Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Coating Process
2.3. Bacterial Culture
2.4. Biofilm Adhesion and Coverage
2.5. Analysis of Nanotube Surface by SEM
2.6. Cell Analyses
2.6.1. Cytotoxicity
2.6.2. Cell Attachment
3. Results
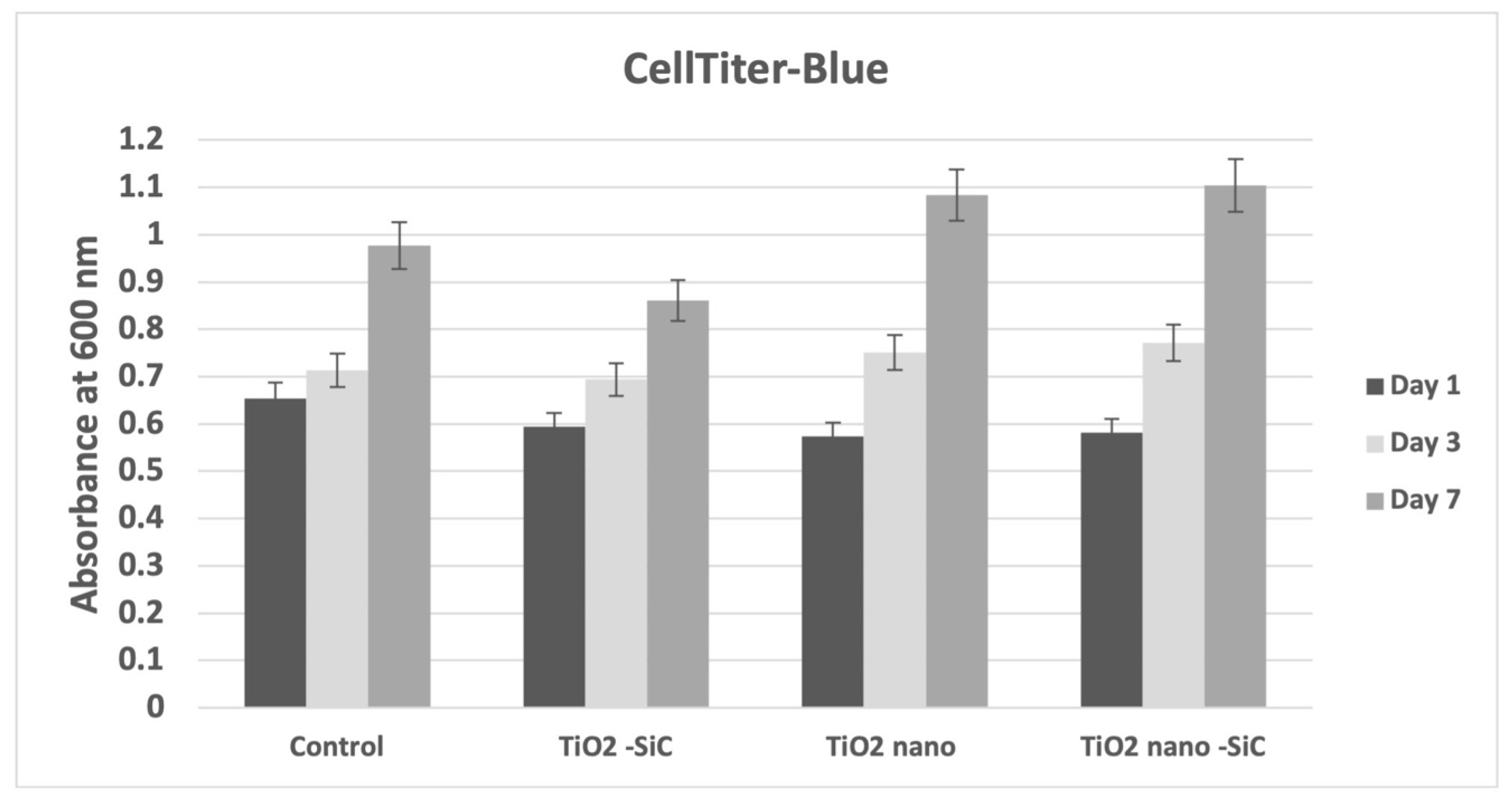
3.1. Cytotoxicity and Cell Proliferation
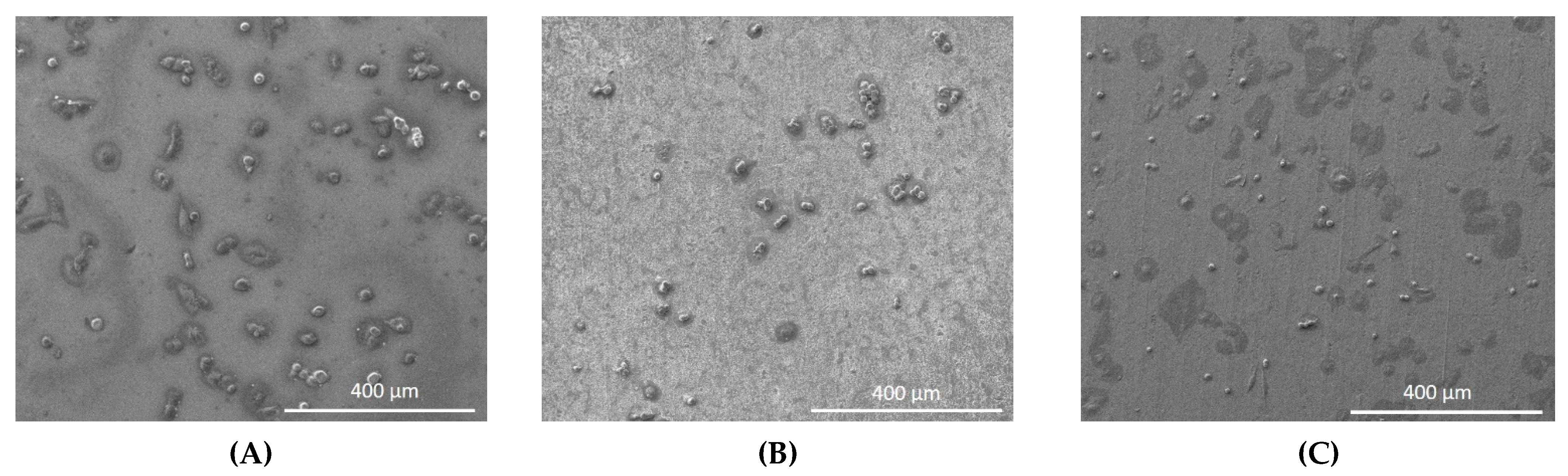
3.2. Cell Attachment
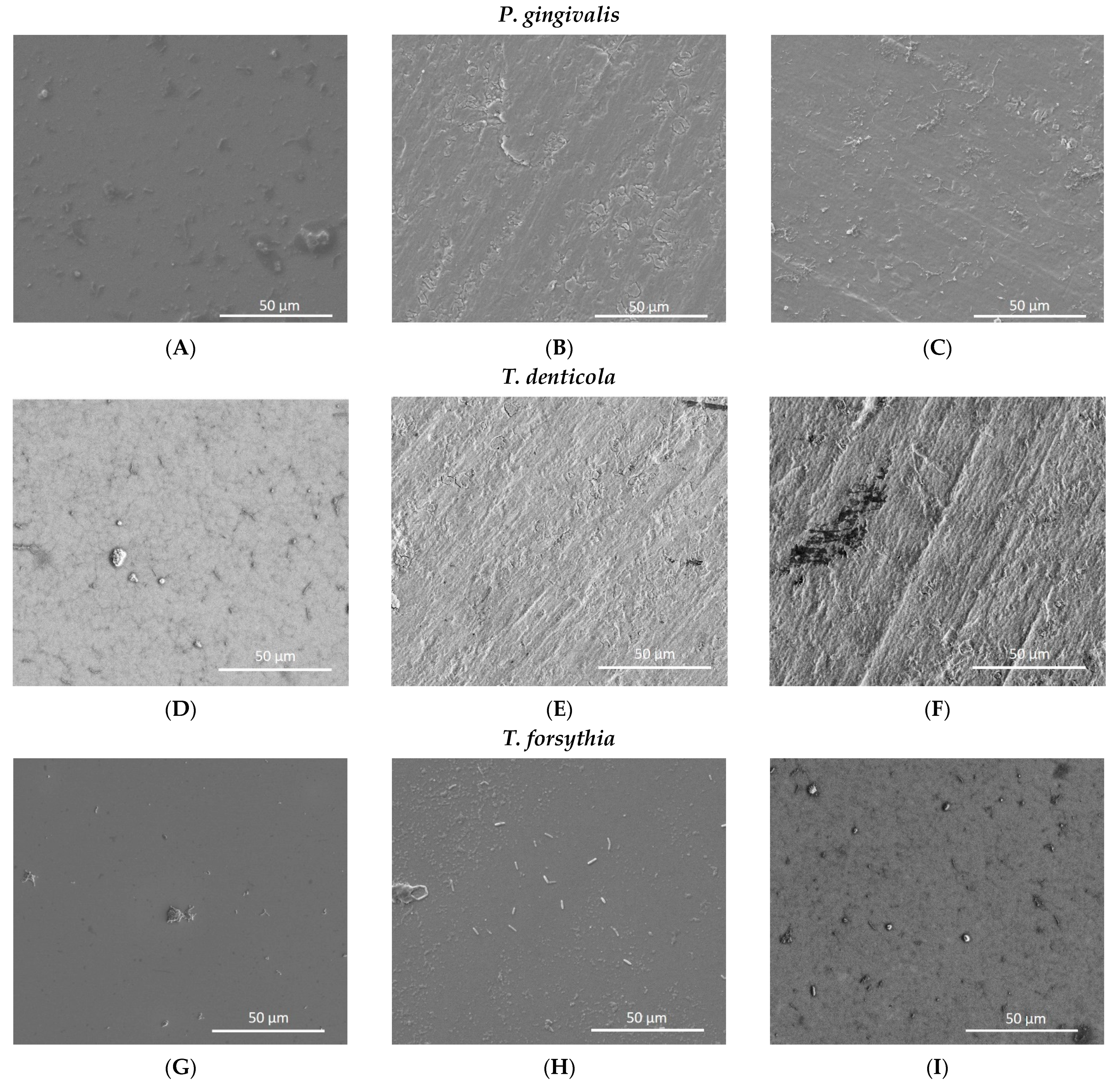
3.3. Biofilm Adhesion
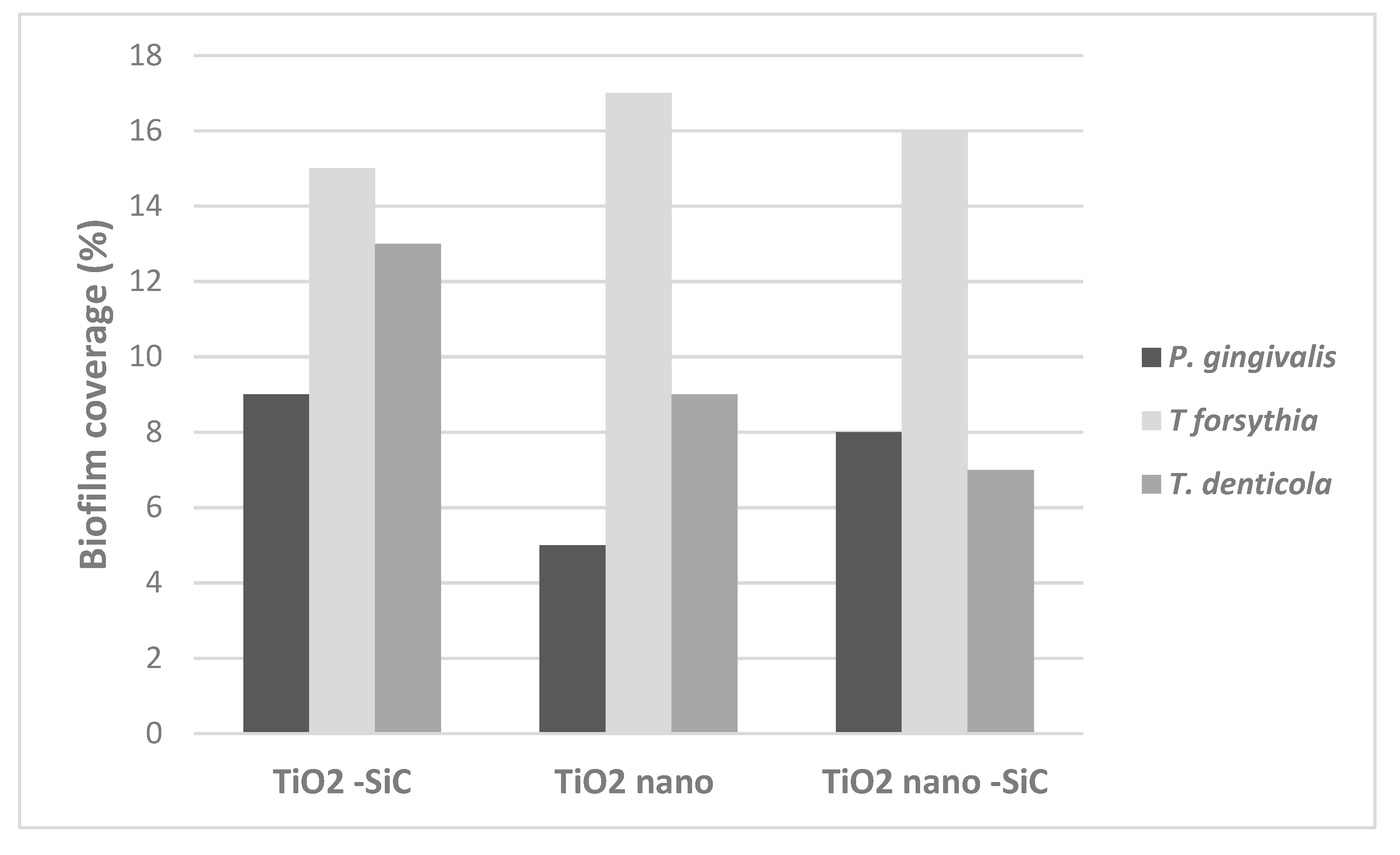
3.4. Biofilm Coverage
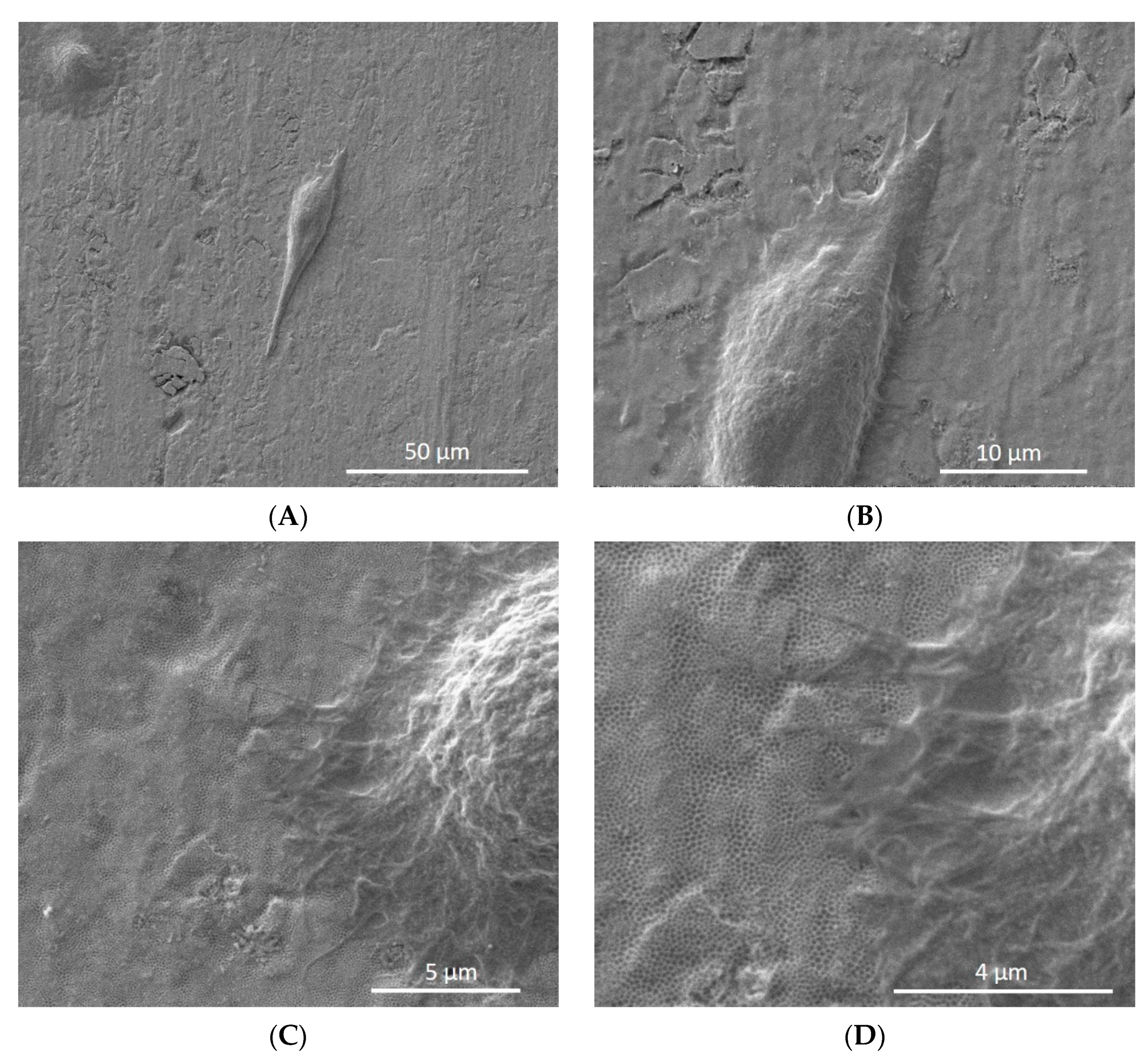
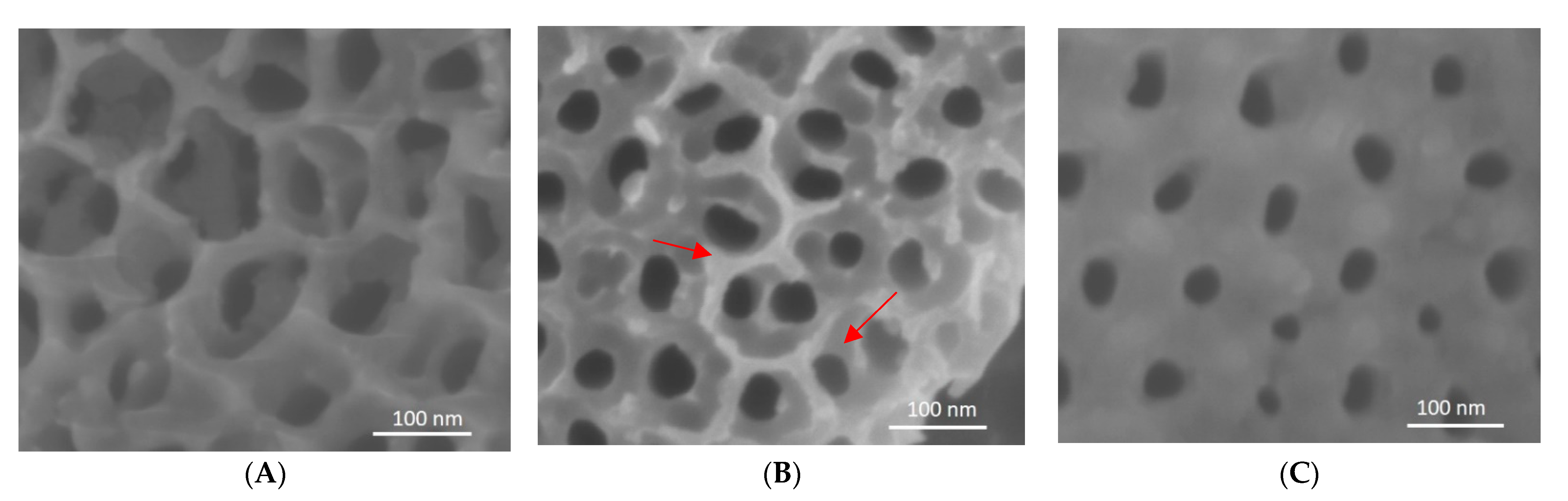
3.5. Analysis of Nanotube Surface by SEM after Bacterial Inoculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutouzis, T.; Eastman, C.; Chukkapalli, S.; Larjava, H.; Kesavalu, L. A Novel Rat Model of Polymicrobial Peri-Implantitis: A Preliminary Study. J. Periodontol. 2017, 88, e32–e41. [Google Scholar] [CrossRef]
- De Waal, Y.C.M.; Eijsbouts, H.V.L.C.; Winkel, E.; Van Winkelhoff, A. Microbial Characteristics of Peri-Implantitis: A Case-Control Study. J. Periodontol. 2017, 88, 209–217. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Roy, T.; Xia, X.; Fares, C.; Hsu, S.-M.; Ren, F.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Novel Coatings to Minimize Corrosion of Titanium in Oral Biofilm. Materials 2021, 14, 342. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Tantalum coated on titanium dioxide nanotubes by plasma spraying enhances cytocompatibility for dental implants. Surf. Coatings Technol. 2020, 382, 125161. [Google Scholar] [CrossRef]
- Capellato, P.; Riedel, N.A.; Williams, J.D.; Machado, J.P.; Popat, K.C.; Claro, A.P.R.A. Ion Bean Etching on Ti-30Ta Alloy for Biomedical Application. Mater. Sci. Forum 2014, 805, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Claro, A.P.A. Cellular Functionality on Nanotubes of Ti-30Ta Alloy. Mater. Sci. Forum 2014, 805, 61–64. [Google Scholar] [CrossRef]
- Im, S.-Y.; Kim, K.-M.; Kwon, J.-S. Antibacterial and Osteogenic Activity of Titania Nanotubes Modified with Electrospray-Deposited Tetracycline Nanoparticles. Nanomaterials 2020, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surfaces B Biointerfaces 2015, 130, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Marquez, D.; Gulati, K.; Carty, C.P.; Stewart, R.A.; Ivanovski, S. Determining the relative importance of titania nanotubes characteristics on bone implant surface performance: A quality by design study with a fuzzy approach. Mater. Sci. Eng. C 2020, 114, 110995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, J.; Say, C.; Dorit, R.L.; Queeney, K. Deconvoluting the effects of surface chemistry and nanoscale topography: Pseudomonas aeruginosa biofilm nucleation on Si-based substrates. J. Colloid Interface Sci. 2018, 519, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Wu, K.; Chen, Z.; Wang, X.; Lin, C.; Lai, Y. Progress in TiO2 nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef]
- Harada, R.; Takemoto, S.; Kinoshita, H.; Yoshinari, M.; Kawada, E. Influence of sulfide concentration on the corrosion behavior of titanium in a simulated oral environment. Mater. Sci. Eng. C 2016, 62, 268–273. [Google Scholar] [CrossRef]
- Harada, R.; Kokubu, E.; Kinoshita, H.; Yoshinari, M.; Ishihara, K.; Kawada, E.; Takemoto, S. Corrosion behavior of titanium in response to sulfides produced by Porphyromonas gingivalis. Dent. Mater. 2018, 34, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Capellato, P.; Camargo, S.E.A.; Silva, G.; Sachs, D.; Vilela, F.B.; Zavaglia, C.A.D.C.; Popat, K.C.; Claro, A.P.A. Coated Surface on Ti-30Ta Alloy for Biomedical Application: Mechanical and in-vitro Characterization. Mater. Res. 2020, 23, 20200305. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, Y.; Wu, D.; Huang, W.; Huang, S.; Zhou, L.; Chen, J. Increased Mesenchymal Stem Cell Response and DecreasedStaphylococcus aureusAdhesion on Titania Nanotubes without Pharmaceuticals. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Fares, C.; Hsu, S.-M.; Xian, M.; Xia, X.; Ren, F.; Mecholsky, J.J.J.; Gonzaga, L.; Esquivel-Upshaw, J. Demonstration of a SiC Protective Coating for Titanium Implants. Materials 2020, 13, 3321. [Google Scholar] [CrossRef]
- Kesavalu, L.; Sathishkumar, S.; Bakthavatchalu, V.; Matthews, D.; Dawson, C.; Steffen, M.; Ebersole, J.L. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 2007, 75, 1704–1712. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.F.; Lee, J.Y.; Aneja, M.; Goswami, V.; Liu, L.; Velsko, I.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Chen, H.; Lucas, A.R.; et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS ONE 2013, 8, e57178. [Google Scholar] [CrossRef] [Green Version]
- Chukkapalli, S.S.; Ambadapadi, S.; Varkoly, K.; Jiron, J.; Aguirre, J.I.; Bhattacharyya, I.; Morel, L.M.; Lucas, A.R.; Kesavalu, L.; Lakshmyya, K. Impaired innate immune signaling due to combined Toll-like receptor 2 and 4 deficiency affects both periodontitis and atherosclerosis in response to polybacterial infection. Pathog. Dis. 2018, 76, 76. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are Titania Photocatalysts and Titanium Implants Safe? Review on the Toxicity of Titanium Compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef]
- Xu, Z.; He, Y.; Zeng, X.; Zeng, X.; Huang, J.; Lin, X.; Chen, J. Enhanced Human Gingival Fibroblast Response and Reduced Porphyromonas gingivalis Adhesion with Titania Nanotubes. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-Doped Titanium Dioxide Nanotubes Promoted Bone Formation on Titanium Implants. Int. J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef] [Green Version]
- Egawa, M.; Miura, T.; Kato, T.; Saito, A.; Yoshinari, M. In vitro adherence of periodontopathic bacteria to zirconia and titanium surfaces. Dent. Mater. J. 2013, 32, 101–106. [Google Scholar] [CrossRef]
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; Van Der Mei, H.C.; Choi, C.-H. Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 12118–12129. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas gingivalis biofilm formation in different titanium surfaces, an in vitro study. Clin. Oral Implants Res. 2016, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Maminskas, J.; Pilipavicius, J.; Staisiunas, E.; Baranovas, G.; Alksne, M.; Daugela, P.; Juodžbalys, G. Novel Yttria-Stabilized Zirconium Oxide and Lithium Disilicate Coatings on Titanium Alloy Substrate for Implant Abutments and Biomedical Application. Materials 2020, 13, 2070. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Roy, T.; Iv, P.H.C.; Fares, C.; Ren, F.; Clark, A.E.; Esquivel-Upshaw, J.F. Novel Coatings to Minimize Bacterial Adhesion and Promote Osteoblast Activity for Titanium Implants. J. Funct. Biomater. 2020, 11, 42. [Google Scholar] [CrossRef]
- Azem, F.A.; Kiss, A.; Birlik, I.; Braic, V.; Luculescu, C.; Vladescu, A. The corrosion and bioactivity behavior of SiC doped hydroxyapatite for dental applications. Ceram. Int. 2014, 40, 15881–15887. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Ren, F.; Chen, Z.; Kim, M.; Fares, C.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Novel Coating to Minimize Corrosion of Glass-Ceramics for Dental Applications. Materials 2020, 13, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.-M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. https://doi.org/10.3390/ma14164357
Camargo SEA, Xia X, Fares C, Ren F, Hsu S-M, Budei D, Aravindraja C, Kesavalu L, Esquivel-Upshaw JF. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials. 2021; 14(16):4357. https://doi.org/10.3390/ma14164357
Chicago/Turabian StyleCamargo, Samira Esteves Afonso, Xinyi Xia, Chaker Fares, Fan Ren, Shu-Min Hsu, Dragos Budei, Chairmandurai Aravindraja, Lakshmyya Kesavalu, and Josephine F. Esquivel-Upshaw. 2021. "Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium" Materials 14, no. 16: 4357. https://doi.org/10.3390/ma14164357
APA StyleCamargo, S. E. A., Xia, X., Fares, C., Ren, F., Hsu, S.-M., Budei, D., Aravindraja, C., Kesavalu, L., & Esquivel-Upshaw, J. F. (2021). Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials, 14(16), 4357. https://doi.org/10.3390/ma14164357










