A Novel Method for the Detection of SARS-CoV-2 Based on Graphene-Impedimetric Immunosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
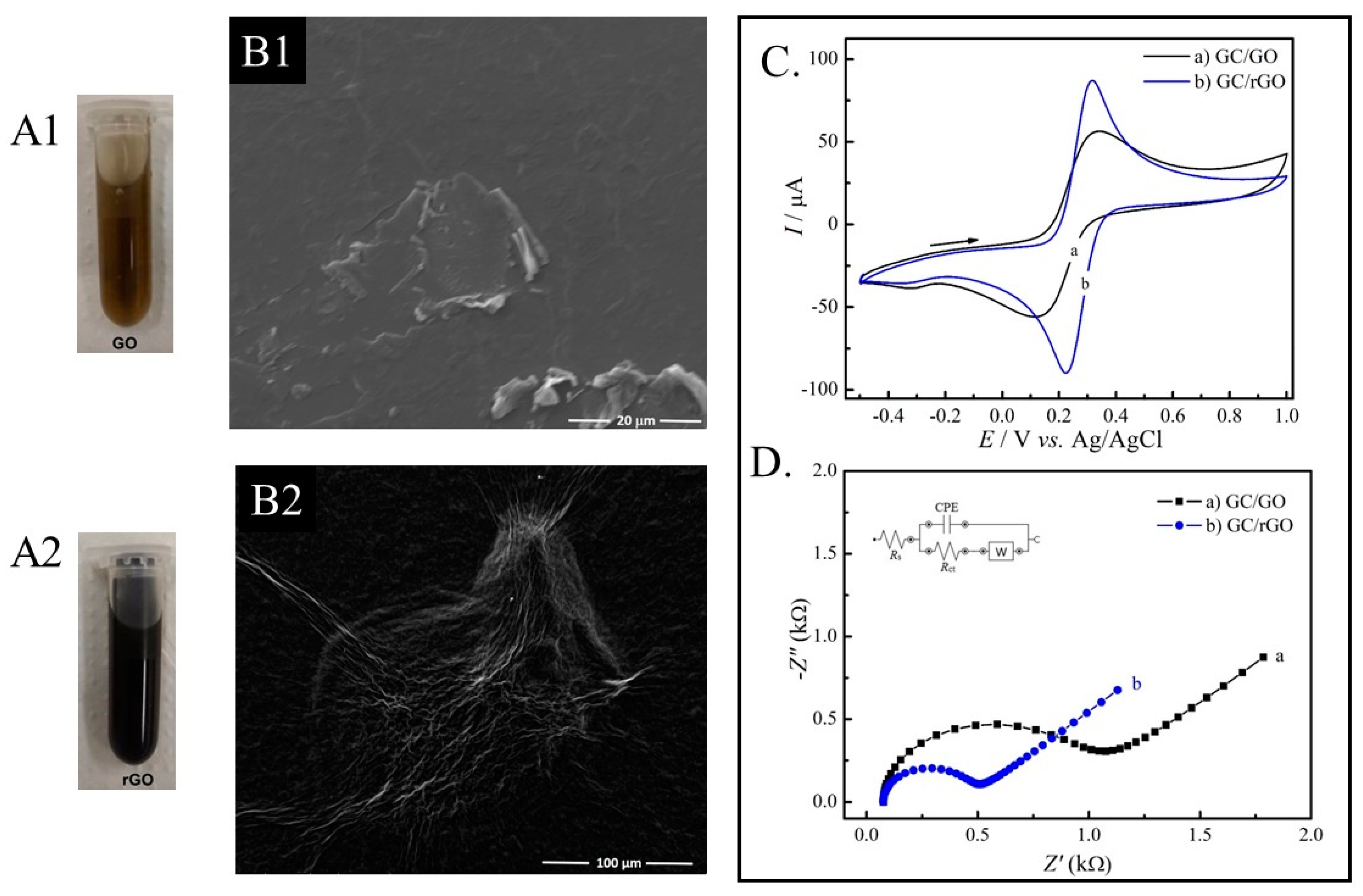
2.2. Production of Reduced Graphene Oxide
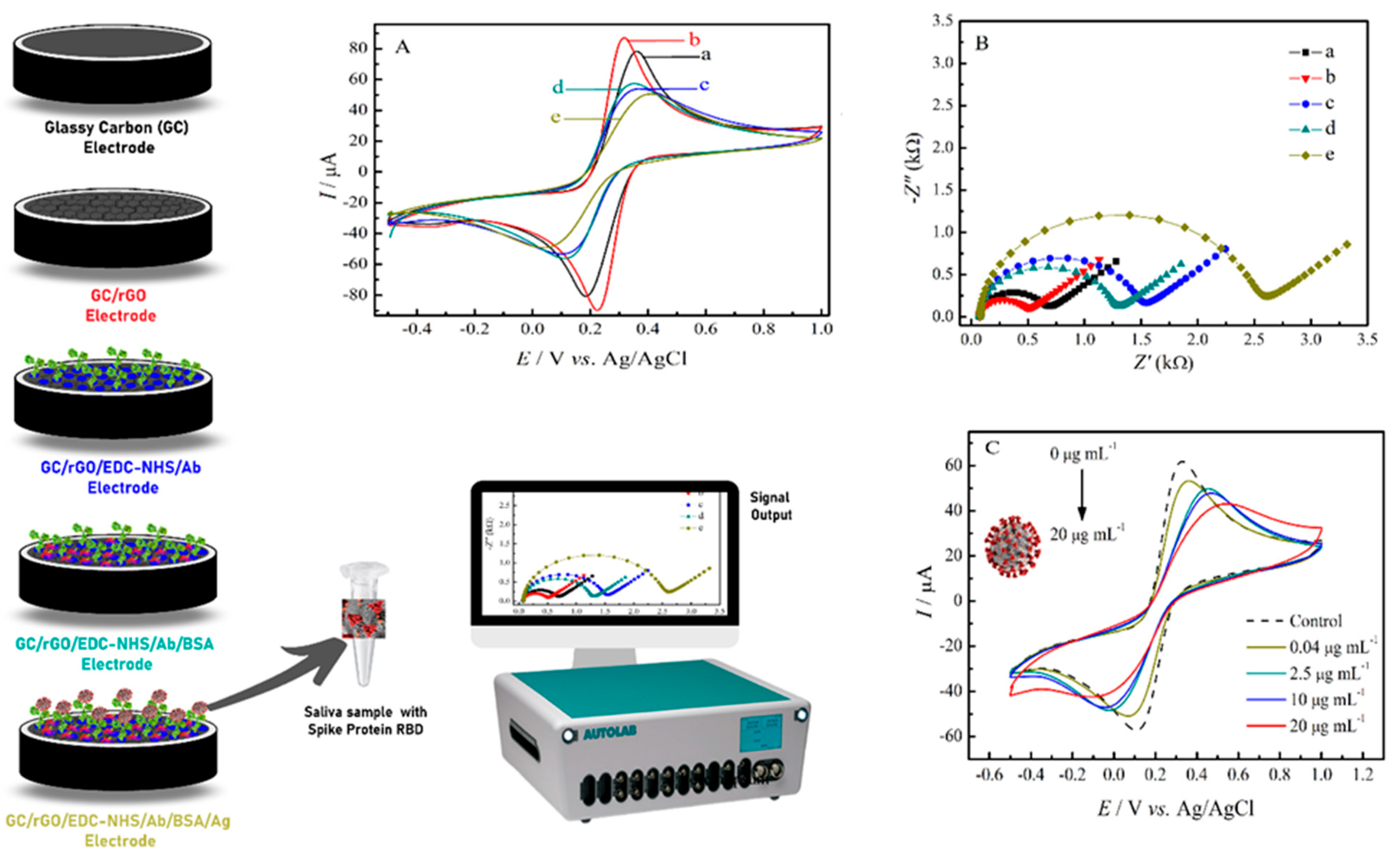
2.3. SARS-CoV-2 Immunosensor Fabrication
2.4. Scanning Electron Microscopy
2.5. Electrochemical Measurements
2.6. Analysis of Spike Protein RBD in Saliva Samples
3. Results and Discussion
3.1. Morphological and Electrochemical Characterization of the rGO and the SARS-CoV-2 Immunosensor
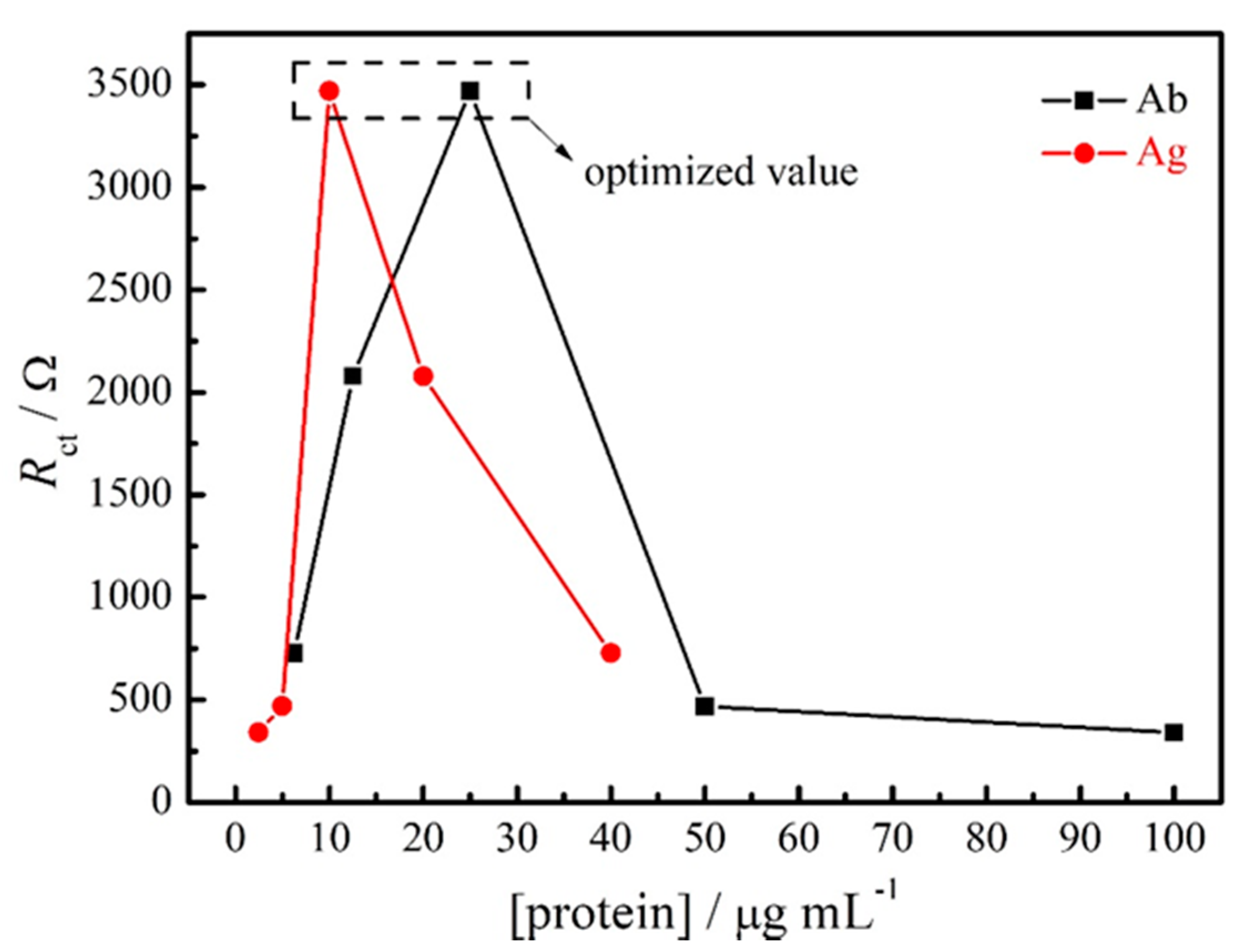
3.2. Optimization and Stability of the Impedimetric Immunosensor for SARS-CoV-2 Spike Protein RBD
3.3. Analytical Performance of the SARS-CoV-2 Immunosensor
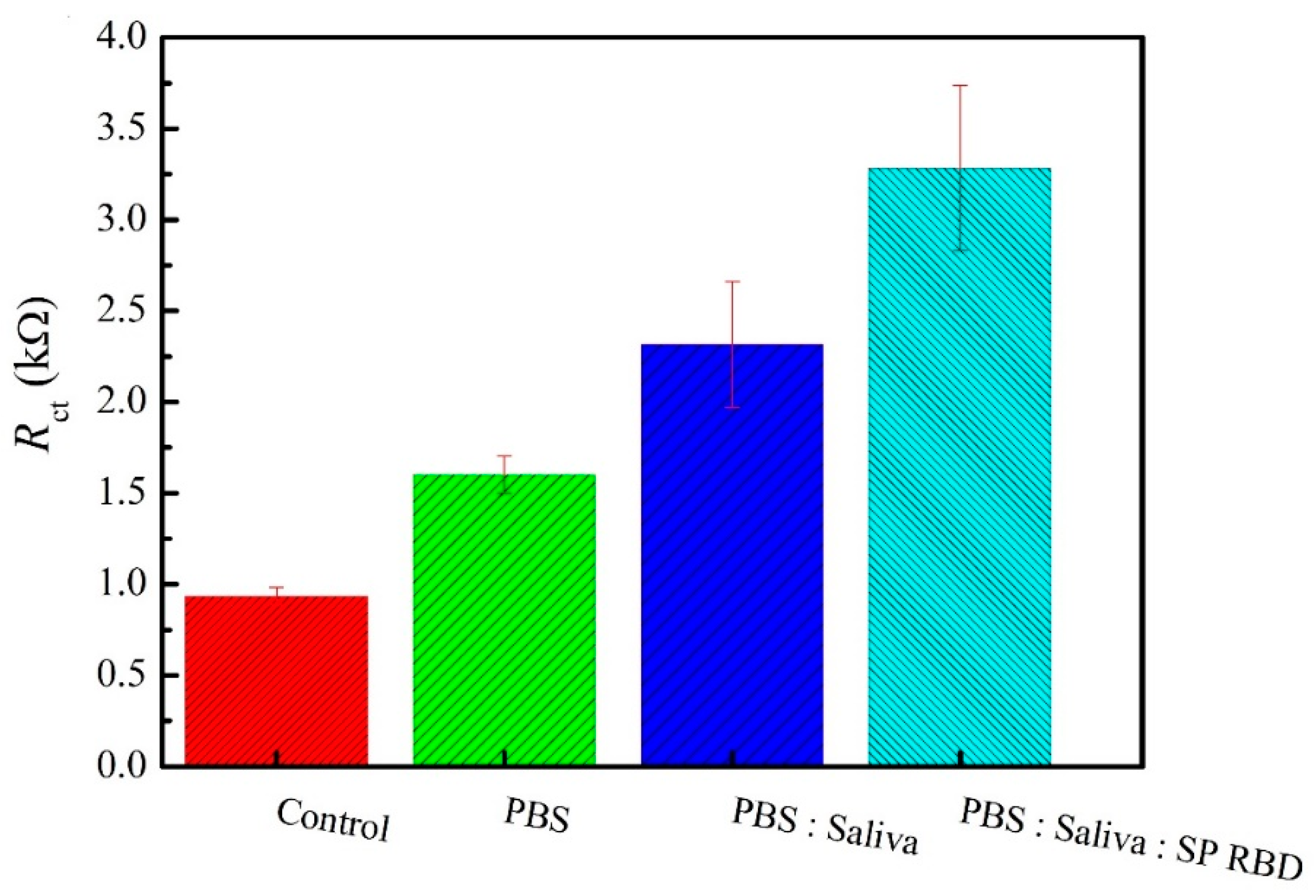
3.4. SARS-CoV-2 Spike Protein RBD Analysis in Saliva Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, D.S.; Prasad, R.; Srivastava, R.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Comprehensive Review on Current Interventions, Diagnostics, and Nanotechnology Perspectives against SARS-CoV-2. Bioconjug. Chem. 2020, 31, 2021–2045. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudr, J.; Michalek, P.; Ilieva, L.; Adam, V.; Zitka, O. COVID-19: A challenge for electrochemical biosensors. TrAC Trends Anal. Chem. 2021, 136, 116192. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef]
- Chandra, P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sens. Int. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Choi, J.R. Development of Point-of-Care Biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]
- Torres, M.D.T.; de Araujo, W.R.; de Lima, L.F.; Ferreira, A.L.; de la Fuente-Nunez, C. Low-cost Biosensor for Rapid Detection of SARS-CoV-2 at the Point-of-Care. Matter 2021, 4, 2403–2416. [Google Scholar] [CrossRef]
- Bandala, E.R.; Kruger, B.R.; Cesarino, I.; Leao, A.L.; Wijesiri, B.; Goonetilleke, A. Impacts of COVID-19 pandemic on the wastewater pathway into surface water: A review. Sci. Total Environ. 2021, 774, 145586. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Applications of graphene in electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2016, 76, 1–14. [Google Scholar] [CrossRef]
- Prattis, I.; Hui, E.; Gubeljak, P.; Kaminski Schierle, G.S.; Lombardo, A.; Occhipinti, L.G. Graphene for Biosensing Applications in Point-of-Care Testing. Trends Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, J.; Li, H.; Wang, L.; Cao, D.; Feng, X.; Liu, Y.; Ma, Y.; Wang, L. Recent Progress on Graphene-based Electrochemical Biosensors. Chem. Rec. 2016, 16, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Donini, C.A.; Silva, M.K.L.; Bronzato, G.R.; Leão, A.L.; Cesarino, I. Evaluation of a biosensor based on reduced graphene oxide and glucose oxidase enzyme on the monitoring of second-generation ethanol production. J. Solid State Electrochem. 2019, 1–8. [Google Scholar] [CrossRef]
- Kohori, N.A.; da Silva, M.K.L.; Cesarino, I. Evaluation of graphene oxide and reduced graphene oxide in the immobilization of laccase enzyme and its application in the determination of dopamine. J. Solid State Electrochem. 2018, 22, 141–148. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.K.L.; Vanzela, H.C.; Defavari, L.M.; Cesarino, I. Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sens. Actuators B Chem. 2018, 277, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Rocha, G.S.; Silva, M.K.L.; Cesarino, I. Reduced Graphene Oxide-Based Impedimetric Immunosensor for Detection of Enterotoxin A in Milk Samples. Materials 2020, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.K.L.; Cesarino, I. Graphene functionalization and nanopolymers. In Carbon Nanostructures; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 157–178. ISBN 978-981-32-9057-0. [Google Scholar]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zhuo, Y.; Yuan, P.X.; Yuan, R.; Chai, Y.Q.; Hong, C.L. Bienzyme functionalized three-layer composite magnetic nanoparticles for electrochemical immunosensors. Biomaterials 2009, 30, 2284–2290. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.; Li, A.; Wang, X.; Hou, P.; Wang, C.; Chen, K.; Zhao, C. A highly sensitive electrochemical impedance immunosensor for indole-3-acetic acid and its determination in sunflowers under salt stress. RSC Adv. 2017, 7, 54416–54421. [Google Scholar] [CrossRef] [Green Version]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.K.L.; Plana Simões, R.; Cesarino, I. Evaluation of Reduced Graphene Oxide Modified with Antimony and Copper Nanoparticles for Levofloxacin Oxidation. Electroanalysis 2018, 30, 2066–2076. [Google Scholar] [CrossRef]
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef]
- Liu, X.; Duckworth, P.A.; Wong, D.K.Y. Square wave voltammetry versus electrochemical impedance spectroscopy as a rapid detection technique at electrochemical immunosensors. Biosens. Bioelectron. 2010, 25, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- European Union Communication from the Commission—Guidelines on COVID-19 In Vitro Diagnostic Tests and Their Performance. Available online: https://op.europa.eu/en/publication-detail/-/publication/9d8c5572-7f12-11ea-aea8-01aa75ed71a1/language-en (accessed on 16 May 2021).
- Shen, Y.; Anwar, T.B.; Mulchandani, A. Current status, advances, challenges and perspectives on biosensors for COVID-19 diagnosis in resource-limited settings. Sens. Actuators Rep. 2021, 3, 100025. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Geng, B.; Muenker, M.C.; Moore, A.J.; Vogels, C.B.F.; et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv 2020, 3. [Google Scholar] [CrossRef] [Green Version]


| Steps of Immunosensor Fabrication | Rct (Ω) | Rs (Ω) | CPE (µF sα−1) | α |
|---|---|---|---|---|
| GC | 720 | 69.5 | 1.49 | 0.95 |
| GC/rGO | 550 | 91 | 1.30 | 0.97 |
| GC/rGO-EDC-NHS/Ab | 1464.5 | 67.6 | 0.79 | 0.97 |
| GC/rGO-EDC-NHS/Ab/BSA | 1241.7 | 60 | 0.81 | 0.94 |
| GC/rGO-EDC-NHS/Ab/BSA/Ag | 2398.8 | 81.7 | 0.75 | 1.00 |
| Dilution | Rct (Ω) | |
|---|---|---|
| Ab | Ag | |
| 1:1600 | 1:10 | 727.81 |
| 1:800 | 1:20 | 2078.5 |
| 1:400 | 1:40 | 3470.4 |
| 1:200 | 1:80 | 468.63 |
| 1:100 | 1:160 | 340.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccariotto, G.C.; Silva, M.K.L.; Rocha, G.S.; Cesarino, I. A Novel Method for the Detection of SARS-CoV-2 Based on Graphene-Impedimetric Immunosensor. Materials 2021, 14, 4230. https://doi.org/10.3390/ma14154230
Zaccariotto GC, Silva MKL, Rocha GS, Cesarino I. A Novel Method for the Detection of SARS-CoV-2 Based on Graphene-Impedimetric Immunosensor. Materials. 2021; 14(15):4230. https://doi.org/10.3390/ma14154230
Chicago/Turabian StyleZaccariotto, Gabriel C., Martin K. L. Silva, Giovanna S. Rocha, and Ivana Cesarino. 2021. "A Novel Method for the Detection of SARS-CoV-2 Based on Graphene-Impedimetric Immunosensor" Materials 14, no. 15: 4230. https://doi.org/10.3390/ma14154230









