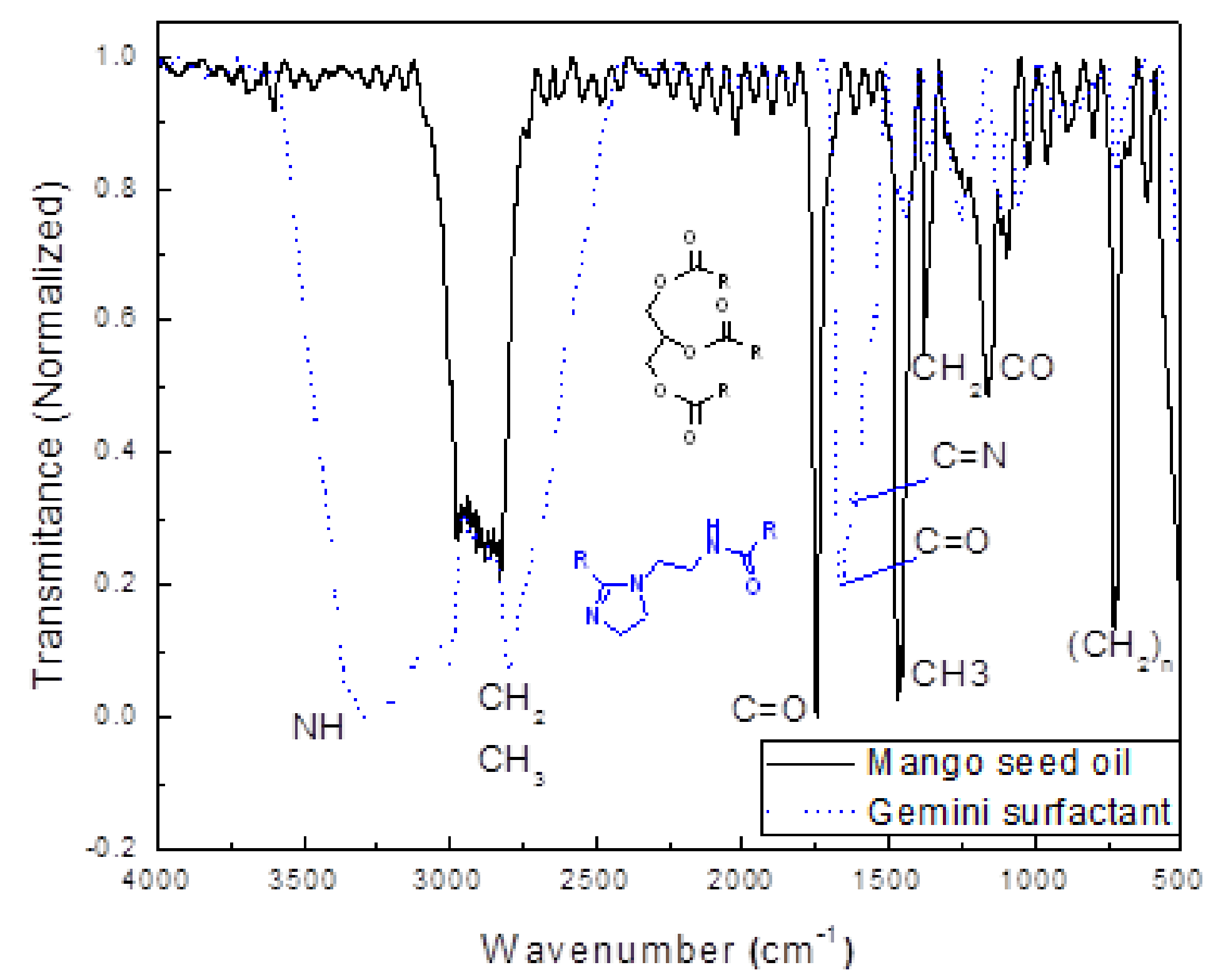
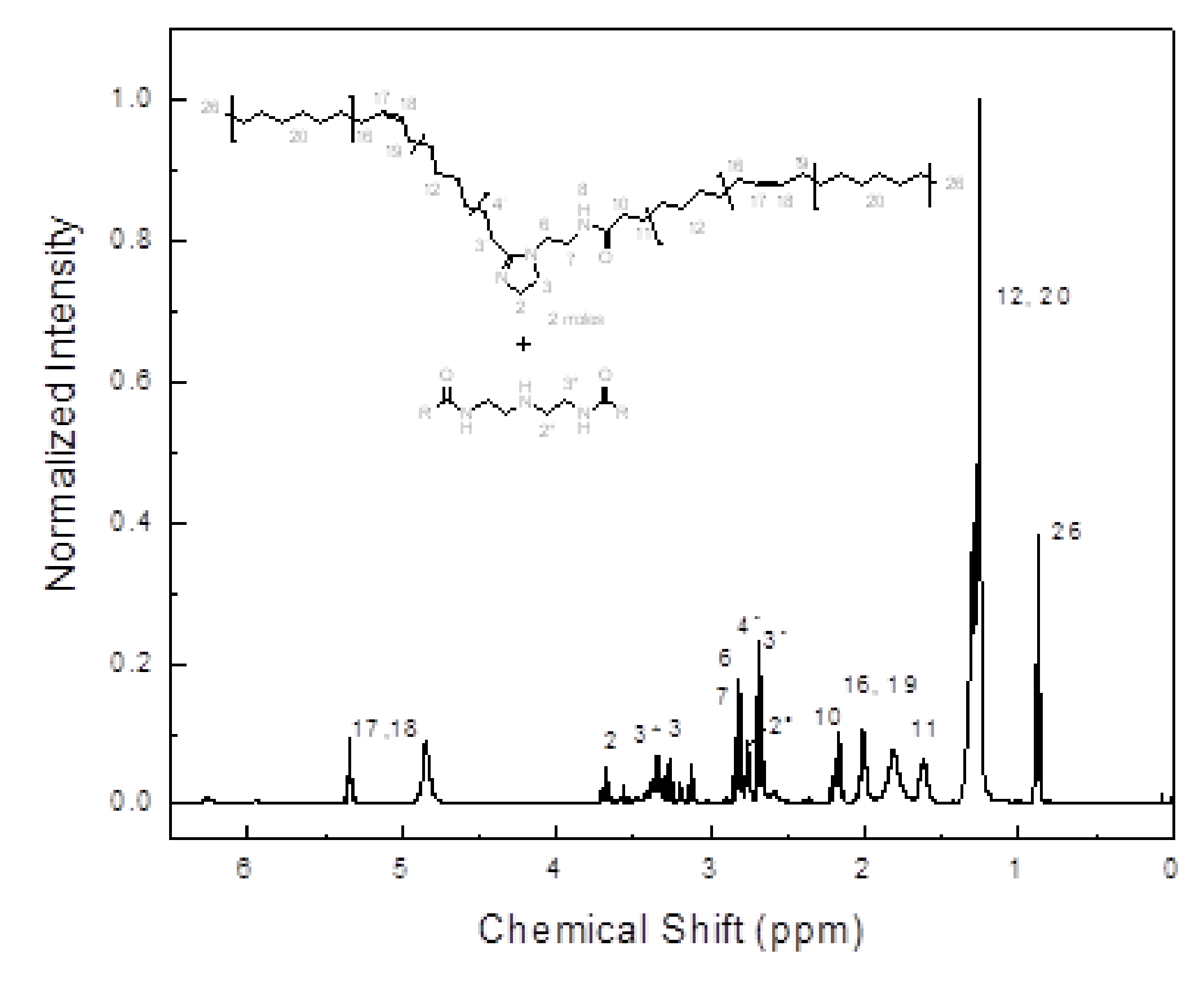
3.2. Characterization of Mango Oil and Corrosion Inhibitor
3.3. Potentiodynamic Polarization Curves
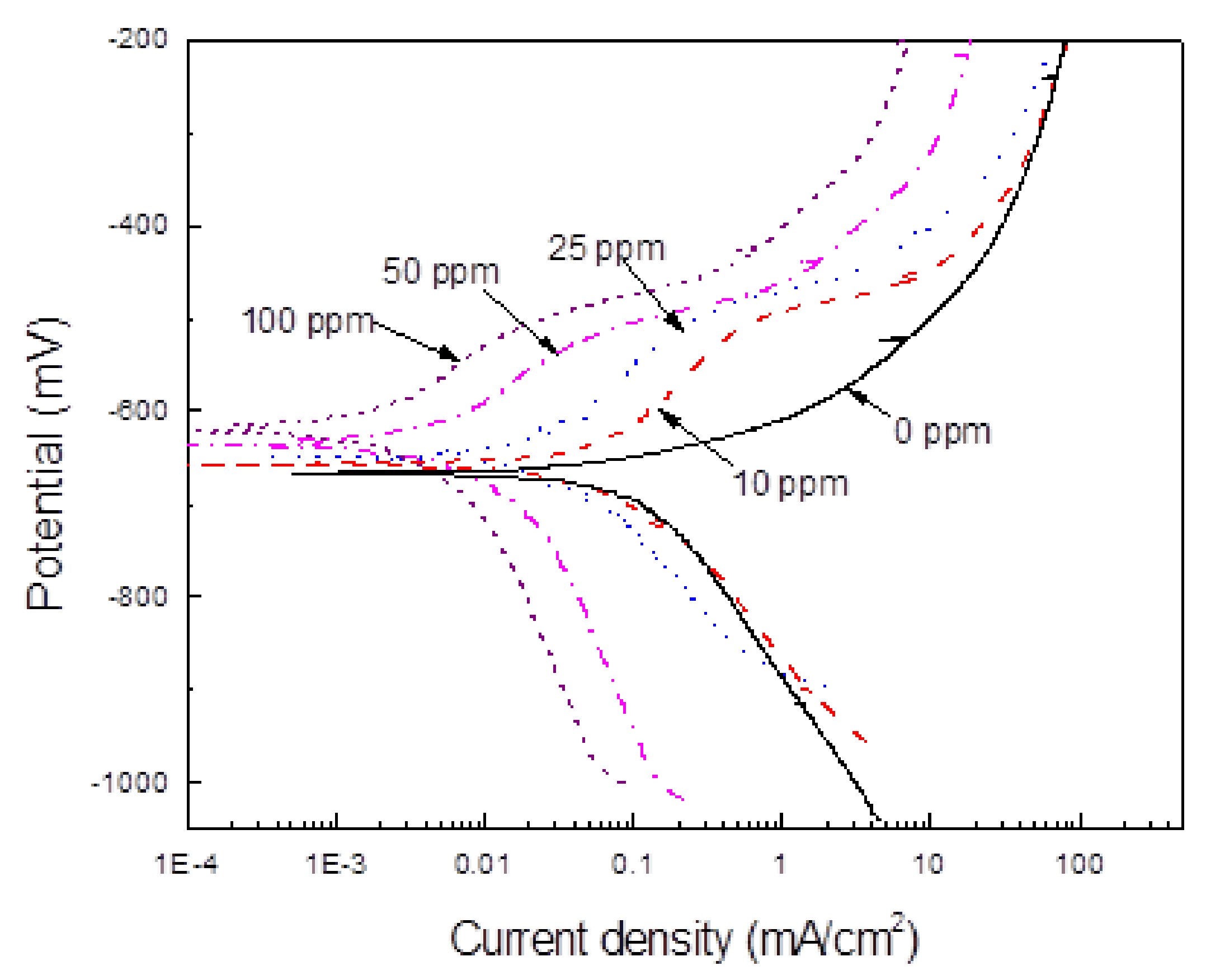
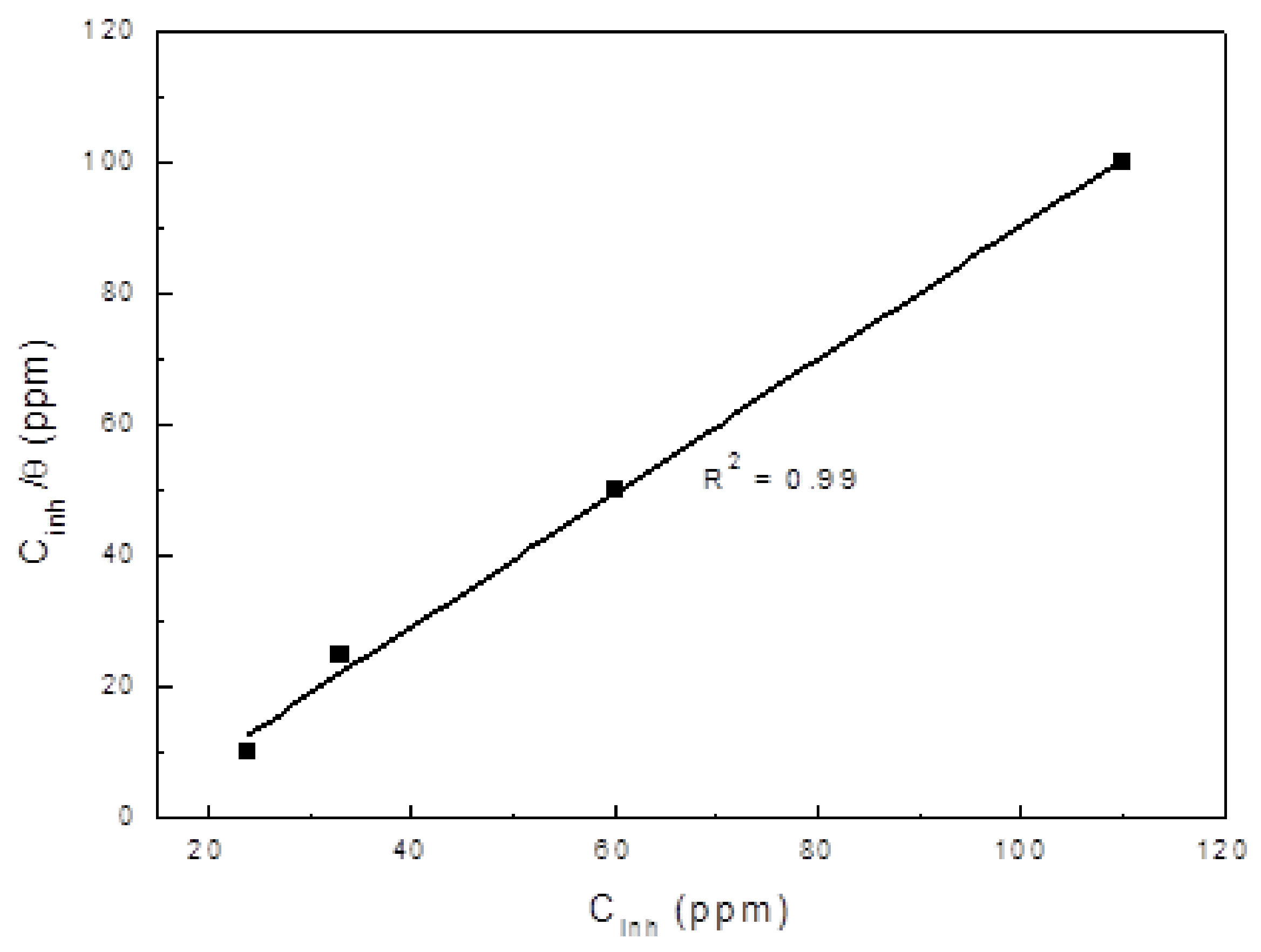
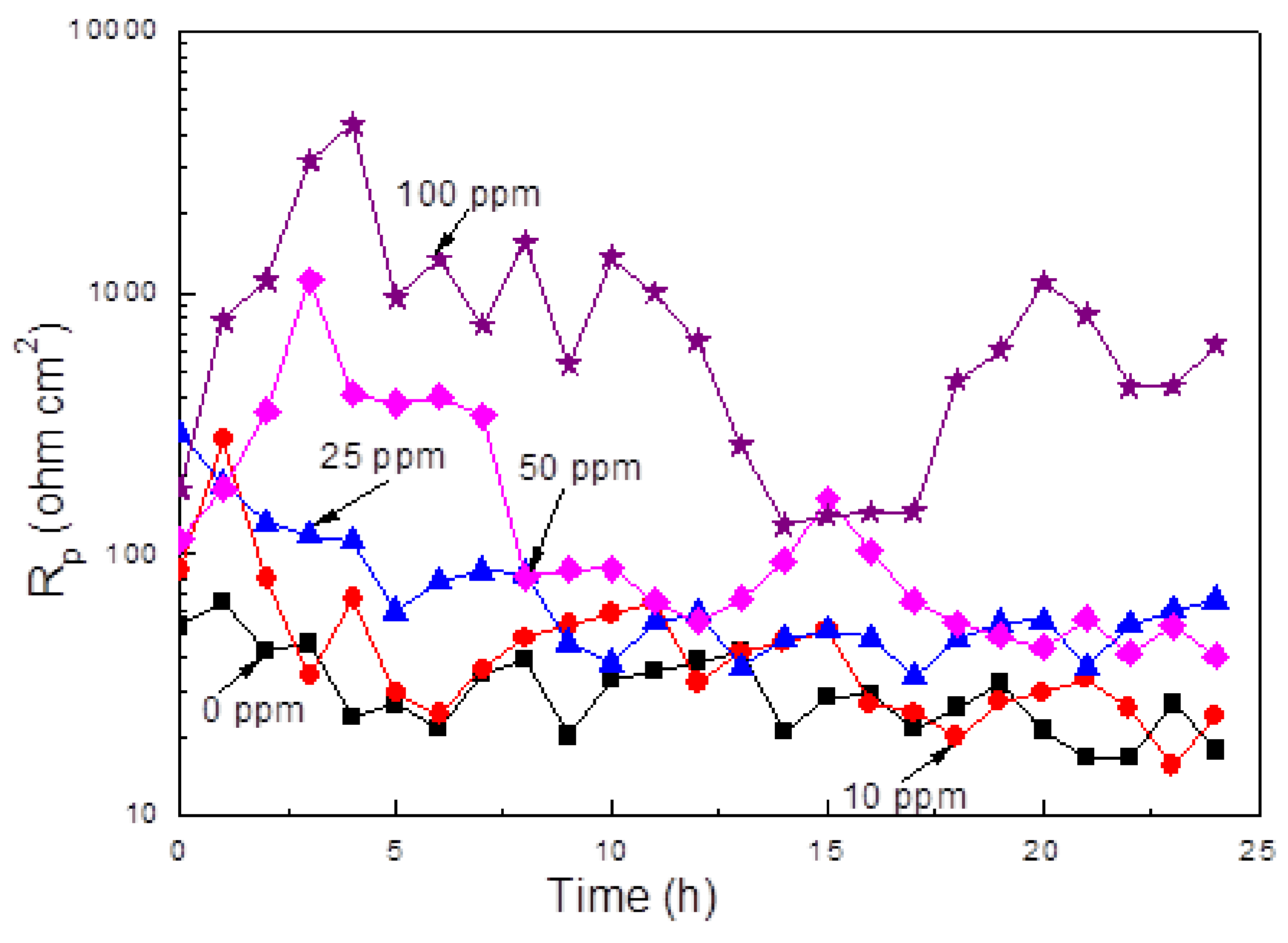
3.4. LPR Measurements
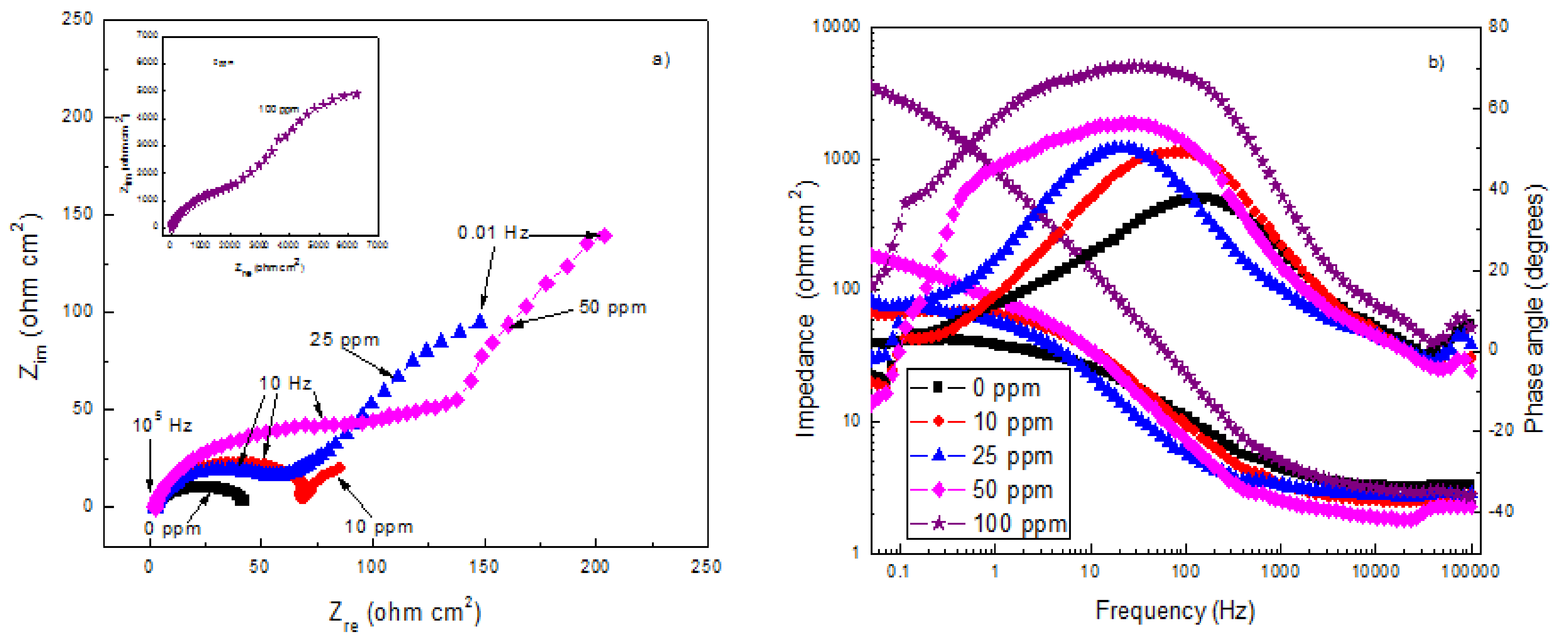
3.5. EIS Measurements
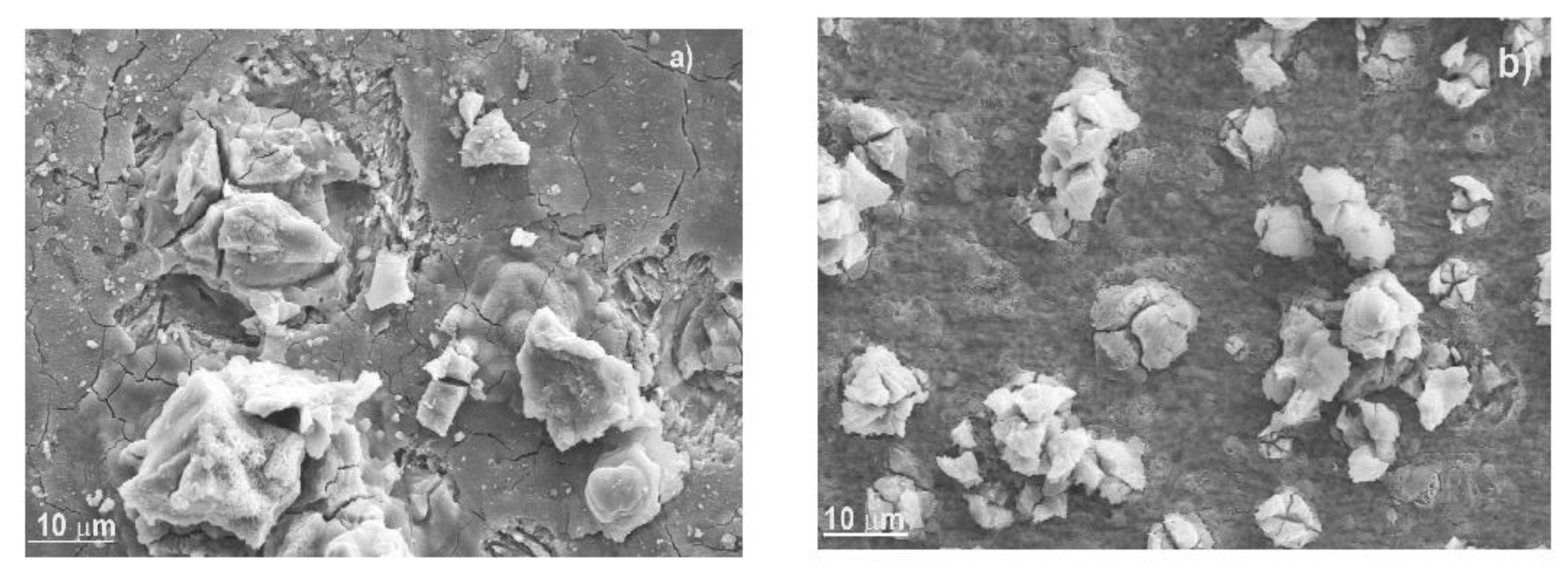
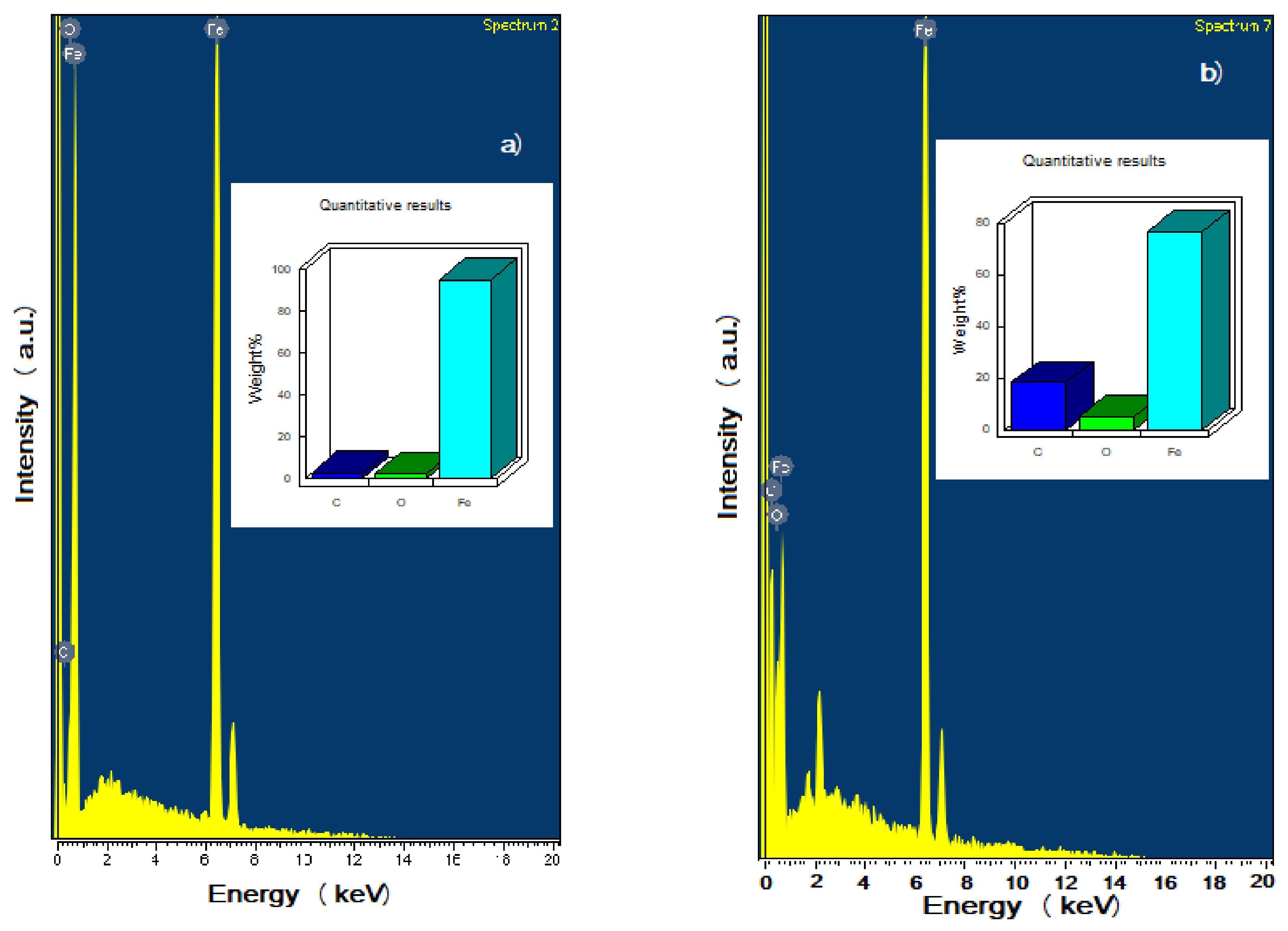
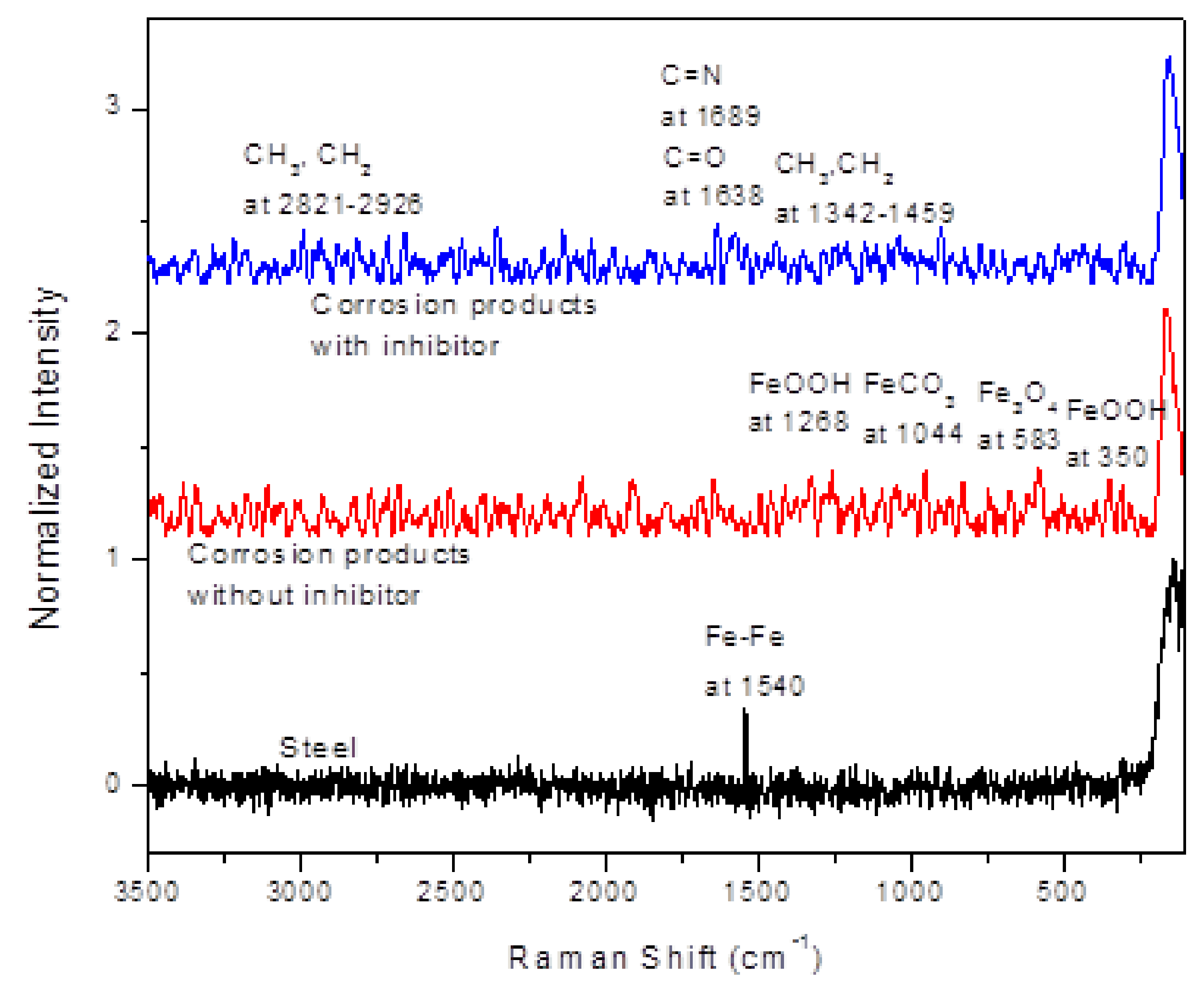
3.6. Surface Analysis
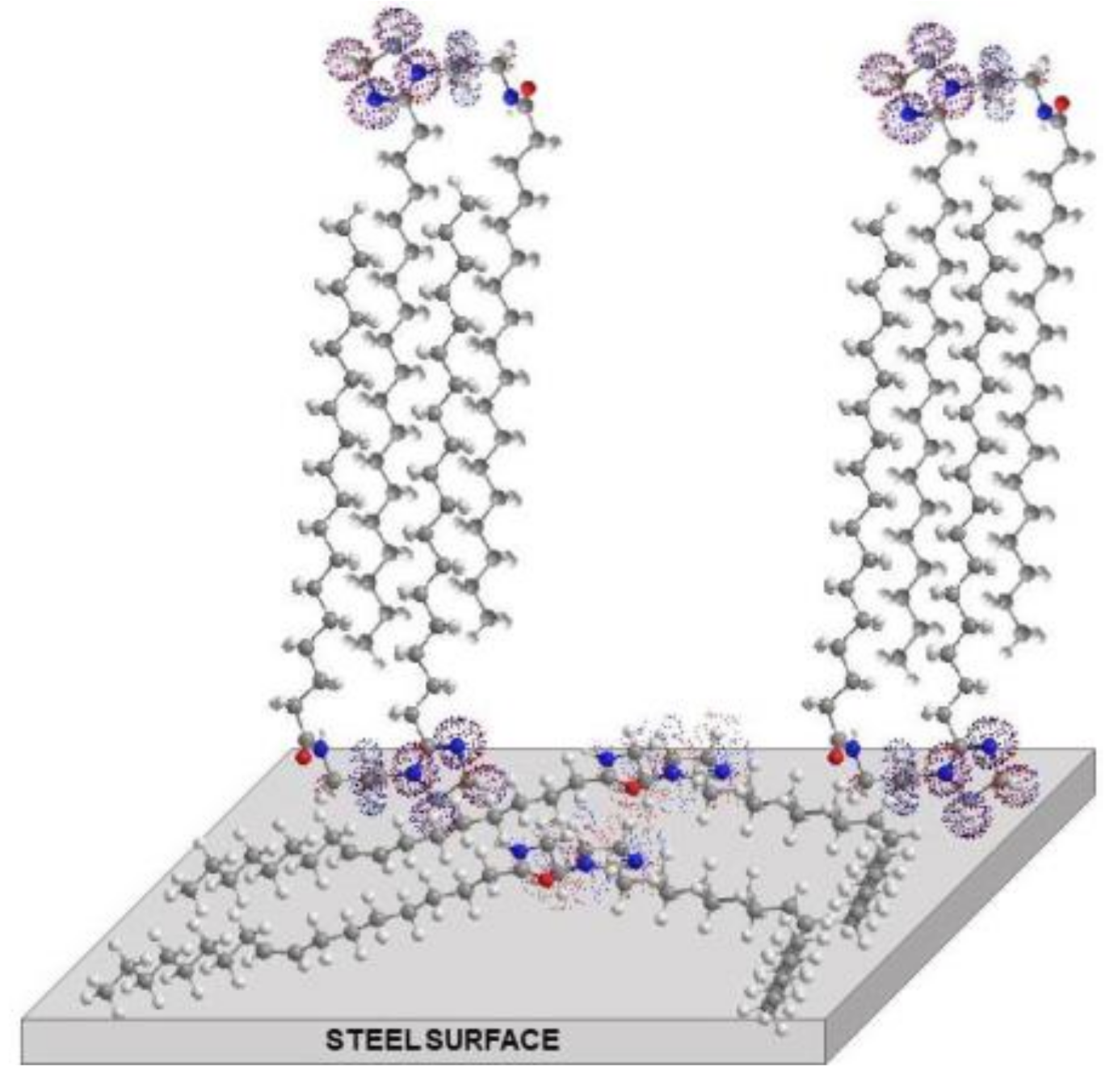
3.7. Corrosion Inhibition Mechanism by the Gemini Surfactant Derived of Mango Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayram, T.C.; Orbey, N.; Adhikari, R.; Tuominen, M. FP-based formulations as protective coatings in oil/gas pipelines. Prog. Org. Coat. 2015, 88, 54–63. [Google Scholar] [CrossRef]
- Zhu, S.; Fu, A.; Miao, J.; Yin, Z.; Zhou, G.; Wei, J. Corrosion of N80 carbon steel in oil field formation water containing CO2 in the absence and presence of acetic acid. Corros. Sci. 2011, 53, 3156–3165. [Google Scholar] [CrossRef]
- Liu, Q.; Mao, L.; Zhou, S. Effects of chloride content on CO2 corrosion of carbon steel in simulated oil and gas well environments. Corros. Sci. 2014, 84, 165–171. [Google Scholar] [CrossRef]
- Barker, R.; Hu, X.; Neville, A.; Cushnaghan, S. Assessment of Preferential Weld Corrosion of Carbon Steel Pipework in CO2-Saturated Flow-Induced. Corrosion 2013, 69, 193–203. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Ebenso, E.E.; Liu, W.; Pan, J.; Huang, B. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5% NaCl solution. J. Ind. Eng. Chem. 2015, 24, 219–228. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M. Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater. Lett. 2008, 62, 2977–2979. [Google Scholar] [CrossRef]
- Rivera-Grau, L.M.; Casales, M.; Regla, I.; Ortega-Toledo, D.M.; Gonzalez-Rodriguez, J.G.; Martinez Gomez, L. CO2 Corrosion Inhibition by Imidazoline Derivatives Based on Coconut Oil. Int. J. Electrochem. Sci. 2012, 7, 13044–13057. [Google Scholar]
- Zhang, X.; Wang, F.; He, Y.; Du, Y. Study of the inhibition mechanism of imidazoline amide on CO2 corrosion of Armco iron. Corros. Sci. 2001, 43, 1417–1431. [Google Scholar] [CrossRef]
- Paolinelli, L.; Pérez, T.; Simison, S. The effect of pre-corrosion and steel microstructure on inhibitor performance in CO2 corrosion. Corros. Sci. 2008, 50, 2456–2464. [Google Scholar] [CrossRef]
- Heydari, M.; Javidi, M.M. Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52 steel in CO2-saturated solution and synergistic effect of iodide ions. Corros. Sci. 2012, 61, 148–155. [Google Scholar] [CrossRef]
- Labena, A.; Hegazy, M.A.; Sami, R.M.; Hozzein, W.N. Multiple Applications of a Novel Cationic Gemini Surfactant: Anti-Microbial, Anti-Biofilm, Biocide, Salinity Corrosion Inhibitor, and Biofilm Dispersion (Part II). Molecules 2020, 25, 1348. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.A.; Ahmed, M.A.; Arida, H.A.; Kandemirli, F.; Saracoglu, M.; Arslan, T.; Basaran, M.A. Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series–Part III. Immersion time effects and theoretical studies. Corros. Sci. 2011, 53, 1895–1909. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Elkholy, A.E. Gemini Corrosion Inhibitors for Carbon Steel. J. Mol. Liq. 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Zana, R.; Xia, J. Gemini Surfactants: Synthesis, Interfacial and Solution-Phase Behavior, and Applications; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- El_Lateef, H.A.; Abo-Riya, M.A.; Tantawy, A.H. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros. Sci. 2016, 108, 94–110. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, X.; Zhu, W.; Zhang, Y.; Sun, D.; Zhang, R.; Wu, C. Imidazoline Gemini Surfactants as Corrosion Inhibitors for Carbon Steel X70 in NaCl Solution. ACS Omega 2021, 6, 5653–5660. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, J.; Zhang, Z.; Xin, X.; Xu, G. The comparison of imidazolium Gemini surfactant [C14-4-C14im]Br2 and its corresponding monomer as corrosion inhibitors for A3 carbon steel in hydrochloric acid solutions: Experimental and quantum chemical studies. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 575, 57–65. [Google Scholar] [CrossRef]
- Yin, C.; Kong, M.; Zhang, J.; Wang, Y.; Ma, Q.; Chen, Q.; Liu, H. Influence of Hydroxyl Groups on the Inhibitive Corrosion of GeminiSurfactant for Carbon Steel. ACS Omega 2020, 5, 2620–2629. [Google Scholar] [CrossRef]
- Abdallah, M.; Hegazy, M.; Alfakeer, M.; Ahmed, H. Adsorption and inhibition performance of the novel cationic Gemini surfactant as a safe corrosion inhibitor for carbon steel in hydrochloric acid. Green Chem. Lett. Rev. 2018, 11, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Deyab, M.A.; Mohsen, Q. Inhibitory influence of cationic Gemini surfactant on the dissolution rate of N80 carbon steel in 15% HCl solution. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Li, P.; Zeng, X.; Guo, B.; Pan, J.; Hou, L.; Yin, X. Novel Schiff base-based cationic Gemini surfactants as corrosion inhibitors for Q235 carbon steel and printed circuit boards. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 623, 126717. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Deyab, M.A.; Osman, M.M.; Nessim, M.I.; Elkholy, A.E. Synthesis and assessment of new cationic gemini surfactants as inhibitors for carbon steel corrosion in oilfield water. RSC Adv. 2017, 7, 47335–47352. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Lin, Y.; Ansari, K.; Quraishi, M.; Ebenso, E.E.; Chen, S.; Liu, W. Electrochemical and surface studies of some Porphines as corrosion inhibitor for J55 steel in sweet corrosion environment. Appl. Surf. Sci. 2015, 359, 331–339. [Google Scholar] [CrossRef]
- Dominguez, O.J.; Brown, B.; Young, D.; Nešić, S. Effect of Corrosion Inhibitor Alkyl Tail Length on the Electrochemical Process Governing CO2 Corrosion of Mild Steel. Corrosion 2019, 75, 137–139. [Google Scholar]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandán, S.A.; Gervasi, C.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Hernández, D.P.; Parra, E.R.; Arango, P.A.; Giraldo, B.S.; Medina, C.A. Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris. Materials 2021, 14, 2234. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Torres-Acosta, A.A.; González-Calderón, P.Y. Opuntia Ficus-Indica (OFI) Mucilage as Corrosion Inhibitor of Steel in CO2-Contaminated Mortar. Materials 2021, 14, 1316. [Google Scholar] [CrossRef]
- El Hamdani, N.; Fdil, R.; Tourabi, M.; Fouad-Bentiss, C.J. Alkaloids extract of Retama monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution:Electrochemical and surface studies. Appl. Surf. Sci. 2015, 357, 1294–1305. [Google Scholar] [CrossRef]
- Sun, X.; Yu, L. Investigation of polyacrylamide containing capsaicin monomer as a novel corrosion inhibitor for mild steel in hydrochloric acid. Mater. Corros. 2018, 69, 1095–1103. [Google Scholar] [CrossRef]
- Carmona-Hernandez, A.; Velez, E.V.; Uruchurtu-Chavarin, J.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Use of an imidazol synthetized from palm oil as a corrosion inhibitor for a supermartensitic stainless steel in H2S. Green Chem. Lett. Rev. 2019, 12, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Zabalegui, A.; Vazquez-Velez, E.; Galicia-Aguilar, G.; Díaz, M.C.; Lopez-Sesenes, R.; Gonzalez-Rodriguez, J.; Martinez-Gomez, L. Use of a non-ionic gemini-surfactant synthesized from the wasted avocado oil as a CO2-corrosion inhibitor for X-52 steel. Ind. Crop. Prod. 2019, 133, 203–211. [Google Scholar] [CrossRef]
- Gomez-Guzman, N.; De La Escalera, D.M.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.; Martinez-Gomez, L. Performance of an amide-based inhibitor derived from coffee bagasse oil as corrosion inhibitor for X70 steel in CO2-saturated brine. Green Chem. Lett. Rev. 2019, 12, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Dorantes, E.; Zúñiga-Díaz, J.; Quinto-Hernández, A.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Pedraza-Basulto, G.K.; Martínez-Gomez, L. Rice Bran as Source for the Synthesis of Imidazoline-type Inhibitors: Synthesis and Corrosion Performance. Int. J. Electrochem. Sci. 2018, 13, 101–118. [Google Scholar] [CrossRef]
- Fahimdanesh, M.; Bahrami, M.E. Evaluation of Physicochemical Properties of Iranian Mango Seed. Int. Food Res. J. 2013, 53, 9–18. [Google Scholar]
- Abdalla, A.E.; Darwish, S.M.; Ayad, E.H.; El-Hamahmy, R.M. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem. 2007, 103, 1134–1140. [Google Scholar] [CrossRef]
- Garg, N.; Bajpai, J.; Ashfaque, M.; Yadav, P. Extraction and Characterization of Oil from Unripe Mango Kernel. In Proceedings of the National Conference on Conservation Horticulture Organized by Indian Society of Horticulture Research and Development, Dehradun, India, 21–23 March 2010; G.B. Pant University of Agriculture and Technology, Pantnagar and Department of Horticulture and Food Processing: Pantnagar, India; Department of Horticulture and Food Processing: Dehradun, Uttarakhand, India, 2010; Volume 276, pp. 21–23. [Google Scholar]
- Ebenso, E.; Ekpe, U.; Ita, B.; Offiong, O.; Ibok, U. Effect of molecular structure on the efficiency of amides and thiosemicarbazones used for corrosion inhibition of mild steel in hydrochloric acid. Mater. Chem. Phys. 1999, 60, 79–90. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Regla, I.; Vazquez-Velez, E.; De La Escalera, L.M.M.; Canto, J.; Díaz, M.C. Effect of the Unsaturation of the Hydrocarbon Chain of Fatty-Amides on the CO2 Corrosion of Carbon Steel Using EIS and Real-Time Corrosion Measurement. J. Spectrosc. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Velazquez-Torres, N.; Martinez, H.; Porcayo-Calderon, J.; Velez, E.V.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. Use of an amide-type corrosion inhibitor synthesized from the coffee bagasse oil on the corrosion of Cu in NaCl. Green Chem. Lett. Rev. 2017, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Von Teichman, I.; Robbertse, P.J.; Schoonraad, E. The structure of the seed of Mangifera indica L. and notes on seed characters of the tribe Mangiferreae. S. Afr. Tydskr. Plantk. 1988, 54, 472–476. [Google Scholar]
- Kaushlesh, K.; Yadav, N.G.; Verma, A.; Kumar, S.; Trivedi, M. Optimization and extraction of oil from mango seed kernel (Mangifera indica). Indian J. Agric. Sci. 2017, 87, 943–946. [Google Scholar]
- Lazzari, E.; Schena, T.; Primaz, C.T.; Maciel, G.P.D.S.; Machado, M.E.; Cardoso, C.A.L.; Jacques, R.; Caramão, E.B. Production and chromatographic characterization of bio-oil from the pyrolysis of mango seed waste. Ind. Crop. Prod. 2016, 83, 529–536. [Google Scholar] [CrossRef]
- Obota, I.B.; Onyeachu, I.B.; Umoren, S.A.; Quraishi, M.A.; Sorour, A.A.; Chen, T.; Aljeaban, N.; Wang, Q. High-temperature sweet corrosion and inhibition in the oil and gas industry: Progress, challenges and future perspectives. J. Petrol. Sci. Eng. 2020, 185, 106469. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Liu, X.; Qin, W.; Yin, C.; Zhang, J. A highly efficient corrosion inhibitor by use of Gemini imidazoline. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Woodlands, TX, USA, 25 January 2016. [Google Scholar]
- Jovanciecevic, V.; Ramachandran, S.; Prince, P. Inhibition of CO2 corrosion of mild steel by imidazolines and their precursors. Corrosion 1998, 55, 449–455. [Google Scholar] [CrossRef]
- Betancourt-Jimenez, D.; Youngblood, J.P.; Martinez, C.J. Synthesis and Characterization of Fatty Acid Amides from Commercial Vegetable Oils and Primary Alkyl Amines for Phase Change Material Applications. ACS Sustain. Chem. Eng. 2020, 8, 13683–13691. [Google Scholar] [CrossRef]
- Mezzetta, A.; Łuczak, J.; Woch, J.J.; Chiappe, C.; Nowicki, J.; Guazzelli., L. Surface active fatty acid ILs: Influence of the hydrophobic tail and/or the imidazolium hydroxyl functionalization on aggregates formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Pang, X.; Zhou, M.; Liu, C.; Wei, L.; Gao, K. The behavior of pre-corrosion effect on the performance of imidazoline-based inhibitor in 3 wt.% NaCl solution saturated with CO2. Appl. Surf. Sci. 2015, 356, 63–72. [Google Scholar] [CrossRef]
- Burkle, D.; De Motte, R.; Taleb, W.; Kleppe, A.; Comyn, T.; Vargas, S.; Neville, A.; Barker, R. In situ SR-XRD study of FeCO3 precipitation kinetics onto carbon steel in CO2-containing environments: The influence of brine pH. Electrochim. Acta 2017, 255, 127–144. [Google Scholar] [CrossRef] [Green Version]
- Nešić, S.; Solvi, G.T.; Enerhaug, J. Comparison of the Rotating Cylinder and Pipe Flow Tests for Flow-Sensitive Carbon Dioxide Corrosion. Corrosion 1995, 51, 773–787. [Google Scholar] [CrossRef]
- Jia, Z.; Li, X.; Du, C.; Liu, Z.; Gao, J. Effect of acetic acid on CO2 corrosion of 3Crlow-alloy steel. Mater. Chem. Phys. 2012, 132, 258–271. [Google Scholar] [CrossRef]
- Song, F. A comprehensive model for predicting CO2 corrosion rate in oil and gas production and transportation systems. Electrochim. Acta 2010, 55, 689–700. [Google Scholar] [CrossRef]
- Chen, G.; Li, L.; Ouyang, J.; Zhu, Z.; Wang, F.; Wang, Y.; Xue, J.; Zhao, J. The synergistic inhibition effect between imidazoline and the oxide film on L360 steel in CO2/H2S systems. Anti-Corros. Methods Mater. 2015, 62, 143–148. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Rivera-Muñoz, E.; Peza-Ledesma, C.; Díaz, M.C.; De La Escalera, L.M.; Canto, J.; Martinez-Gomez, L. Sustainable Development of Palm Oil: Synthesis and Electrochemical Performance of Corrosion Inhibitors. J. Electrochem. Sci. Technol. 2017, 8, 133–145. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Wu, Y.; Wang, Y.-M.; Jiang, X. Synergistic effect between cationic gemini surfactant and chloride ion for the corrosion inhibition of steel in sulphuric acid. Corros. Sci. 2008, 50, 576–582. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.S.; Ahamad, I.; Quraishi, M. Electrochemical behavior of steel/acid interface: Adsorption and inhibition effect of oligomeric aniline. RSC Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO2−4. Eng. Fail. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Hua, Y.; Xu, S.; Wang, Y.; Taleb, W.; Sun, J.; Zhang, L.; Barker, R.; Neville, A. The formation of FeCO3 and Fe3O4 on carbon steel and their protective capabilities against CO2 corrosion at elevated temperature and pressure. Corros. Sci. 2019, 157, 392–405. [Google Scholar] [CrossRef]

| Fe | C | N | Si | Mn | P | S | Cr |
| Bal. | 0.027 | 0.006 | 0.24 | 1.00 | 0.003 | 0.005 | 0.4 |
| Mo | Ni | Al | Co | Cu | Nb | Ti | |
| 0.18 | 1.35 | 0.045 | 0.004 | 0.0107 | 0.024 | 0.154 |
| Mango Kind | Recovery Oil Yield (%) | Particle Size (mm) | Duration of Extraction (h) | Recovery Oil Yield (%) | M:V Relationship (g:mL) | Recovery Oil Yield (%) |
|---|---|---|---|---|---|---|
| Manila | 10.34 | 2.5–0.3 | 6 | 14.95 | 1:7 | - |
| Ataulfo | 7.53 | 1.19 | 6 | 7.38 | 1:7 | - |
| Haden | 11.54 | 0.45 | 4 | 8.2 | 1:3 | 8.79 |
| 5 | 8.84 | 1:5 | 8.99 | |||
| Criollo | 11.22 | 6 | 8.95 | 1:7 | 9.45 | |
| Panameño | 14.95 | 7 | 9.45 | 1:9 | 9.49 | |
| 8 | 9.63 | 1:11 | 9.40 |
| Fatty Acids | Structure | Fatty Acids (wt %) |
|---|---|---|
| Palmitic Acid | C16:0 | 10 |
| Stearic Acid | C18:0 | 33 |
| Oleic Acid | C18:1 | 49 |
| Linoleic Acid | C18:2 | 4 |
| Arachidic Acid | C20:0 | 3 |
| Cinh (ppm). | Ecorr (mV) | Icorr (mA/cm2) | βa (mV/dec) | −βc (mV/dec) | I.E. (%) | θ |
|---|---|---|---|---|---|---|
| 0 | −670 | 0.12 | 45 | 230 | - | - |
| 10 | −650 | 0.07 | 110 | 180 | 41 | 0.41 |
| 25 | −635 | 0.03 | 100 | 160 | 75 | 0.75 |
| 50 | −620 | 0.007 | 90 | 150 | 83 | 0.83 |
| 100 | −610 | 0.001 | 90 | 140 | 98 | 0.98 |
| Cinh (ppm) | Rs (ohm m2) | Cdl (μF cm−2) | Rct (ohm m2) | Cf (μF cm−2) | Rf (ohm m2) |
|---|---|---|---|---|---|
| 0 | 3.2 | 2.3 × 10−4 | 43 | - | - |
| 10 | 2.8 | 1.4 × 10−4 | 55 | 6.3 × 10−4 | 175 |
| 25 | 2.7 | 7.5 × 10−5 | 69 | 1.2 × 10−4 | 310 |
| 50 | 2.4 | 3.1 × 10−5 | 132 | 7.05 × 10−5 | 450 |
| 100 | 3.1 | 5.5 × 10−6 | 2100 | 1.4 × 10−5 | 7100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Salazar, E.; Vazquez-Velez, E.; Uruchurtu, J.; Porcayo-Calderon, J.; Casales, M.; Rosales-Cadena, I.; Lopes-Cecenes, R.; Gonzalez-Rodriguez, J.G. Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel. Materials 2021, 14, 4206. https://doi.org/10.3390/ma14154206
Sanchez-Salazar E, Vazquez-Velez E, Uruchurtu J, Porcayo-Calderon J, Casales M, Rosales-Cadena I, Lopes-Cecenes R, Gonzalez-Rodriguez JG. Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel. Materials. 2021; 14(15):4206. https://doi.org/10.3390/ma14154206
Chicago/Turabian StyleSanchez-Salazar, E., E. Vazquez-Velez, J. Uruchurtu, J. Porcayo-Calderon, M. Casales, I. Rosales-Cadena, R. Lopes-Cecenes, and J. G. Gonzalez-Rodriguez. 2021. "Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel" Materials 14, no. 15: 4206. https://doi.org/10.3390/ma14154206
APA StyleSanchez-Salazar, E., Vazquez-Velez, E., Uruchurtu, J., Porcayo-Calderon, J., Casales, M., Rosales-Cadena, I., Lopes-Cecenes, R., & Gonzalez-Rodriguez, J. G. (2021). Use of a Gemini-Surfactant Synthesized from the Mango Seed Oil as a CO2-Corrosion Inhibitor for X-120 Steel. Materials, 14(15), 4206. https://doi.org/10.3390/ma14154206








