High Temperature Oxidation Behavior of an Equimolar Cr-Mn-Fe-Co High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
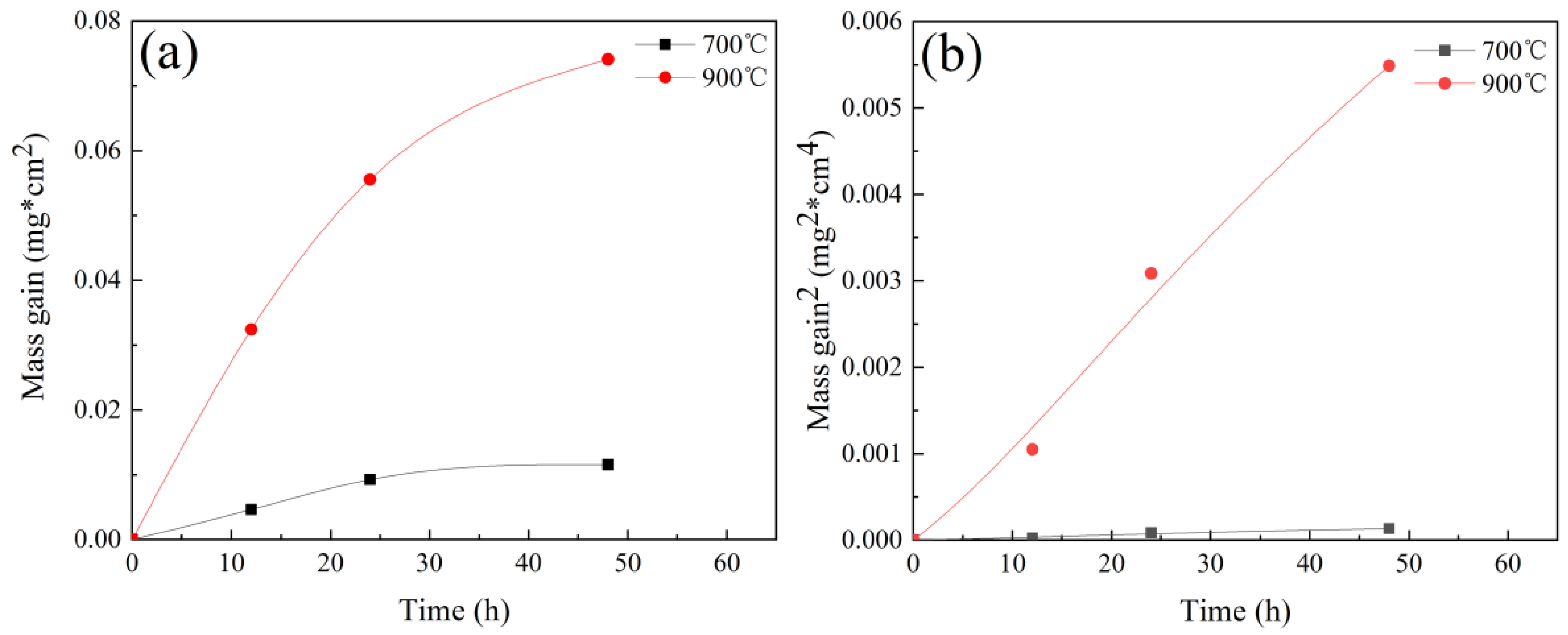
3.1. Oxidation Properties of Cr-Mn-Fe-Co HEA
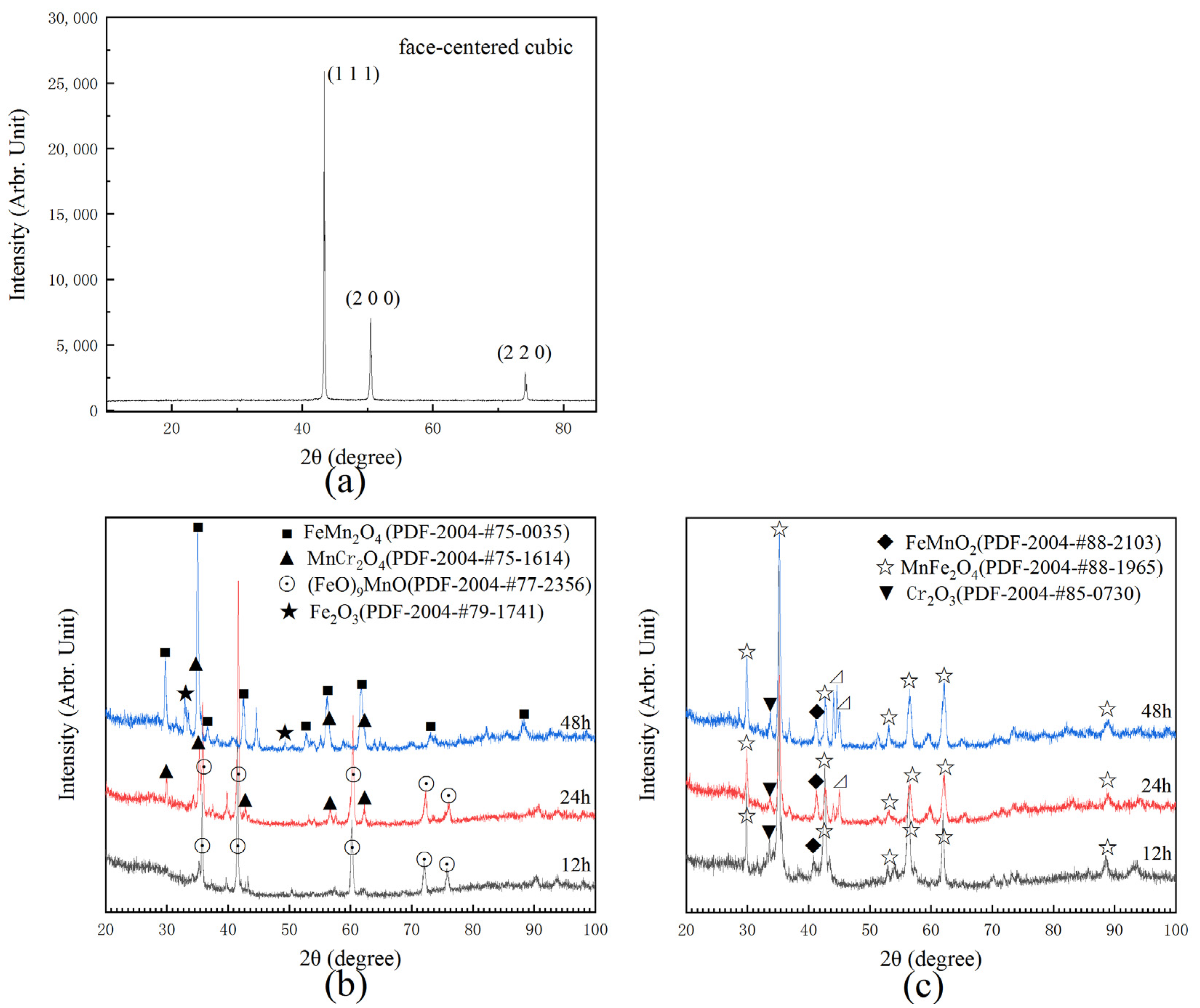
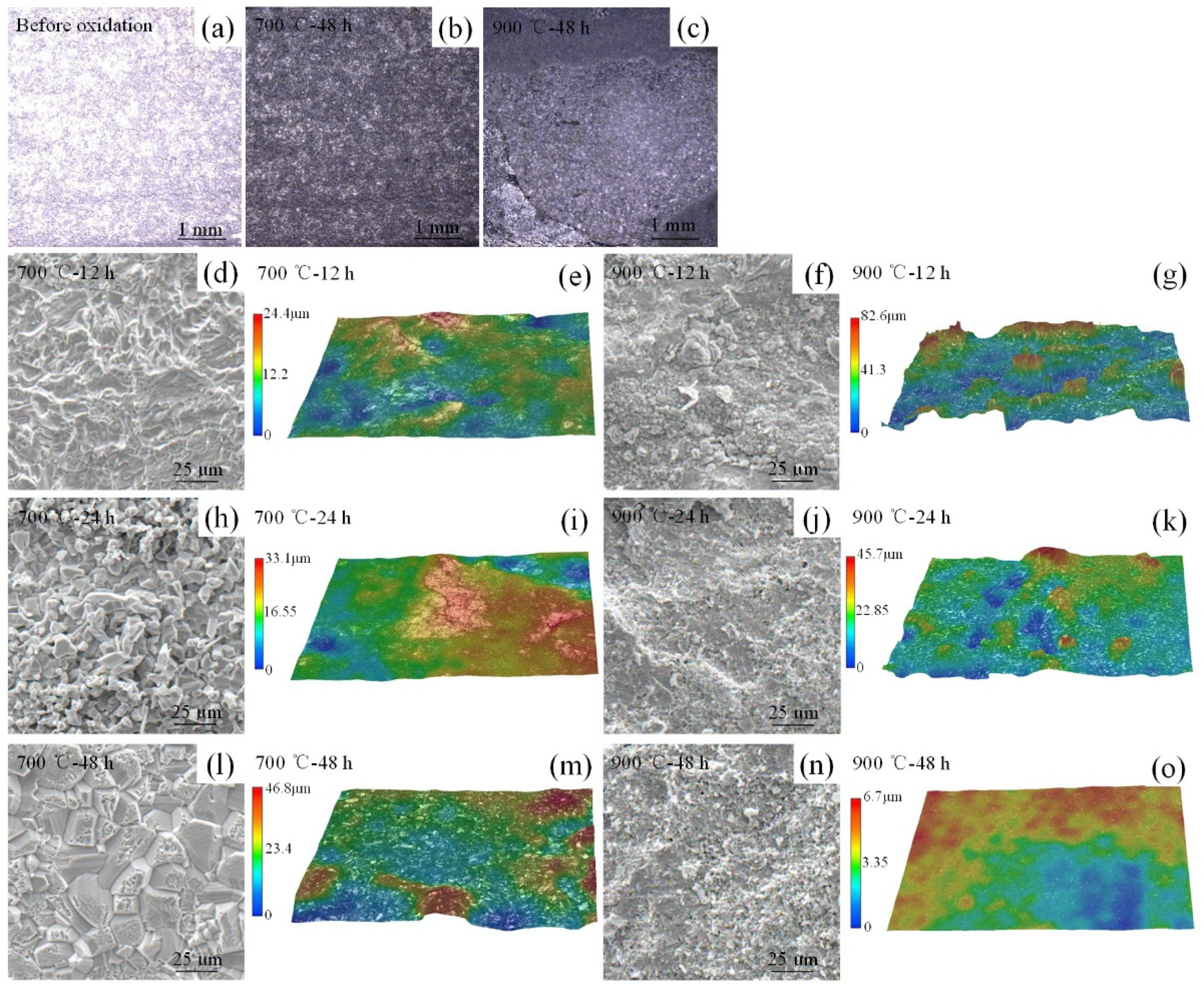
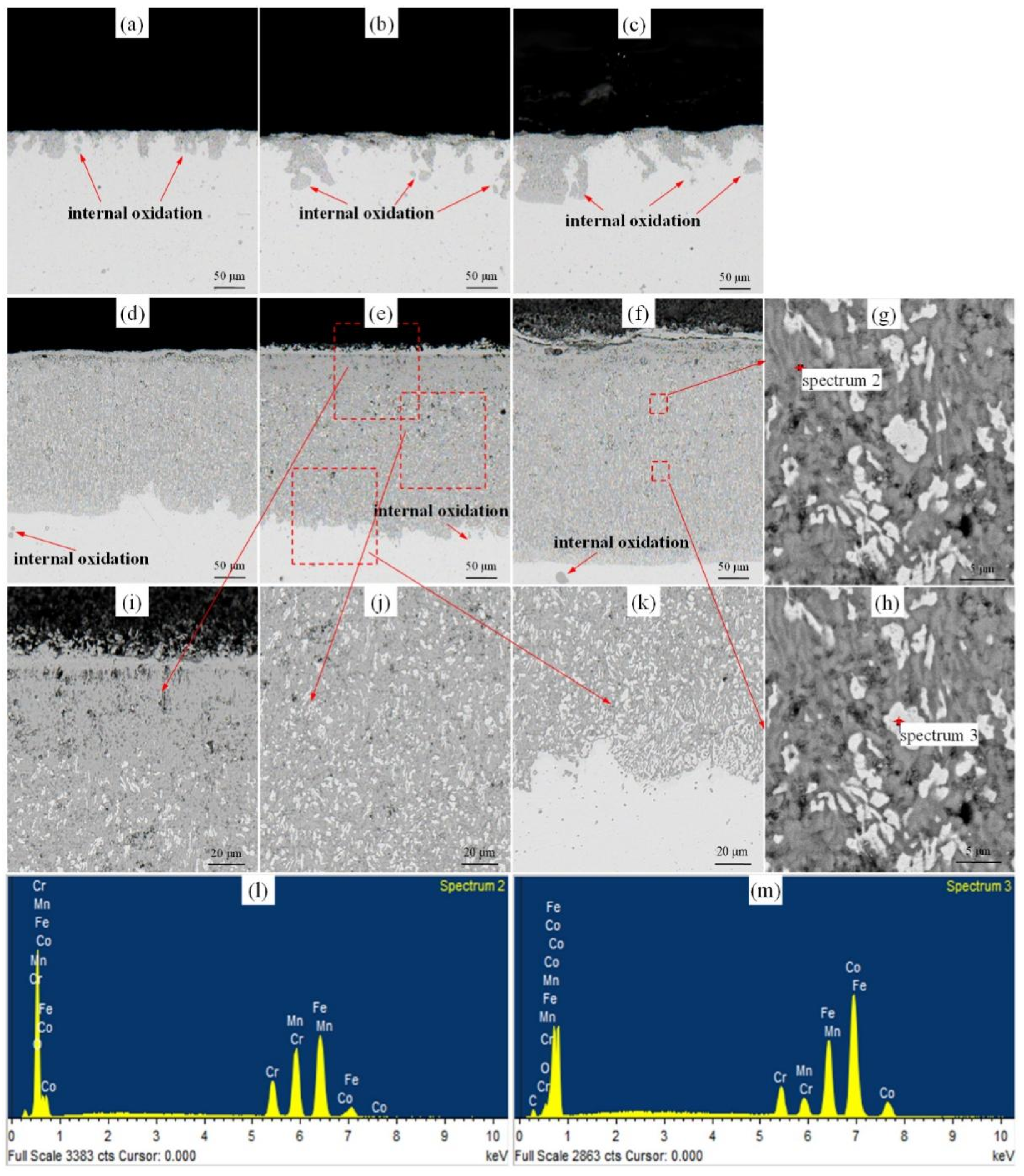
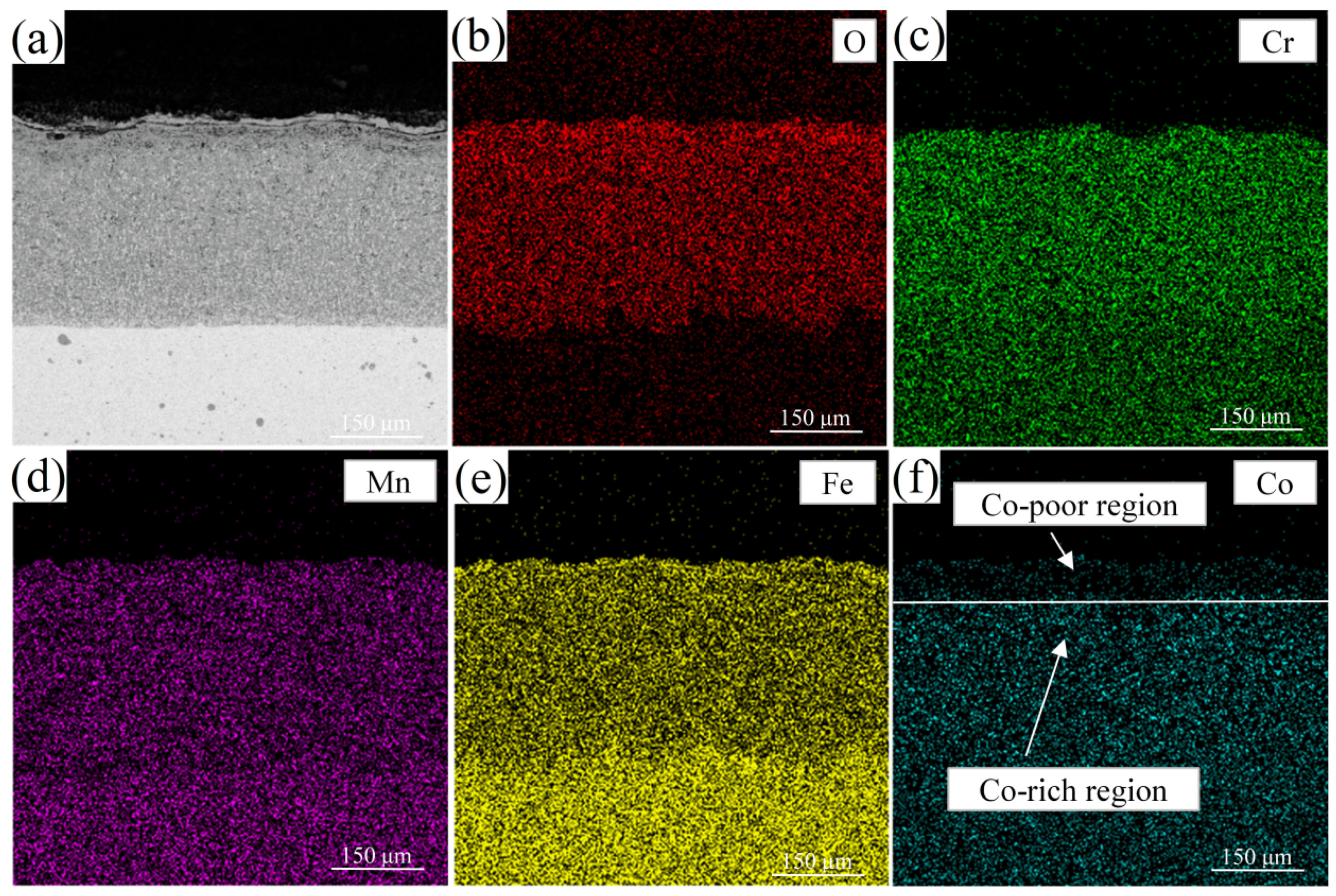
3.2. High-Temperature Surface Oxidation Mechanism of Cr-Mn-Fe-Co HEA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high-entropy alloys with multiple principal elements, novel alloy design, conceptes and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Bhatta, L.; Kong, C.; Yu, H. Study of texture analysis on asymmetric cryorolled and annealed CoCrNi medium entropy alloy. Crystals 2020, 10, 1154. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Li, C.; Gan, B.; Kong, C.; Wang, Y.; Yu, H. Annealing effect on mechanical properties of asymmetric cryorolled CrCoNi medium entropy alloy. Mater. Today Comm. 2020. [Google Scholar] [CrossRef]
- Vaidyal, M.; Muralikrishnal, G.; Murty, B. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Tsai, K.; Tsai, M.; Yeh, J. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.; George, E.; Ritchie, R. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, F.; Dlouhy, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Sang, H.; Byung, J.; Sun, I. Correlation between mechanical properties and thermodynamic parameters of dual-fcc-phase CoCrFeCuxNi (x = 1, 1.71) and CoCu1.71FeMnNi. Mater. Lett. 2020, 272, 127866. [Google Scholar] [CrossRef]
- Keil, T.; Bruder, E.; Laurent-Brocq, M.; Durst, K. From diluted solid solutions to high entropy alloys: Saturation grain size and mechanical properties after high pressure torsion. Scripta Mater. 2021, 192, 43–48. [Google Scholar] [CrossRef]
- Kai, W.; Li, C.; Cheng, F.; Chu, K.; Huang, R.; Tsay, L.; Kai, J. The oxidation behavior of an equimolar Fe-Co-Ni-Cr-Mn high-entropy alloy at 950 °C in various oxygen-containing atmospheres. Corr. Sci. 2016, 108, 209–214. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloy. Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Sang, H.; Yong, K.; Rizi, M.; Sun, I. Microstructure evolution and mechanical properties of (CoCrNi)90(AlTiZr)5(CuFeMo)5 multicomponent alloy: A pathway through multicomponent alloys toward new superalloys. J. Alloy. Compd. 2021, 860, 158412. [Google Scholar] [CrossRef]
- Yong, K.; Byung, J.; Soon-Ku, H.; Sun, I. Strengthening and fracture of deformation-processed dual fcc-phase CoCrFeCuNi and CoCrFeCu1.71Ni high entropy alloys. Mater. Sci. Eng. A 2020, 781, 139241. [Google Scholar] [CrossRef]
- Kim, Y.; Joo, Y.; Kim, H.; Lee, K. High temperature oxidation behavior of Cr-Mn-Fe-Co-Ni high entropy alloy. Intermetallics 2018, 98, 45–53. [Google Scholar] [CrossRef]
- Wagner, C. Passivity and inhibition during the oxidation of metals at elevated temperatures. Corr. Sci. 1965, 5, 751–764. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Wu, Y.; Gesmundo, F. The third-element effect in the oxidation of Ni-xCr-7Al (x = 0, 5, 10, 15 at.%) alloys in 1 atm O2 at 900–1000 °C. Corr. Sci. 2006, 48, 4020–4036. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Ma, S.; Liaw, P.; Tang, Z.; Cheng, Y.; Gao, M. Guidelines in predicting phase formation of high-entropy alloys. MRS Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Chen, J.; Rogers, P.; Little, J. Oxidation behavior of several chromia-forming commercial nickel-base superalloys. Oxid. Met. 1997, 47, 381–410. [Google Scholar] [CrossRef]
- Jacob, Y.; Haanappel, V.; Stroosnijder, M.; Buscail, H.; Fielitz, P.; Borchardt, G. The effect of gas composition on the isothermal oxidation behaviour of PM chromium. Corros. Sci. 2002, 44, 2027–2039. [Google Scholar] [CrossRef]
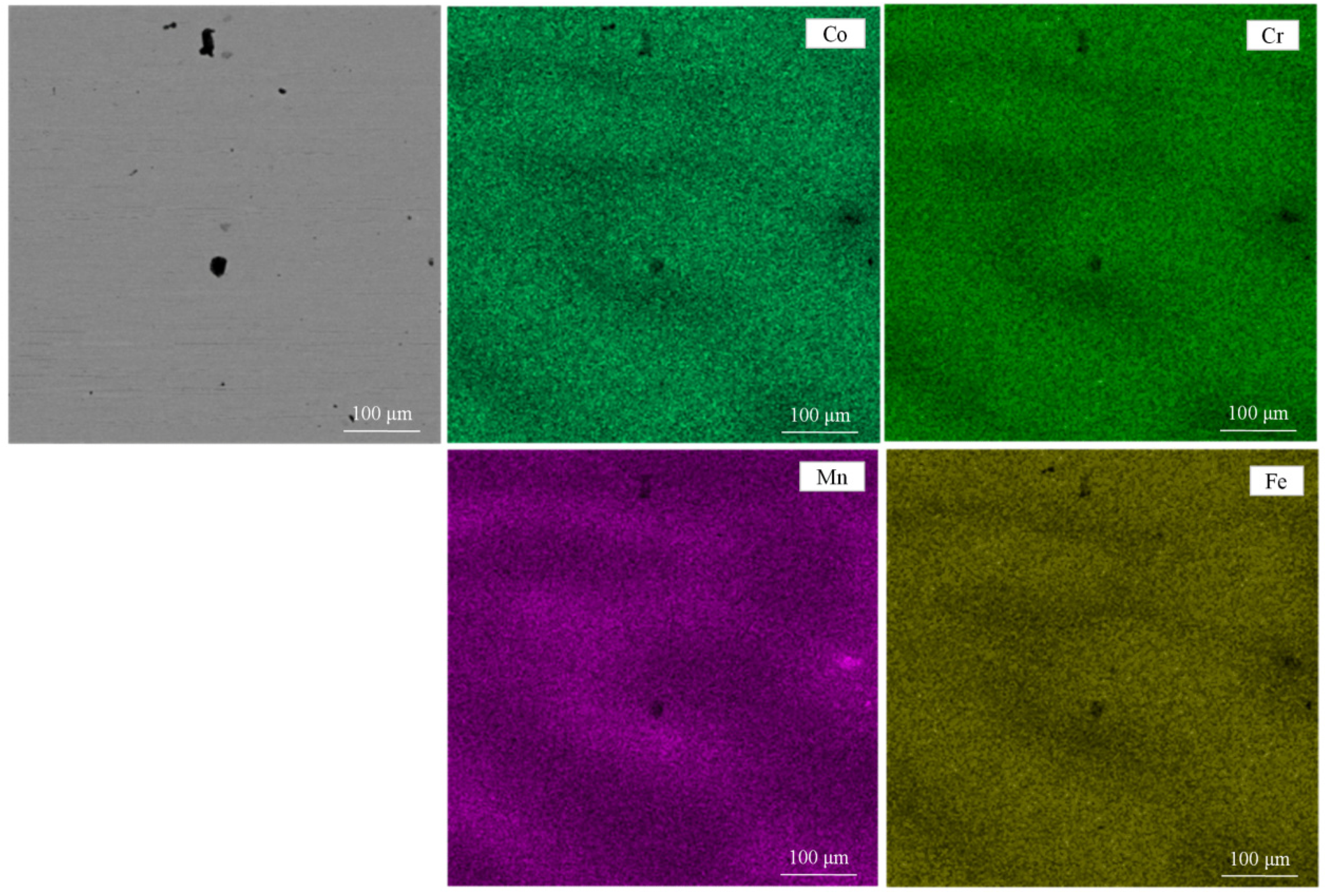
| Composition (wt.%) | Co | Cr | Fe | Mn |
|---|---|---|---|---|
| The initial HEA | 24.67 | 24.25 | 25.72 | 25.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zeng, Q.; Xie, Z.; Zhang, Y.; Gao, H. High Temperature Oxidation Behavior of an Equimolar Cr-Mn-Fe-Co High-Entropy Alloy. Materials 2021, 14, 4259. https://doi.org/10.3390/ma14154259
Wang L, Zeng Q, Xie Z, Zhang Y, Gao H. High Temperature Oxidation Behavior of an Equimolar Cr-Mn-Fe-Co High-Entropy Alloy. Materials. 2021; 14(15):4259. https://doi.org/10.3390/ma14154259
Chicago/Turabian StyleWang, Lin, Quanqing Zeng, Zhibao Xie, Yun Zhang, and Haitao Gao. 2021. "High Temperature Oxidation Behavior of an Equimolar Cr-Mn-Fe-Co High-Entropy Alloy" Materials 14, no. 15: 4259. https://doi.org/10.3390/ma14154259
APA StyleWang, L., Zeng, Q., Xie, Z., Zhang, Y., & Gao, H. (2021). High Temperature Oxidation Behavior of an Equimolar Cr-Mn-Fe-Co High-Entropy Alloy. Materials, 14(15), 4259. https://doi.org/10.3390/ma14154259







