Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review
Abstract
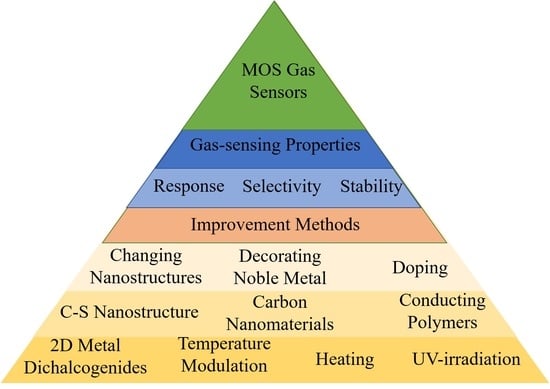
:1. Introduction
2. The Properties of MOS Gas Sensors
3. The Methods to Improve the Properties
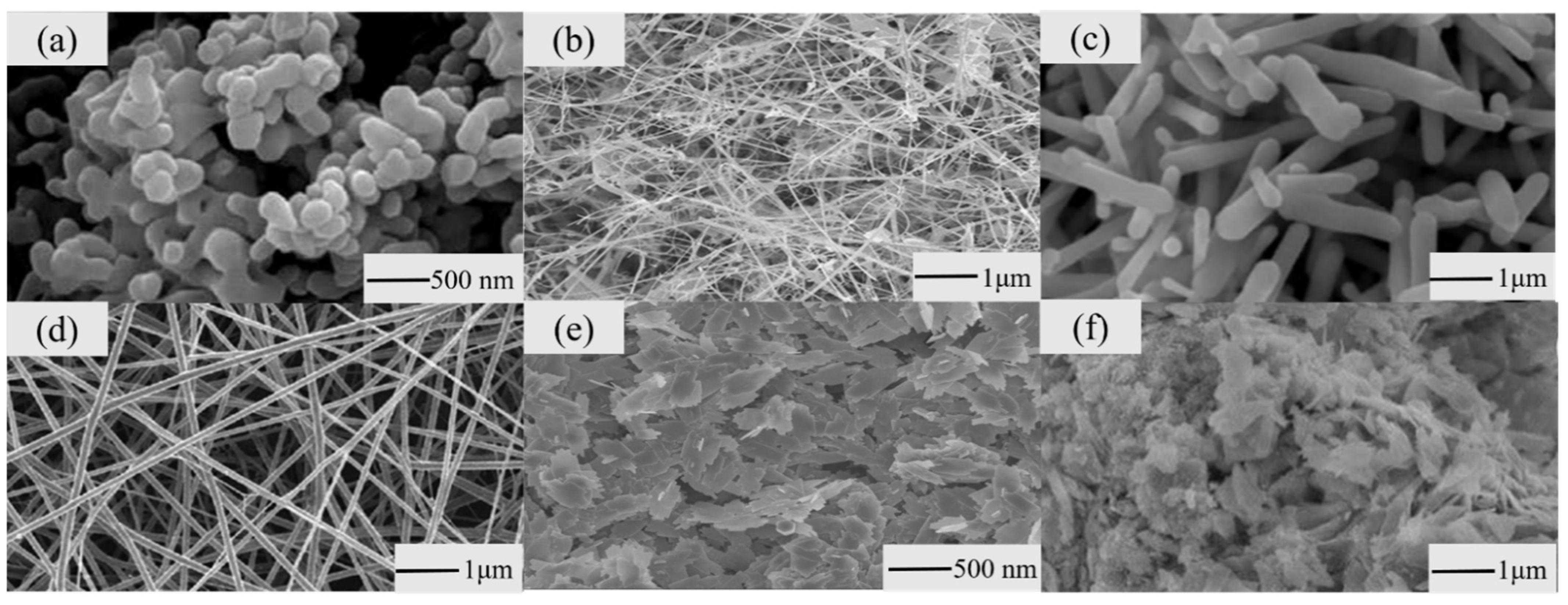
3.1. Changing the Morphology of Nanostructures
3.1.1. Nanoparticles
3.1.2. Nanowires
3.1.3. Nanorods
3.1.4. Nanofibers
3.1.5. Nanosheets
3.1.6. Nanoflowers
3.2. Noble Metal Decorating
3.3. Doping
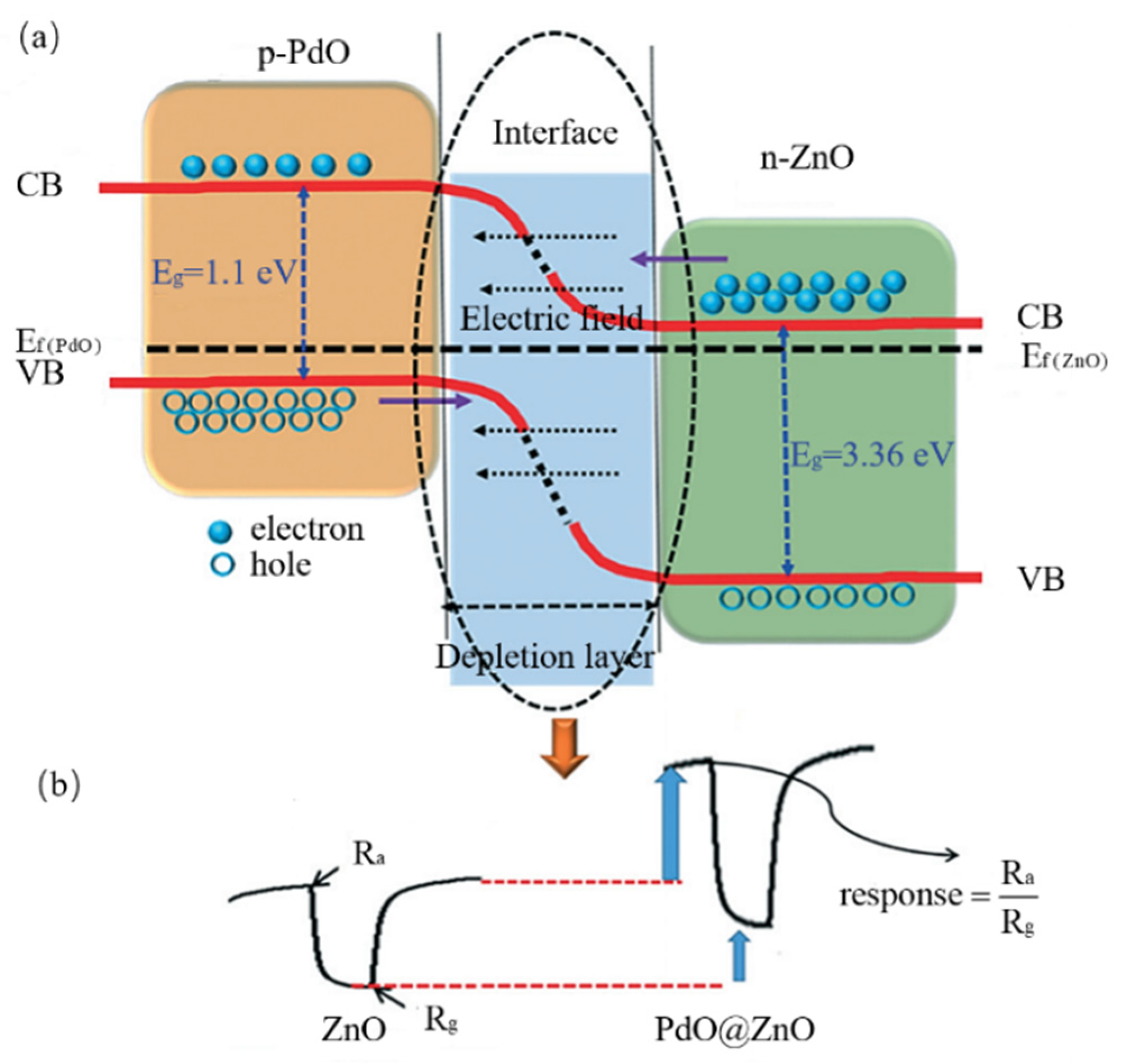
3.4. Core-Shell (C-S) Nanostructures
3.4.1. Overview
The Definition of C-S Nanostructure
The Composition of C-S Nanostructures
- (1)
- Metal oxide/metal oxide
- (2)
- Metal oxide/metal sulfide
- (3)
- Metal oxide/noble metal
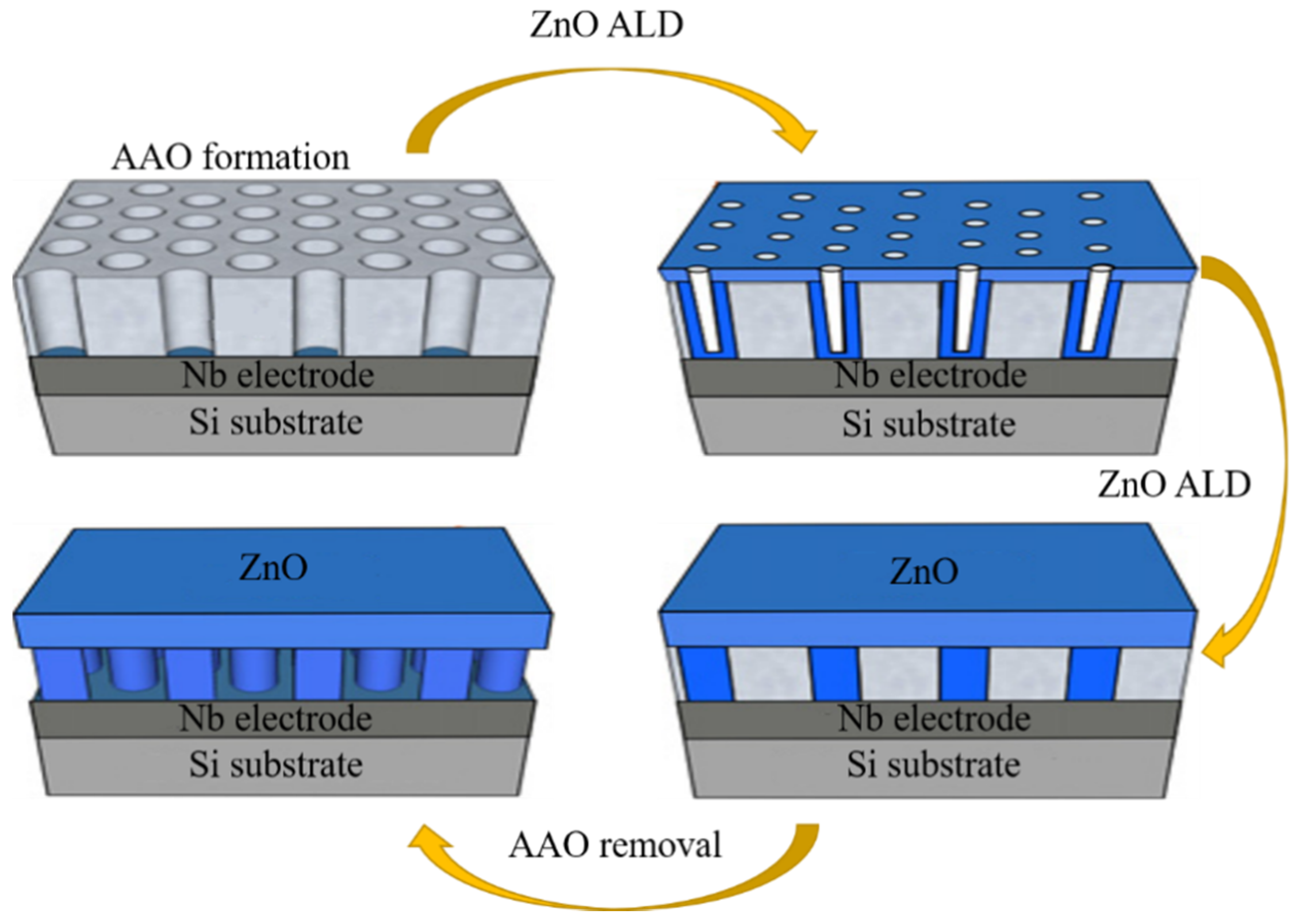
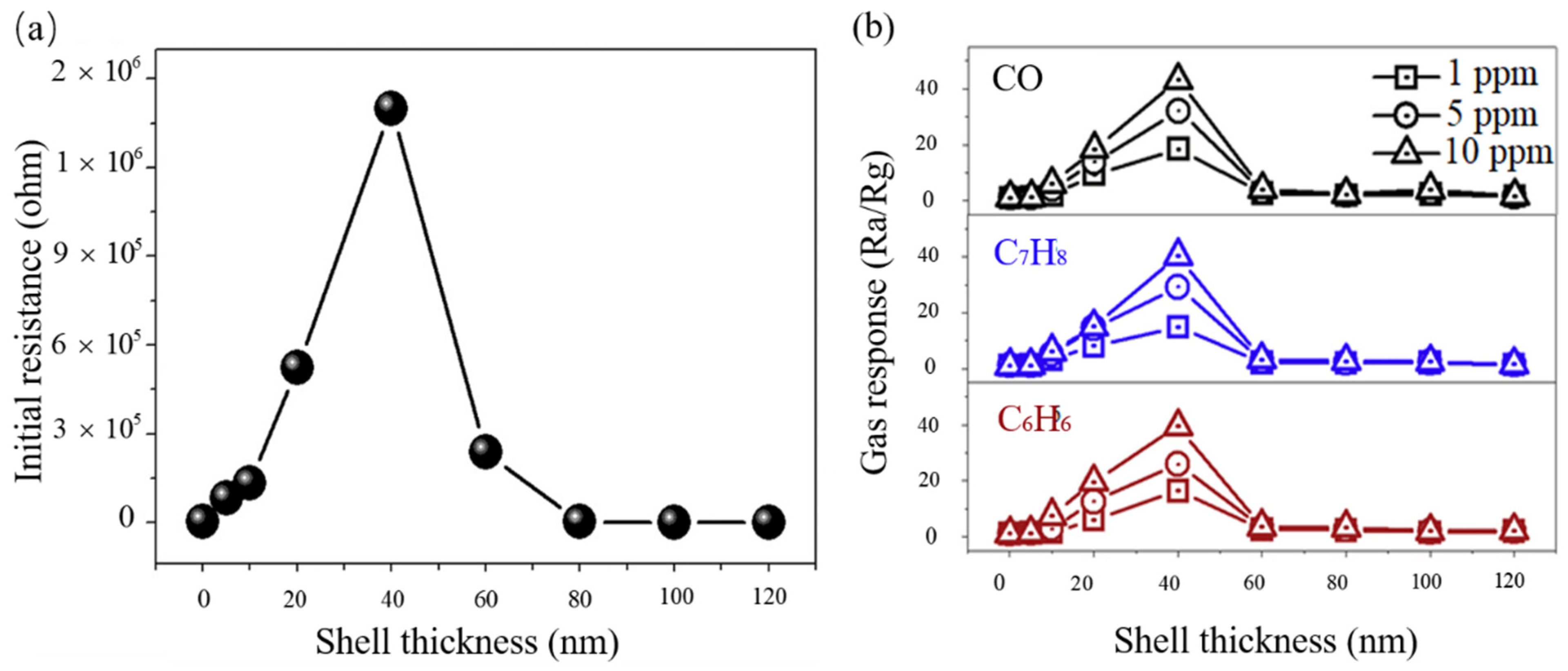
3.4.2. The Thickness of Shell Layer
3.4.3. The Manufacture of C-S Nanostructures
3.4.4. The Application of C-S Nanostructures
Metal Oxide/Metal Oxide
Metal Oxide/Metal Sulfide
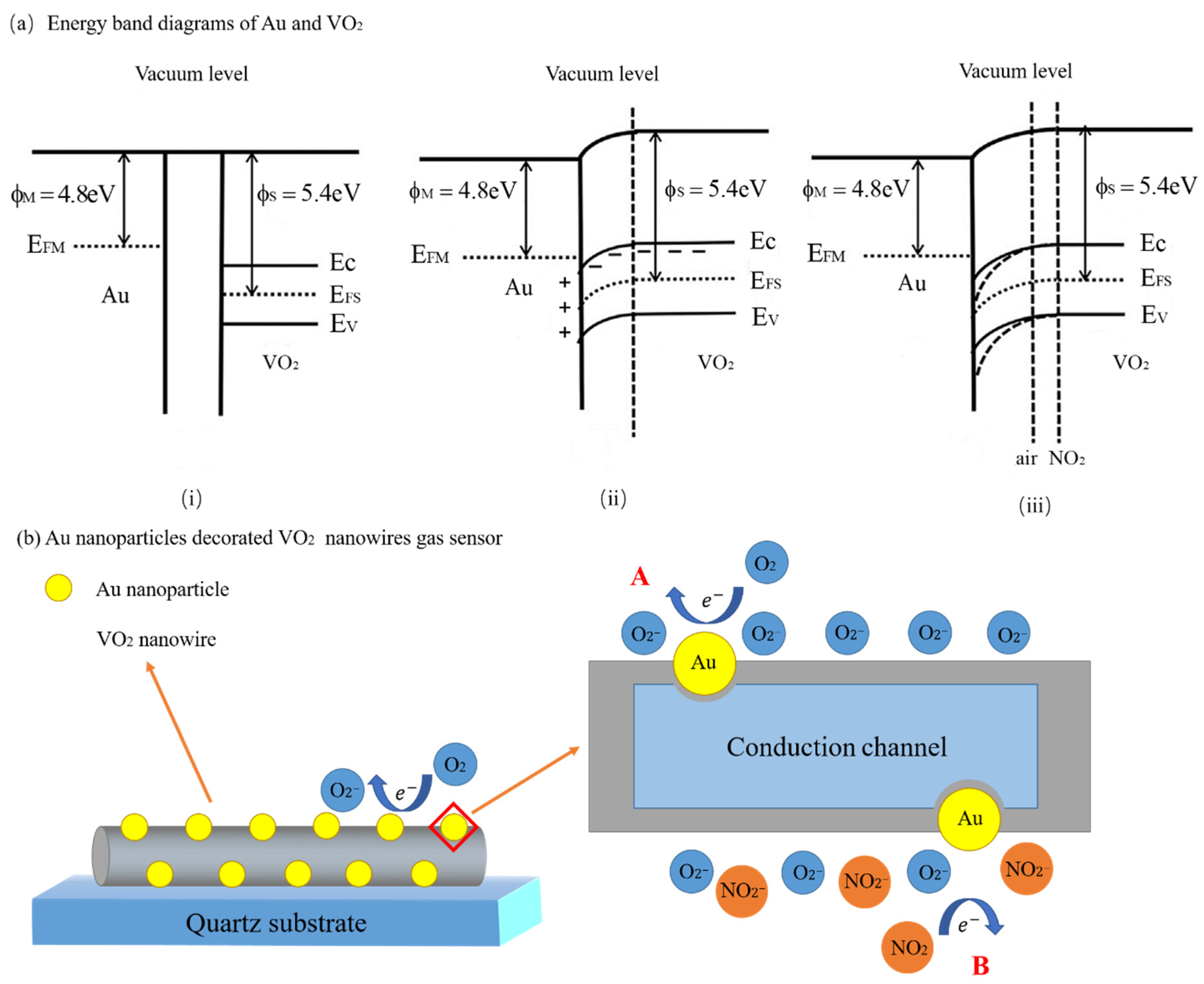
Metal Oxide/Noble Metal
3.4.5. C-S Nanostructure and Noble Metal Decorating/Doping
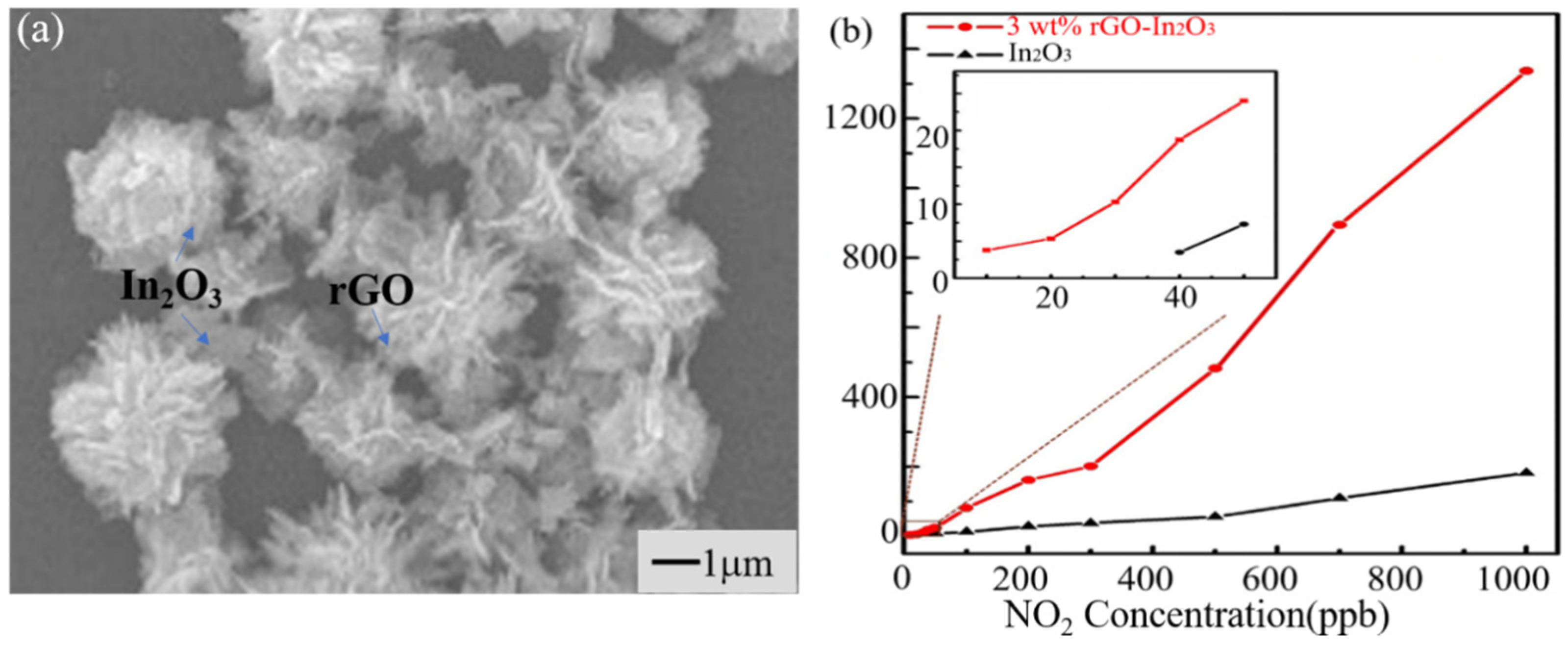
3.5. Carbon Nanomaterials
3.6. Conducting Polymers
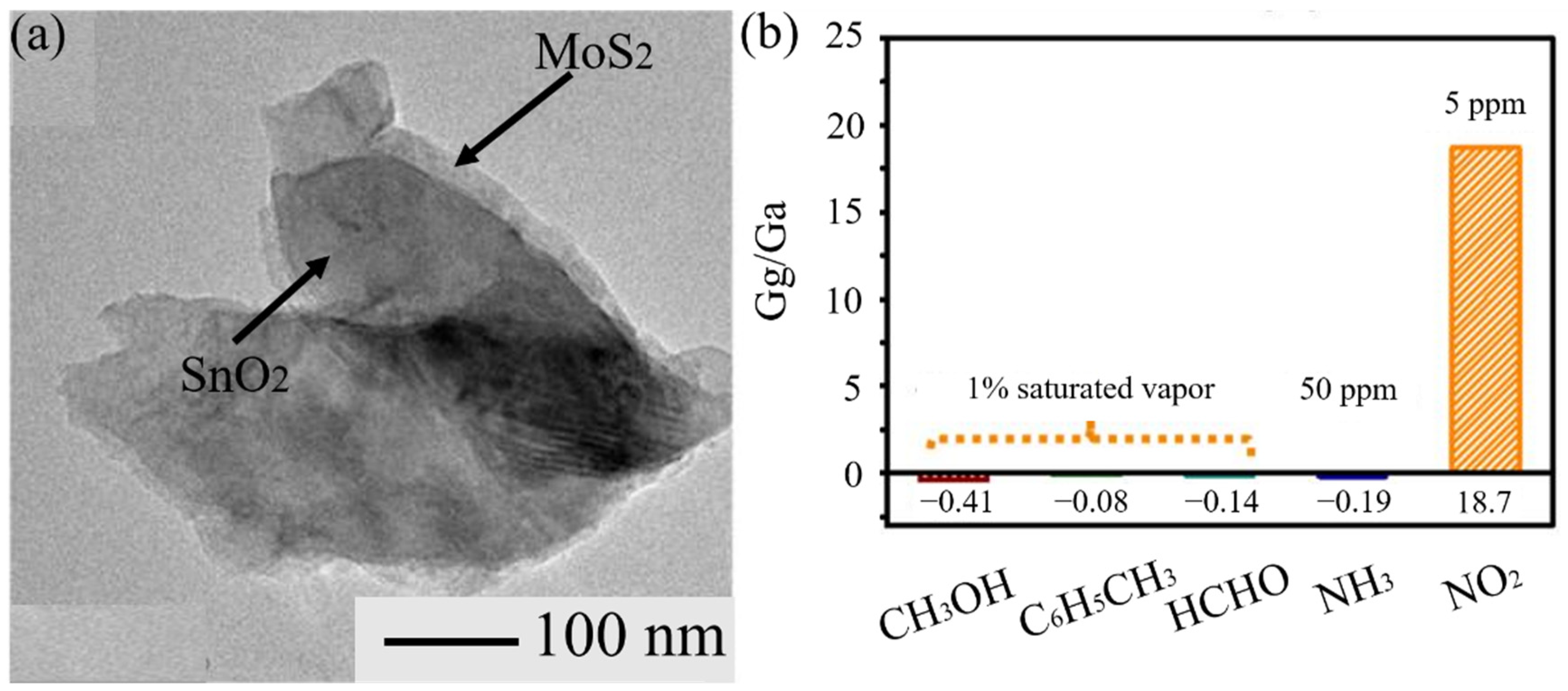
3.7. 2D Metal Dichalcogenides
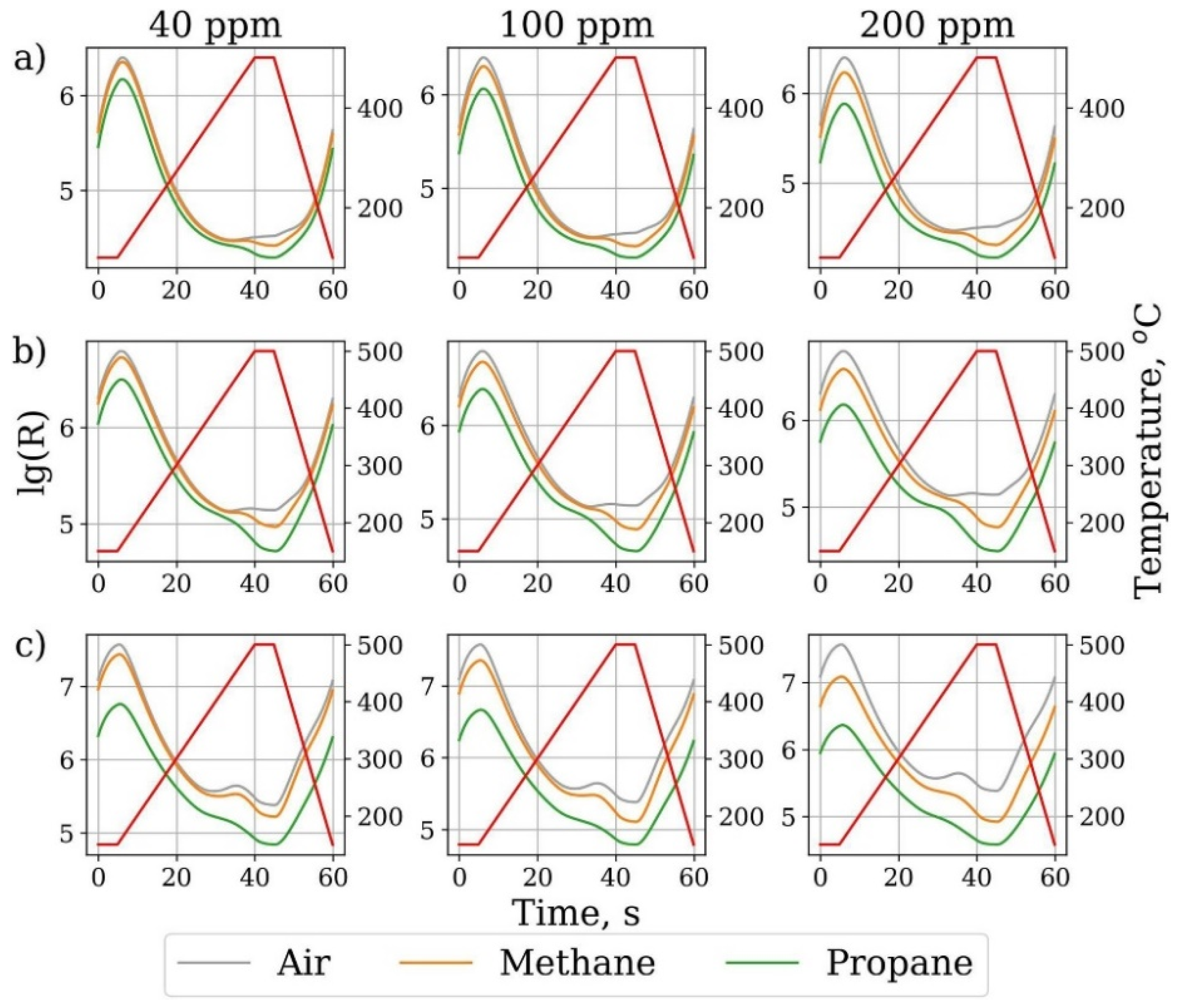
3.8. Temperature Modulating
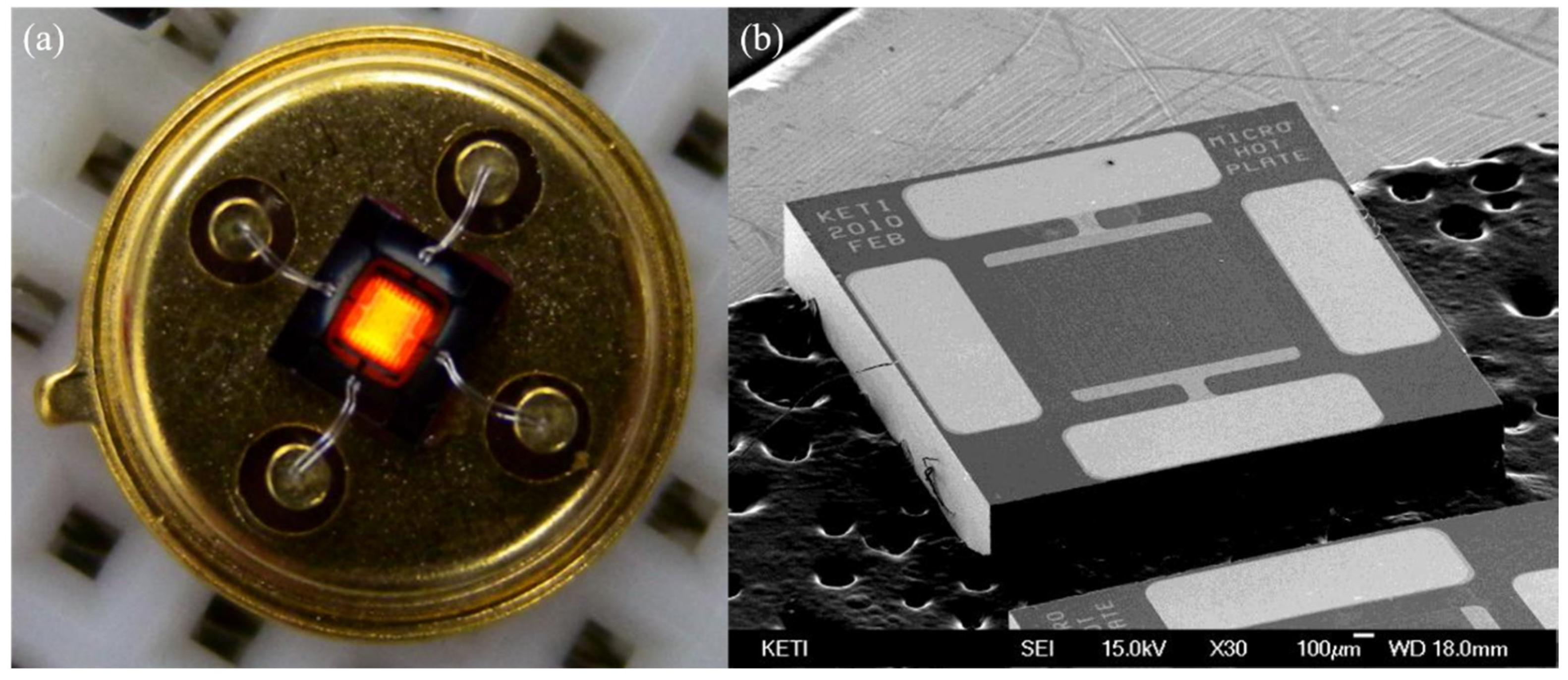
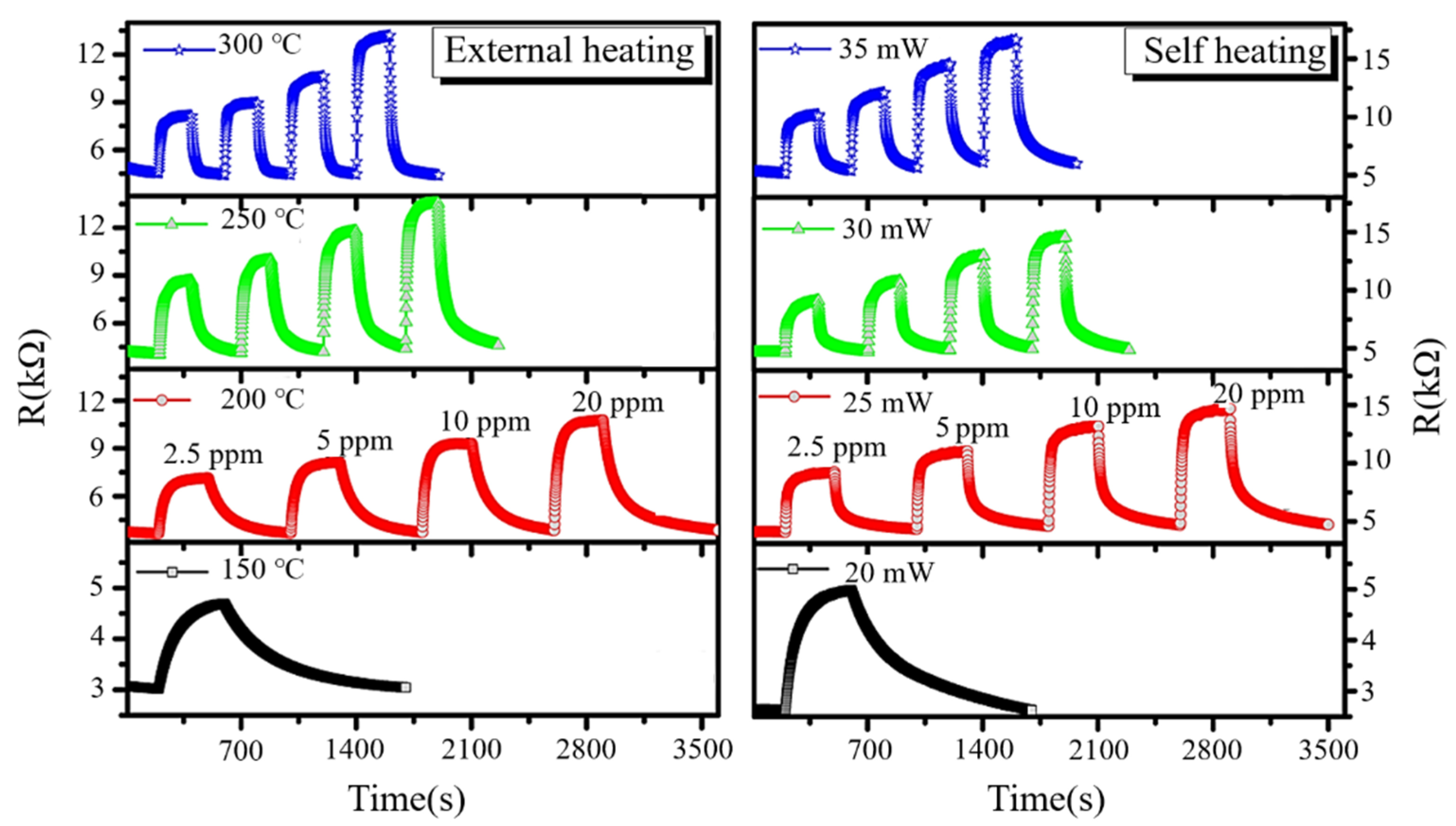
3.9. Heating
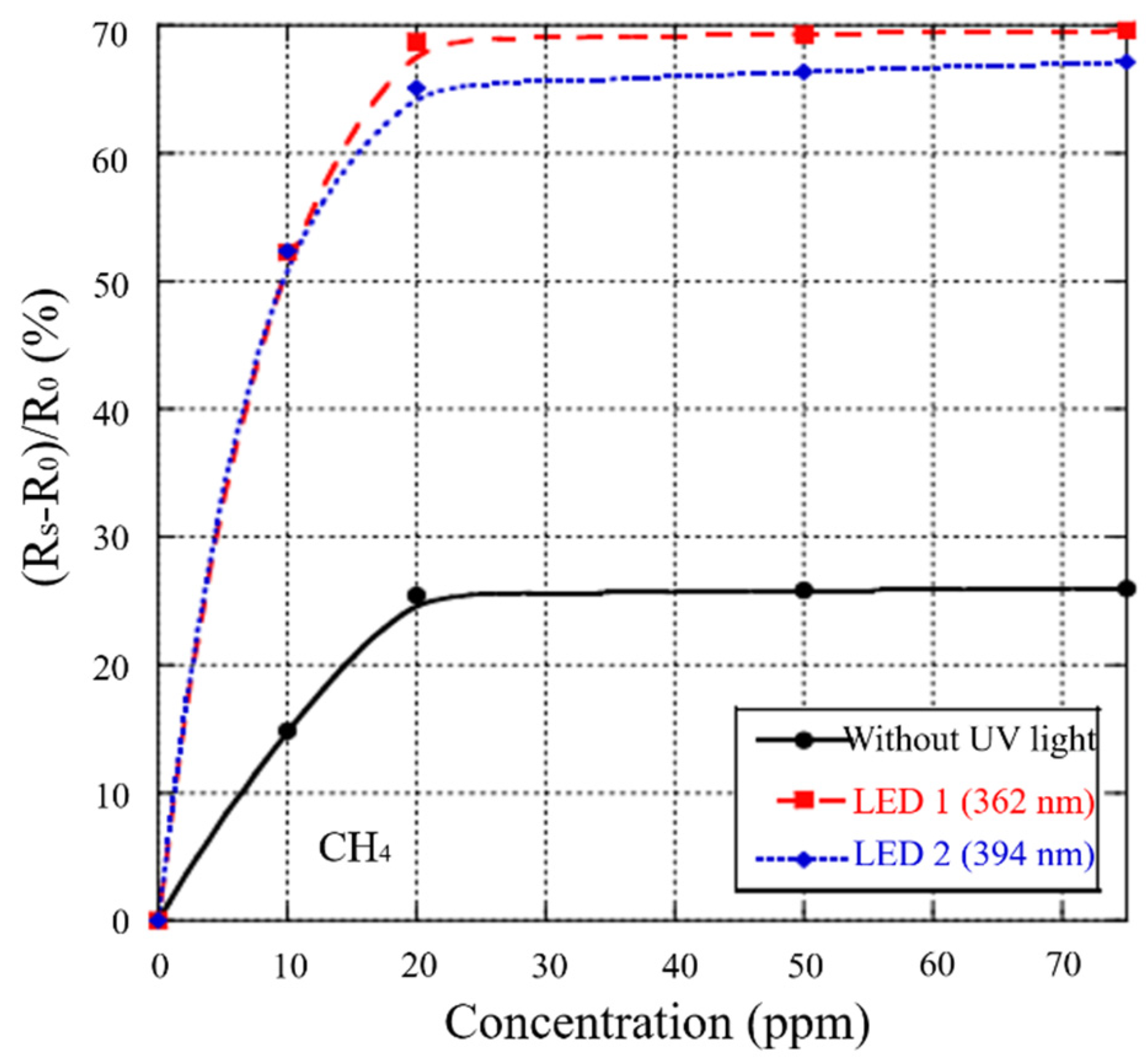
3.10. UV Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [Green Version]
- Park, S. Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloy. Compd. 2017, 696, 655–662. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low-Voltage-Driven Sensors Based on ZnO Nanowires for Room-Temperature Detection of NO2 and CO Gases. ACS Appl. Mater. Interfaces 2019, 11, 24172–24183. [Google Scholar] [CrossRef]
- Brattain, W.H.; Bardeen, J. Surface Properties of Germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fukiishi, K.S.; Nagatini, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.L.; Zeng, Q.; Hui, Y.; Alhai, M.E.E.; Qin, S.J.; Wu, T.Z. Fast Polymerization of Polydopamine Based on Titanium Dioxide for High-Performance Flexible Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 14495–14506. [Google Scholar] [CrossRef] [PubMed]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Rossi, R.; Cassano, G.; Di Franco, C.; Palmisano, F.; Torsi, L.; Cioffi, N. Gas sensing properties of MWCNT layers electrochemically decorated with Au and Pd nanoparticles. Beilstein J. Nanotechnol. 2017, 8, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Hayasaka, T.; Kubota, Y.; Liu, Y.; Lin, L. The influences of temperature, humidity, and O2 on electrical properties of graphene FETs. Sens. Actuators B 2019, 285, 116–122. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Patil, S.P.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Patil, P.S. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Hwang, I.-S.; Park, J.-G.; Choi, K.-J.; Park, J.-H.; Lee, J.-H. Novel fabrication of a SnO2 nanowire gas sensor with high gas response. Nanotechnology 2008, 19, 095508. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef]
- Mirzaei, A.; Hashemi, B.; Janghorban, K. α-Fe2O3 based nanomaterials as gas sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 3109–3144. [Google Scholar] [CrossRef]
- Liu, X.H.; Ma, T.T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Wang, Y.R.; Liu, B.; Xiao, S.H.; Wang, X.H.; Sun, L.M.; Li, H.; Xie, W.Y.; Li, Q.H.; Zhang, Q.; Wang, T.H. Low-Temperature H2S Detection with Hierarchical Cr-Doped WO3 Microspheres. ACS Appl. Mater. Interfaces 2016, 8, 9674–9683. [Google Scholar] [CrossRef]
- Joshi, N.; Silva, L.F.D.; Jadhav, H.; M’Peko, J.C.; Torres, B.B.M.; Aguir, K.; Mastelaro, V.R.; Oliveira Jr, O.N. One-step approach for preparing ozone gas sensors based on hierarchical NiCo2O4 structures. RSC Adv. 2016, 6, 92655–92662. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Silva, L.F.D.; Jadhav, H.S.; Shimizu, F.M.; Suman, P.H.; M’Peko, J.C.; Orlandi, M.O.; Seo, J.G.; Mastelaro, V.R.; Oliveira, O.N., Jr. Yolk-shelled ZnCo2O4 microspheres: Surface properties and gas sensing application. Sens. Actuators B 2018, 257, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, H.R.; Hashemi, B.; Mirzaei, A.; Roshan, H.; Sheikhi, M.H. Effect of Ag on the ZnO nanoparticles properties as an ethanol vapor sensor. Mater. Sci. Semicond. Process. 2020, 117, 105172. [Google Scholar] [CrossRef]
- Manasa, M.V.; Sarala Devi, G.; Prasada Reddy, P.S.; Sreedhar, B. High performance CO2 gas sensor based on noble metal functionalized semiconductor nanomaterials for health and environmental safety. Mater. Res. Express. 2019, 6, 125041. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: A case study on the {221} high-index faceted SnO2 octahedra. Cryst. Eng. Comm. 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Kim, J.H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly Selective Sensing of CO, C6H6, and C7H8 Gases by Catalytic Functionalization with Metal Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Song, S.; Wang, P.; Zhao, Q.; Chuai, M.; Zhang, M. Promoting effects of Ag on In2O3 nanospheres of sub-ppb NO2 detection. Sens. Actuators B 2017, 241, 489–497. [Google Scholar] [CrossRef]
- Han, S.D.; Moon, H.G.; Noh, M.-S.; Pyeon, J.J.; Shim, Y.-S.; Nahm, S.; Kim, J.-S.; Yo, K.S.; Kang, C.-Y. Self-doped nanocolumnar vanadium oxides thin films for highly selective NO2 gas sensing at low temperature. Sens. Actuators B 2017, 241, 40–47. [Google Scholar] [CrossRef]
- Bayata, F.; Saruhan-Brings, B.; Urgen, M. Hydrogen gas sensing properties of nanoporous Al-doped titania. Sens. Actuators B 2014, 204, 109–118. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Extremely sensitive and selective sub-ppm CO detection by the synergistic effect of Au nanoparticles and core–shell nanowires. Sens. Actuators B 2017, 249, 177–188. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Kim, J.-S. Recent advances on H2 sensor technologies based on MOX and FET devices: A review. Sens. Actuators B 2018, 262, 758–770. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Enhancement of CO and NO2 sensing in n-SnO2-p-Cu2O core-shell nanofibers by shell optimization. J. Hazard. Mater. 2019, 376, 68–82. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Adv. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on alpha-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [PubMed]
- Liu, Z.; Yamazaki, T.; Shen, Y.; Kikuta, T.; Nakatani, N.; Li, Y. O2 and CO sensing of Ga2O3 multiple nanowire gas sensors. Sens. Actuators B 2008, 129, 666–670. [Google Scholar] [CrossRef]
- Lim, Y.T.; Son, J.Y.; Rhee, J.S. Vertical ZnO nanorod array as an effective hydrogen gas sensor. Ceram. Int. 2013, 39, 887–890. [Google Scholar] [CrossRef]
- Zhang, J.-j.; Guo, E.-j.; Wang, L.-p.; Yue, H.-y.; Cao, G.-j.; Song, L. Effect of annealing treatment on morphologies and gas sensing properties of ZnO nanorods. Trans. Nonferrous Met. Soc. 2014, 24, 736–742. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Wang, W.; Li, Z.; Zhang, H.; Wang, Y.; Wang, Z.; Wang, C. A highly sensitive and fast-responding sensor based on electrospun In2O3 nanofibers. Sens. Actuators B 2009, 142, 61–65. [Google Scholar] [CrossRef]
- Jia, X.; Fan, H.; Yang, W. Hydrothermal Synthesis and Primary Gas Sensing Properties of CuO Nanosheets. J. Dispers. Sci. Technol. 2010, 31, 866–869. [Google Scholar] [CrossRef]
- Song, L.; Lukianov, A.; Butenko, D.; Li, H.; Zhang, J.; Feng, M.; Liu, L.; Chen, D.; Klyui, N.I. Facile Synthesis of Hierarchical Tin Oxide Nanoflowers with Ultra-High Methanol Gas Sensing at Low Working Temperature. Nanoscale Res. Lett. 2019, 14, 84. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Ko, H.; Jin, C.; Lee, C. Synthesis of nanograined ZnO nanowires and their enhanced gas sensing properties. ACS Appl. Mater. Interfaces 2012, 4, 3650–3656. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, S.-J.; Hsueh, T.-J. A low-temperature ZnO nanowire ethanol gas sensor prepared on plastic substrate. Mater. Res. Express. 2016, 3, 095002. [Google Scholar] [CrossRef]
- Jung, S.H.; Oh, E.; Lee, K.H.; Park, W.; Jeong, S.H. A Sonochemical Method for Fabricating Aligned ZnO Nanorods. Adv. Mater. 2007, 19, 749–753. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 286, 229–235. [Google Scholar] [CrossRef]
- Choi, P.G.; Izu, N.; Shirahata, N.; Masuda, Y. Fabrication and H2-Sensing Properties of SnO2 Nanosheet Gas Sensors. ACS Omega 2018, 3, 14592–14596. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Fu, M.; Zhai, C.; Wang, Z. Hydrothermal synthesis and gas-sensing properties of ultrathin hexagonal ZnO nanosheets. Ceram. Int. 2014, 40, 2295–2298. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, L.; Deng, Z.; Liao, J.H. Electrically Transduced Gas Sensors Based on Semiconducting Metal Oxide Nanowires. Sensors 2020, 20, 6781. [Google Scholar] [CrossRef]
- Xie, F.; Li, W.H.; Zhang, Q.Y.; Zhang, S.P. Highly Sensitive and Selective CO/NO/H2/NO2 Gas Sensors Using Noble Metal (Pt, Pd) Decorated MOx (M = Sn, W) Combined with SiO2 Membrane. IEEE Sens. J. 2019, 19, 10674–10679. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yange, M.J.; Liang, K.Y.; Turak, A.; Zhang, B.X.; Meng, D.; Wang, C.X.; Qu, F.D.; Cheng, W.L.; Yang, M.H. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Improving the hydrogen sensing properties of SnO2 nanowire-based conduc-tometric sensors by Pd-decoration. Sens. Actuators B 2019, 285, 358–367. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Effect of Noble Metal Addition on Co3O4-Based Gas Sensors for Selective NO Detection. Sens. Mater. 2016, 28, 1191–1201. [Google Scholar]
- Zhou, Q.; Hong, C.X.; Li, Z.G.; Peng, S.D.; Wu, G.L.; Wang, Q.; Zhang, Q.Y.; Xu, L.N. Facile Hydrothermal Synthesis and Enhanced Methane Sensing Properties of Pt-Decorated ZnO Nanosheets. J. Nanosci. Nanotechol. 2018, 18, 3335–3340. [Google Scholar] [CrossRef]
- Ma, J.H.; Ren, Y.; Zhou, X.R.; Liu, L.L.; Zhu, Y.H.; Cheng, X.W.; Xu, P.C.; Li, X.X.; Deng, Y.H.; Zhao, D.Y. Pt Nanoparticles Sensitized Ordered Mesoporous WO3 Semiconductor: Gas Sensing Performance and Mechanism Study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar]
- Li, Y.; Hua, Z.Q.; Wu, Y.; Zeng, Y.; Qiu, Z.L.; Tian, X.M.; Wang, M.J. Surface Modification of Pt-loaded WO3 Nanosheets for Acetone Sensing Application. Chem. Lett. 2018, 47, 167–170. [Google Scholar] [CrossRef]
- Liu, L.; Song, P.; Wei, Q.; Zhong, X.; Yang, Z.X.; Wang, Q. Synthesis of porous SnO2 hexagon nanosheets loaded with Au na-noparticles for high performance gas sensors. Mater. Lett. 2017, 201, 211–215. [Google Scholar] [CrossRef]
- Liang, J.R.; Zhu, K.L.; Yang, R.; Hu, M. Room temperature NO2 sensing properties of Au-decorated vanadium oxide nanowires sensor. Ceram. Int. 2018, 44, 2261–2268. [Google Scholar] [CrossRef]
- Xue, D.P.; Zhang, Z.Y.; Wang, Y. Enhanced methane sensing performance of SnO2 nanoflowers based sensors decorated with Au nanoparticles. Mater. Chem. Phys. 2019, 237, 121864. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.D.; Yi, G.Y.; Zhou, L.X.; Cao, J.L.; Sun, G.; Chen, Z.H.; Bala, H.; Zhang, Z.Y. Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol-sensing properties. Mater. Sci. Semicond. Process. 2018, 75, 327–333. [Google Scholar] [CrossRef]
- Kamble, C.; Panse, M.; Nimbalkar, A. Ag decorated WO3 sensor for the detection of sub-ppm level NO2 concentration in air. Mater. Sci. Semicond. Process. 2019, 103, 104613. [Google Scholar] [CrossRef]
- Wang, Y.L.; Cui, X.B.A.; Yang, Q.Y.; Liu, J.; Gao, Y.; Sun, P.; Lu, G.Y. Preparation of Ag-loaded mesoporous WO3 and its en-hanced NO2 sensing performance. Sens. Actuators B 2016, 255, 544–552. [Google Scholar] [CrossRef]
- Tan, Y.; Lei, Y. Atomic layer deposition of Rh nanoparticles on WO3 thin film for CH4 gas sensing with enhanced detection characteristics. Ceram. Int. 2020, 46, 9936–9942. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wang, Y.Y.; Zhu, X.G.; Li, Y.; Cai, W.P. Bilayer Au nanoparticle-decorated WO3 porous thin films: On-chip fabrication and enhanced NO2 gas sensing performances with high selectivity. Sens. Actuators B 2019, 280, 192–200. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Zhang, J.J.; Meng, F.L.; Li, Y.; Li, R.; Chang, Y.L.; Zhao, J.P.; Han, E.C.; Wang, S.Y. Highly Sensitive Ammonia Sensors Based on Ag-Decorated WO3 Nanorods. IEEE Trans. Nanotechnol. 2018, 17, 1252–1258. [Google Scholar] [CrossRef]
- Meng, F.L.; Zheng, H.X.; Chang, Y.L.; Zhao, Y.; Li, M.Q.; Wang, C.; Sun, Y.F.; Liu, J.H. One-Step Synthesis of Au/SnO2/RGO Nanocomposites and Their VOC Sensing Properties. IEEE Trans. Nanotechnol. 2018, 17, 212–219. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Lee, S.; Lee, C. Acetone Gas-sensing Properties of Multiple-networked Pd-decorated Bi2O3 Nanorod Sensors. Bull. Korean Chem. Soc. 2015, 36, 468–472. [Google Scholar]
- Na, C.W.; Woo, H.S.; Kim, I.D.; Lee, J.H. Selective detection of NO2, C2H5OH using a Co3O4-decorated ZnO nanowire network sensor. Chem. Commun. 2011, 47, 5148–5150. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Kim, K.M.; Kwon, Y.J.; Choi, H.; Park, J.K.; Jeong, Y.K. ALD-assisted synthesis of V2O5 nanoislands on SnO2 nanowires for improving NO2 sensing performance. Appl. Surf. Sci. 2020, 509, 144821. [Google Scholar] [CrossRef]
- Jian, Y.Y.; Hu, W.W.; Zhao, Z.H.; Cheng, P.F.; Haick, H.; Yao, M.S.; Wu, W.W. Gas Sensors Based on Chemi Resistive Hybrid Functional Nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.L.; Hua, Z.Q.; Li, Y.; Wang, M.J.; Huang, D.; Tian, C.; Zhang, C.S.; Tian, X.M.; Li, E.P. Acetone Sensing Properties and Mechanism of Rh-Loaded WO3 Nanosheets. Front. Chem. 2018, 6, 385. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Wang, Y.H.; Ghanshyam, C.; Kapur, P. Fast response time alcohol gas sensor using nanocrystalline F-doped SnO2 films derived via sol-gel method. Bull. Mater. Sci. 2013, 36, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.M.; Fan, X.H.; Cao, L.; Qi, L.J.; Yan, W. Gas sensing enhancement of aluminum-doped ZnO nanovase structure with many gas facile diffusivity paths. Appl. Surf. Sci. 2013, 265, 108–113. [Google Scholar]
- Du, W.; Si, W.; Du, W.; Ouyang, T.; Wang, F.; Gao, M.; Wu, L.; Liu, J.; Qian, Z.; Liu, W. Unraveling the promoted nitrogen dioxide detection performance of N-doped SnO2 microspheres at low temperature. J. Alloy. Compd. 2020, 834, 155209. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Jayababu, N.; Poloju, M.; Ramana Reddy, M.V. Facile synthesis of SnO2-Fe2O3 core-shell nanostructures and their 2-methoxyethanol gas sensing characteristics. J. Alloy. Compd. 2019, 780, 523–533. [Google Scholar] [CrossRef]
- Yang, J.-H.; Yuan, K.-P.; Zhu, L.-Y.; Hang, C.-Z.; Li, X.-X.; Tao, J.-J.; Ma, H.-P.; Jiang, A.-Q.; Lu, H.-L. Facile synthesis of α-Fe2O3/ZnO core-shell nanowires for enhanced H2S sensing. Sens. Actuators B 2020, 307, 127617. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B 2020, 302, 127150. [Google Scholar] [CrossRef]
- Yang, X.; Fu, H.; Zhang, L.; An, X.; Xiong, S.; Jiang, X.; Yu, A. Enhanced gas sensing performance based on the fabrication of polycrystalline Ag@TiO2 core-shell nanowires. Sens. Actuators B 2019, 286, 483–492. [Google Scholar] [CrossRef]
- Sun, G.-J.; Kheel, H.; Choi, S.; Hyun, S.K.; Lee, C. Prominent Gas Sensing Performance of TiO2-Core/NiO-Shell Nanorod Sensors. J. Nanosci. Nanotechol. 2017, 17, 4099–4102. [Google Scholar] [CrossRef]
- Park, B.G.; Reddeppa, M.; Kim, Y.H.; Kim, S.G.; Kim, M.D. Hydrogenation-produced In2O3/InN core-shell nanorod and its effect on NO2 gas sensing behavior. Nanotechnology 2020, 31, 335503. [Google Scholar] [CrossRef]
- Bonyani, M.; Lee, J.K.; Sun, G.-J.; Lee, S.; Ko, T.; Lee, C. Benzene sensing properties and sensing mechanism of Pd-decorated Bi2O3-core/ZnO-shell nanorods. Thin Solid Film. 2017, 636, 257–266. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Huang, B.Y.; Zhang, Z.X.; Zhao, C.H.; Cairang, L.M.; Bai, J.L.; Zhang, Y.X.; Mu, X.M.; Du, J.W.; Wang, H.; Pan, X.J.; et al. Enhanced gas-sensing performance of ZnO@In2O3 core@shell nanofibers prepared by coaxial electrospinning. Sens. Actuators B 2018, 255, 2248–2257. [Google Scholar] [CrossRef]
- Li, F.; Zhang, T.; Gao, X.; Wang, R.; Li, B. Coaxial electrospinning heterojunction SnO2/Au-doped In2O3 core-shell nanofibers for acetone gas sensor. Sens. Actuators B 2017, 252, 822–830. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Sun, G.J.; Kim, H.W.; Kim, S.S. Mechanism and prominent enhancement of sensing ability to reducing gases in p/n core-shell nanofiber. Nanotechnology 2014, 25, 175501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of Au-decorated WS2 nanosheets as low power-consumption and selective gas sensors. Sens. Actuators B 2019, 296, 126659. [Google Scholar] [CrossRef]
- Yin, M.; Wang, F.; Fan, H.; Xu, L.; Liu, S. Heterojunction CuO@ZnO microcubes for superior p-type gas sensor application. J. Alloy. Compd. 2016, 672, 374–379. [Google Scholar] [CrossRef]
- Song, H.; Luo, Z.; Liu, M.; Zhang, G.; Peng, W.; Wang, B.; Zhu, Y. Centrifugal Deposited Au-Pd Core-Shell Nanoparticle Film for Room-Temperature Optical Detection of Hydrogen Gas. Sensors 2018, 18, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Liu, J.; Liu, Q.; Chen, R.; Zhang, H.; Yu, J.; Song, D.; Li, J.; Zhang, M.; Wang, J. Core-shell structure of ZnO/Co3O4 composites derived from bimetallic-organic frameworks with superior sensing performance for ethanol gas. Appl. Surf. Sci. 2019, 475, 700–709. [Google Scholar] [CrossRef]
- Diao, Q.; Yin, Y.; Zhang, X.; Li, J.; Jiao, M.; Cao, J.; Qin, Q.; Yang, K.; Zhu, G.; Xu, X. Fabrication of ZnO@CeO2 core–shell hetero-structural nanofibers and enhanced gas sensing performance for acetone. Funct. Mater. Lett. 2020, 13, 2050013. [Google Scholar] [CrossRef]
- Diao, K.; Xiao, J.; Zheng, Z.; Cui, X. Enhanced sensing performance and mechanism of CuO nanoparticle-loaded ZnO nanowires: Comparison with ZnO-CuO core-shell nanowires. Appl. Surf. Sci. 2018, 459, 630–638. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.; Qiao, X.; Li, K.; Xiong, Y.; Li, X.; Guo, T.; Zhu, L.; Xue, Q. Metal-organic frameworks derived ZnO@MoS2 nanosheets core/shell heterojunctions for ppb-level acetone detection: Ultra-fast response and recovery. Sens. Actuators B 2020, 304, 127430. [Google Scholar] [CrossRef]
- Chang, X.; Qiao, X.; Li, K.; Wang, P.; Xiong, Y.; Li, X.; Xia, F.; Xue, Q. UV assisted ppb-level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature. Sens. Actuators B 2020, 317, 128208. [Google Scholar] [CrossRef]
- Majhi, S.M.; Naik, G.K.; Lee, H.-J.; Song, H.-G.; Lee, C.-R.; Lee, I.-H.; Yu, Y.-T. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhong, X.; Han, C.; Zhao, Q.; Wei, D. Design of Au@WO3 core−shell structured nanospheres for ppb-level NO2 sensing. Sens. Actuators B 2019, 282, 917–926. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Wang, Y.S.; Bhat, K.S.; Mahmoudi, T.; Yu, Y.T.; Suh, E.K.; Hahn, Y.B. Improved selectivity and low concentration hydrogen gas sensor application of Pd sensitized heterojunction n-ZnO/p-NiO nanostructures. J. Alloy. Compd. 2019, 797, 456–464. [Google Scholar] [CrossRef]
- Raza, M.H.; Kaur, N.; Comini, E.; Pinna, N. Toward Optimized Radial Modulation of the Space-Charge Region in One-Dimensional SnO2-NiO Core-Shell Nanowires for Hydrogen Sensing. ACS Appl. Mater. Interfaces 2020, 12, 4594–4606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, F.; Liu, J.; Wang, Y.; Zhou, J.; Ruan, S. Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B 2015, 209, 515–523. [Google Scholar] [CrossRef]
- Yuan, K.P.; Zhu, L.Y.; Yang, J.H.; Hang, C.Z.; Tao, J.J.; Ma, H.P.; Jiang, A.Q.; Zhang, D.W.; Lu, H.L. Precise preparation of WO3@SnO2 core shell nanosheets for efficient NH3 gas sensing. J. Colloid Interf. Sci. 2020, 568, 81–88. [Google Scholar] [CrossRef]
- Kim, W.; Baek, M.; Yong, K. Fabrication of ZnO/CdS, ZnO/CdO core/shell nanorod arrays and investigation of their ethanol gas sensing properties. Sens. Actuators B 2016, 223, 599–605. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Yuan, K.; Yang, J.-G.; Ma, H.-P.; Wang, T.; Ji, X.-M.; Feng, J.-J.; Devi, A.; Lu, H.-L. Fabrication of heterostructured p-CuO/n-SnO2 core-shell nanowires for enhanced sensitive and selective formaldehyde detection. Sens. Actuators B 2019, 290, 233–241. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.; Barsan, N. Design of Core-Shell Heterostructure Nanofibers with Different Work Function and Their Sensing Properties to Trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Z.; Wang, R.; Liu, S.; Fei, T.; Wang, L.; Zhang, T. Core–shell Co3O4/α-Fe2O3 heterostructure nanofibers with enhanced gas sensing properties. RSC Adv. 2015, 5, 36340–36346. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; He, Y.; Zhao, B.; Ju, X.; Chen, W.; Lu, B.; Li, H.; Li, Y.; Liu, L.; et al. Gas sensors based on pearl-necklace-shaped In2O3 nanotubes with highly enhanced formaldehyde-sensing performance. J. Mater. Sci. Mater. Electron. 2019, 30, 18362–18373. [Google Scholar] [CrossRef]
- Peng, X.; Santulli, A.C.; Sutter, E.; Wong, S.S. Fabrication and enhanced photocatalytic activity of inorganic core–shell nanofibers produced by coaxial electrospinning. Chem. Sci. 2012, 3, 1262–1272. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 nanosheet composites: Hydrothermal synthesis and gas sensing mechanism. J. Alloy. Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Yu, Y.-T.; Majhi, S.M.; Song, H.-G. Synthesis and Gas Sensing Properties of Au@In2O3 Core-shell Nanoparticles. Proc. Eng. 2016, 168, 227–230. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Hu, W.; Zhou, Q.; He, K.; Xu, K.; Yang, Y.; Yu, T.; Yuan, C. Synthesis and gas-sensing properties of ZnO@NiCo2O4 core@shell nanofibers. Mater. Res. Bull. 2019, 114, 1–9. [Google Scholar] [CrossRef]
- Majhi, S.M.; Lee, H.-J.; Choi, H.-N.; Cho, H.-Y.; Kim, J.-S.; Lee, C.-R.; Yu, Y.-T. Construction of novel hybrid PdO–ZnO p–n heterojunction nanostructures as a high-response sensor for acetaldehyde gas. Cryst. Eng. Comm. 2019, 21, 5084–5094. [Google Scholar] [CrossRef]
- Xu, K.; Duan, S.; Tang, Q.; Zhu, Q.; Zhao, W.; Yu, X.; Yang, Y.; Yu, T.; Yuan, C. P-N heterointerface-determined acetone sensing characteristics of α-MoO3@NiO core@shell nanobelts. Cryst. Eng. Comm. 2019, 21, 5834–5844. [Google Scholar] [CrossRef]
- Jayababu, N.; Poloju, M.; Shruthi, J.; Reddy, M.V.R. Ultrasensitive resistivity-based ethanol sensor based on the use of CeO2-Fe2O3 core-shell microclusters. Mikrochim. Acta 2019, 186, 712. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Wang, D.; Wang, F.; Li, H.; Xu, J.; Wang, X.; Yang, J. Hierarchical In2O3@SnO2 Core-Shell Nanofiber for High Efficiency Formaldehyde Detection. ACS Appl. Mater. Interfaces 2019, 11, 45214–45225. [Google Scholar] [CrossRef]
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing: A review. Sens. Actuators B 2019, 295, 127–143. [Google Scholar] [CrossRef]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Zhang, Z.C.; Xu, Q.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Near Room Temperature, Fast-Response, and Highly Sensitive Triethylamine Sensor Assembled with Au-Loaded ZnO/SnO2 Core-Shell Nanorods on Flat Alumina Substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wu, X.; Zhou, X.; Li, X.; Han, N.; Chen, Y. High acetone sensitive and reversible P- to N-type switching NO2 sensing properties of Pt@Ga-ZnO core-shell nanoparticles. Sens. Actuators B 2019, 289, 114–123. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Bang, J.H.; Kim, H.W.; Kim, S.S. Selective H2S sensing without external heat by a synergy effect in self-heated CuO-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2019, 300, 126981. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3 and SnO2-based ozone sensors: Design and characterization. Crit. Rev. Sol. St. Mater. Sci. 2017, 43, 83–132. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Umar, A.; Wang, Y.; Li, H.; Zhou, G. Three-Dimensional Crumpled Graphene-Based Nanosheets with Ultrahigh NO2 Gas Sensibility. ACS Appl. Mater. Interfaces 2017, 9, 11819–11827. [Google Scholar] [CrossRef]
- Karaduman, I.; Er, E.; Çelikkan, H.; Erk, N.; Acar, S. Room-temperature ammonia gas sensor based on reduced graphene oxide nanocomposites decorated by Ag, Au and Pt nanoparticles. J. Alloy. Compd. 2017, 722, 569–578. [Google Scholar] [CrossRef]
- Lu, X.; Song, X.; Gu, C.; Ren, H.; Sun, Y.; Huang, J. Freeze drying-assisted synthesis of Pt@reduced graphene oxide nanocomposites as excellent hydrogen sensor. J. Phys. Chem. Solid. 2018, 116, 324–330. [Google Scholar] [CrossRef]
- Lupan, O.; Schutt, F.; Postica, V.; Smazna, D.; Mishra, Y.K.; Adelung, R. Sensing performances of pure and hybridized carbon nanotubes-ZnO nanowire networks: A detailed study. Sci. Rep. 2017, 7, 14715. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, X.; Cheng, H.; Liu, Z.; Zhao, L.; Yang, T.; Dai, Z.; Cheng, Z.; Shi, E.; Yang, L.; et al. Graphene Reinforced Carbon Nanotube Networks for Wearable Strain Sensors. Adv. Funct. Mater. 2016, 26, 2078–2084. [Google Scholar] [CrossRef]
- Duy, N.V.; Hoa, N.D.; Dat, N.T.; Le, D.T.T.; Hieu, N.V. Ammonia-Gas-Sensing Characteristics of WO3/Carbon Nanotubes Nanocomposites: Effect of Nanotube Content and Sensing Mechanism. Sci. Adv. Mater. 2016, 8, 524–533. [Google Scholar]
- Wang, P.; Wang, D.; Zhang, M.; Zhu, Y.; Xu, Y.; Ma, X.; Wang, X. ZnO nanosheets/graphene oxide nanocomposites for highly effective acetone vapor detection. Sens. Actuators B 2016, 230, 477–484. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J.; Gaskov, A.M. Reduced graphene oxide (rGO) encapsulated Co3O4 composite nanofibers for highly selective ammonia sensors. Sens. Actuators B 2016, 222, 864–870. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Xie, T.; Yuan, Z.; Liu, C.; Jiang, Y. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B 2016, 223, 149–156. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Bai, S.; Sun, X.; Han, N.; Shu, X.; Pan, J.; Guo, H.; Liu, S.; Feng, Y.; Luo, R.; Li, D.; et al. rGO modified nanoplate-assembled ZnO/CdO junction for detection of NO2. J. Hazard. Mater. 2020, 394, 121832. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, B.; Wang, Y.; Gao, Y.; Liang, X.; Wang, Y.; Lu, G. Flower-like In2O3 modified by reduced graphene oxide sheets serving as a highly sensitive gas sensor for trace NO2 detection. J. Colloid Interf. Sci. 2017, 504, 206–213. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, T.; Tan, Q.; Guo, Q.; Lu, F.; Xiong, J. A Room-Temperature CNT/Fe3O4 Based Passive Wireless Gas Sensor. Sensors 2018, 18, 3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutt, F.; Postica, V.; Adelung, R.; Lupan, O. Single and Networked ZnO-CNT Hybrid Tetrapods for Selective Room-Temperature High-Performance Ammonia Sensors. Acs Appl. Mater. Interfaces 2017, 9, 23107–23118. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Nagaraju, P. Synthesis and characterization of ZnO-MWCNT nanocomposites for 1-butanol sensing application at room temperature. Phys. B 2019, 570, 139–147. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.H.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Funct. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Wang, L.L.; Huang, H.; Xiao, S.H.; Cai, D.P.; Liu, Y.; Liu, B.; Wang, D.D.; Wang, C.X.; Li, H.; Wang, Y.R.; et al. Enhanced sensitivity and stability of room-temperature NH3 sensors using core-shell CeO2 nanoparticles@cross-linked PANI with p-n heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 14131–14140. [Google Scholar] [CrossRef]
- Jiang, T.T.; Wang, Z.J.; Li, Z.Y.; Wang, W.; Xu, X.R.; Liu, X.C.; Wang, J.F.; Wang, C. Synergic effect within n-type inorganic–p-type organic nano-hybrids in gas sensors. J. Mater. Chem. C 2013, 1, 3017–3025. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.T.; Ma, Y.J.; Liu, Y.; Xu, S.S.; Chen, X.W.; Zeng, M.; Hu, N.T.; Su, Y.J.; Zhou, Z.H.; Yang, Z. Construction of MoS2/SnO2 heterostructures for sensitive NO2 detection at room temperature. Appl. Sur. Sci. 2019, 493, 613–619. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Bang, J.H.; Kim, Y.W.; Kim, S.S. Achievement of self-heated sensing of hazardous gases by WS2 (core)–SnO2 (shell) nanosheets. J. Hazard. Mater. 2021, 412, 125196. [Google Scholar] [CrossRef]
- Abun, A.; Huang, B.R.; Saravanan, A.; Kathiravan, D.; Hong, P.D. Exfoliated MoSe2 Nanosheets Doped on the Surface of ZnO Nanorods for Hydrogen Sensing Applications. Acs Appl. Nano Mater. 2020, 3, 12139–12147. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Han, E.C.; Meng, F.L.; Zuo, K.Y. Detection and Identification of Volatile Organic Compounds Based on Temperature-Modulated ZnO Sensors. IEEE Trans. Instrum. Meas. 2020, 69, 4533–4544. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Liu, Y.; Zhang, J.J.; Meng, F.L.; Zhang, H. Rose-Like MoO₃/MoS₂/rGO Low-Temperature Ammonia Sensors Based on Multigas Detection Methods. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar]
- Krivetskiy, V.V.; Andreev, M.D.; Efitorov, A.O.; Gaskov, A.M. Statistical shape analysis pre-processing of temperature modulated metal oxide gas sensor response for machine learning improved selectivity of gases detection in real atmospheric conditions. Sens. Actuators B 2021, 329, 129187. [Google Scholar] [CrossRef]
- Hwang, W.J.; Shin, K.S.; Roh, J.H.; Lee, D.S.; Choa, S.H. Development of Micro-Heaters with Optimized Temperature Compensation Design for Gas Sensors. Sensors 2011, 11, 2580–2591. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.S.; Kim, B.J.; Lee, H.J.; Choi, J.W.; Kim, S.D.; Min, N.K. Study on the micro-heater geometry in In2O3 micro electro mechanical systems gas sensor platforms and effects on NO2 gas detecting performances. J. Nanosci. Nanotechnol. 2012, 12, 1170–1173. [Google Scholar] [CrossRef]
- Moon, S.E.; Lee, H.K.; Choi, N.J.; Lee, J.; Yang, W.S.; Kim, J.; Jong, J.J.; Yoo, D.J. Low-power-Consumption metal oxide NO2 gas sensor based on micro-heater and screen printing technology. J. Nanosci. Nanotechnol. 2012, 12, 5543–5546. [Google Scholar] [CrossRef] [PubMed]
- Rajput, G.Y.; Gofane, M.S.; Dhobale, S. Design of Micro-heater on 3D-SnO2 Gas Sensor, Computing. Commun. Signal. Process. 2019, 621–629. [Google Scholar]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-functionalized core-shell composite nanowires for self-heating, sensitive, and benzene-selective gas sensors. Sens. Actuators A 2020, 308, 112011. [Google Scholar] [CrossRef]
- Zhu, L.F.; She, J.C.; Luo, J.Y.; Deng, S.Z.; Chen, J.; Ji, X.W.; Xu, N.S. Self-heated hydrogen gas sensors based on Pt-coated W18O49 nanowire networks with high sensitivity, good selectivity and low power consumption. Sens. Actuators B 2011, 153, 354–360. [Google Scholar] [CrossRef]
- Tan, H.M.; Hung, C.M.; Ngoc, T.M.; Nguyen, H.; Hoa, D.; Van Duy, N.; Van Hieu, N. Novel Self-Heated Gas Sensors Using on-Chip Networked Nanowires with Ultralow Power Consumption. ACS Appl. Mater. Interfaces 2017, 9, 6153–6162. [Google Scholar] [CrossRef]
- Sharma, S.; Madou, M. A new approach to gas sensing with Nanotechnology. Phil. Trans. R. Soc. A 2012, 370, 2448–2473. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mat. Sci. 2016, 42, 416–432. [Google Scholar] [CrossRef]
- Karaduman, I.; Yıldız, D.E.; Sincar, M.M.; Acar, S. UV light activated gas sensor for NO2 detection. Mater. Sci. Semicond. Process. 2014, 28, 43–47. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Trawka, M.; Smulko, J.; Hasse, L.; Granqvist, C.-G.; Annanouch, F.E.; Ionescu, R. Fluctuation enhanced gas sensing with WO3-based nanoparticle gas sensors modulated by UV light at selected wavelengths. Sens. Actuators B 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light activation of nanocrystalline metal oxides for gas sensing: Principles, achievements, challenges. Nanomaterials 2021, 11, 892. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.C.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]




| Noble Metal Catalysts | Technique | Content | Material Structure | Operation Temperature (°C) | Target Gas | Gas Concentration (ppm) | Response (Ra/Rg) | Reference |
|---|---|---|---|---|---|---|---|---|
| Pd | Decorating | 0.5 mol% | SnO2 Films | 150 | NO | 0.5 | 542.8 | [48] |
| Decorating | / | SnO2 Nanowires | 300 | H2 | 1 | 8.02 | [50] | |
| Loading | 10 wt% | Co3O4 Membranes | 150 | H2 | 100 | 2.95 | [51] | |
| Loading | / | Fe2O3 Nanocubes | 139 | Acetone | 100 | 25.7 | [49] | |
| Pt | Decorating | 6 % | ZnO Nanosheets | 240 | CH4 | 50 | 63.45 | [52] |
| Decorating | 0.5 mol% | SnO2 Films | 300 | CO | 150 | 406.2 | [48] | |
| Loading | 0.5 wt% | WO3 Mesoporous | 125 | CO | 100 | 10 ± 1 | [53] | |
| Loading | 2 wt% | WO3 Nanosheets | 300 | Acetone | 1.5 | 5.1 | [54] | |
| Au | Decorating | 4 wt% | SnO2 Nanosheets | 260 | Ethanol | 100 | 70.2 | [55] |
| Decorating | 10% | VO2 Nanowires | 25 | NO2 | 5 | 3.22 | [56] | |
| Decorating | 1.5 wt% | SnO2 Nanoflowers | 120 | CH4 | 100 | 4.973 | [57] | |
| Ag | Decorating | 1 wt% | ZnO Nanorods | 360 | Ethanol | 50 | 21.5 | [58] |
| Decorating | 0.5% | WO3 Films | 200 | NO2 | 3 | 12.22 | [59] | |
| Loading | 0.5% | WO3 Mesoporous | 75 | NO2 | 1 | 44 | [60] | |
| Rh | Decorating | 2 deposition cycles | WO3 Films | 350 | CH4 | 5 | 63.1 | [61] |
| Semiconductor Type | Majority Carrier | Target Gas | Conductivity Performance |
|---|---|---|---|
| n-type | Free Electron | Oxidizing Gas | Reduce |
| Reducing Gas | Increase | ||
| p-type | Hole | Oxidizing Gas | Increase |
| Reducing Gas | Reduce |
| C-S Heterostructure | Shell Deposition Technique | Nanostructure | Operation Temperature (°C) | Target Gas | Gas Concentration (ppm) | Response (Ra/Rg) | Reference |
|---|---|---|---|---|---|---|---|
| CuO-SnO2 | ALD | Nanowires | 250 | HCHO | 50 | 2.42 | [101] |
| SnO2-NiO | ALD | Nanowires | 500 | H2 | 500 | 114 | [97] |
| α-Fe2O3-ZnO | ALD | Nanowires | 250 | H2S | 5 | 5.98 | [76] |
| WO3-SnO2 | ALD | Nanosheets | 200 | NH3 | 15 | 1.55 | [99] |
| Ag-TiO2 | Sol–gel | Nanowires | 240 | NH3 | 100 | 9 | [78] |
| SnO2-Fe2O3 | Sol–gel | Nanoparticles | Room Temperature | 2-methoxyethanol | 100 | 2080 | [75] |
| Au-In2O3 | Hydrothermal | Nanoparticles | 300 | H2 | 100 | 34.4 | [107] |
| TiO2-NiO | Hydrothermal | Nanorods | 400 | Acetone | 200 | 9.81 | [79] |
| WO3-SnO2 | Hydrothermal | Nanosheets | 260 | Acetone | 50 | 32.1 | [106] |
| In2O3-SnO2 | Coaxial Electrospinning | Nanofibers | 280 | TMA | 10 | 7.11 | [102] |
| ZnO@In2O3 | Coaxial Electrospinning | Nanofibers | 225 | Ethanol | 100 | 31.87 | [83] |
| Co3O4-α-Fe2O3 | Coaxial Electrospinning | Nanofibers | 240 | Acetone | 50 | 11.7 | [103] |
| ZnO-CeO2 | Coaxial Electrospinning | Nanofibers | 370 | Acetone | 1 | 8.2 | [90] |
| Heterojunction Type | Main Carrier | Target Gas | Main Depletion Layer Types | Layer Thickness |
|---|---|---|---|---|
| p–n Type | Free Electrons and Holes | Oxidizing Gas | Electron Depletion Layer | Increase |
| Hole Depletion Layer | Reduce | |||
| Reducing Gas | Electron Depletion Layer | Reduce | ||
| Hole Depletion Layer | Increase | |||
| n–n Type | Free Electrons | Oxidizing Gas | Electron Depletion Layer | Increase |
| Reducing Gas | Reduce | |||
| p–p Type | Holes | Oxidizing Gas | Hole Depletion Layer | Reduce |
| Reducing Gas | Increase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, S.; Cao, S.; Huang, Z.; Yang, D.; Zhang, G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials 2021, 14, 4263. https://doi.org/10.3390/ma14154263
Xue S, Cao S, Huang Z, Yang D, Zhang G. Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials. 2021; 14(15):4263. https://doi.org/10.3390/ma14154263
Chicago/Turabian StyleXue, Shirui, Sicheng Cao, Zhaoling Huang, Daoguo Yang, and Guoqi Zhang. 2021. "Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review" Materials 14, no. 15: 4263. https://doi.org/10.3390/ma14154263
APA StyleXue, S., Cao, S., Huang, Z., Yang, D., & Zhang, G. (2021). Improving Gas-Sensing Performance Based on MOS Nanomaterials: A Review. Materials, 14(15), 4263. https://doi.org/10.3390/ma14154263