Influence of Intermediate Annealing Treatment on the Kinetics of Bainitic Transformation in X37CrMoV5-1 Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results
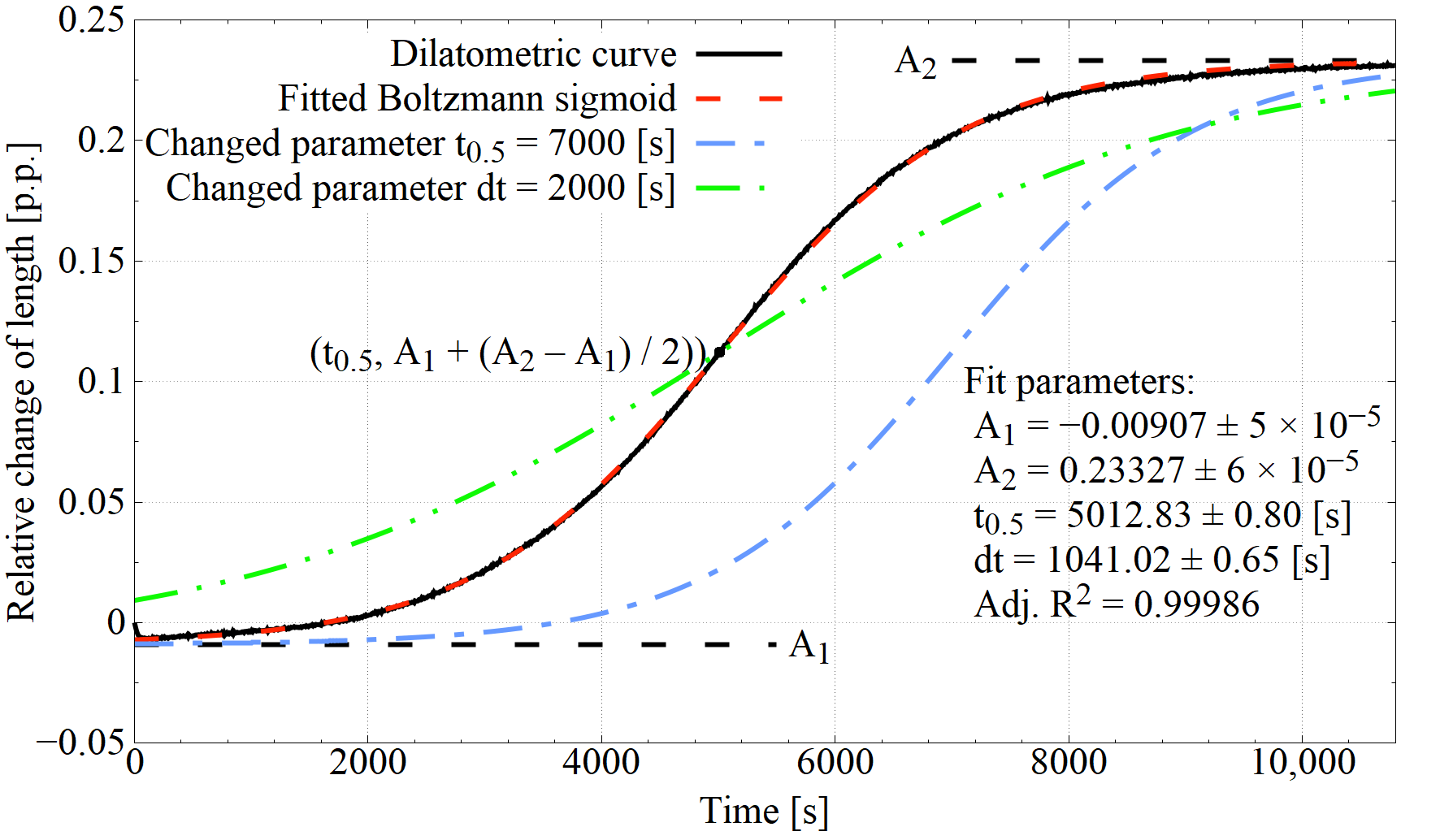
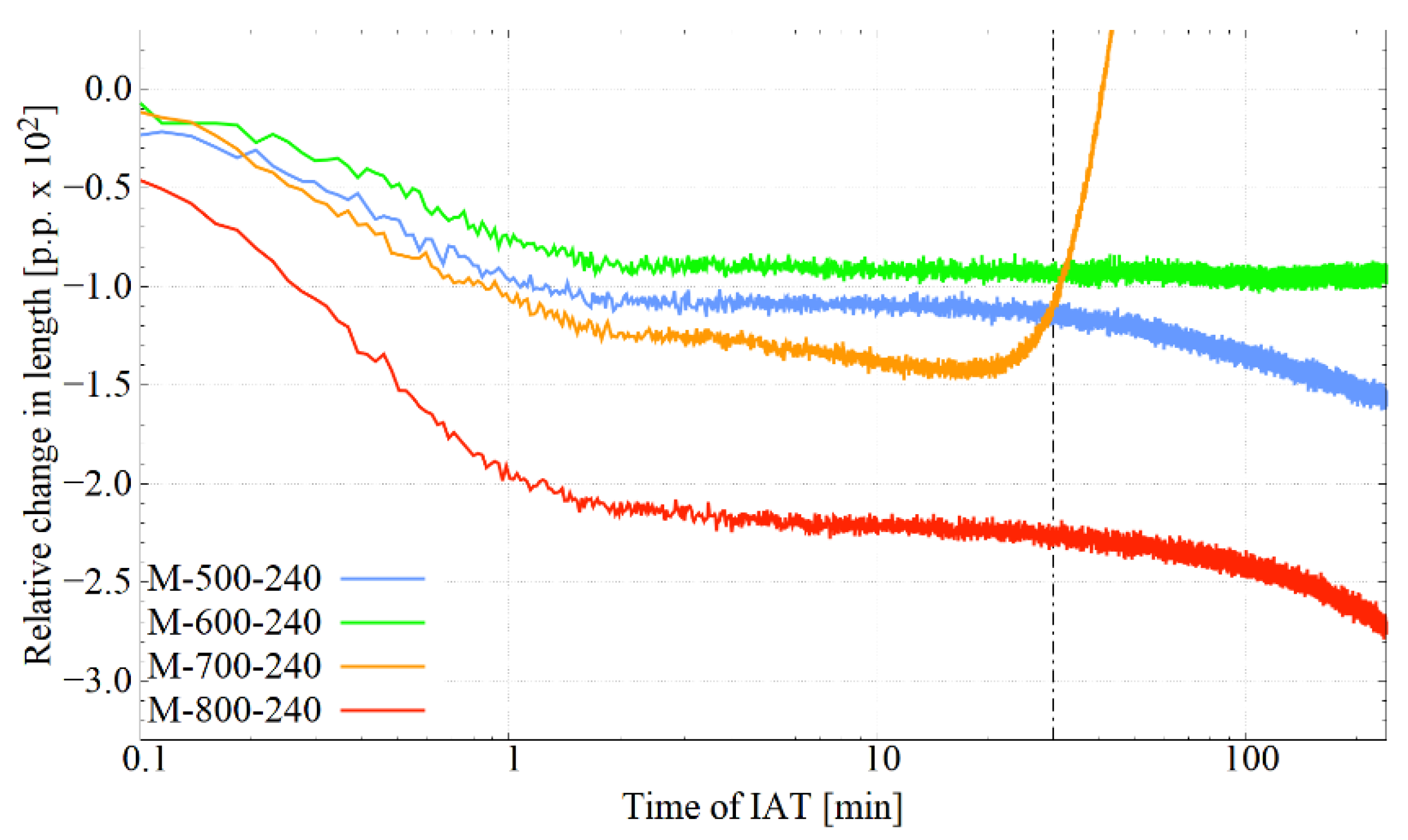
3.1. Dilatometric Study of IAT
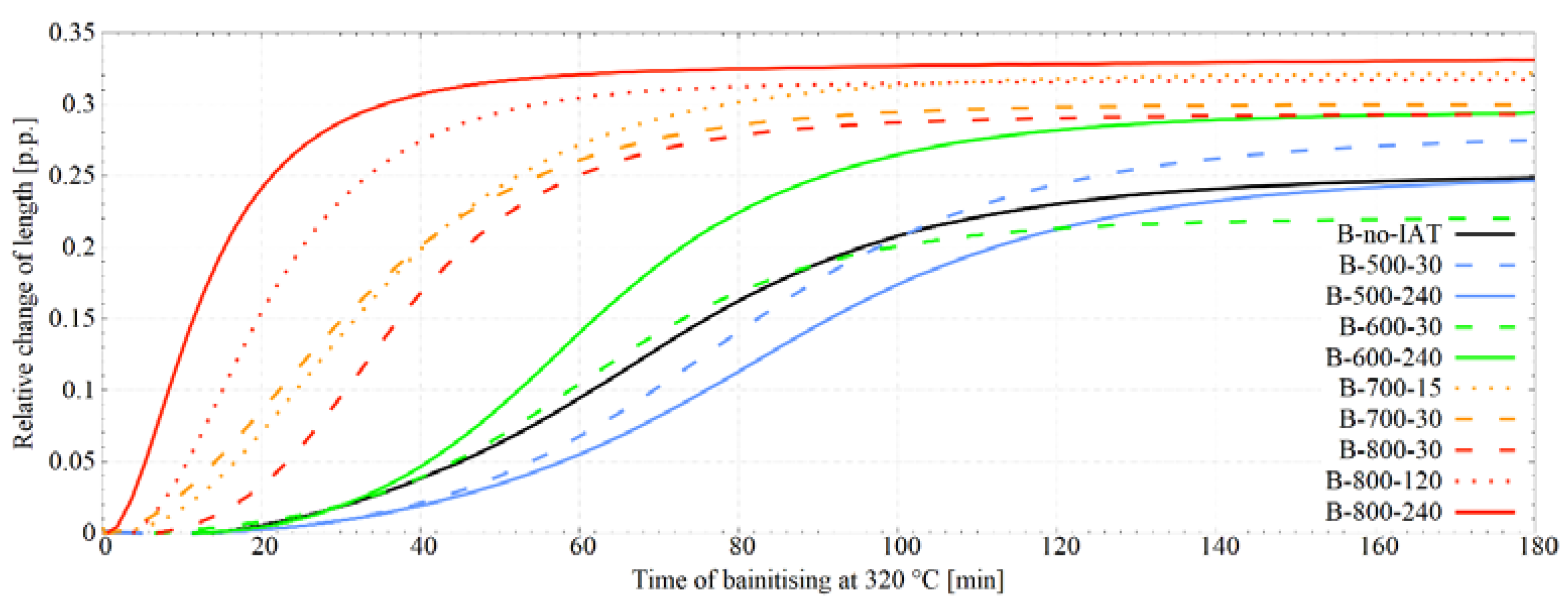
3.2. Bainitic Transformation Influenced by IAT
3.3. Microstructural Changes
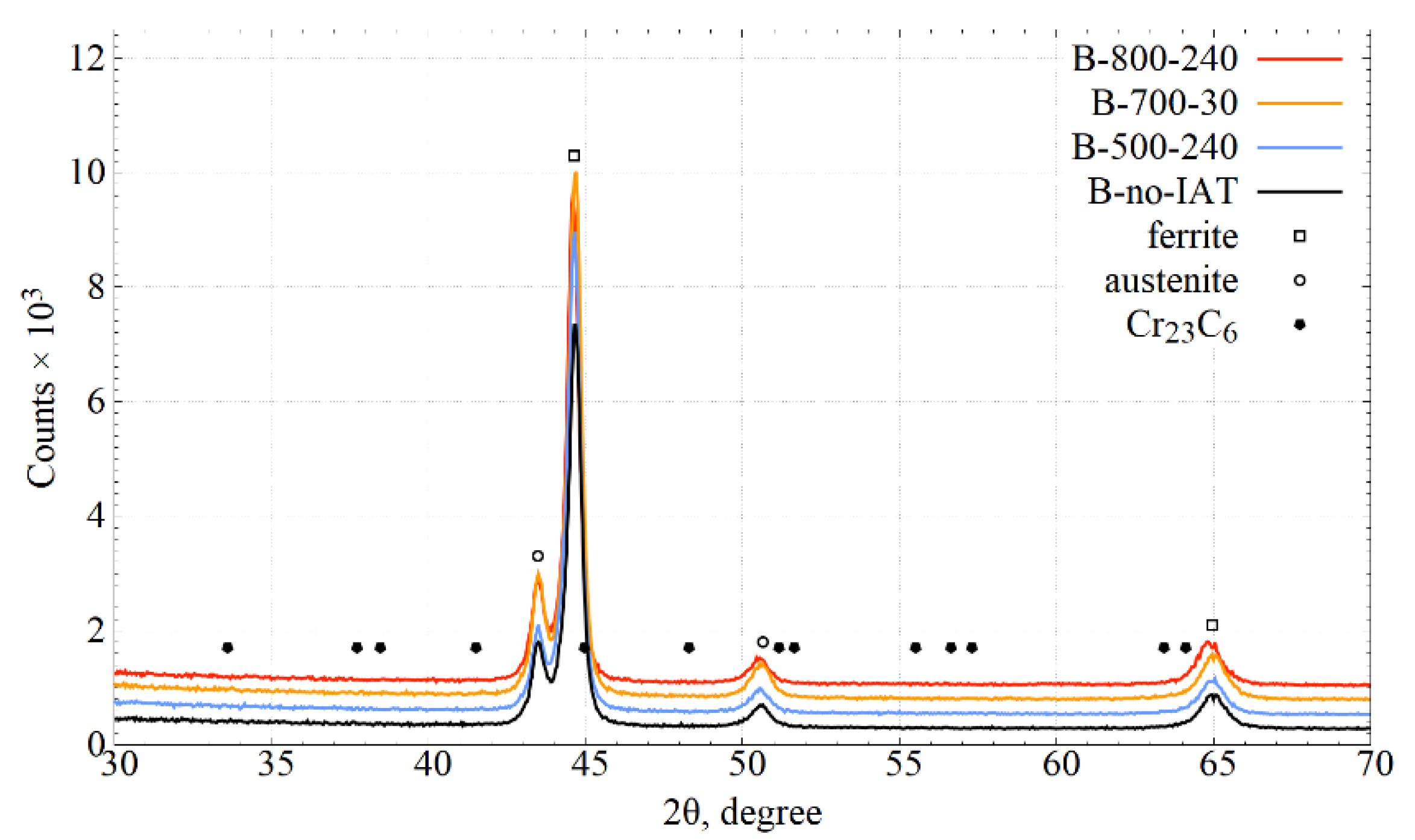
3.3.1. X-ray Diffraction
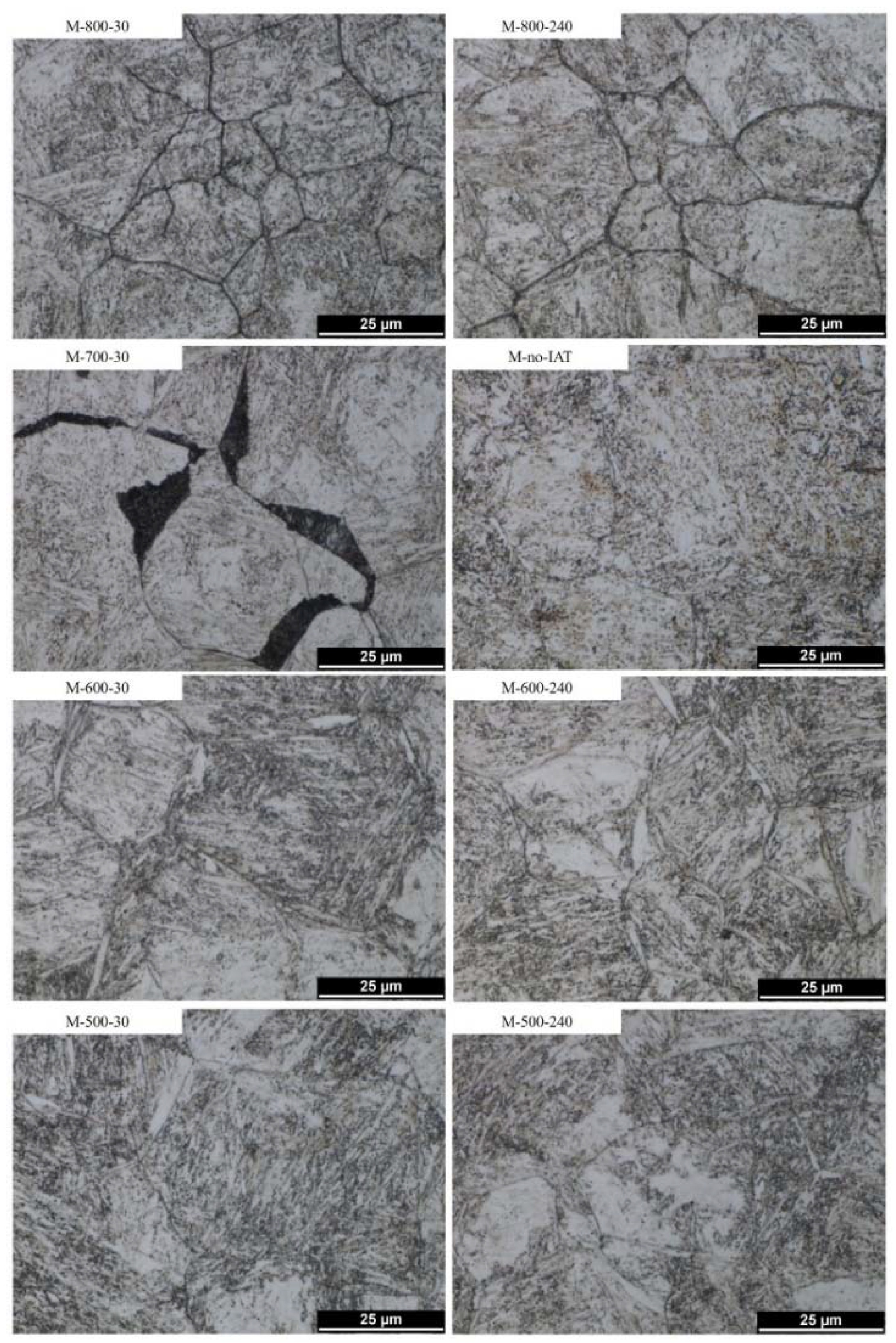
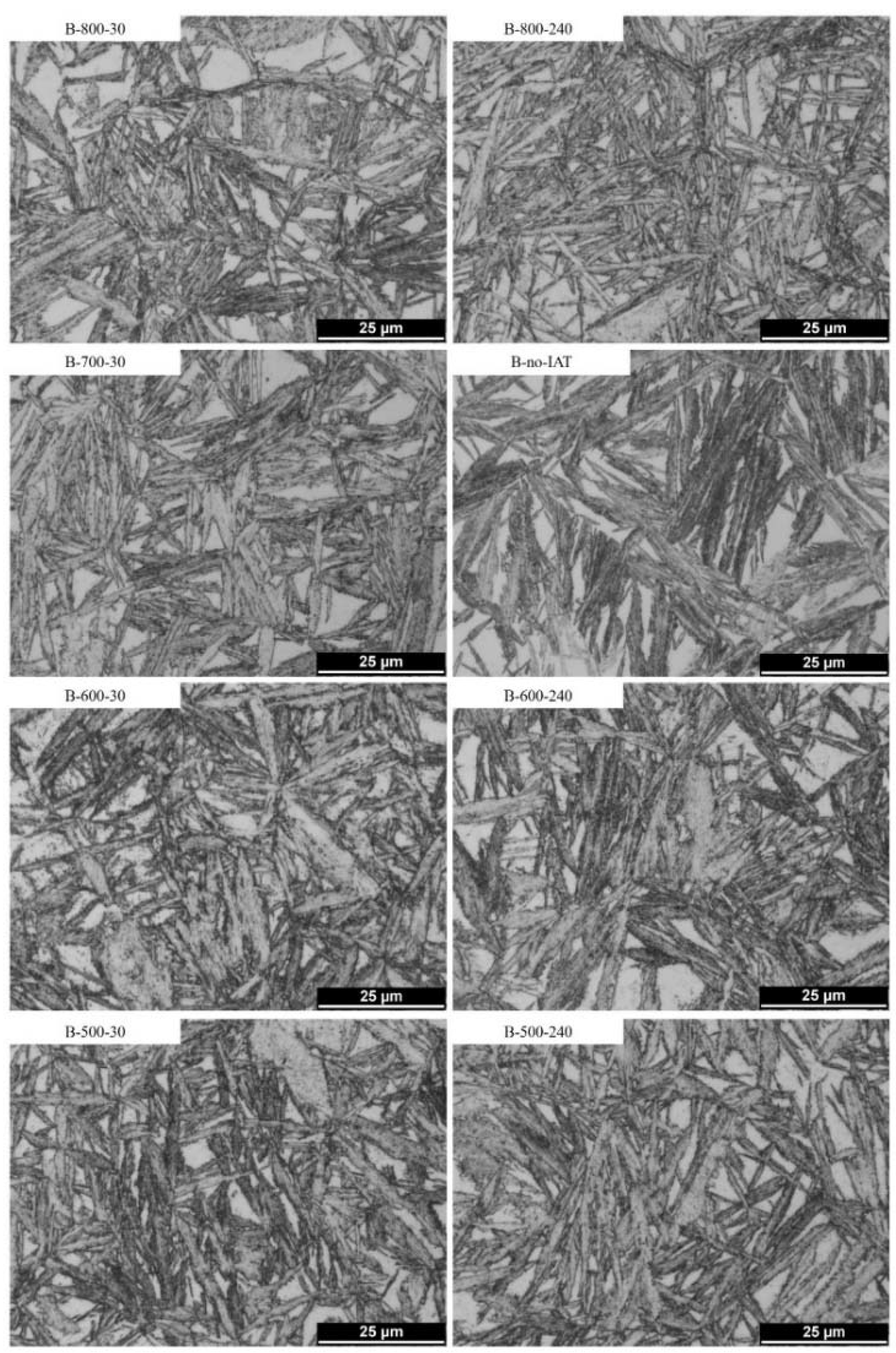
3.3.2. Light Microscopy
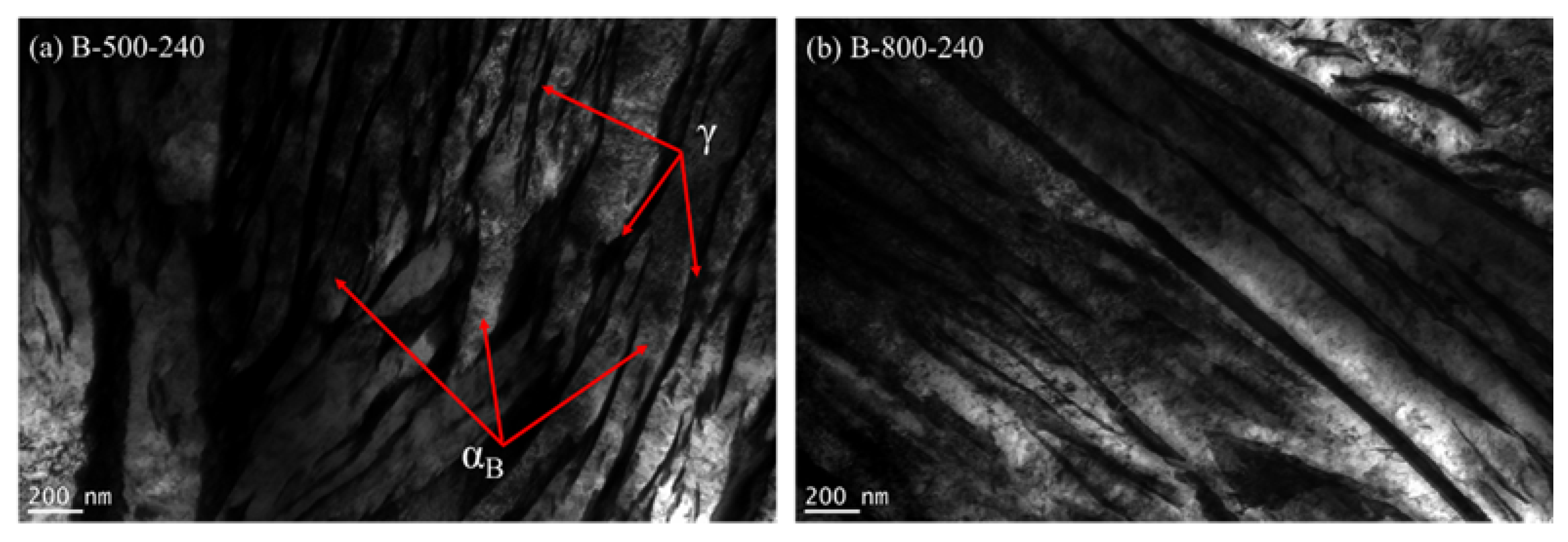
3.3.3. Transmission Electron Microscopy
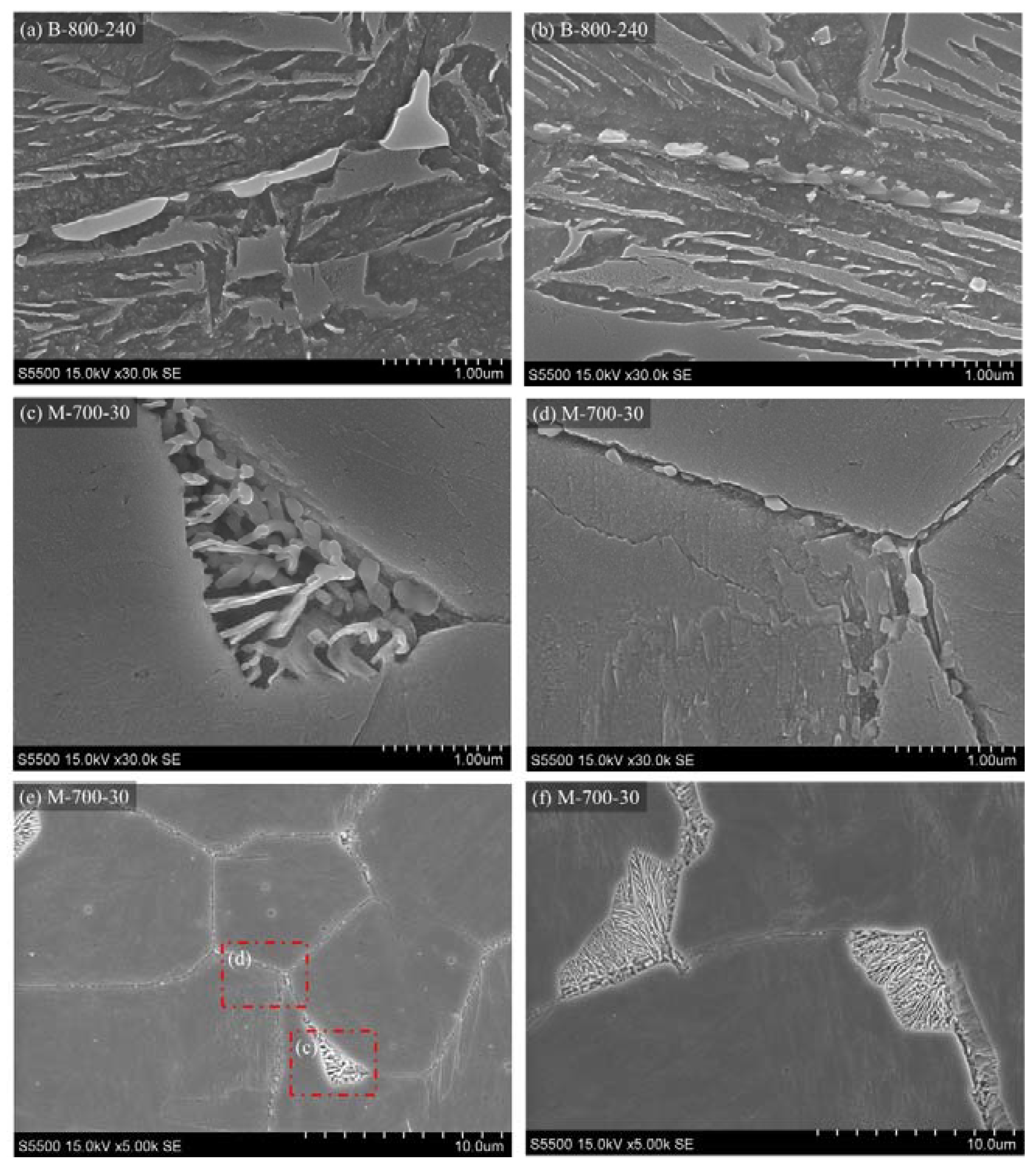
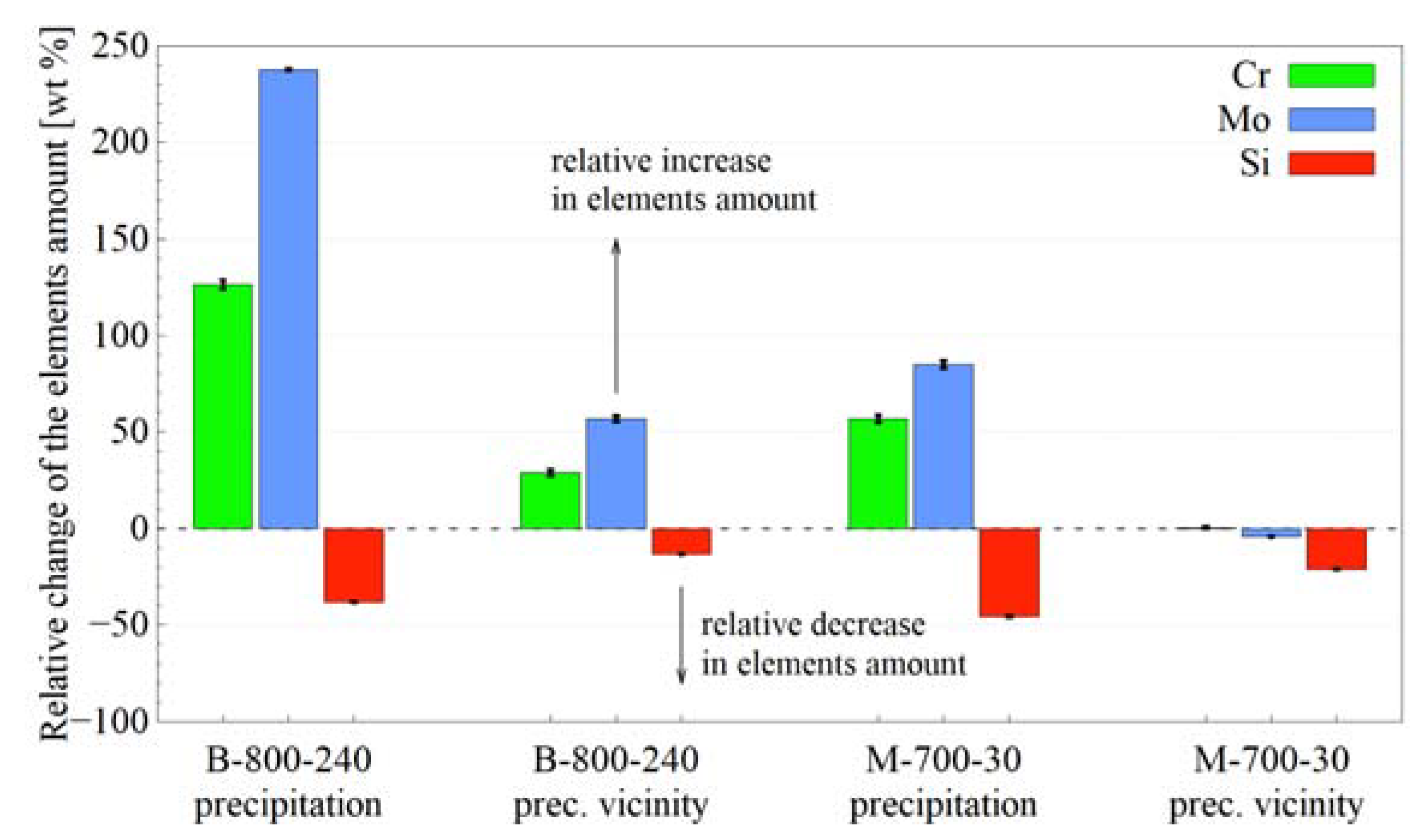
3.3.4. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
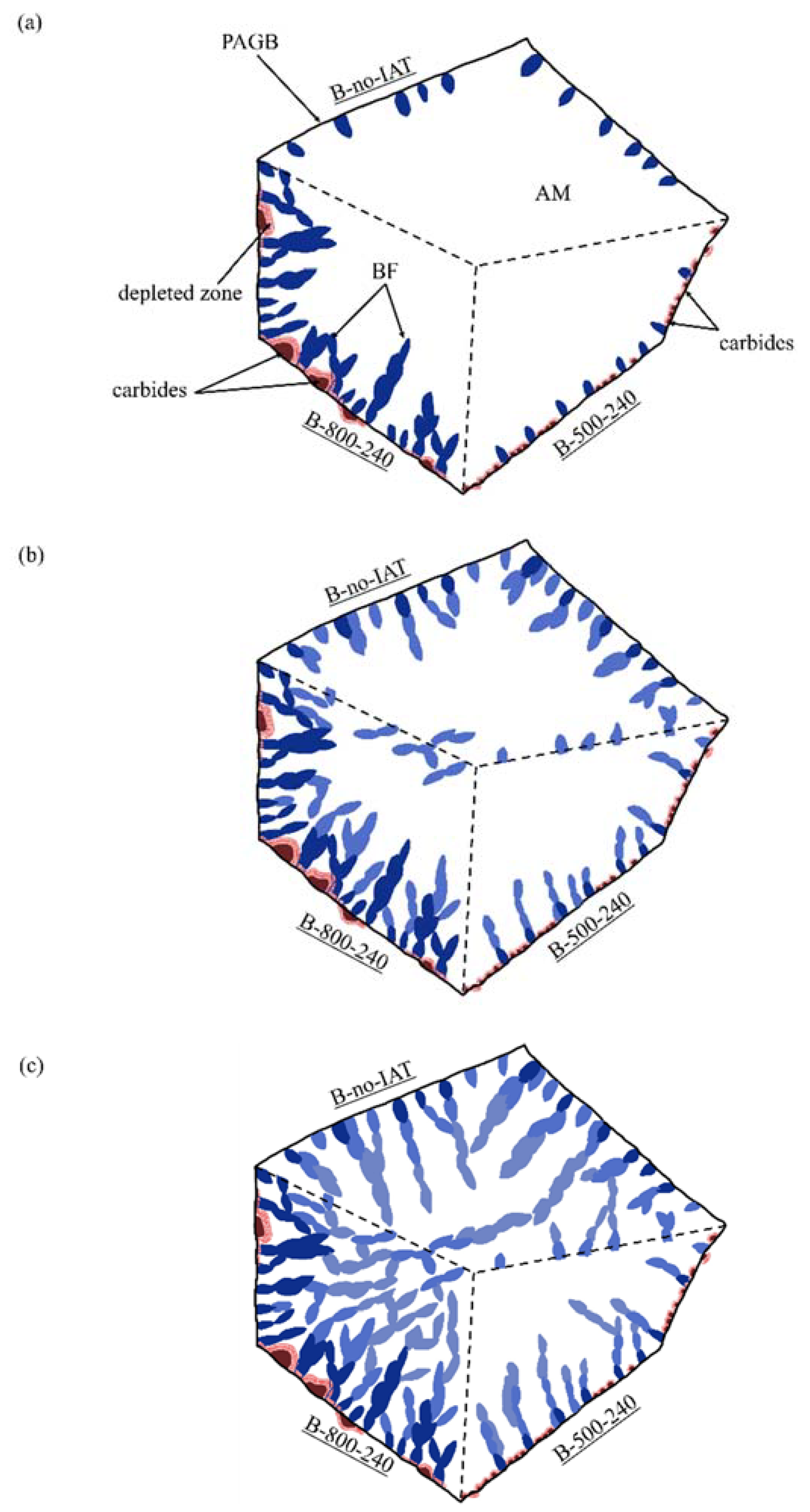
4. Discussion
4.1. Phase Transformations during IAT
4.2. Impact of IAT on Incubation of Bainitic Transformation
4.3. Impact of IAT on the Autocatalytic Phase of Bainitic Transformation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References and Note
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Dworecka, J.; Jezierska, E.; Rożniatowski, K.; Światnicki, W. Characterization of nanobainitic structure obtained in 100CrMnSi6-4 steel after industrial heat treatment. Arch. Metall. Mater. 2014, 59, 1637–1640. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H.; Mawella, K.J.A.; Jones, D.G.; Brown, P. Design of novel high strenght bainitic steels: Part 1. J. Mater. Sci. Technol. 2001, 17, 512–516. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Beladi, H.; Xiong, X.Y.; Adachi, Y.; Hodgson, P.D. Nanoscale microstructural characterization of a nanobainitic steel. Acta Mater. 2011, 59, 5511–5522. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, F.C.; Wang, T.S. A novel bainitic steel comparable to maraging steel in mechanical properties. Scr. Mater. 2013, 68, 763–766. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Miller, M.K. Design of novel bainitic steels: Moving from ultrafine to nanoscale structures. JOM 2014, 66, 747–755. [Google Scholar] [CrossRef]
- Yoozbashi, M.N.; Yazdani, S.; Wang, T.S. Design of a new nanostructured, high-Si bainitic steel with lower cost production. Mater. Des. 2011, 32, 3248–3253. [Google Scholar] [CrossRef]
- Sourmail, T.; Caballero, F.G.; Garcia-Mateo, C.; Smanio, V.; Ziegler, C.; Kuntz, M.; Elvira, R.; Leiro, A.; Vuorinen, E.; Teeri, T. Evaluation of potential of high Si high C steel nanostructured bainite for wear and fatigue applications. Mater. Sci. Technol. 2013, 29, 1166–1173. [Google Scholar] [CrossRef]
- Das Bakshi, S.; Leiro, A.; Prakash, B.; Bhadeshia, H.K.D.H. Dry rolling/sliding wear of nanostructured bainite. Wear 2014, 316, 70–78. [Google Scholar] [CrossRef]
- Rementeria, R.; Morales-Rivas, L.; Kuntz, M.; Garcia-Mateo, C.; Kerscher, E.; Sourmail, T.; Caballero, F.G. On the role of microstructure in governing the fatigue behaviour of nanostructured bainitic steels. Mater. Sci. Eng. A 2015, 630, 71–77. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Development of hard bainite. ISIJ Int. 2003, 43, 1238–1243. [Google Scholar] [CrossRef]
- Singh, S.B.; Bhadeshia, H.K.D.H. Estimation of bainite plate-thickness in low-alloy steels. Mater. Sci. Eng. A 1998, 245, 72–79. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, Z. Development of and perspective on high-performance nanostructured bainitic bearing steel. Engineering 2019, 5, 319–328. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of low-temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Zhang, F.; Long, X.; Chen, C. Acceleration of bainitic transformation by introducing AlN in medium carbon steel. Mater. Sci. Technol. 2019, 35, 147–154. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Harjo, S.; Su, Y.H.; Aizawa, K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015, 85, 243–249. [Google Scholar] [CrossRef]
- Zhao, H.; Wynne, B.P.; Palmiere, E.J. Effect of austenite grain size on the bainitic ferrite morphology and grain refinement of a pipeline steel after continuous cooling. Mater. Charact. 2017, 123, 128–136. [Google Scholar] [CrossRef]
- Hu, H.; Zurob, H.S.; Xu, G.; Embury, D.; Purdy, G.R. New insights to the effects of ausforming on the bainitic transformation. Mater. Sci. Eng. A 2015, 626, 34–40. [Google Scholar] [CrossRef]
- He, J.; Zhao, A.; Zhi, C.; Fan, H. Acceleration of nanobainite transformation by multi-step ausforming process. Scr. Mater. 2015, 107, 71–74. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, G.; Hu, H.; Yuan, Q.; Tian, J. Comprehensive analysis on the effects of different stress states on the bainitic transformation. Mater. Sci. Eng. A 2017, 704, 427–433. [Google Scholar] [CrossRef]
- Jaramillo, R.A.; Babu, S.S.; Ludtka, G.M.; Kisner, R.A.; Wilgen, J.B.; Mackiewicz-Ludtka, G.; Nicholson, D.M.; Kelly, S.M.; Murugananth, M.; Bhadeshia, H.K.D.H. Effect of 30 T magnetic field on transformations in a novel bainitic steel. Scr. Mater. 2005, 52, 461–466. [Google Scholar] [CrossRef]
- Ravi, A.M.; Sietsma, J.; Santofimia, M.J. The role of grain-boundary cementite in bainite formation in high-carbon steels. Scr. Mater. 2020, 185, 7–11. [Google Scholar] [CrossRef]
- Ravi, A.M.; Kumar, A.; Herbig, M.; Sietsma, J.; Santofimia, M.J. Impact of austenite grain boundaries and ferrite nucleation on bainite formation in steels. Acta Mater. 2020, 188, 424–434. [Google Scholar] [CrossRef]
- Skołek, E.; Kamiński, J.; Marciniak, S.; Świątnicki, W.A. Corrosion resistance of the nanostructured X37CrMoV5-1 steel. Arch. Metall. Mater. 2020, 65, 133–140. [Google Scholar] [CrossRef]
- Marciniak, S.; Skołek, E.; Świątnicki, W. The effect of stepped austempering on phase composition and mechanical properties of nanostructured X37CrMoV5-1 steel. Arch. Metall. Mater. 2015, 60, 517–521. [Google Scholar] [CrossRef]
- Sourmail, T.; Too, C.H.; Bhadeshia, H.K.D.H. Sensitisation and evolution of chromium-depleted zones in Fe-Cr-Ni-C systems. ISIJ Int. 2014, 43, 1814–1820. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Ding, Z.; Dong, L.; Liang, B.; Xu, Z.; Ding, Z.; Liang, B. Characterization of M23C6 carbides precipitating at grain boundaries in 100Mn13 steel. Metall. Mater. Trans. A 2016, 47, 4862–4868. [Google Scholar] [CrossRef]
- Hu, Z.F.; Zhang, Z. Investigation the effect of precipitating characteristics on the creep behavior of HR3C austenitic steel at 650 °C. Mater. Sci. Eng. A 2019, 742, 451–463. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Hong, J.; Gao, J.; Zhang, H.; Li, J.; Wang, Q. The evolution of precipitates of 22Cr-25Ni-Mo-Nb-N heat-resistant austenitic steel in long-term creep. Mater. Sci. Eng. A 2010, 527, 4424–4430. [Google Scholar] [CrossRef]
- Kaneko, K.; Fukunaga, T.; Yamada, K.; Nakada, N.; Kikuchi, M.; Saghi, Z.; Barnard, J.S.; Midgley, P.A. Formation of M23C6-type precipitates and chromium-depleted zones in austenite stainless steel. Scr. Mater. 2011, 65, 509–512. [Google Scholar] [CrossRef]
- Sasmal, B. Mechanism of the formation of lamellar M23C6 at and near twin boundaries in austenitic stainless steels. Metall. Mater. Trans. A 1999, 30, 2791–2801. [Google Scholar] [CrossRef]
- Trillo, E.A.; Murr, L.E. A TEM investigation of M23C6 carbide precipitation behaviour on varying grain boundary misorientations in 304 stainless steels. J. Mater. Sci. 1998, 33, 1263–1271. [Google Scholar] [CrossRef]
- Weiss, B.; Stickler, R. Phase instabilities during high temperature exposure of 316 austenitic stainless steel. Metall. Mater. Trans. B 1972, 3, 851–866. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, X.; Kang, X.; Li, D. Precipitation of M23C6 and its effect on tensile properties of 0.3C-20Cr-11Mn-1Mo-0.35N steel. Mater. Des. 2015, 78, 42–50. [Google Scholar] [CrossRef]
- Gu, J.; Li, J.; Chang, R. Enhanced refinement of Cr23C6 by heterogeneous nucleation in annealed nitrogen-alloyed 4Cr5Mo2V die steel. Metall. Mater. Trans. A 2019, 50, 518–522. [Google Scholar] [CrossRef]
- Sasmal, B. Mechanism of the formation of M23C6 plates around undissolved NbC particles in a stabilized austenitic stainless steel. J. Mater. Sci. 1997, 32, 5439–5444. [Google Scholar] [CrossRef]
- Spadotto, J.C.; Dille, J.; Watanabe, M.; Solórzano, I.G. Grain boundary precipitation phenomena in an alloy 33 (Cr-Fe-Ni-N) subjected to direct-aging treatments (700 °C and 900 °C). Mater. Charact. 2018, 140, 113–121. [Google Scholar] [CrossRef]
- Hong, S.P.; Il Kim, S.; Ahn, T.Y.; Hong, S.T.; Kim, Y.W. Effects of extended heat treatment on carbide evolution in Cr-Mo steels. Mater. Charact. 2016, 115, 8–13. [Google Scholar] [CrossRef]
- Cheng, W.C.; Li, Y.C. The coexistence of two different pearlites, lamellae of (Ferrite + M3C), and lamellae of (Ferrite + M23C6) in a Mn-Al steel. Metall. Mater. Trans. A 2012, 43, 1817–1825. [Google Scholar] [CrossRef]
- Hong, H.U.; Rho, B.S.; Nam, S.W. Correlation of the M23C6 precipitation morphology with grain boundary characteristics in austenitic stainless steel. Mater. Sci. Eng. 2001, 318, 285–292. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, B.; Dong, X.; Sun, F.; Zhang, L. Preferential growth of coherent precipitates at grain boundary. Mater. Lett. 2020, 261, 126984. [Google Scholar] [CrossRef]
- Souissi, M.; Sluiter, M.H.F.; Matsunaga, T.; Tabuchi, M.; Mills, M.J.; Sahara, R. Direct observation and modeling of growth-induced stacking fault in chromium-rich γ-M23C6 carbides. Scr. Mater. 2020, 178, 290–294. [Google Scholar] [CrossRef]
- Du, J.K.; Wang, C.H.; Wang, K.C.; Chen, K.K. TEM analysis of 2205 duplex stainless steel to determine orientation relationship between M23C6 carbide and austenite matrix at 950 °C. Intermetallics 2014, 45, 80–83. [Google Scholar] [CrossRef]
- Singhal, L.K.; Martin, J.W. The nucleation and growth of Widmannstätten M23C6 precipitation in an austenitic stainless steel. Acta Metall. 1968, 16, 1159–1165. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Guo, J.; Shi, Z.; Ren, X.; Yang, Q. Thermal-mismatch-stress induced deformation zone on primary-carbide/austenite interface. Mater. Lett. 2019, 248, 55–59. [Google Scholar] [CrossRef]
- Mújica Roncery, L.; Weber, S.; Theisen, W. Nucleation and precipitation kinetics of M23C6 and M2N in an Fe-Mn-Cr-C-N austenitic matrix and their relationship with the sensitization phenomenon. Acta Mater. 2011, 59, 6275–6286. [Google Scholar] [CrossRef]
- Fukunaga, T.; Kaneko, K.; Kawano, R.; Ueda, K.; Yamada, K.; Nakada, N.; Kikuchi, M.; Barnard, J.S.; Midgley, P.A. Formation of intergranular M23C6 in sensitized type-347 stainless steel. ISIJ Int. 2014, 54, 148–152. [Google Scholar] [CrossRef]
- Thorvaldsson, T.; Dunlop, G.L. Grain boundary Cr-depleted zones in Ti and Nb stabilized austenitic stainless steels. J. Mater. Sci. 1983, 18, 793–803. [Google Scholar] [CrossRef]
- Dub, V.A.; Rodin, A.; Bokstein, B.; Belikov, S.; Kozlov, P.; Schepkin, I.; Dub, V.S. Modeling of the carbide growth kinetics in the low alloyed steels. Mater. Lett. 2018, 215, 134–136. [Google Scholar] [CrossRef]
- Hall, E.L.; Briant, C.L. Chromium depletion in the vicinity of carbides in sensitized austenitic stainless steels. Metall. Trans. A 1984, 15, 793–811. [Google Scholar] [CrossRef]
- Grybėnas, A.; Makarevičius, V.; Baltušnikas, A.; Lukošiūtė, I.; Kriūkienė, R. Correlation between structural changes of M23C6 carbide and mechanical behaviour of P91 steel after thermal aging. Mater. Sci. Eng. A 2017, 696, 453–460. [Google Scholar] [CrossRef]
- Wieczerzak, K.; Bala, P.; Dziurka, R.; Tokarski, T.; Cios, G.; Koziel, T.; Gondek, L. The effect of temperature on the evolution of eutectic carbides and M7C3 → M23C6carbides reaction in the rapidly solidified Fe-Cr-C alloy. J. Alloy. Compd. 2017, 698, 673–684. [Google Scholar] [CrossRef]
- Zhang, X.F.; Komizo, Y. Direct observation of thermal stability of M 23C 6 carbides during reheating using in situ synchrotron X-ray diffraction. Philos. Mag. Lett. 2013, 93, 9–17. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Nanostructured bainite. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 3–18. [Google Scholar] [CrossRef]
- Caballero, F.G.; Miller, M.K.; Garcia-Mateo, C.; Cornide, J. New experimental evidence of the diffusionless transformation nature of bainite. J. Alloy. Compd. 2013, 577 (Suppl. S1), S626–S630. [Google Scholar] [CrossRef]
- Muddle, B.C.; Nie, J.F. Formation of bainite as a diffusional-displacive phase transformation. Scr. Mater. 2002, 47, 187–192. [Google Scholar] [CrossRef]
- Ravi, A.M.; Sietsma, J.; Santofimia, M.J. Exploring bainite formation kinetics distinguishing grain-boundary and autocatalytic nucleation in high and low-Si steels. Acta Mater. 2016, 105, 155–164. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.; Wu, Q.; Gao, B.; Zhang, X. Research on nucleation mechanism of the nanoscale bainite ferrite in a high carbon steel Fe-0.88C-1.35Si-1.03Cr-0.43Mn. J. Mater. Res. 2016, 31, 1510–1517. [Google Scholar] [CrossRef]
- Catteau, S.D.; Van Landeghem, H.P.; Teixeira, J.; Dulcy, J.; Dehmas, M.; Denis, S.; Redjaïmia, A.; Courteaux, M. Carbon and nitrogen effects on microstructure and kinetics associated with bainitic transformation in a low-alloyed steel. J. Alloy. Compd. 2016, 658, 832–838. [Google Scholar] [CrossRef]
- Quidort, D. unpublished research cited after [62].
- Miyamoto, G.; Yokoyama, K.; Furuhara, T. Quantitative analysis of Mo solute drag effect on ferrite and bainite transformations in Fe-0.4C-0.5Mo alloy. Acta Mater. 2019, 177, 187–197. [Google Scholar] [CrossRef]
- Quidort, D.; Bréchet, Y. The role of carbon on the kinetics of bainite transformation in steels. Scr. Mater. 2002, 47, 151–156. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Luo, C.; Liu, Z. Microstructure, crystallography of phase transformations and multiple precipitations in PH 15-7Mo stainless steel. J. Alloy. Compd. 2016, 672, 386–392. [Google Scholar] [CrossRef]
- Kaneshita, T.; Miyamoto, G.; Furuhara, T. Variant selection in grain boundary nucleation of bainite in Fe-2Mn-C alloys. Acta Mater. 2017, 127, 368–378. [Google Scholar] [CrossRef]
- Guo, H.; Gao, X.; Bai, Y.; Enomoto, M.; Yang, S.; He, X. Variant selection of bainite on the surface of allotriomorphic ferrite in a low carbon steel. Mater. Charact. 2012, 67, 34–40. [Google Scholar] [CrossRef]
- Gómez, L.; García, N.; Vitelli, V.; Vega, D.A. Phase nucleation in curved space. Nat. Commun. 2015, 6, 6856. [Google Scholar] [CrossRef]
- Loder, D.; Michelic, S.K.; Bernhard, C. Acicular ferrite formation and its influencing factors—A review. J. Mater. Sci. Res. 2016, 6, 24–43. [Google Scholar] [CrossRef]
- Liu, C.; Di, X.; Chen, C.; Guo, X.; Xue, Z. A bainite transformation kinetics model and its application to X70 pipeline steel. J. Mater. Sci. 2015, 50, 5079–5090. [Google Scholar] [CrossRef]
- Ławrynowicz, Z. An investigation of the mechanism of bainite transformation in experimental 0.2c-1v-2mn steel. Adv. Mater. Sci. 2013, 12, 19–30. [Google Scholar] [CrossRef]
- Hu, H.; Xu, G.; Zhang, Y.; Xue, Z.; Zhou, M. Dynamic observation of bainite transformation in a Fe-C-Mn-Si superbainite steel. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 818–821. [Google Scholar] [CrossRef]
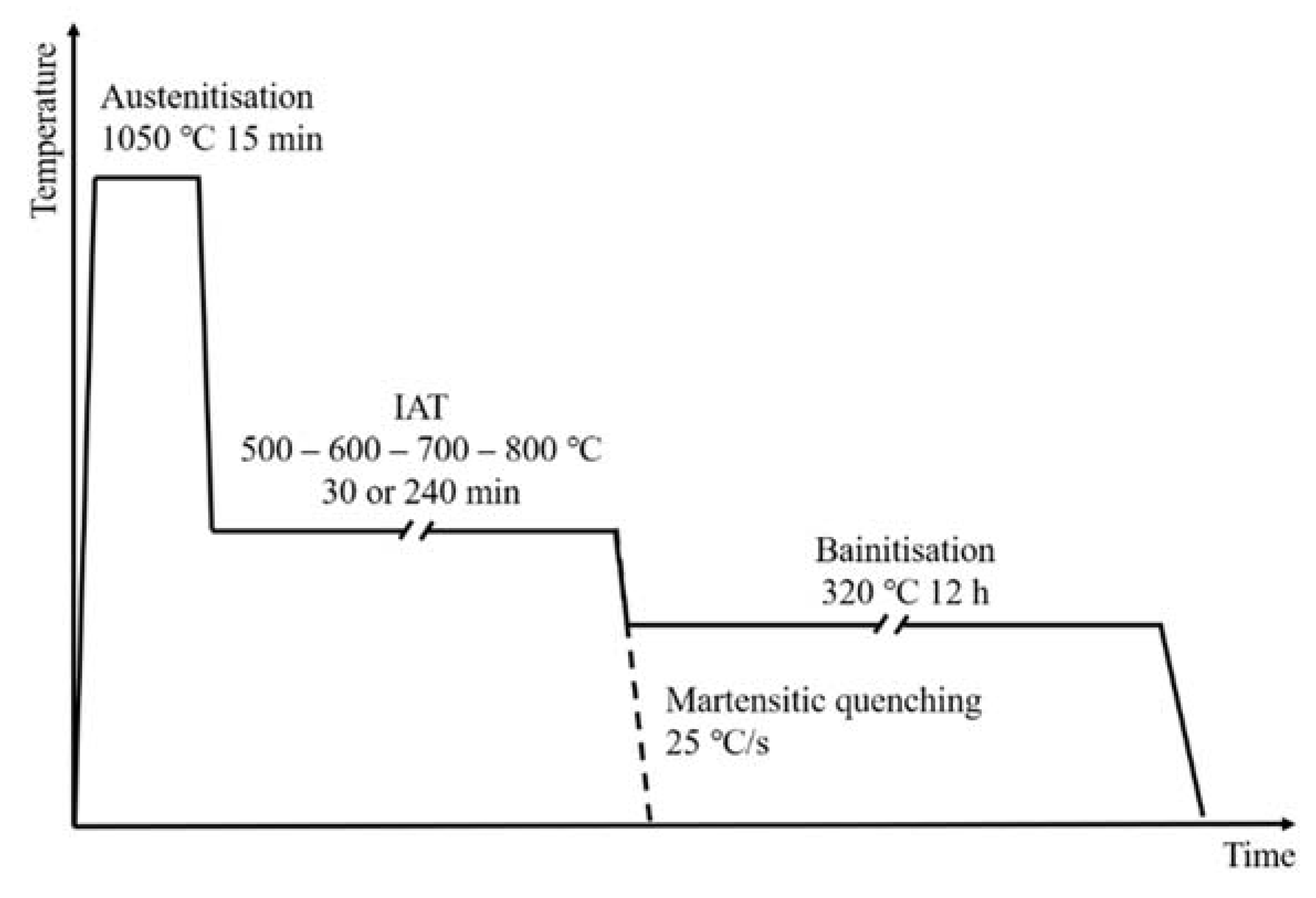

| C | Si | Cr | Mn | Mo | Ni | V |
|---|---|---|---|---|---|---|
| 0.37 | 1.16 | 4.95 | 0.43 | 1.22 | 0.26 | 0.40 |
| Treatment | IAT Temperature and Time | Designation |
|---|---|---|
| quenching | 800 °C, 240 min | M-800-240 |
| 800 °C, 30 min | M-800-30 | |
| 700 °C, 240 min | M-700-240 | |
| 700 °C, 30 min | M-700-30 | |
| 600 °C, 240 min | M-600-240 | |
| 600 °C, 30 min | M-600-30 | |
| 500 °C, 240 min | M-500-240 | |
| 500 °C, 30 min | M-500-30 | |
| no IAT | M-no-IAT | |
| austempering | 800 °C, 240 min | B-800-240 |
| 800 °C, 120 min | B-800-120 | |
| 800 °C, 30 min | B-800-30 | |
| 700 °C, 30 min | B-700-30 | |
| 700 °C, 15 min 40 s | B-700-15 | |
| 600 °C, 240 min | B-600-240 | |
| 600 °C, 30 min | B-600-30 |
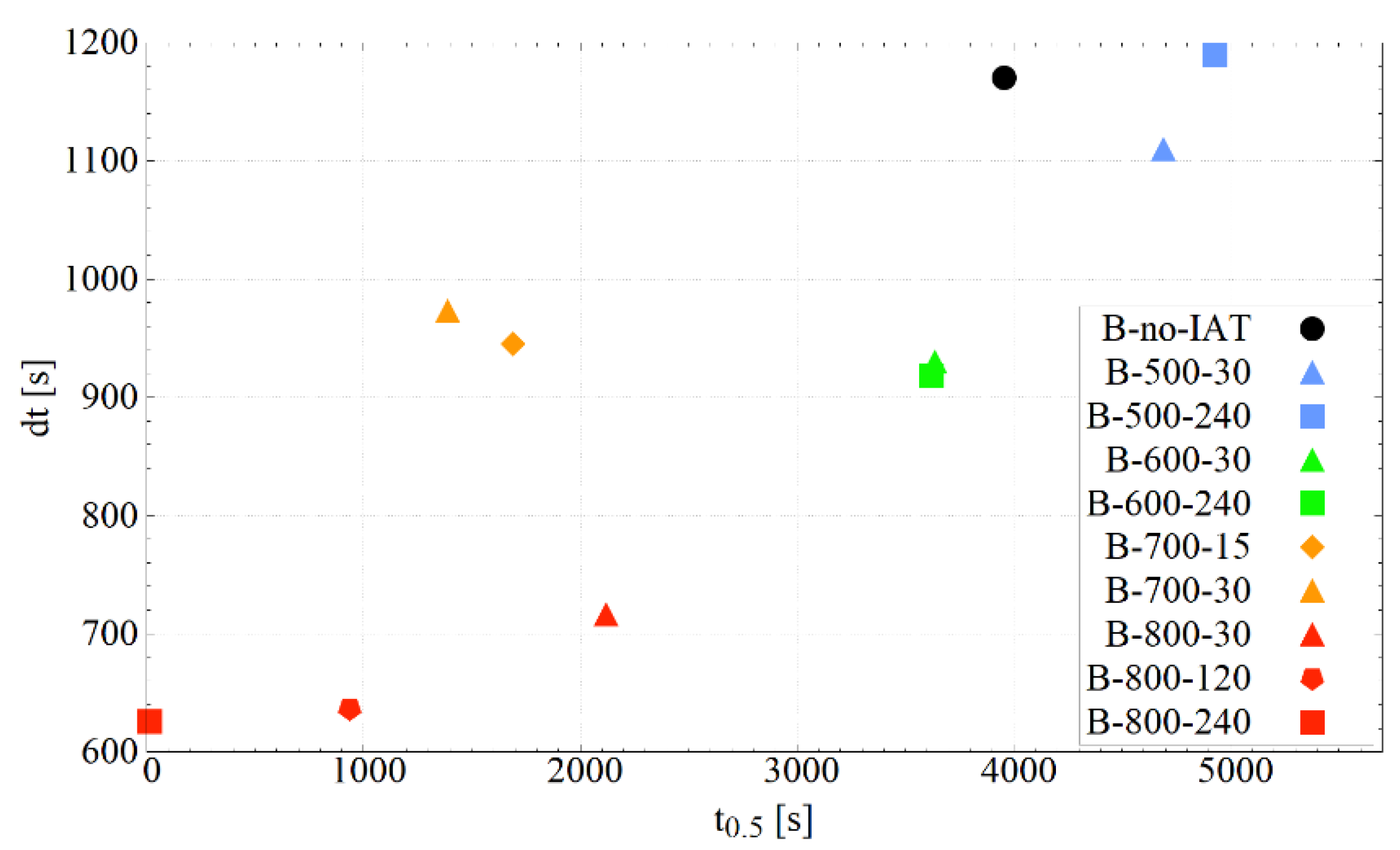
| IAT Variant | dt [s] | t0.5 [s] | Adj. R2 |
|---|---|---|---|
| B-800-240 | 626.1 ± 4.6 | 12.7 ± 21.4 | 0.99694 |
| B-800-120 | 636.2 ± 1.6 | 934.7 ± 3.7 | 0.99822 |
| B-800-30 | 715.7 ± 2.8 | 2120.2 ± 3.9 | 0.99864 |
| B-700-30 | 973.1 ± 3.2 | 1386.9 ± 7.1 | 0.99947 |
| B-700-15 | 945.0 ± 1.8 | 1691.0 ± 3.4 | 0.99895 |
| B-600-240 | 917.9 ± 3.1 | 3623.0 ± 3.6 | 0.99932 |
| B-600-30 | 930.1 ± 1.4 | 3637.1 ± 1.6 | 0.99986 |
| B-500-240 | 1190.1 ± 1.5 | 4930.4 ± 1.5 | 0.99987 |
| B-500-30 | 1109.9 ± 2.3 | 4690.0 ± 2.4 | 0.99975 |
| B-no-IAT | 1170.0 ± 1.3 | 3957.2 ± 1.5 | 0.99987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukaszewicz, G.; Wasiak, K.; Skołek, E.K.; Diduszko, R.; Świątnicki, W.A. Influence of Intermediate Annealing Treatment on the Kinetics of Bainitic Transformation in X37CrMoV5-1 Steel. Materials 2021, 14, 4411. https://doi.org/10.3390/ma14164411
Łukaszewicz G, Wasiak K, Skołek EK, Diduszko R, Świątnicki WA. Influence of Intermediate Annealing Treatment on the Kinetics of Bainitic Transformation in X37CrMoV5-1 Steel. Materials. 2021; 14(16):4411. https://doi.org/10.3390/ma14164411
Chicago/Turabian StyleŁukaszewicz, Grzegorz, Krzysztof Wasiak, Emilia K. Skołek, Ryszard Diduszko, and Wiesław A. Świątnicki. 2021. "Influence of Intermediate Annealing Treatment on the Kinetics of Bainitic Transformation in X37CrMoV5-1 Steel" Materials 14, no. 16: 4411. https://doi.org/10.3390/ma14164411
APA StyleŁukaszewicz, G., Wasiak, K., Skołek, E. K., Diduszko, R., & Świątnicki, W. A. (2021). Influence of Intermediate Annealing Treatment on the Kinetics of Bainitic Transformation in X37CrMoV5-1 Steel. Materials, 14(16), 4411. https://doi.org/10.3390/ma14164411






