Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Test Methods
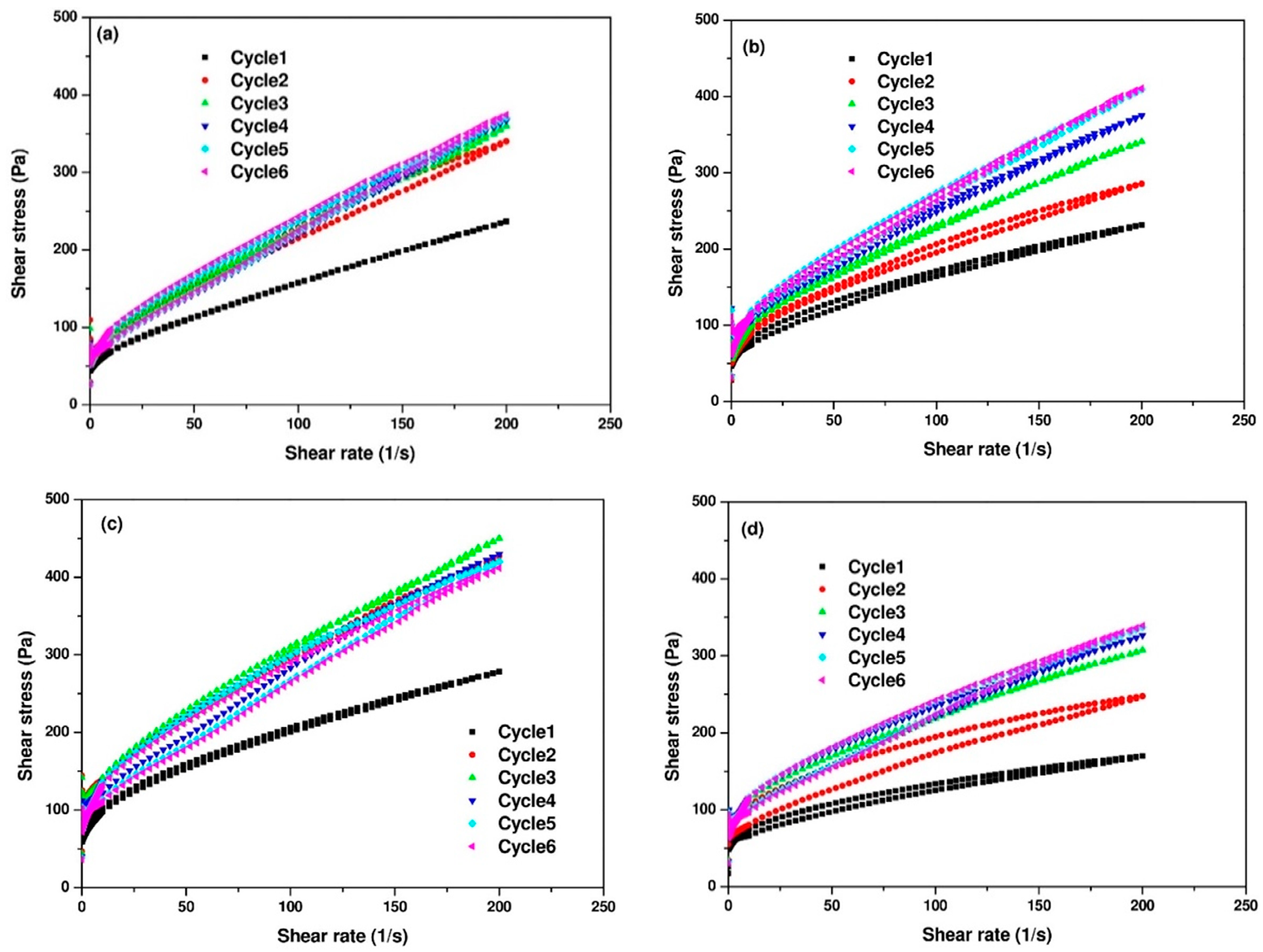
- Rheological tests: The flow curves of the pastes were obtained by the shear protocol presented in Figure 3 for determining viscosity and yield stress. The rheological parameters were determined by considering the descending part of the curves. The downward curve of all mixtures followed a modified Bingham model (Equation (1)) and was used to determine the dynamic yield stress and plastic viscosity of the mixtures.where, is the shear stress in Pa, is the yield stress in Pa, is the plastic viscosity in Pa·s and is the shear rate in 1/s, is the second order term (Pa·s2). All rheological tests were carried out on three fresh samples.
- Setting times: The initial and the final setting times were determined by an automatic Vicat apparatus according to EN196-3:2005 [16] on paste samples. The initial setting time was determined by the elapsed time from the first contact of the slag particles with the alkaline activator to the time at which the distance between the needle and the base-plate was (6 ± 3) mm. The final setting time was determined by the time at which the needle can penetrate only 0.5 mm from the surface.
- Mechanical properties: The compressive and flexural strength of the mixtures were measured on mortar samples. Standard CEN sand was used as aggregate, and an aggregate to binder ratio of three (by mass) was kept constant for all mortar mixtures. The fresh mortar specimens were wrapped in plastic foil and stored in the moisture room for 24 h at 20 °C and 90% RH. The specimens were stored in the same environmental conditions after demolding until the mechanical tests at 2, 7, and 28 days. The compressive strength of the specimens was determined on the two broken portions of prisms after the flexural test according to EN 1015-11 [17]. At least three samples for each mixture were tested to achieve reproducibility.
- Mercury intrusion porosimetry (MIP): At the testing ages (2, 7, and 28 d), the paste samples were crushed into small pieces with dimensions of around 1 cm3, and then the small pieces of the samples were immersed in isopropanol for at least one week to stop the reaction of the slag and dried in a 40 °C oven for 1 h. Then the dried samples were stored in a low vacuum desiccator before analysis. A Pascal 440 mercury porosimeter with a maximum load capacity of 420 MPa was used in the MIP test. However, the maximum pressure was limited to 200 MPa in order to avoid cracks induced by the mercury pressure [18]. The adopted mercury surface tension and contact angle between the mercury and the solid surface were 0.482 N/m and 142°, respectively.
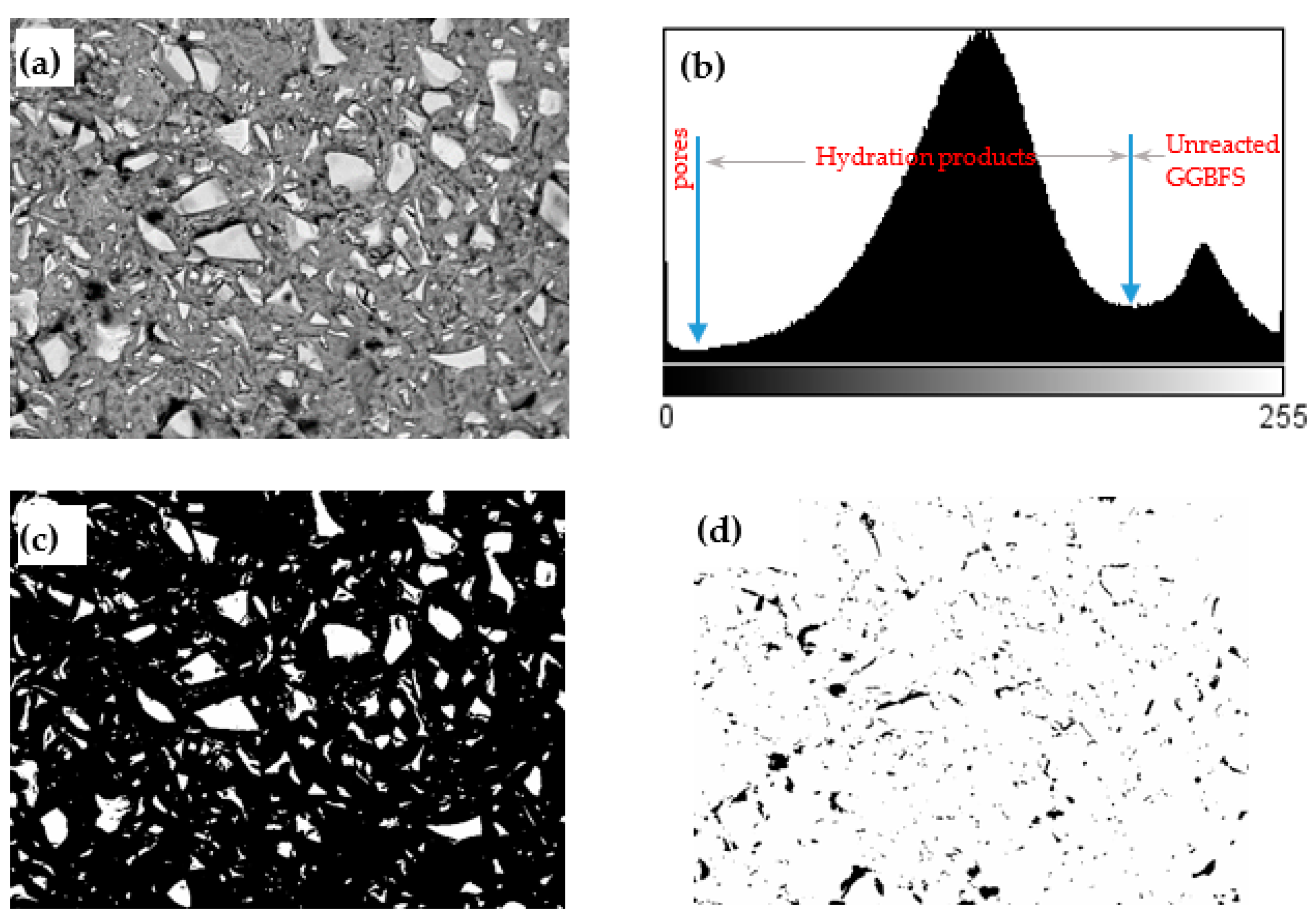
- Scanning electron microscopy (SEM) and image analysis (IA): Paste samples at the age of 28 days were immersed into epoxy and then polished up to 0.25 μm surface fineness using diamond paste. Afterward, the polished samples were observed by an SEM in backscattering electron (BSE) mode at an acceleration voltage of 15.0 kV under low vacuum. The magnification of each image was 500×. A representative BSE image and the analytical procedure for calculating the reaction degree from BSE images are illustrated in Figure 4. The discrimination between hydrated/anhydrous regions using the original BSE image was not easy, since no distinctive peaks according to gray level are observable from the gray-level histogram. The Bilateral filter available in the ImageJ software (https://imagej.nih.gov/ij/ accessed on 20 January 2021) was used to make the BSE images smoother and keep the particle edges in BSE images. The gray-scale histogram (Figure 4b) was obtained from the cropped/filtered image. As can be seen from Figure 4b, there were two distinct regions allowing the determination of the thresholds for quantifying the areas corresponding to unreacted GGBFS and hydrated phases, as well as cracks or pores. By applying an appropriate threshold value (Figure 4b), the areas corresponding to the unreacted GGBFS was obtained as shown in Figure 4c. The first principle of stereology (or the Delesse Principe [19]), states that a determination of the area fraction of a phase in a random section, is an unbiased estimator of the volume fraction of this phase. As such, the degree of reaction of GGBFS can be estimated as follows:where is the degree of reaction of GGBFS at age t, and are the volume fraction of unreacted slag relative to the total volume of mixture at given curing time and at the initial state, respectively, and and are the area fraction of unreacted slag relative to the total cross section of mixture at a given curing time and at the initial state, respectively, as obtained by the BSE image analysis. The initial volume fraction of the GGBFS was calculated as 45.16% and 45.35% for Mix 1 and 4, respectively. It has been reported that an image analysis based on 12 or more BSE images can enable a 95% degree of confidence [20]. Consequently, a total number of 20 images on randomly selected locations for each sample were used in image analysis.
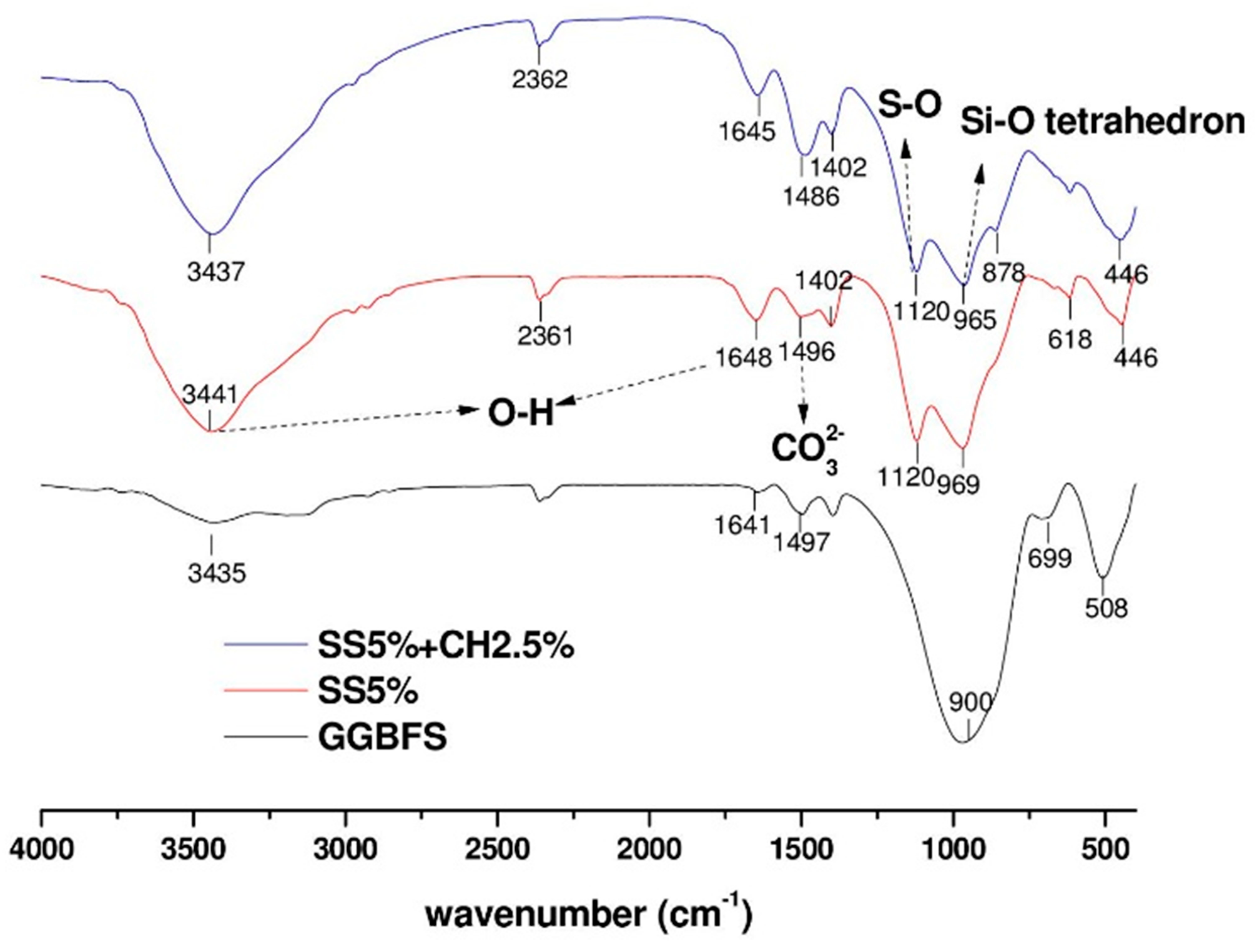
- Fourier transform infrared (FTIR) spectroscopy: The samples were taken from the hardened paste samples at an age of 28 days. Following the RILEM TC-238 [21] methodology, after crushing the hardened pieces to a size of 125 μm to 1 mm, 3 g of the powder was mixed with 100 mL isopropanol for 15 min. The suspension was filtered and rinsed with isopropanol and diethyl ether before drying at 40 °C for 8 min. Then the dried samples were stored in a low vacuum desiccator prior to analysis. KBr pellets were prepared by mixing 1 mg of sample and 100 mg of KBr. The FTIR tests were conducted on a Perkin Elmer spectrum BX FT-IR system, in the frequency range of 400–4000 cm−1 with a 4 cm−1 resolution.
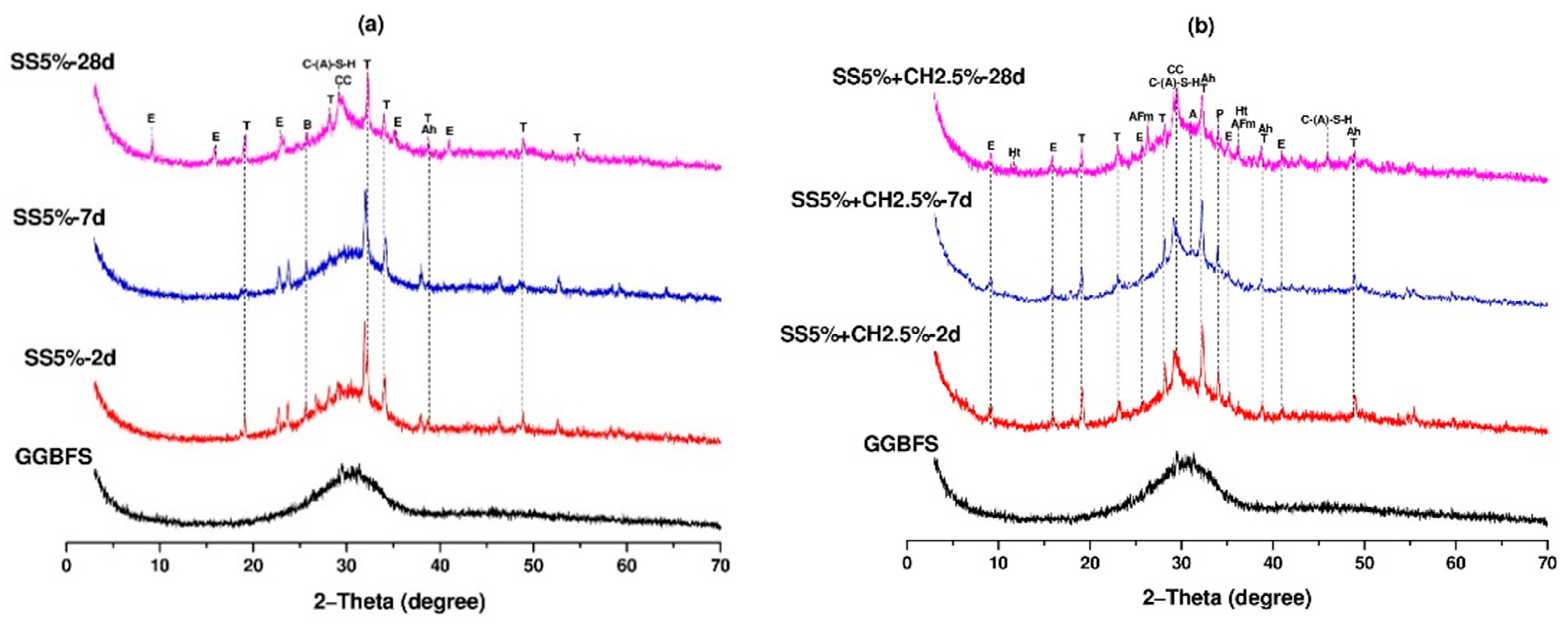
- X-ray diffraction (XRD): The sample preparation procedure was the same as those for FTIR. The XRD measurements were conducted on a Rigaku D/Max-2200/PC X-ray diffractometer with CuKα radiation (λ = 0.1542 nm) at 40 kV and 36 mA, scanning from 5° to 70° 2θ with a 0.02° step size.
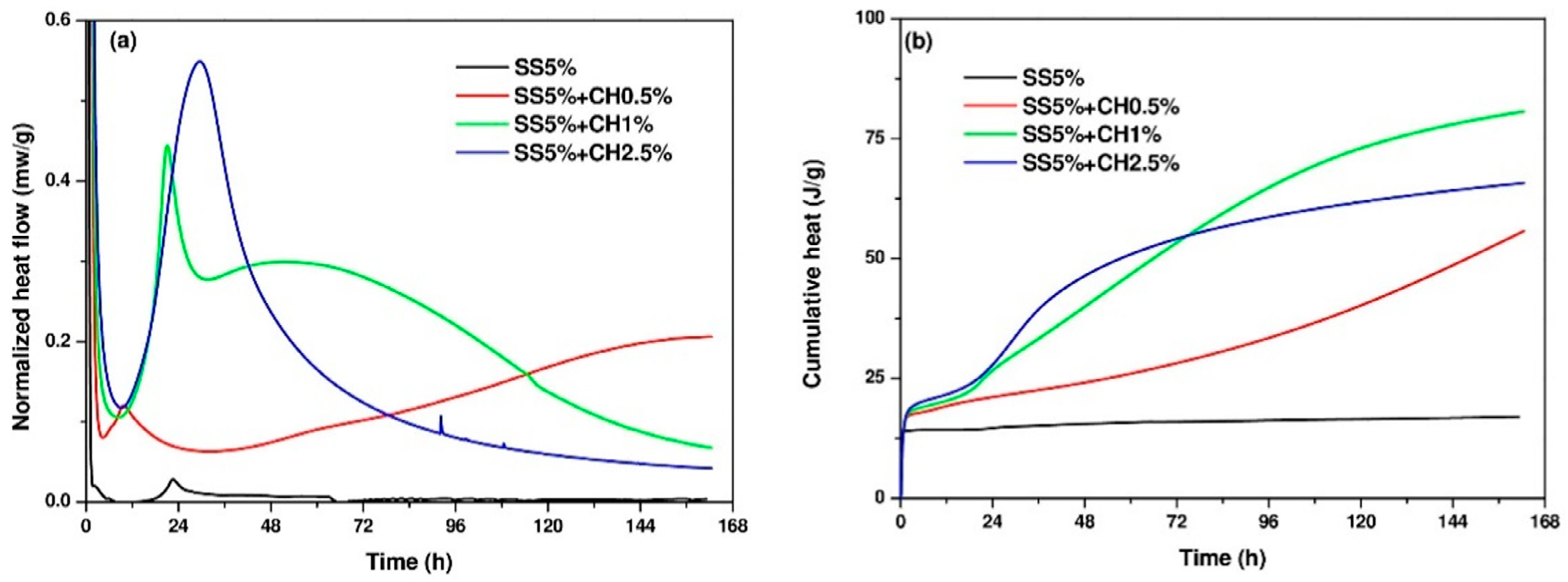
- Isothermal calorimetry: The heat of the hydration of the pastes was measured using a TAM air calorimeter with eight channels. Immediately after mixing the activator solution with GGBFS for 3 min, 14 g of paste sample was poured into a glass ampoule bottle and then the sealed bottle was placed into the calorimeter. The calorimetric measurements were performed at 20 ± 0.02 °C for 7 d.
3. Results and Discussions
3.1. Isothermal Calorimetry of AAC Pastes
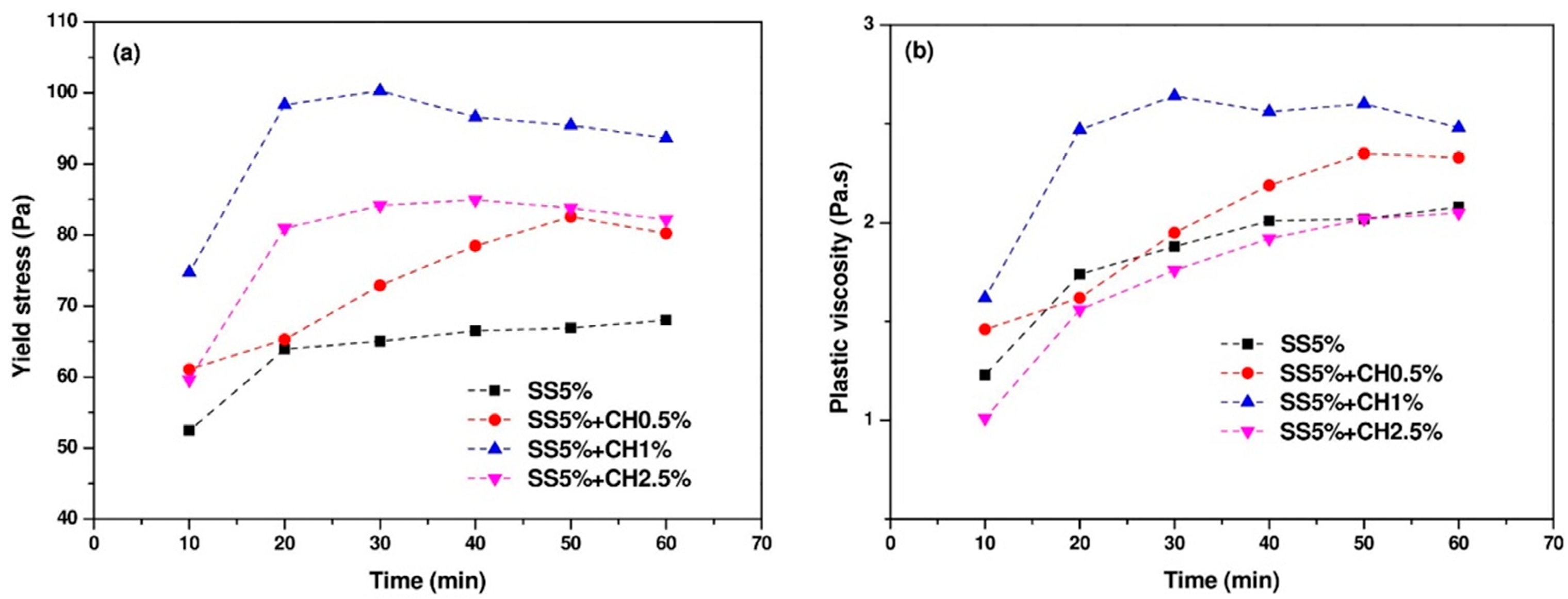
3.2. Flow Curves of AAC Pastes
3.3. Initial and Final Setting Times of AAC Pastes
3.4. Compressive and Flexural Strength of AAC Mortars
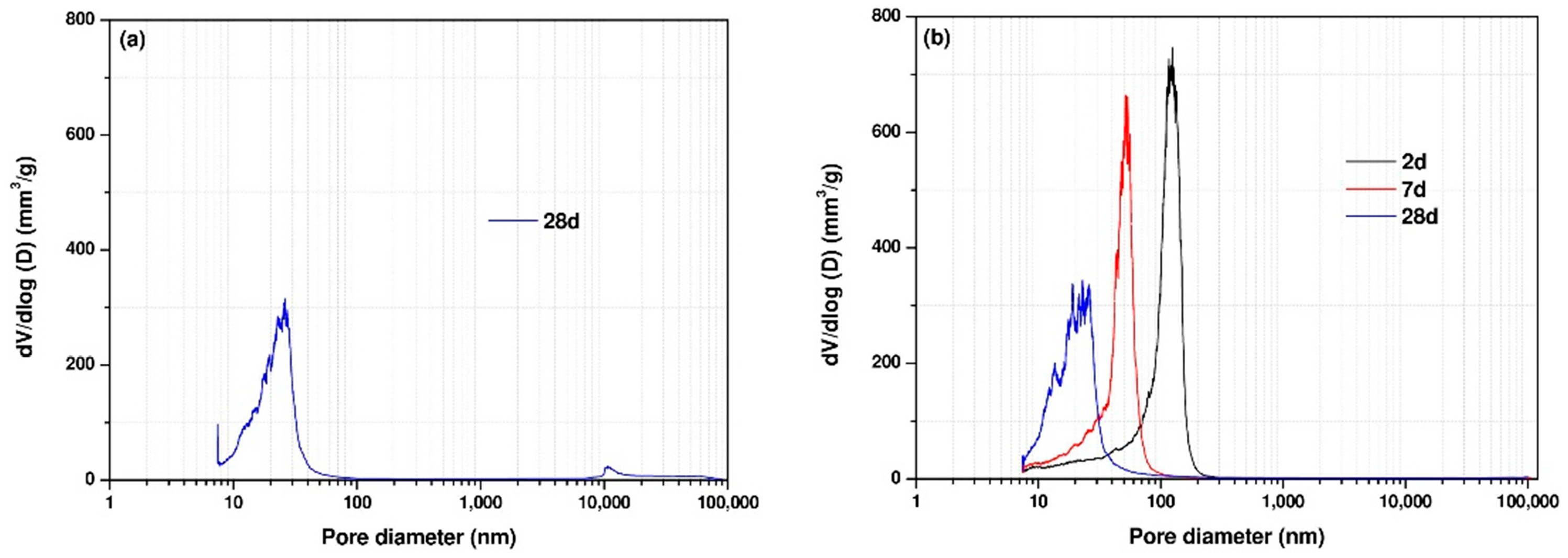
3.5. Pore Structure of AAC Pastes
3.6. Morphology of AAC Pastes
3.7. FTIR and XRD Analyses of AAC Pastes
4. Conclusions
- The AAC mixture activated by the sole sodium sulfate activator solution could not develop strength until the age of 7 days. Early age strength development of sodium sulfate activated slag mixtures could be improved by Ca(OH)2 addition to the activator solution.
- The very long setting times of the sole sodium sulfate activated AAC mixture were significantly shortened by the addition of Ca(OH)2. The yield stress and plastic viscosity could also be significantly improved by the addition of an appropriate amount of Ca(OH)2.
- The binary usage of Ca(OH)2 and sodium sulfate activators exhibited a higher reaction degree at the early ages compared with sole usage of sodium sulfate activator. However, the addition of Ca(OH)2 higher than 1% led to a more porous microstructure at later ages. The porosity of the mixture with the 2.5% Ca(OH)2 addition at later ages was found to be slightly higher than the mixture activated by only sodium sulfate activator.
- The FTIR and XRD analyses confirmed that the main reaction products in sodium sulfate activated AAC mixtures with or without Ca(OH)2 were C-A-S-H and ettringite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, C.; Pavel, V.; Krivenko, D.R. Alkali-Activated Cements and Concretes; CRC Press: London, UK, 2006; ISBN 0419245502. [Google Scholar]
- Habert, G.; Ouellet-Plamondon, C. Recent update on the environmental impact of geopolymers. RILEM Tech. Lett. 2016, 1, 17. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated Colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A. New cementitious materials based on alkali-activated fly ash: Performance at high temperatures. J. Am. Ceram. Soc. 2008, 91, 3308–3314. [Google Scholar] [CrossRef] [Green Version]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External sulfate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Colangelo, F.; Ciof, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C.; Ii, F. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B Eng. 2017, 129. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Mobasher, N.; Bernal, S.A.; Provis, J.L. Structural evolution of an alkali sulfate activated slag cement. J. Nucl. Mater. 2016, 468, 97–104. [Google Scholar] [CrossRef]
- Mobasher, N.; Bernal, S.A.; Hussain, O.H.; Apperley, D.C.; Kinoshita, H.; Provis, J.L. Characterisation of Ba(OH)2-Na2SO4-blast furnace slag cement-like composites for the immobilisation of sulfate bearing nuclear wastes. Cem. Concr. Res. 2014, 66, 64–74. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Collier, N.C.; Milestone, N.B. Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem. Concr. Res. 2012, 42, 333–343. [Google Scholar] [CrossRef]
- Fu, J.; Jones, A.M.; Bligh, M.W.; Holt, C.; Keyte, L.M.; Moghaddam, F.; Foster, S.J.; Waite, T.D. Mechanisms of enhancement in early hydration by sodium sulfate in a slag-cement blend—Insights from pore solution chemistry. Cem. Concr. Res. 2020, 135, 106110. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; Bao, M.; Liu, X.; Luo, Z.; Wang, P. Low carbon cementitious materials: Sodium sulfate activated ultra-fine slag/fly ash blends at ambient temperature. J. Clean. Prod. 2021, 280, 124363. [Google Scholar] [CrossRef]
- BS EN 196-3:2005 +A1:2008 Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness; British Standards Institution: London, UK, 2003; Volume 3.
- EN 1015-11: Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; European Committee for Standardization: Brussels, Belgium, 2019; ISBN 0000105058.
- Alderete, N.; Villagrán, Y.; Mignon, A.; Snoeck, D.; Belie, N. De Pore structure description of mortars containing ground granulated blast-furnace slag by mercury intrusion porosimetry and dynamic vapour sorption. Constr. Build. Mater. 2017, 145, 157–165. [Google Scholar] [CrossRef]
- Mandarim-de-lacerda, C.A. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 2003, 75, 469–486. [Google Scholar] [CrossRef]
- Lee, D.; Chen, M. An Image-Based Method for Obtaining Pore-Size Distribution of Porous Media. Environ. Sci. Technol. 2009, 43, 3248–3253. [Google Scholar]
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. RILEM TC-238 SCM Recommendation on hydration stoppage by solvent exchange for the study of hydrate assemblages. Mater. Struct. 2018, 51, 172. [Google Scholar] [CrossRef]
- Uppalapati, S.; Vandewalle, L.; Cizer, Ö. Monitoring the setting process of alkali-activated slag-fly ash cements with ultrasonic P-wave velocity. Constr. Build. Mater. 2021, 271, 121592. [Google Scholar] [CrossRef]
- Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; De Schutter, G. Effects of activator properties and GGBFS/FA ratio on the structural build-up and rheology of AAC. Cem. Concr. Res. 2020, 138, 106253. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; Schutter, G. De Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Day, R.L.; Shi, C. Influence of the fineness of pozzolan on the strength of lime natural-pozzolan cement pastes. Cem. Concr. Res. 1994, 24, 1485–1491. [Google Scholar] [CrossRef]
- Yang, K.; Cho, A.; Song, J.; Nam, S.; Al, S. Hydration products and strength development of calcium hydroxide-based alkali-activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Acceleration of strength gain of lime-pozzolan cements by thermal activation. Cem. Concr. Res. 1993, 23, 824–832. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Chemical Activation of Lime-Slag Blends. ACI Symp. Publ. 1995, 153, 1165–1178. [Google Scholar] [CrossRef]
- Eun, J.; Jun, Y.; Park, J.; Ha, J. Influence of four additional activators on hydrated-lime [Ca(OH)2] activated ground granulated blast-furnace slag. Cem. Concr. Compos. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Cook, R.A.; Hover, K.C. Mercury porosimetry of hardened cement pastes. Cem. Concr. Res. 1999, 29, 933–943. [Google Scholar] [CrossRef]
- Diamond, S. A critical comparison of mercury porosimetry and capillary condensation pore size distributions of portland cement pastes. Cem. Concr. Res. 1971, 1, 531–545. [Google Scholar] [CrossRef]
- Zuo, Y.; Ye, G. Pore Structure Characterization of Sodium Hydroxide Activated Slag Using Mercury Intrusion Porosimetry, Nitrogen Adsorption, and Image Analysis. Materials 2018, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Banthia, N.; Zhang, Y.; Zhang, Z. Interpreting the early-age reaction process of alkali-activated slag by using combined embedded ultrasonic measurement, thermal analysis, XRD, FTIR and SEM. Compos. Part B 2020, 186, 107840. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Shi, C.; Wan, S.; Li, N.; Zhang, Z. Effect of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. the activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Guimarães, D.; de A. Oliveira, V.; Leão, V.A. Kinetic and thermal decomposition of ettringite synthesized from aqueous solutions. J. Therm. Anal. Calorim. 2016, 124, 1679–1689. [Google Scholar] [CrossRef]
- Scholtzová, E.; Kucková, L.; Kožíšek, J.; Tunega, D. Structural and spectroscopic characterization of ettringite mineral-combined DFT and experimental study. J. Mol. Struct. 2015, 1100, 215–224. [Google Scholar] [CrossRef]





| Precursor | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | K2O | Na2O | Fe2O3 | MnO | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GGBFS | 40.8 | 33.3 | 12.3 | 7.84 | 2.30 | 1.29 | 0.67 | 0.44 | 0.39 | 0.36 | 0.31 |
| Mix | Mixture Notation | W/SB * | Na2O % ** | Ca(OH)2 % ** |
|---|---|---|---|---|
| 1 | SS5% *** | 0.42 | 5 | 0 |
| 2 | SS5% + CH0.5% | 0.42 | 5 | 0.5 |
| 3 | SS5% + CH1% | 0.42 | 5 | 1.0 |
| 4 | SS5% + CH2.5% | 0.42 | 5 | 2.5 |
| Mixture | Mixture Notation | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|---|
| 1 | SS5% | 2940 | 3960 |
| 2 | SS5% + CH0.5% | 402 | 1251 |
| 3 | SS5% + CH1.0% | 330 | 738 |
| 4 | SS5% + CH2.5% | 396 | 990 |
| Mixture | Mixture Notation | Flexural Strength (MPa) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|---|
| 2-Day | 7-Day | 28-Day | 2-Day | 7-Day | 28-Day | ||
| 1 | SS5% | 0 | 0 | 4.9 ± 0.4 | 0 | 0 | 26.0 ± 0.5 |
| 2 | SS5% + CH0.5% | 1.0 ± 0.4 | 5.0 ± 0.4 | 6.8 ± 0.3 | 3.7 ± 0.4 | 23.2 ± 0.6 | 45.2 ± 1.3 |
| 3 | SS5% + CH1.0% | 4.1 ± 0.2 | 7.3 ± 0.2 | 8.6 ± 0.5 | 12.3 ± 0.3 | 31.3 ± 0.8 | 45.2 ± 2.3 |
| 4 | SS5% + CH2.5% | 4.0 ± 0.1 | 6.6 ± 0.1 | 7.2 ± 0.2 | 9.7 ± 0.2 | 17.3 ± 0.2 | 23.4 ± 0.1 |
| Mixture | Mixture Notation | 2 d Porosity (%) | 7 d Porosity (%) | 28 d Porosity (%) |
|---|---|---|---|---|
| 1 | SS5% | not hardened | not hardened | 19.8 |
| 4 | SS5% + CH2.5% | 28.0 | 23.5 | 20.2 |
| Mixture | Mixture Notation | 2 d Reaction Degree (%) | 7 d Reaction Degree (%) | 28 d Reaction Degree (%) |
|---|---|---|---|---|
| 1 | SS5% | not hardened | not hardened | 61.5 ± 0.2 |
| 4 | SS5% + CH2.5% | 47.8 ± 1.2 | 52.5 ± 0.5 | 60.2 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; Schutter, G.D. Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag. Materials 2021, 14, 4266. https://doi.org/10.3390/ma14154266
Dai X, Aydın S, Yardımcı MY, Lesage K, Schutter GD. Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag. Materials. 2021; 14(15):4266. https://doi.org/10.3390/ma14154266
Chicago/Turabian StyleDai, Xiaodi, Serdar Aydın, Mert Yücel Yardımcı, Karel Lesage, and Geert De Schutter. 2021. "Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag" Materials 14, no. 15: 4266. https://doi.org/10.3390/ma14154266
APA StyleDai, X., Aydın, S., Yardımcı, M. Y., Lesage, K., & Schutter, G. D. (2021). Effect of Ca(OH)2 Addition on the Engineering Properties of Sodium Sulfate Activated Slag. Materials, 14(15), 4266. https://doi.org/10.3390/ma14154266









