Fabrication of MNPs/rGO/PMMA Composite for the Removal of Hazardous Cr(VI) from Tannery Wastewater through Batch and Continuous Mode Adsorption
Abstract
:1. Introduction
2. Experimental Procedure
2.1. List of Chemicals and Standards
2.2. List of Instruments
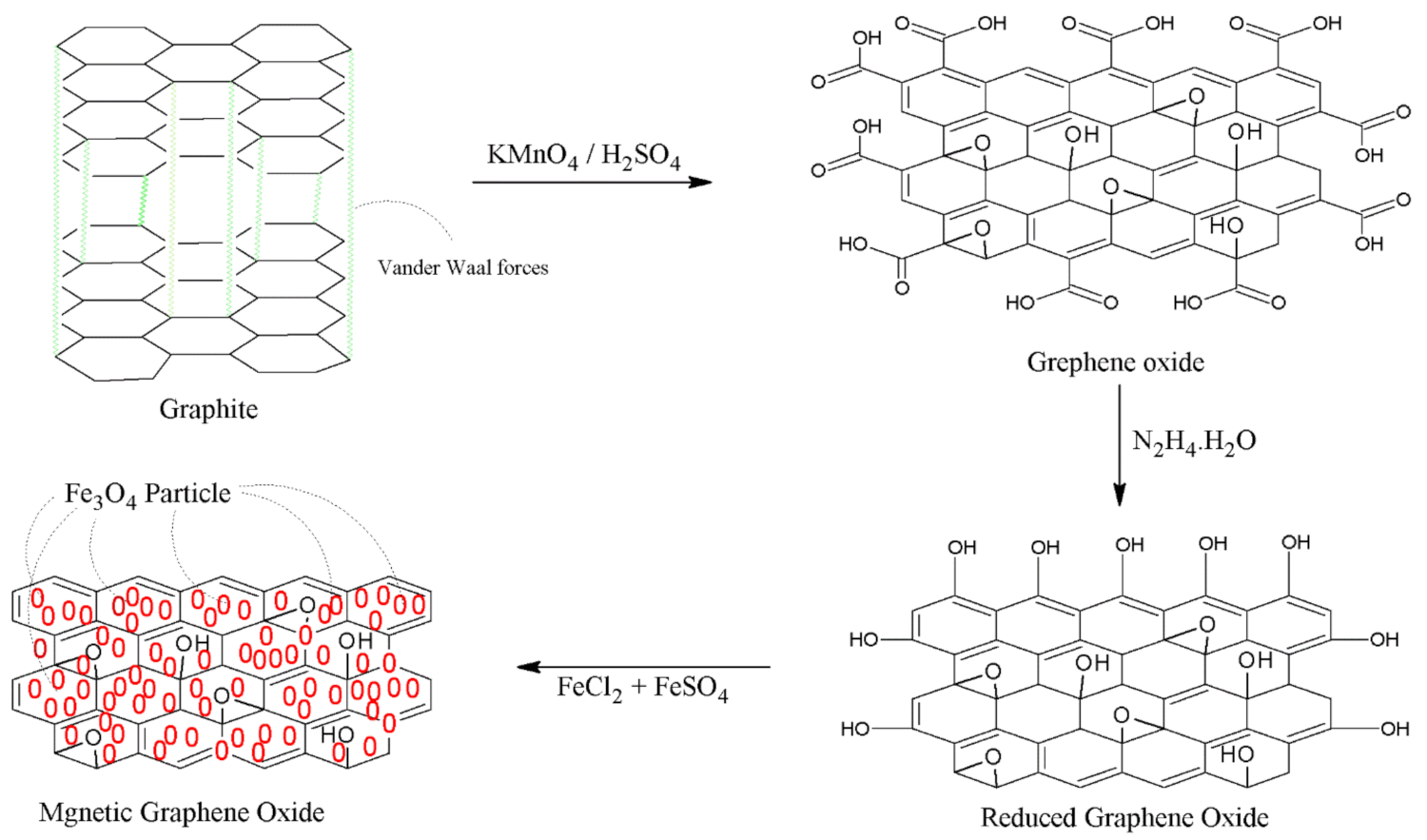
2.3. Synthesis of MNPs/rGO/PMMA Composite
2.3.1. Synthesis of GO
2.3.2. Synthesis of rGO
2.3.3. Preparation of MNPs/GO
2.3.4. Preparation of MNPs/rGO and PMMA Composite
2.4. Batch Adsorption and Desorption Studies
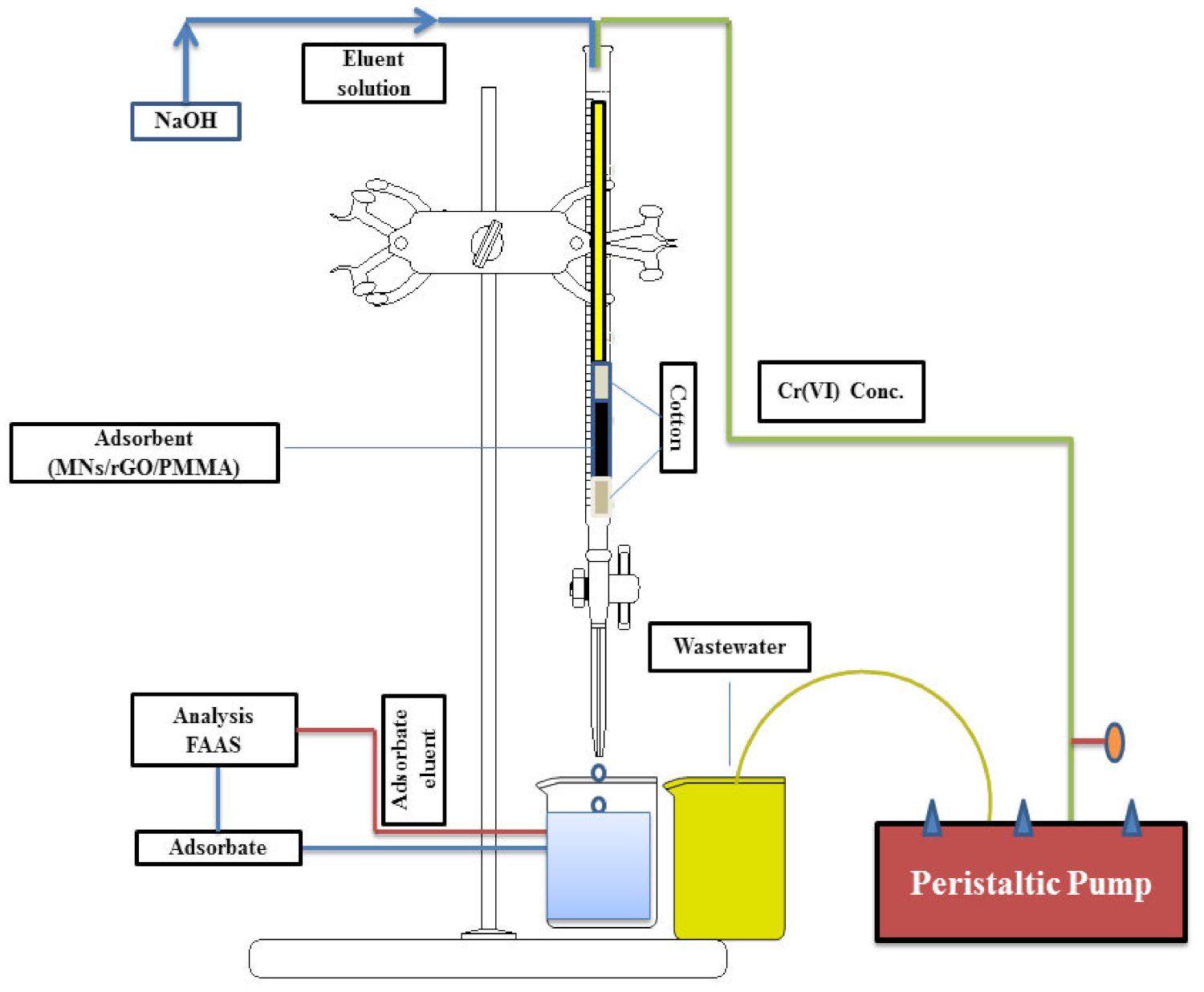
2.5. Fixed-Bed Column Experiments
3. Results and Discussions
3.1. Characteristics of Adsorbent
3.2. Physiochemical Characterization of Wastewater
4. Adsorption Experiment
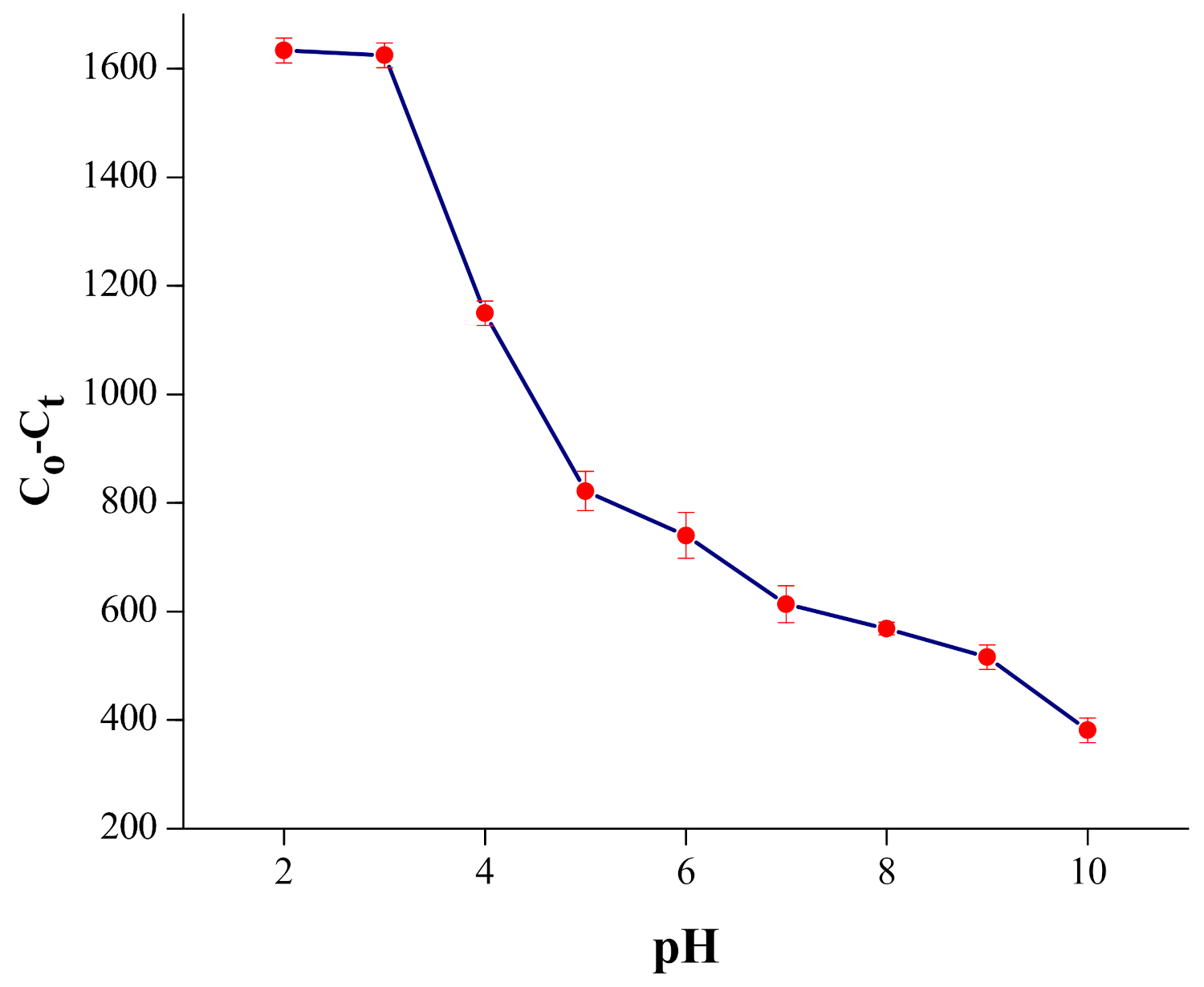
4.1. pH Investigation
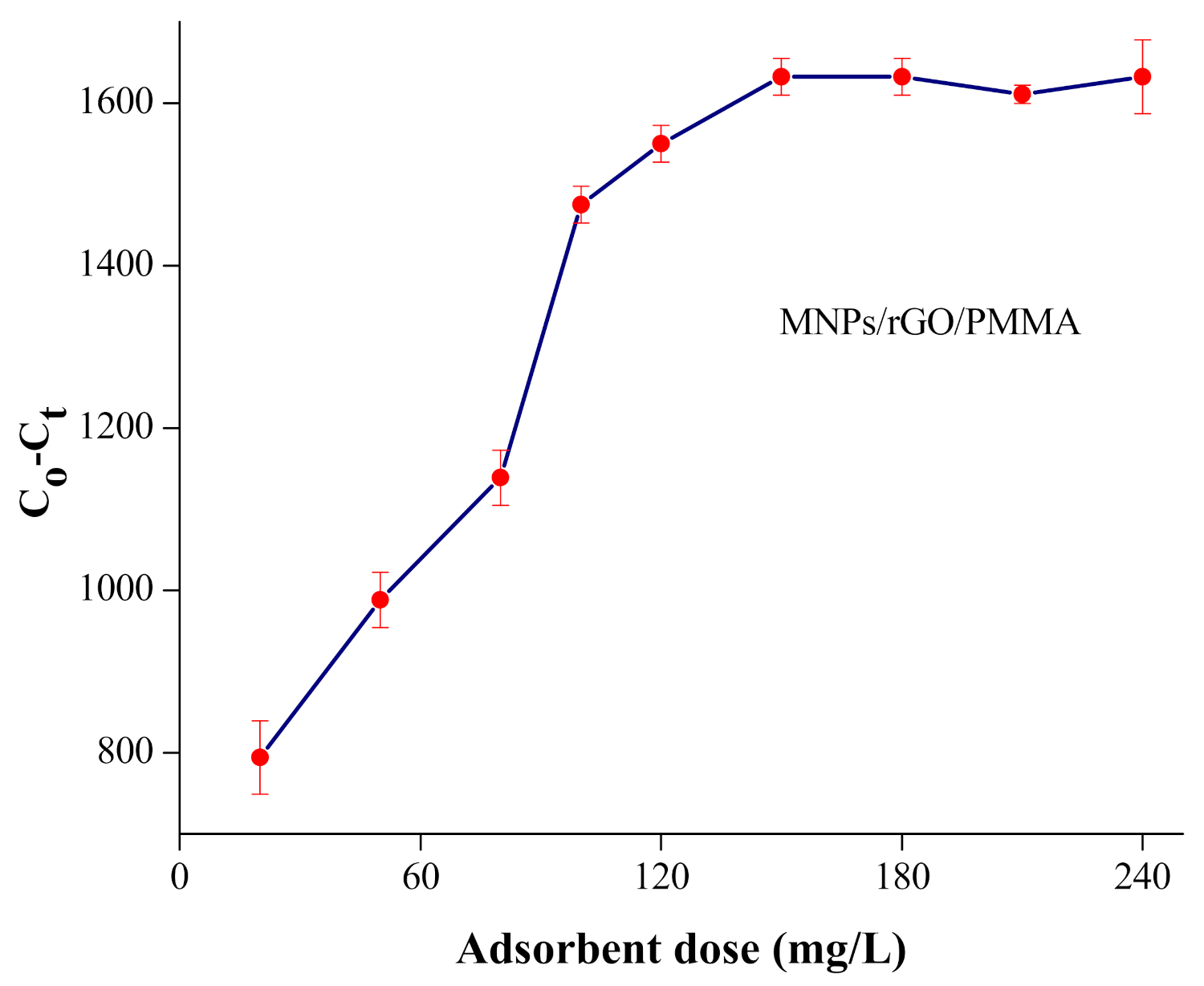
4.2. Adsorbent Dose Investigation
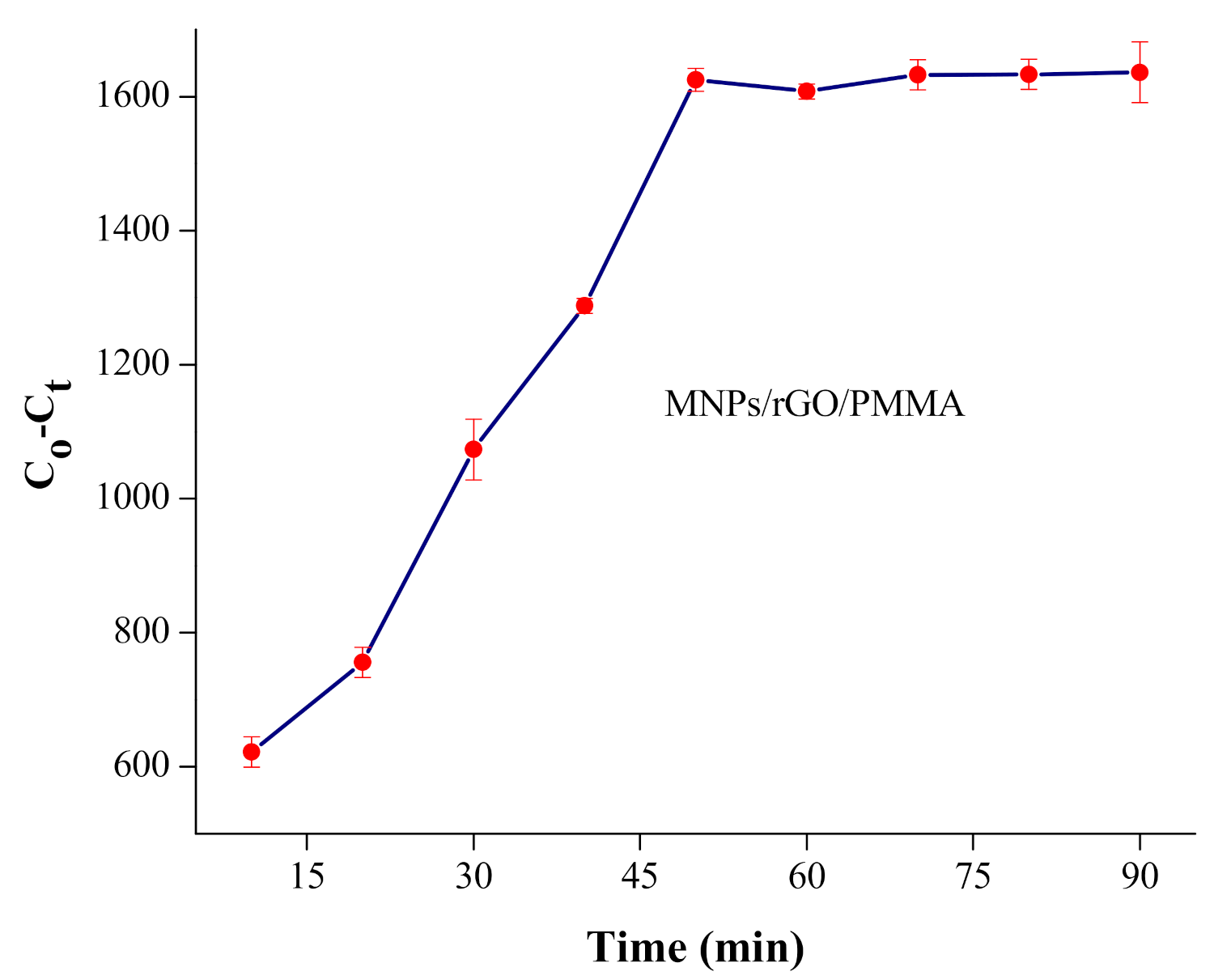
4.3. Kinetic Investigation
4.4. Adsorption Isotherms
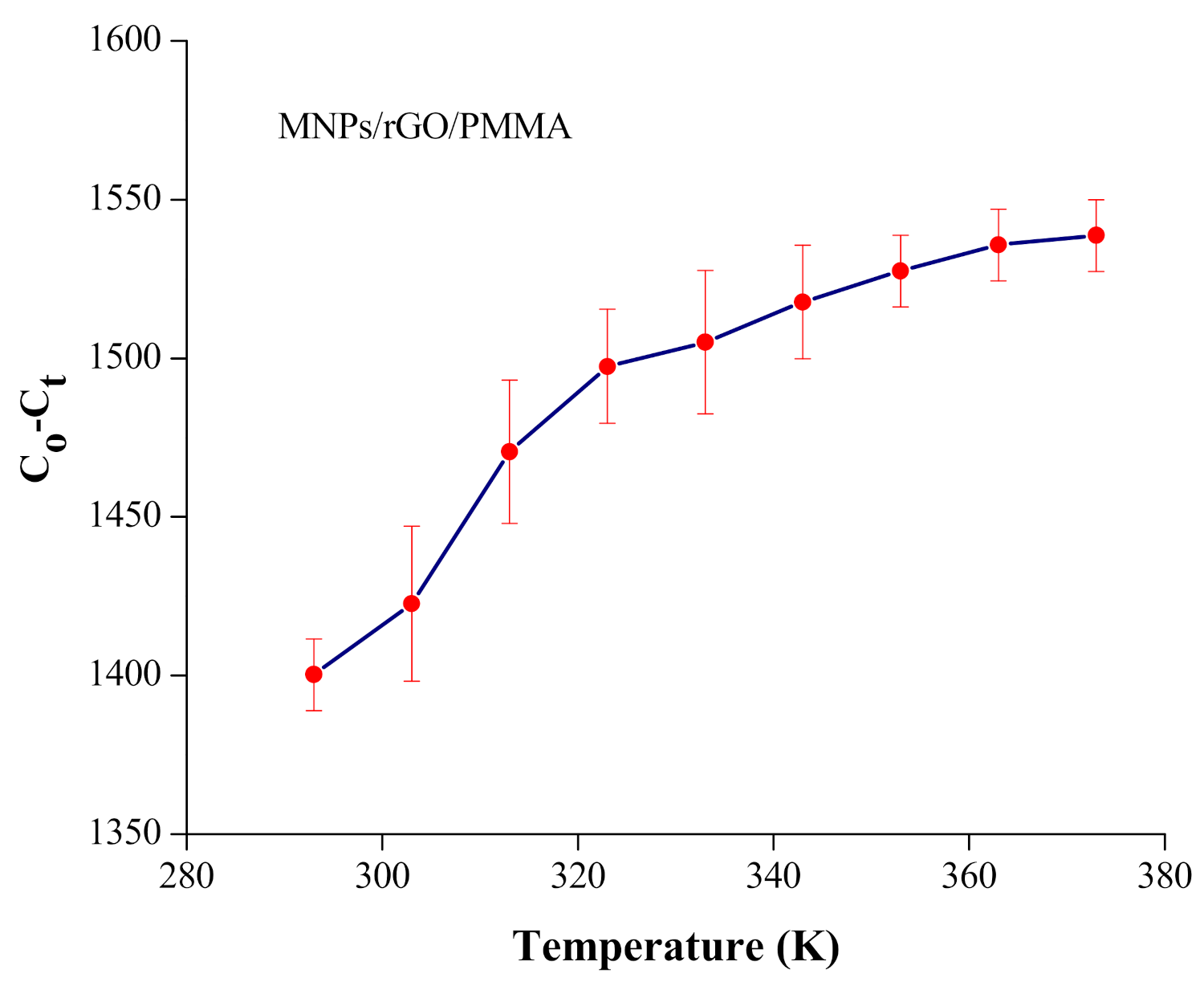
4.5. Thermodynamic Investigation
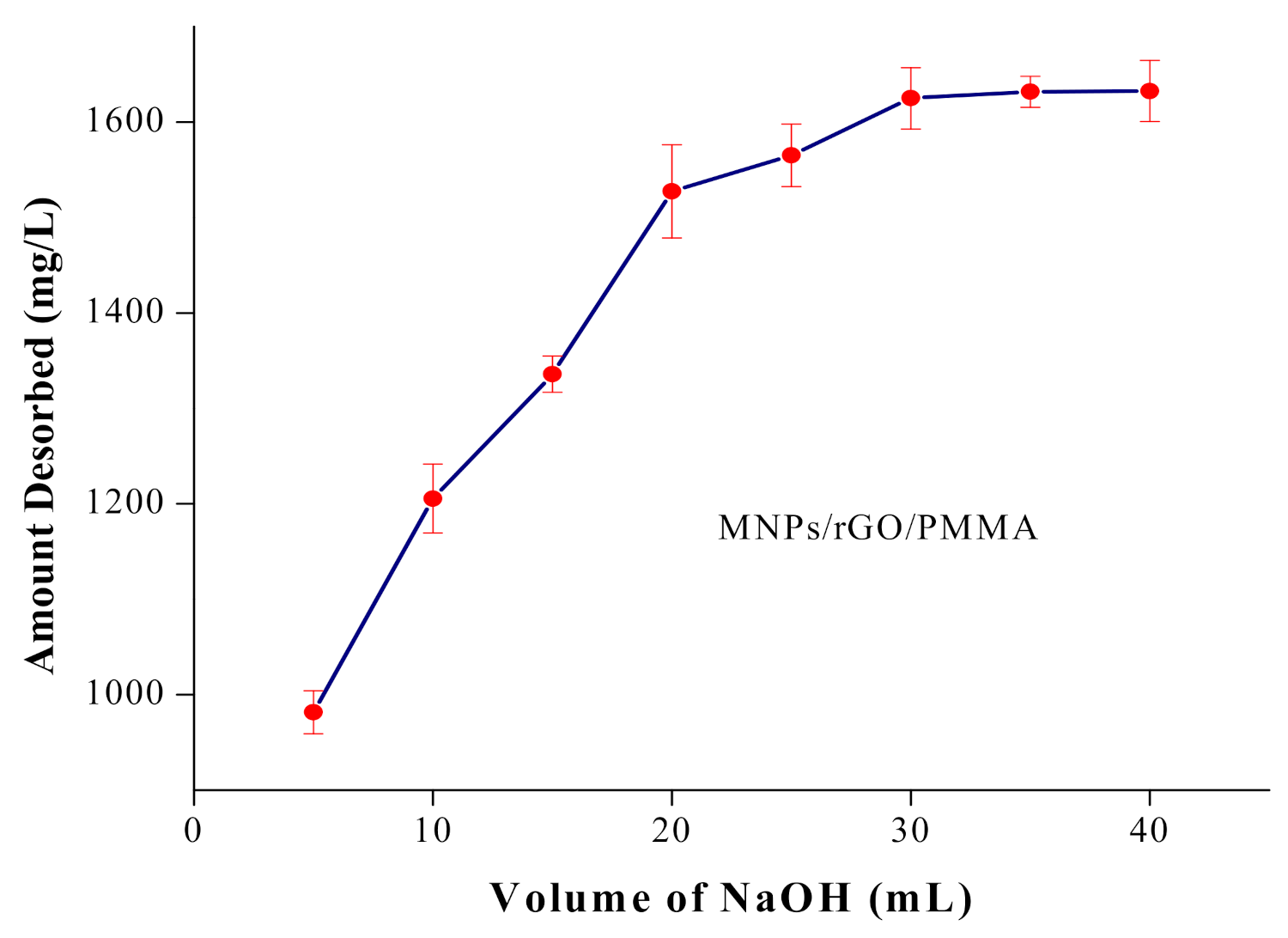
4.6. Volume of Eluent Optimization and Desorption Studies
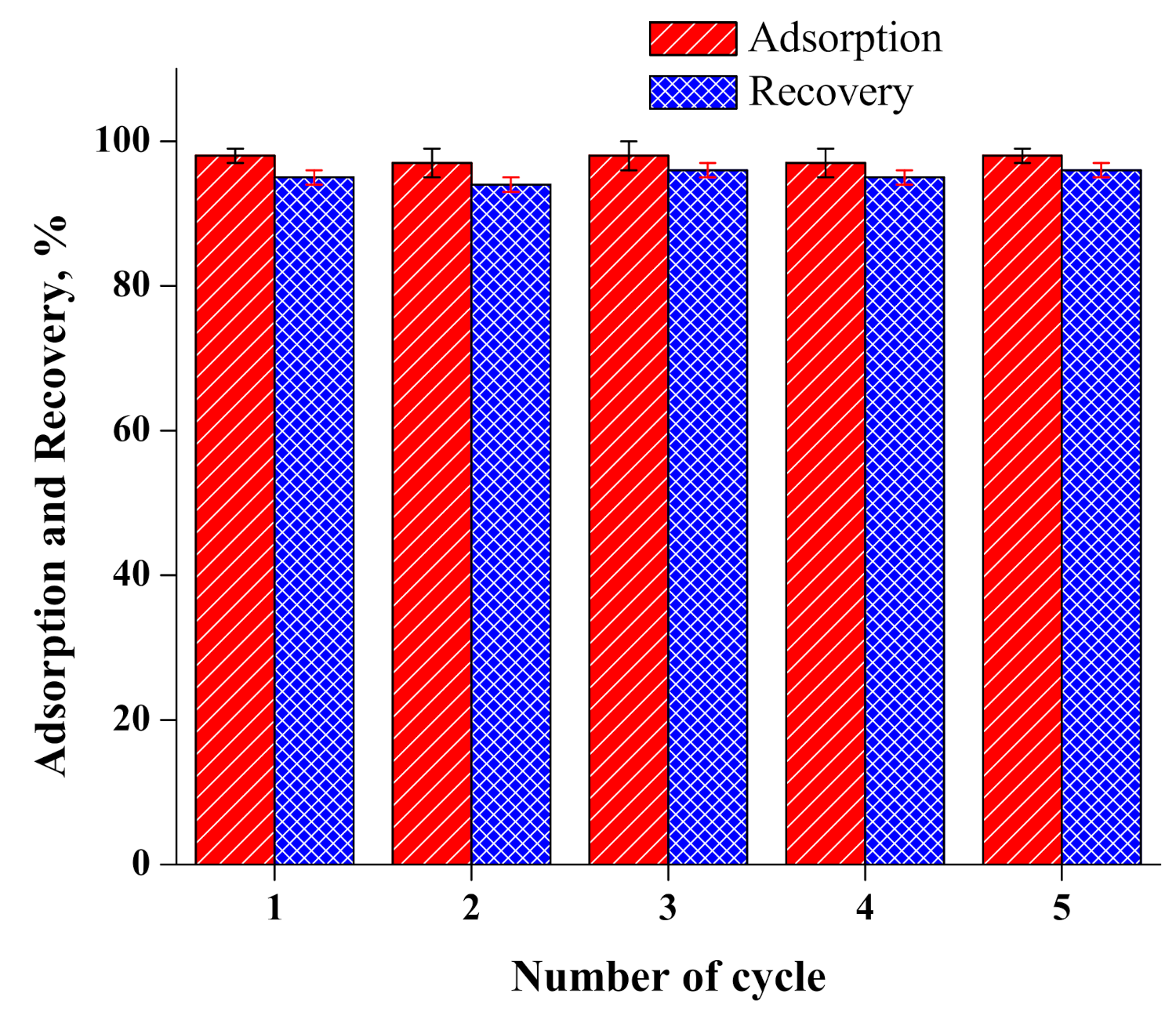
4.7. Recovery of Cr(VI), Recycling and Regeneration of the Adsorbent
5. Theoretical Analysis of Fixed-Bed Column Data
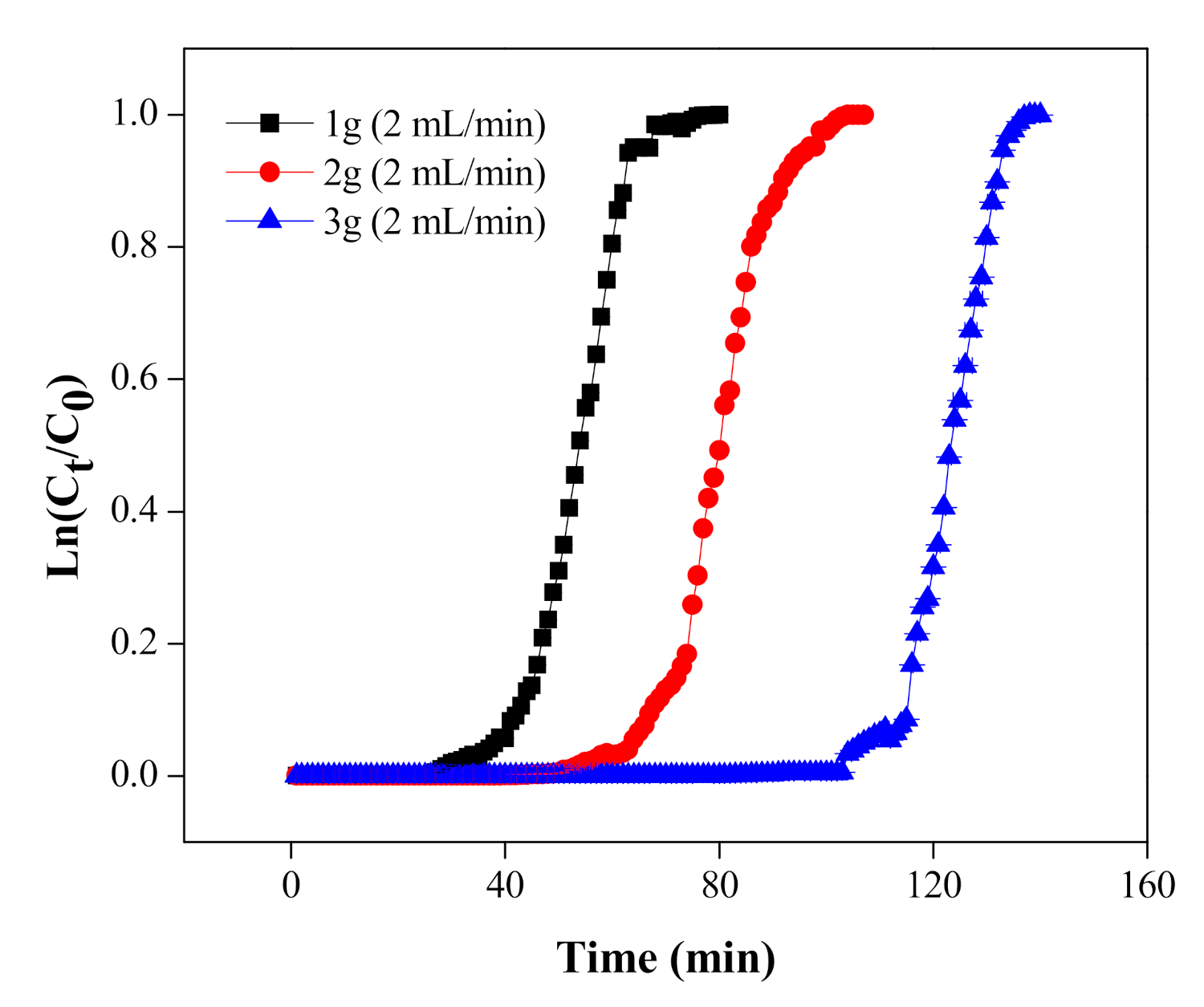
5.1. Effect of Mass of MNPs/rGO/PMMA Composite on the Breakthrough Curve
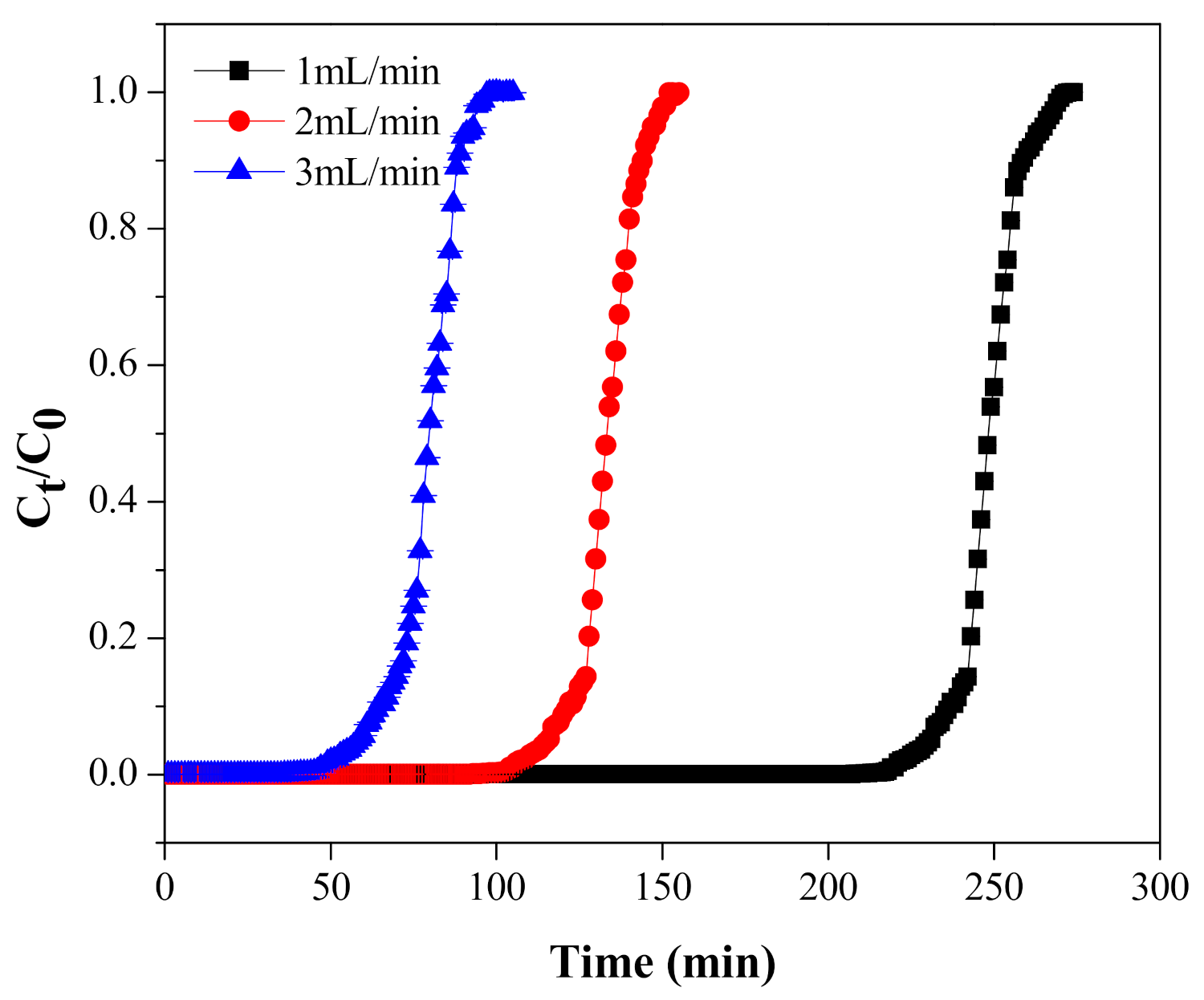
5.2. Effect of Flow Rate
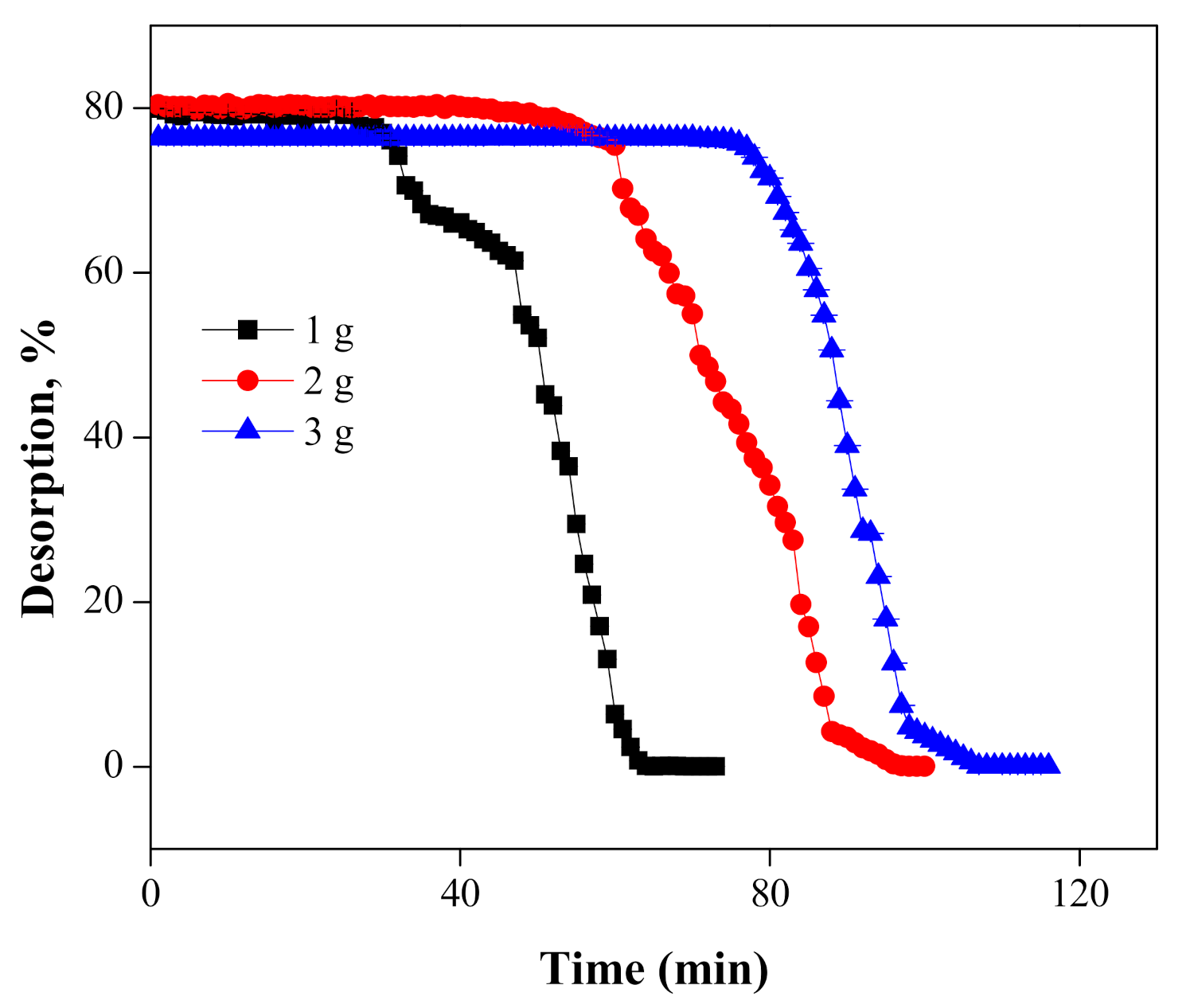
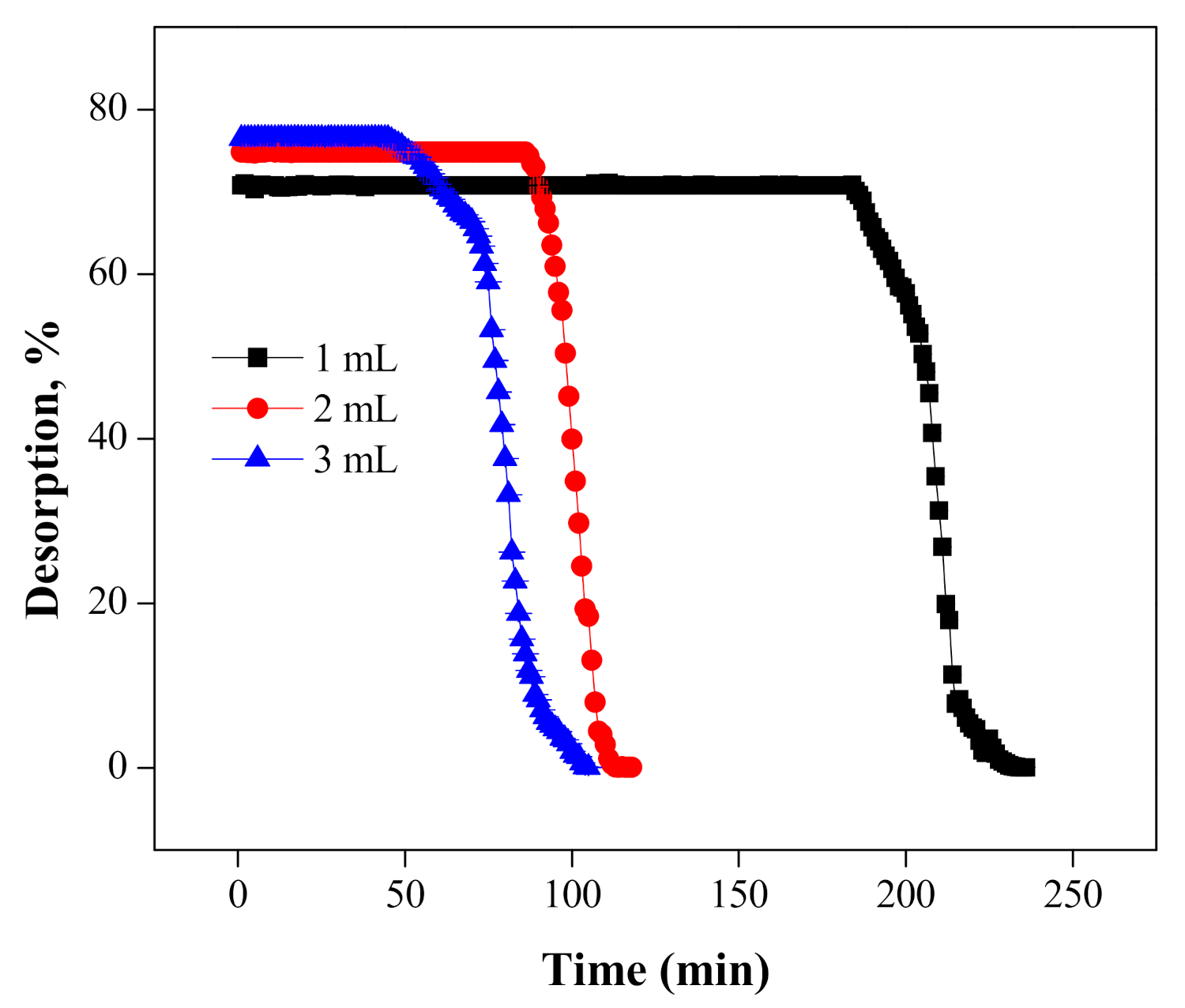
5.3. Desorption and Regeneration of the Column
5.4. Theoretical Modeling of the Breakthrough Curve
5.4.1. Thomas Model
5.4.2. Yoon–Nelson Model
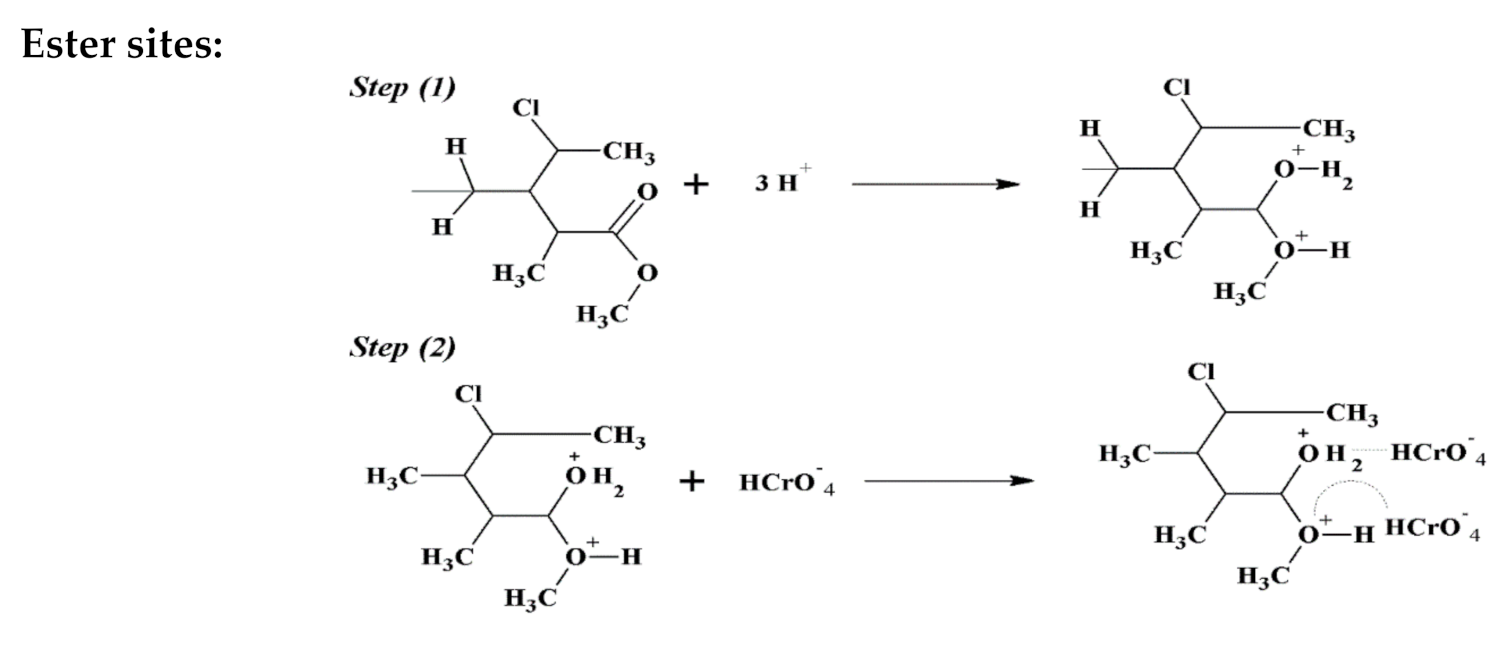
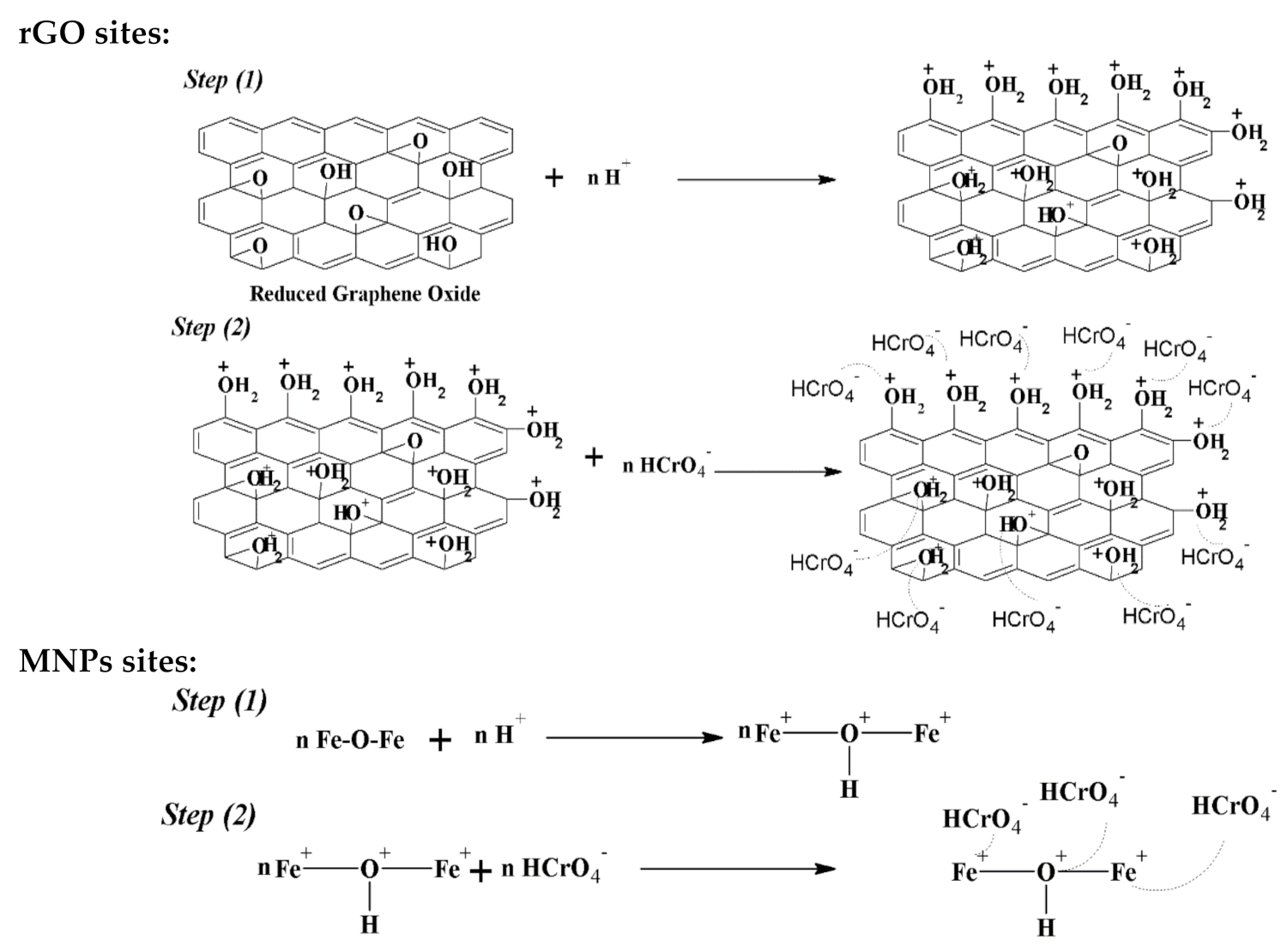
6. Adsorption Mechanism
7. Physicochemical Study of Tannery Wastewater
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.; Wei, W.; Wu, L.; Ni, B.-J. Zero-valent iron mediated biological wastewater and sludge treatment. Chem. Eng. J. 2021, 426, 130821. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Li, Z. Husbandry waste derived coralline-like composite biomass material for efficient heavy metal ions removal. Bioresour. Technol. 2021, 337, 125408. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Rajoriya, S.; Singh, S.R.; Kumar, P. Adsorption of Cr (VI) ion from tannery wastewater on tea waste: Kinetics, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2019, 7, 103188. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, G.; Fang, J.; Dou, X. Enhanced chromium recovery from tanning wastewater. J. Clean. Prod. 2006, 14, 75–79. [Google Scholar] [CrossRef]
- Mella, B.; Glanert, A.C.; Gutterres, M. Removal of chromium from tanning wastewater and its reuse. Process. Saf. Environ. Prot. 2015, 95, 195–201. [Google Scholar] [CrossRef]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Wang, J.; Pan, K.; He, Q.; Cao, B. Polyacrylonitrile/polypyrrole core/shell nanofiber mat for the removal of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2013, 244–245, 121–129. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, P.; Wang, Z.; Cui, W.W.; Zhang, Y.; Zheng, S.; Zhang, Y. Sustainable Disposal of Cr(VI): Adsorption–Reduction Strategy for Treating Textile Wastewaters with Amino-Functionalized Boehmite Hazardous Solid Wastes. ACS Sustain. Chem. Eng. 2018, 6, 6811–6819. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in Drinking Water: Sources, Metabolism, and Cancer Risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Hong, J.; Xie, J.; Mirshahghassemi, S.; Lead, J. Metal (Cd, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone-coated magnetite nanoparticles. RSC Adv. 2020, 10, 3266–3276. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochim. Acta 2016, 191, 1044–1055. [Google Scholar] [CrossRef]
- Qasim, W.; Mane, A. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour. Ind. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mubeena, K.; Muthuraman, G. Extraction and stripping of Cr(VI) from aqueous solution by solvent extraction. Desalination Water Treat. 2014, 55, 1–8. [Google Scholar] [CrossRef]
- Alvarado, L.; Torres, I.R.; Chen, A. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater. Sep. Purif. Technol. 2013, 105, 55–62. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Li, Y.; Li, Y.; Yang, R.; Wang, C. Branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane: A novel and effective adsorbent for Cr(vi) remediation in wastewater. J. Mater. Chem. A 2017, 5, 1133–1144. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, Y.; Zheng, X.; Ji, M.; Zhang, Z. Adsorption behavior and mechanism of Cr(VI) using Sakura waste from aqueous solution. Appl. Surf. Sci. 2016, 360, 470–476. [Google Scholar] [CrossRef]
- Deng, H.; Chen, G.Q.; Gras, S.L.; Kentish, S.E. The effect of restriction membranes on mass transfer in an electrodialysis with filtration membrane process. J. Membr. Sci. 2017, 526, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Qaiser, S.; Ullah, R.; Mohamed Jan, B.; Karakassides, M.A.; Salmas, C.E.; Kenanakis, G.; Ikram, R. Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials 2021, 14, 34. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Guo, Y.; He, D.; Qu, W.; Tang, Y.; Zhou, L.; Zhu, R. A novel graphene oxide-dicationic ionic liquid composite for Cr(VI) adsorption from aqueous solutions. J. Hazard. Mater. 2021, 416, 125706. [Google Scholar] [CrossRef]
- Kong, D.; He, L.; Li, H.; Zhang, F.; Song, Z. Preparation and characterization of graphene oxide/chitosan composite aerogel with high adsorption performance for Cr(VI) by a new crosslinking route. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126832. [Google Scholar] [CrossRef]
- Shan, H.; Zeng, C.; Zhao, C.; Zhan, H. Iron oxides decorated graphene oxide/chitosan composite beads for enhanced Cr(VI) removal from aqueous solution. Int. J. Biol. Macromol. 2021, 172, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Pipíška, M.; Zarodňanská, S.; Horník, M.; Ďuriška, L.; Holub, M.; Šafařík, I. Magnetically functionalized moss biomass as biosorbent for efficient Co2+ ions and thioflavin T removal. Materials 2020, 13, 3619. [Google Scholar] [CrossRef]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Influence of reaction media on synthesis of dialdehyde cellulose/GO composites and their adsorption performances on heavy metals. Carbohydr. Polym. 2020, 232, 115781. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.-S.; Angaru, G.K.R.; Karri, R.R.; Yang, J.-K.; Chang, Y.-Y.; Koduru, J.R. Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 2022, 286, 131776. [Google Scholar] [CrossRef]
- Lee, S.; Lingamdinne, L.P.; Yang, J.-K.; Chang, Y.-Y.; Koduru, J.R. Potential electromagnetic column treatment of heavy metal contaminated water using porous Gd2O3-doped graphene oxide nanocomposite: Characterization and surface interaction mechanisms. J. Water Process. Eng. 2021, 41, 102083. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2010, 22, 055705. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.A.A.P.; Barros-Timmons, A.; Bdkin, I.; Singh, M.K.; Emami, N.; Grácio, J. Graphene oxide modified with PMMA via ATRP as a reinforcement filler. J. Mater. Chem. 2010, 20, 9927–9934. [Google Scholar] [CrossRef] [Green Version]
- Hoan, V.; Thu, N.T.; Duc, H.V. Fe3O4/reduced graphene oxide nanocomposite: Synthesis and its application for toxic metal ion removal. J. Chem. 2016, 2016, 2418172. [Google Scholar]
- Chang, Y.-P.; Ren, C.-L.; Qu, J.-C.; Chen, X.-G. Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 2012, 261, 504–509. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, M. High rate capability and long cycle stability Fe3O4–graphene nanocomposite as anode material for lithium ion batteries. J. Alloy Compd. 2013, 551, 53–60. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhang, H.-B.; Li, X.; Gui, C.-X.; Yu, Z.-Z. Enhanced electromagnetic interference shielding efficiency of polystyrene/graphene composites with magnetic Fe3O4 nanoparticles. Carbon 2015, 82, 67–76. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, G.; Zhang, Z.; Yu, J.; Fan, H.; Li, P.; Zeng, H.; Xing, B.; Chen, L.; Zhang, C. Magnetic graphene-based nanocomposites as highly efficient absorbents for Cr(VI) removal from wastewater. Environ. Sci. Pollut. Res. 2021, 28, 14671–14680. [Google Scholar] [CrossRef]
- Amiri, M.; Shabani, A.M.H.; Dadfarnia, S.; Sadjadi, S. Simultaneous Functionalization and Reduction of Magnetic Graphene Oxide by l-Histidine and its Application for Magnetic Separation/Preconcentration of Antioxidant Trace Elements. Biol. Trace Elem. Res. 2018, 190, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Wang, J.-C.; Chen, C.-Y.; Chu, K.; Lin, C.-K. Spherical Composite Powder by Coupling Polymethyl Methacrylate and Boron Nitride via Spray Drying for Cosmetic Application. Materials 2019, 12, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.R.; Shih, K.Y. Facile Microwave Hydrothermal Synthesis of ZnFe2O4/rGO Nanocomposites and Their Ultra-Fast Adsorption of Methylene Blue Dye. Materials 2021, 14, 5394. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.d.C.; Silva, I.A.A.; Alves, J.R. Magnetic hybrids synthesized from agroindustrial byproducts for highly efficient removal of total chromium from tannery effluent and catalytic reduction of 4-nitrophenol. Cellulose 2018, 25, 7409–7422. [Google Scholar] [CrossRef]
- Sharma, M.; Joshi, M.; Nigam, S.; Shree, S.; Avasthi, D.K.; Adelung, R.; Srivastava, S.K.; Mishra, Y. ZnO tetrapods and activated carbon based hybrid composite: Adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution. Chem. Eng. J. 2019, 358, 540–551. [Google Scholar] [CrossRef]
- Cai, W.; Zhu, F.; Liang, H.; Jiang, Y.; Tu, W.; Cai, Z.; Wu, J.; Zhou, J. Preparation of thiourea-modified magnetic chitosan composite with efficient removal efficiency for Cr(VI). Chem. Eng. Res. Des. 2019, 144, 150–158. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Khosravi, R.; Zarei, A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr(VI) from aqueous solution. Ecol. Eng. 2017, 103, 180–190. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Sarin, V.; Pant, K. Removal of chromium from industrial waste by using eucalyptus bark. Bioresour. Technol. 2006, 97, 15–20. [Google Scholar] [CrossRef]
- Garg, U.K.; Kaur, M.; Garg, V.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.-U.-H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, M.; Bukallah, S.B.; Hamour, F.A.; Nasir, A.S. Adsorption of dyes from aqueous solutions onto sand and their kinetic behavior. Chem. Eng. J. 2008, 137, 238–243. [Google Scholar] [CrossRef]
- Samir, L.; Samira, A.; Mekatel, E.H.; Djamel, N. Adsorption of Cr(VI) on Stipa tenacissima L (Alfa): Characteristics, kinetics and thermodynamic studies. Sep. Sci. Technol. 2018, 54, 876–887. [Google Scholar] [CrossRef]
- Hashem, A.; Momen, A.; Hasan, M.; Nur-A-Tomal, S.; Sheikh, H.R. Chromium removal from tannery wastewater using Syzygium cumini bark adsorbent. Int. J. Environ. Sci. Technol. 2019, 16, 1395–1404. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S. Packed bed column optimization and modeling studies for removal of chromium ions using chemically modified Lantana camara adsorbent. J. Water Process. Eng. 2020, 33, 101069. [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Hexavalent chromium removal and total chromium biosorption from aqueous solution by Quercus crassipes acorn shell in a continuous up-flow fixed-bed column: Influencing parameters, kinetics, and mechanism. PLoS ONE 2020, 15, e0227953. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.; Bajpai, A.K. Dynamic Column Adsorption Studies of Toxic Cr(VI) Ions onto Iron Oxide Loaded Gelatin Nanoparticles. J. Dispers. Sci. Technol. 2011, 32, 1353–1362. [Google Scholar] [CrossRef]
- Ajmani, A.; Patra, C.; Subbiah, S.; Narayanasamy, S. Packed bed column studies of hexavalent chromium adsorption by zinc chloride activated carbon synthesized from Phanera vahlii fruit biomass. J. Environ. Chem. Eng. 2020, 8, 103825. [Google Scholar] [CrossRef]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. A fixed bed column study of natural and chemically modified Lagerstroemia speciosa bark for removal of synthetic Cr(VI) ions from aqueous solution. Int. J. Phytoremediation 2020, 22, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Noonpui, S.; Thiravetyan, P.; Nakbanpote, W.; Netpradit, S. Color removal from water-based ink wastewater by bagasse fly ash, sawdust fly ash and activated carbon. Chem. Eng. J. 2010, 162, 503–508. [Google Scholar] [CrossRef]
- Schmuhl, R.; Krieg, H.; Keizer, K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies. Water SA 2004, 27, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-Y.; Hong, K.-J.; Kajiuchi, T.; Yang, J.-W. Synthesis of chitosan-based polymeric surfactants and their adsorption properties for heavy metals and fatty acids. Int. J. Biol. Macromol. 2005, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Yuan, D.-X.; Yan, J.-M.; Li, Q.-L.; Ouyang, T. Electrochemical removal of chromium from aqueous solutions using electrodes of stainless steel nets coated with single wall carbon nanotubes. J. Hazard. Mater. 2011, 186, 473–480. [Google Scholar] [CrossRef] [PubMed]




| Experimental qe (mg·g−1) | Pseudo-1st Order | Pseudo-2nd Order | ||||
|---|---|---|---|---|---|---|
| K1 min−1 | qe (mg·g−1) | R2 | K2 (g.mg−1·min−1) | qe (mg·g−1) | R2 | |
| 109.33 | 0.151 | 23.93 | 0.86 | 0.362 | 166.66 | 0.98 |
| Model Equation | R2 | Kf | 1/n | Kb | qm (mg·g−1) | RL |
|---|---|---|---|---|---|---|
| Langmuir Isotherm | 0.991 | --- | --- | 4.021 | 240.96 | 0.199 |
| Freundlich Isotherm | 0.972 | 6.85 | 0.351 | --- | --- |
| Temperature K | ΔGo KJ.mole−1 | ΔHo KJ.mole−1 | ΔSo KJ.mole−1 |
|---|---|---|---|
| 293 | −14.4944 | 46.0074 | 0.1875 |
| 303 | −15.4132 | ||
| 313 | −16.5329 | ||
| 323 | −17.4261 | ||
| 333 | −18.3889 | ||
| 343 | −19.4415 | ||
| 353 | −20.8456 | ||
| 363 | −22.5964 | ||
| 373 | −24.0239 |
| Basic Solution | Concentration of Eluent mol·L−1 | %, Recovery of Cr(VI) |
|---|---|---|
| NaOH | 1 | 70 ± 1 |
| NaOH | 2 | 95 ± 1 |
| NH4OH | 1 | 65 ± 2 |
| NH4OH | 2 | 78 ± 1 |
| Ions | Concentration (mg/L) | % Adsorption of Cr(VI) |
|---|---|---|
| Cu2+ | 10 | 96 ± 4 |
| Mn2+ | 10 | 98 ± 3 |
| Zn2+ | 100 | 93 ± 1 |
| Cd2+ | 100 | 97 ± 1 |
| Fe3+ | 100 | 96 ± 1 |
| Ni2+ | 10 | 94 ± 1 |
| Ca2+ | 500 | 98 ± 1 |
| Mg2+ | 500 | 98 ± 1 |
| CO32− | 500 | 96 ± 1 |
| F− | 1000 | 99 ± 1 |
| SO42− | 500 | 98 ± 1 |
| Parameters | Adsorbent Capacity (mg/g) | AER (g/L) | |
|---|---|---|---|
| Adsorbent Mass (g) | Flow Rate (mL/min) | ||
| 1 | 2 | 125.16 | 20.00 |
| 2 | 2 | 134.77 | 17.24 |
| 3 | 2 | 152.13 | 14.56 |
| Flow rate (mL/min) | Adsorbent masses (g) | ||
| 1 | 3 | 120.06 | 11.02 |
| 2 | 3 | 111.98 | 10.10 |
| 3 | 3 | 75.01 | 9.80 |
| Parameters | Thomas Model | Yoon–Nelson Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | Rate Flow (mL/min) | Bed Height (cm−1) | pH | KTH (L/min gm) | q (mg/g) | R2 | KYN (min−1) | τ (min) | R2 |
| 1640 | 1 | 2.7 | 2 | 11.58 × 10−2 | 135.31 | 0.999 | 27.19 × 10−2 | 135.31 | 0.998 |
| 1640 | 2 | 2.7 | 2 | 9.14 × 10−2 | 126.51 | 0.993 | 22.03 × 10−2 | 126.51 | 0.997 |
| 1640 | 3 | 2.7 | 2 | 8.82 × 10−2 | 111.48 | 0.972 | 43.53 × 10−2 | 111.48 | 0.982 |
| Parameters | Values | ||
|---|---|---|---|
| Before Treatment | After Batch Mode Adsorption | After Column Adsorption | |
| Cr concentration | 1640 mg/L | 3.51 mg/L | 3.42 mg/L |
| pH | 3.17 | Variable | Variable |
| Chemical oxygen demand (COD) | 1130 mg/L | 110 mg/L | 99 mg/L |
| Biological oxygen demand (BOD) | 396 mg/L | 120 mg/L | 109 mg/L |
| Suspended solids (SS) | 960 mg/L | 0.00 mg/L | 0.00 mg/L |
| Adsorbent | Adsorption Capacity | pH | Adsorption Process | Wastewater | Ref. |
|---|---|---|---|---|---|
| Bagasse fly ash | 29.07 mg/g | 2–3 | Batch | Synthetic | [61] |
| Fe3O4/rGO | 98.1% | 1 | Batch | - | [41] |
| Non-cross-linked chitosan | 80 mg/g | 5 | Batch | Synthetic | [62] |
| Polymeric based surfactant-chitosan | 180 mg/g | 5.3 | Batch | Synthetic | [63] |
| Sawdust | 1.74 mg/g | 3 | Batch | Synthetic | [64] |
| SWCNTs | 96.9 mg/g | 4 | Batch | Synthetic | [65] |
| MNPs/rGO/PMMA | 109.3/135.3 mg/g | 3 | Batch/column | Wastewater | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Ahmad, W.; Yaseen, M.; Khan, M.; Iqbal Khattak, M.; Mohamed Jan, B.; Ikram, R.; Kenanakis, G. Fabrication of MNPs/rGO/PMMA Composite for the Removal of Hazardous Cr(VI) from Tannery Wastewater through Batch and Continuous Mode Adsorption. Materials 2021, 14, 6923. https://doi.org/10.3390/ma14226923
Ullah R, Ahmad W, Yaseen M, Khan M, Iqbal Khattak M, Mohamed Jan B, Ikram R, Kenanakis G. Fabrication of MNPs/rGO/PMMA Composite for the Removal of Hazardous Cr(VI) from Tannery Wastewater through Batch and Continuous Mode Adsorption. Materials. 2021; 14(22):6923. https://doi.org/10.3390/ma14226923
Chicago/Turabian StyleUllah, Rahman, Waqas Ahmad, Muhammad Yaseen, Mansoor Khan, Mehmood Iqbal Khattak, Badrul Mohamed Jan, Rabia Ikram, and George Kenanakis. 2021. "Fabrication of MNPs/rGO/PMMA Composite for the Removal of Hazardous Cr(VI) from Tannery Wastewater through Batch and Continuous Mode Adsorption" Materials 14, no. 22: 6923. https://doi.org/10.3390/ma14226923








