Electrochemically Inert Li2MnO3: The Key to Improving the Cycling Stability of Li-Rich Manganese Oxide Used in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozawa, K. Lithium-Ion Rechargeable Batteries with LiCoO2 and Carbon Electrodes—The LiCoO2/C System. Solid State Ion. 1994, 69, 212–221. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Whittingham, M.S. History, Evolution, and Future Status of Energy Storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Zuo, W.H.; Luo, M.Z.; Liu, X.S.; Wu, J.; Liu, H.D.; Li, J.; Winter, M.; Fu, R.Q.; Yang, W.L.; Yang, Y. Li-rich cathodes for rechargeable Li-based batteries: Reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 2020, 13, 4450–4497. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.L.; Li, J.C.; Xiao, X.C.; Lott, A.; Lu, W.Q.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1–23. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, G.Q.; Deng, W.T.; Huang, Z.D.; Gao, X.; Liu, C.; Yin, S.Y.; Liu, H.Q.; Deng, X.L.; Tian, Y.; et al. Pseudo-Bonding and Electric-Field Harmony for Li-Rich Mn-Based Oxide Cathode. Adv. Funct. Mater. 2020, 30, 2004302. [Google Scholar] [CrossRef]
- Sun, Y.K.; Chen, Z.; Noh, H.J.; Lee, D.J.; Jung, H.G.; Ren, Y.; Wang, S.; Yoon, C.S.; Myung, S.T.; Amine, K. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 2012, 11, 942–947. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, D.J.; Zhang, D.W.; Chen, X.Y.; Tian, X.L. One-step synthesis and electrochemical behavior of LiMnO2 and its composite from MnO2 in the presence of glucose. J. Phys. Chem. Solids 2009, 70, 936–940. [Google Scholar] [CrossRef]
- Saroha, R.; Gupta, A.; Panwar, A.K. Electrochemical performances of Li-rich layered-layered Li2MnO3-LiMnO2 solid solutions as cathode material for lithium-ion batteries. J. Alloys Compd. 2017, 696, 580–589. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Hu, X.; Peng, T. Synthesis of layered xLi2MnO3·(1−x)LiMnO2 nanoplates and its electrochemical performance as Li-rich cathode materials for Li-ion battery. Electrochim. Acta 2015, 165, 182–190. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Chang, H.; Gan, J.; Yue, H.; Yang, Y. Hydrothermal Synthesis of Nanosized LiMnO2–Li2MnO3 Compounds and Their Electrochemical Performances. J. Electrochem. Soc. 2009, 156, A162–A168. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2016, 7, 1601284. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.-M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.-W.; et al. Understanding the Rate Capability of High-Energy-Density Li-Rich Layered Li1.2Ni0.15Co0.1Mn0.55O2 Cathode Materials. Adv. Energy Mater. 2014, 4, 1300950. [Google Scholar] [CrossRef]
- Hy, S.; Felix, F.; Rick, J.; Su, W.N.; Hwang, B.J. Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[NixLi1−2x/3Mn2−x/3]O2 (0≤x≤0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar] [CrossRef]
- Koga, H.; Croguennec, L.; Mannessiez, P.; Menetrier, M.; Weill, F.; Bourgeois, L.; Duttine, M.; Suard, E.; Delmas, C. Li1.20Mn0.54Co0.13Ni0.13O2 with Different Particle Sizes as Attractive Positive Electrode Materials for Lithium-Ion Batteries: Insights into Their Structure. J. Phys. Chem. C 2012, 116, 13497–13506. [Google Scholar] [CrossRef]
- Jarvis, K.A.; Deng, Z.Q.; Allard, L.F.; Manthiram, A.; Ferreira, P.J. Atomic Structure of a Lithium-Rich Layered Oxide Material for Lithium-Ion Batteries: Evidence of a Solid Solution. Chem. Mater. 2011, 23, 3614–3621. [Google Scholar] [CrossRef]
- Rousse, G.; Tarascon, J.M. Sulfate-Based Polyanionic Compounds for Li-Ion Batteries: Synthesis, Crystal Chemistry, and Electrochemistry Aspects. Chem. Mater. 2013, 26, 394–406. [Google Scholar] [CrossRef]
- Yang, J.S.; Li, P.; Zhong, F.P.; Feng, X.M.; Chen, W.H.; Ai, X.P.; Yang, H.X.; Xia, D.G.; Cao, Y.L. Suppressing Voltage Fading of Li-Rich Oxide Cathode via Building a Well-Protected and Partially-Protonated Surface by Polyacrylic Acid Binder for Cycle-Stable Li-Ion Batteries. Adv. Energy Mater. 2020, 10, 1904264. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.M.; Yoon, W.S. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew Chem. Int. Ed. Engl. 2020, 59, 2578–2605. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Assat, G.; Tarascon, J.M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373–386. [Google Scholar] [CrossRef]
- Hy, S.; Liu, H.D.; Zhang, M.H.; Qian, D.N.; Hwang, B.J.; Meng, Y.S. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 2016, 9, 1931–1954. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.S.; Fu, C.C.; Luo, D.; Fan, J.M.; Xie, D.J.; Li, L.P. Balancing stability and specific energy in Li-rich cathodes for lithium ion batteries: A case study of a novel Li-Mn-Ni-Co oxide. J. Mater. Chem. A 2015, 3, 10592–10602. [Google Scholar] [CrossRef]
- Shen, C.Q.; Xu, H.; Liu, L.; Hu, H.S.; Chen, S.Y.; Su, L.W.; Wang, L.B. EDTA-2Na assisted dynamic hydrothermal synthesis of orthorhombic LiMnO2 for lithium ion battery. J. Alloys Compd. 2020, 830, 154599. [Google Scholar] [CrossRef]
- Fang, D.; Xie, J.L.; Hu, H.; Yang, H.; He, F.; Fu, Z.B. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. [Google Scholar] [CrossRef]
- Dong, J.Y.; Lu, G.; Yue, J.S.; Cheng, Z.M.; Kang, X.H. Valence modulation in hollow carbon nanosphere/manganese oxide composite for high performance supercapacitor. Appl. Surf. Sci. 2019, 480, 1116–1125. [Google Scholar] [CrossRef]
- Li, X.H.; Su, Z.; Wang, Y.B. Electrochemical properties of monoclinic and orthorhombic LiMnO2 synthesized by a one-step hydrothermal method. J. Alloys Compd. 2018, 735, 2182–2189. [Google Scholar] [CrossRef]
- Zhang, X.D.; Shi, J.L.; Liang, J.Y.; Yin, Y.X.; Zhang, J.N.; Yu, X.Q.; Guo, Y.G. Suppressing Surface Lattice Oxygen Release of Li-Rich Cathode Materials via Heterostructured Spinel Li4 Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Y.; Shua, F.F.; Zhang, J.W.; Chen, K.F.; Xue, D.F.; Guo, X.W.; Komarneni, S. Role of Hydrothermal parameters on phase purity of orthorhombic LiMnO2 for use as cathode in Li ion battery. Ceram. Int. 2015, 41, 6729–6733. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Hu, X.; Peng, T.; Liu, J. Preparation of Li-rich layered-layered type x Li2MnO3·(1−x)LiMnO2 nanorods and its electrochemical performance as cathode material for Li-ion battery. J. Power Source 2017, 353, 323–332. [Google Scholar] [CrossRef]
- Sun, Y.; Zan, L.; Zhang, Y.X. Enhanced electrochemical performances of Li2MnO3 cathode materials via adjusting oxygen vacancies content for lithium-ion batteries. Appl. Surf. Sci. 2019, 483, 270–277. [Google Scholar] [CrossRef]
- Tang, S.B.; Lai, M.O.; Lu, L. Electrochemical studies of low-temperature processed nano-crystalline LiMn2O4 thin film cathode at 55 °C. J. Power Source 2007, 164, 372–378. [Google Scholar] [CrossRef]
- Croguennec, L.; Deniard, P.; Brec, R.; Biensan, P.; Broussely, M. Electrochemical behavior of orthorhombic LiMnO2: Influence of the grain size and cationic disorder. Solid State Ion. 1996, 89, 127–137. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Pang, W.K.; Lee, J.Y.; Wei, Y.S.; Wu, S.H. Preparation and characterization of Cr-doped LiMnO2 cathode materials by Pechini’s method for lithium ion batteries. Mater. Chem. Phys. 2013, 139, 241–246. [Google Scholar] [CrossRef]
- Jang, Y.I.; Huang, B.; Wang, H.; Sadoway, D.R.; Chiang, Y.M. Electrochemical Cycling-Induced Spinel Formation in High-Charge-Capacity Orthorhombic LiMnO2. J. Electrochem. Soc. 2019, 146, 3217–3223. [Google Scholar] [CrossRef]
- Kim, S.-W.; Pyun, S.-I. Lithium transport through a sol–gel derived LiMn2O4 film electrode: Analyses of potentiostatic current transient and linear sweep voltammogram by Monte Carlo simulation. Electrochim. Acta 2002, 47, 2843–2855. [Google Scholar] [CrossRef]
- Francis Amalraj, S.; Markovsky, B.; Sharon, D.; Talianker, M.; Zinigrad, E.; Persky, R.; Haik, O.; Grinblat, J.; Lampert, J.; Schulz-Dobrick, M.; et al. Study of the electrochemical behavior of the “inactive” Li2MnO3. Electrochim. Acta 2012, 78, 32–39. [Google Scholar] [CrossRef]
- Boulineau, A.; Croguennec, L.; Delmas, C.; Weill, F. Reinvestigation of Li2MnO3 Structure: Electron Diffraction and High Resolution TEM. Chem. Mater. 2009, 21, 4216–4222. [Google Scholar] [CrossRef]
- Uyama, T.; Mukai, K.; Yamada, I. High-pressure synthesis and electrochemical properties of tetragonal LiMnO2. RSC Adv. 2018, 8, 26325–26334. [Google Scholar] [CrossRef] [Green Version]
- Shao-Horn, Y.; Hackney, S.A.; Armstrong, A.R.; Bruce, P.G.; Gitzendanner, R.; Johnson, C.S.; Thackeray, M.M. Structural Characterization of Layered LiMnO2 Electrodes by Electron Diffraction and Lattice Imaging. J. Electrochem. Soc. 1999, 146, 2404–2412. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, T.; Zhan, D.; Hu, X. Synthesis and electrochemical property of xLi2MnO3·(1−x)LiMnO2 composite cathode materials derived from partially reduced Li2MnO3. J. Power Source 2014, 250, 40–49. [Google Scholar] [CrossRef]
- Song, J.; Li, B.; Chen, Y.; Zuo, Y.; Ning, F.; Shang, H.; Feng, G.; Liu, N.; Shen, C.; Ai, X.; et al. A High-Performance Li-Mn-O Li-rich Cathode Material with Rhombohedral Symmetry via Intralayer Li/Mn Disordering. Adv. Mater. 2020, 32, 2000190. [Google Scholar] [CrossRef]
- Zi, Z.Y.; Zhang, Y.T.; Meng, Y.Q.; Gao, G.; Hou, P.Y. Hierarchical Li-rich oxide microspheres assembled from {010} exposed primary grains for high-rate lithium-ion batteries. New J. Chem. 2020, 44, 8486–8493. [Google Scholar] [CrossRef]
- Luo, X.D.; Yin, Y.Z.; Yuan, M.; Zeng, W.; Lin, G.; Huang, B.; Li, Y.W.; Xiao, S.H. High performance composites of spinel LiMn2O4/3DG for lithium ion batteries. RSC Adv. 2018, 8, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Vetter, J.; Novak, P.; Wagner, M.R.; Veit, C.; Moller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Source 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Dang, F.; Hoshino, T.; Oaki, Y.; Hosono, E.; Zhou, H.; Imai, H. Synthesis of Li-Mn-O mesocrystals with controlled crystal phases through topotactic transformation of MnCO3. Nanoscale 2013, 5, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.X.; Hu, Z.L.; Mao, D.L.; Chang, C.K.; Huang, F.Q. One-step hydrothermal routine for pure-phased orthorhombic LiMnO2 for Li ion battery application. Electrochim. Acta 2008, 53, 7298–7302. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, J.; Wang, G.F.; Liu, S.S.; Tan, M.; Liu, X.Q.; Komarneni, S. Facile synthesis of orthorhombic LiMnO2 nanorods by in-situ carbothermal reduction: Promising cathode material for Li ion batteries. Ceram. Int. 2017, 43, 10585–10589. [Google Scholar] [CrossRef]
- Tong, W.M.; Chu, Q.X.; Meng, Y.J.; Wang, X.F.; Yang, B.; Gao, J.J.; Zhao, X.D.; Liu, X.Y. Synthesis of mesoporous orthorhombic LiMnO2 cathode materials via a one-step flux method for high performance lithium-ion batteries. Mater. Res. Express 2018, 5, 065511. [Google Scholar] [CrossRef]
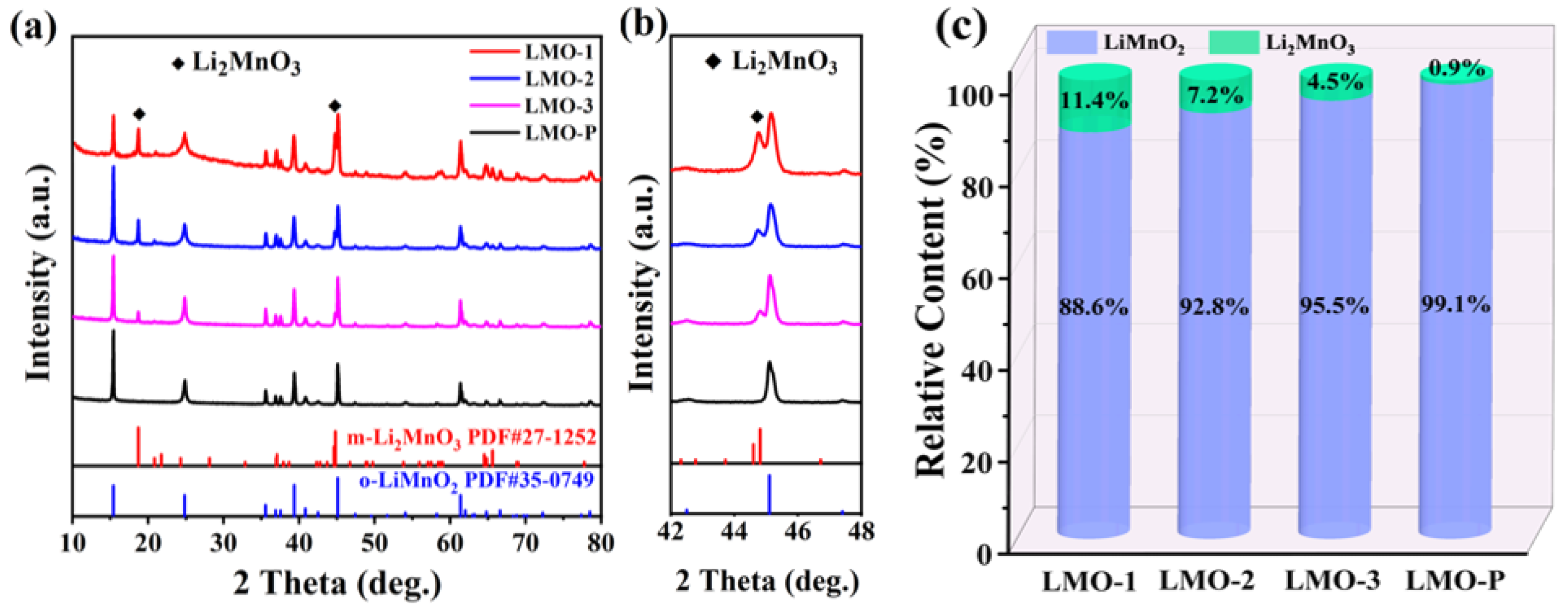
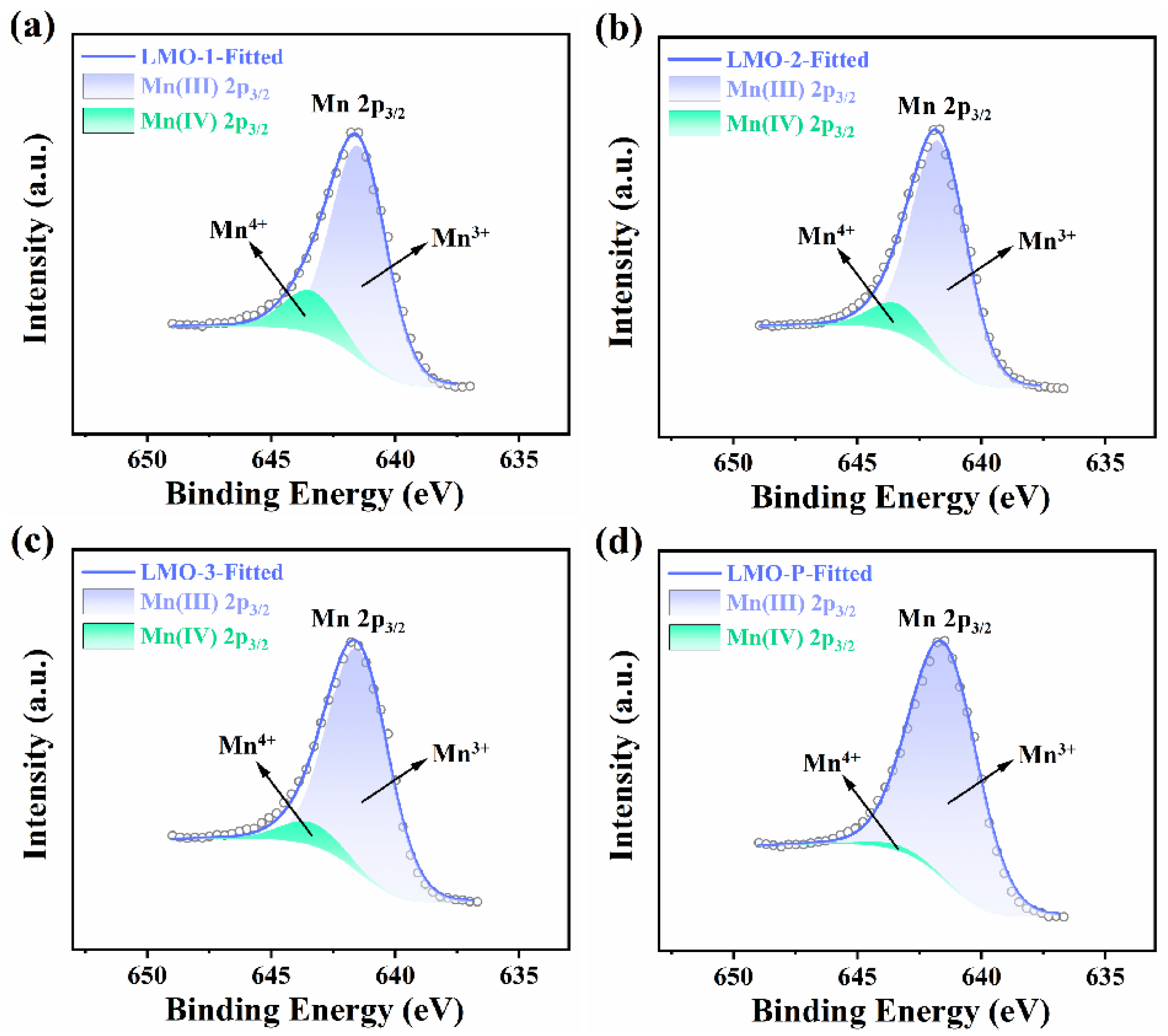
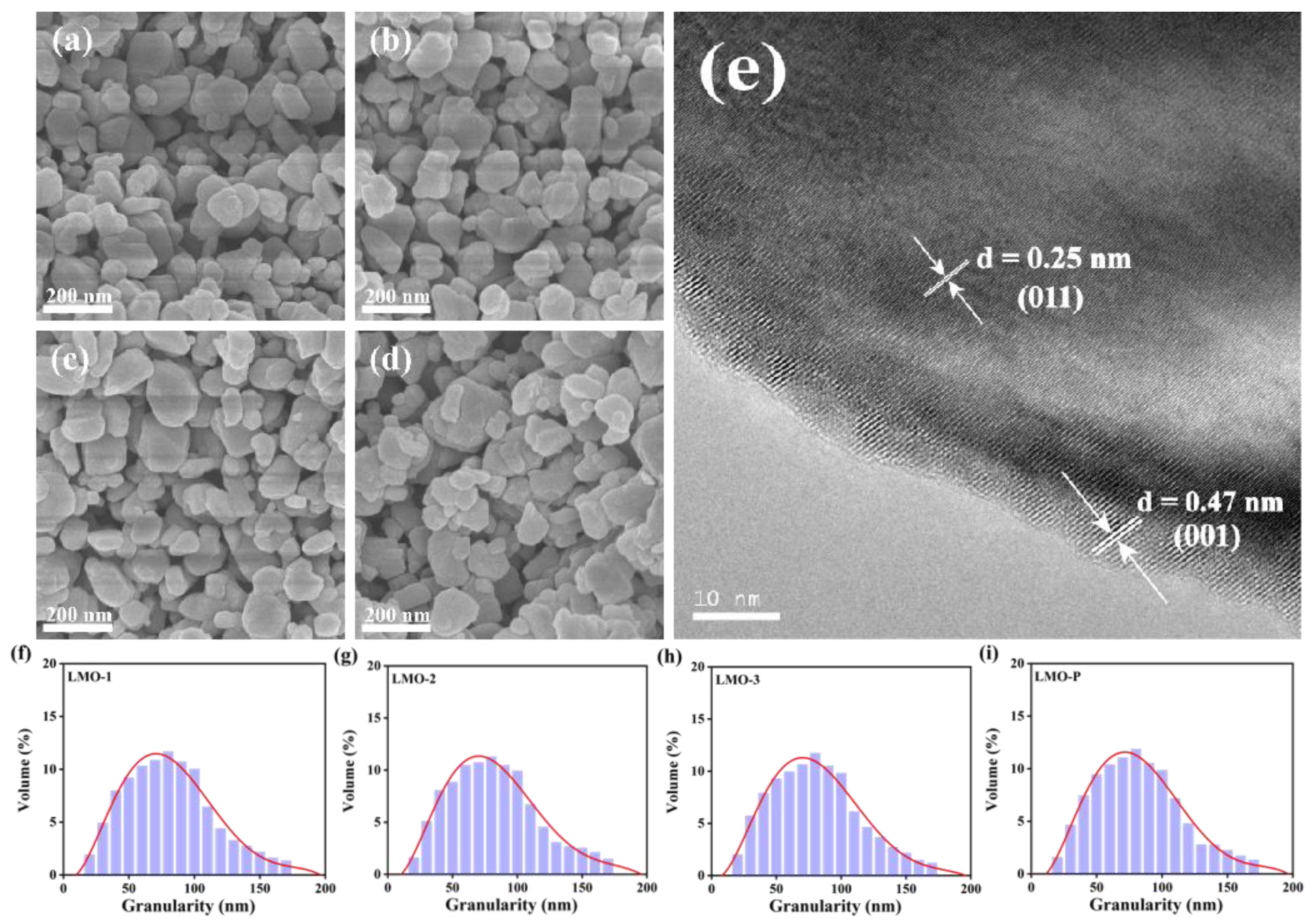

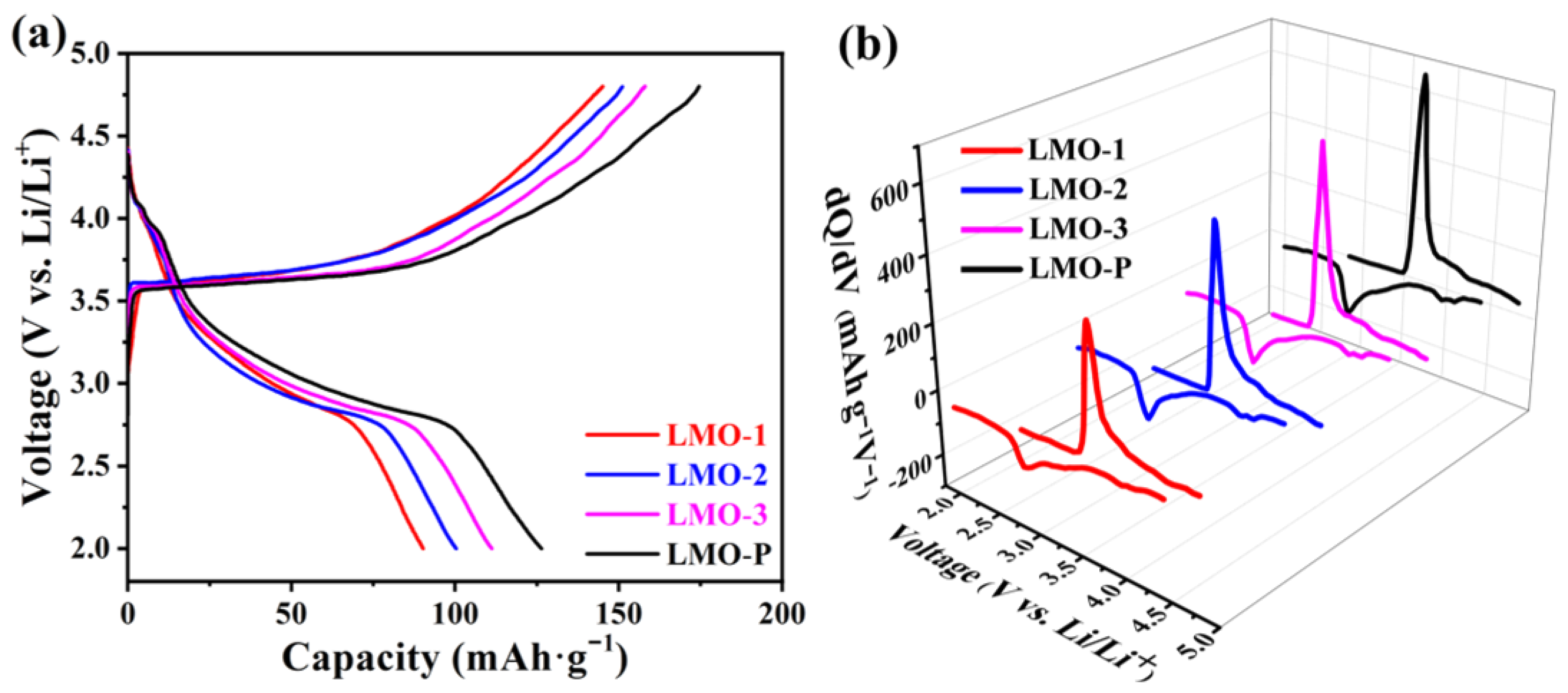
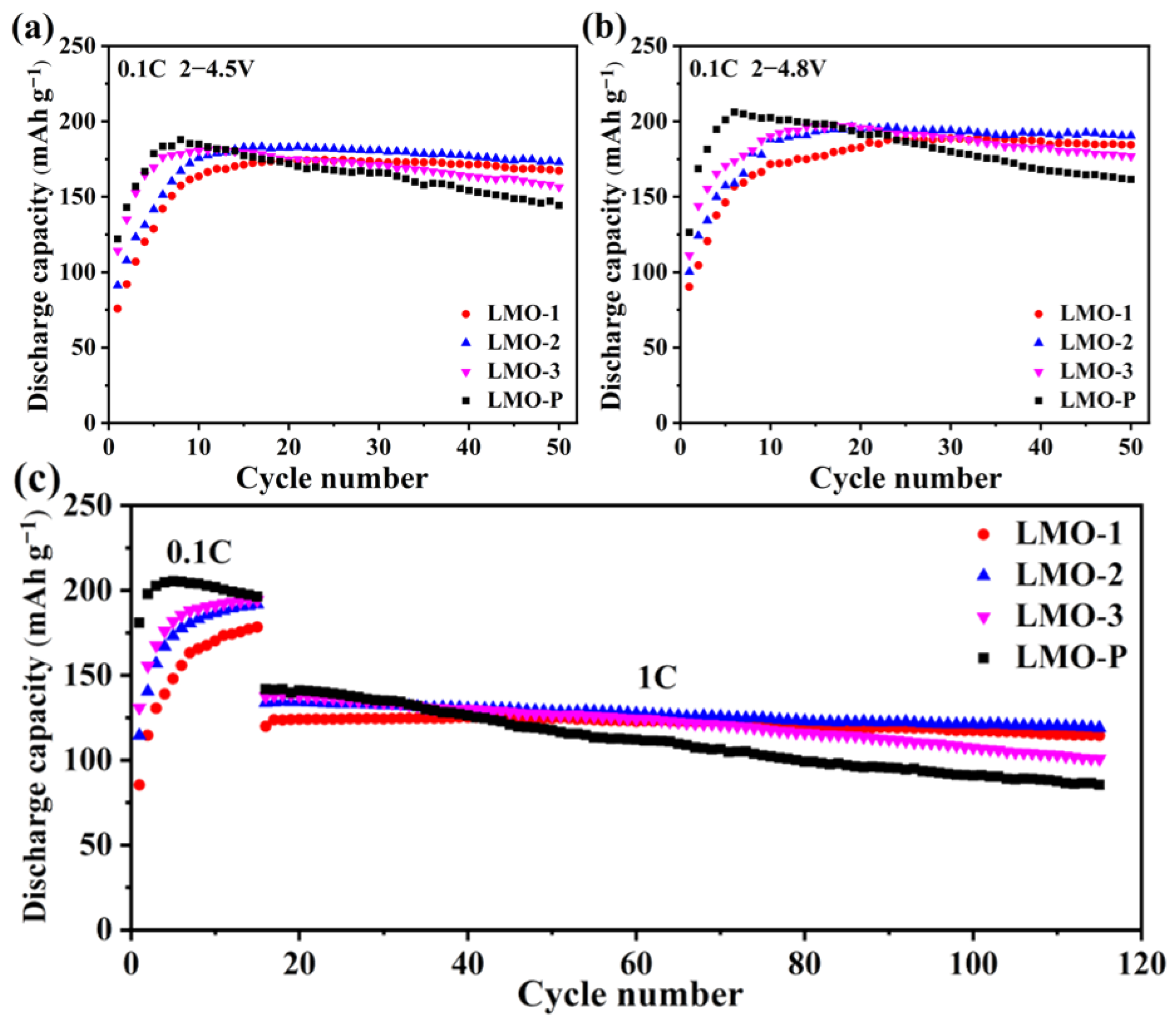
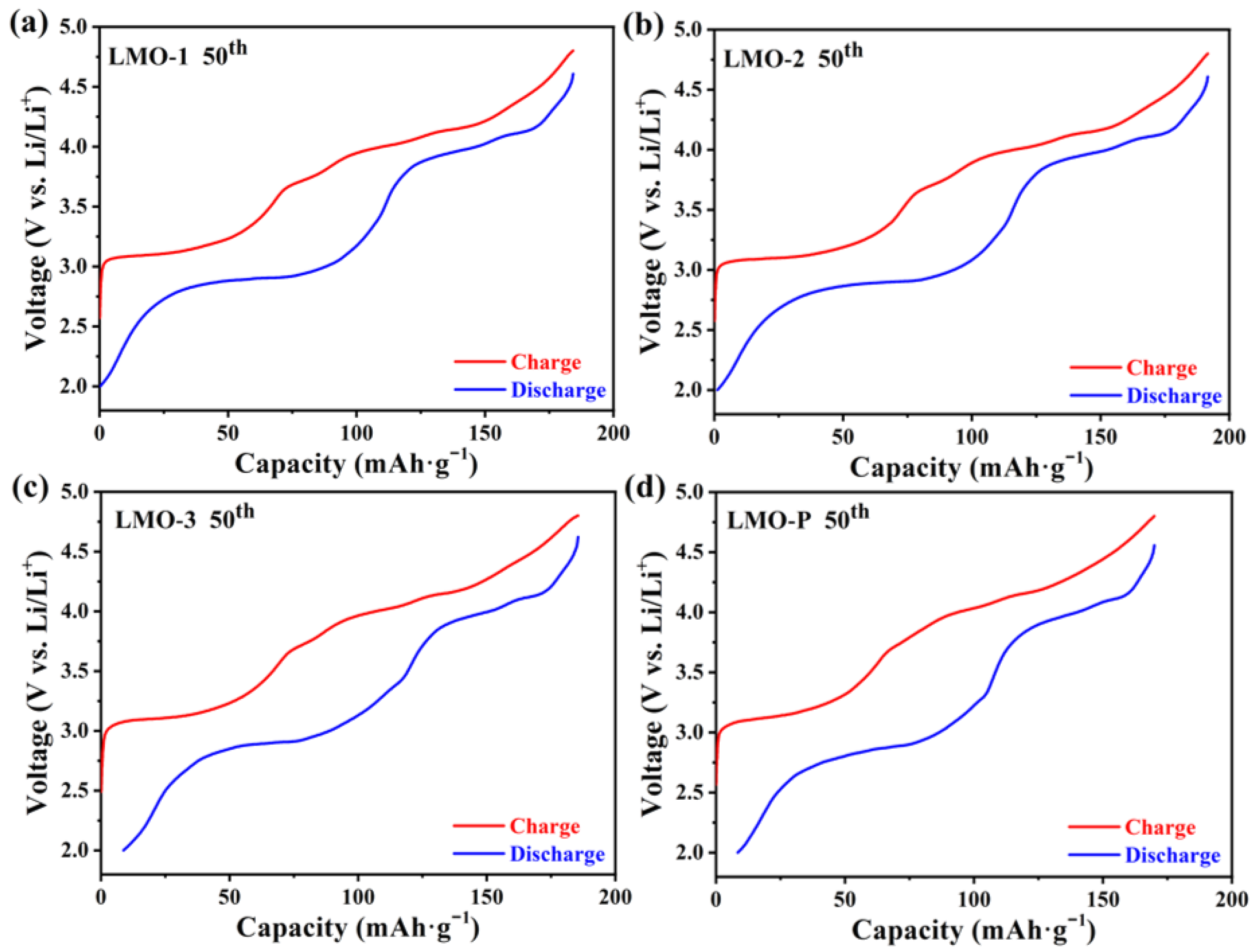
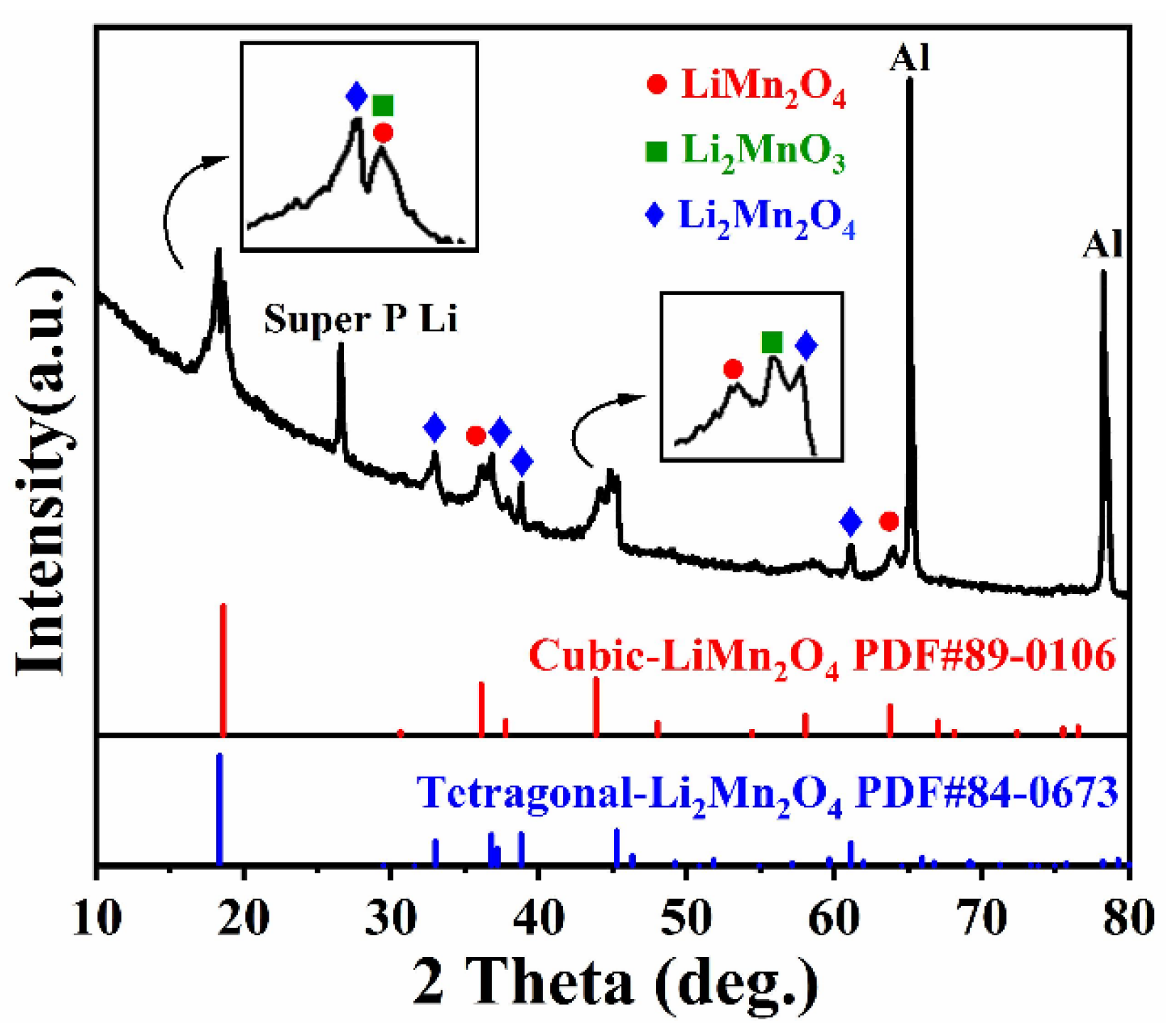
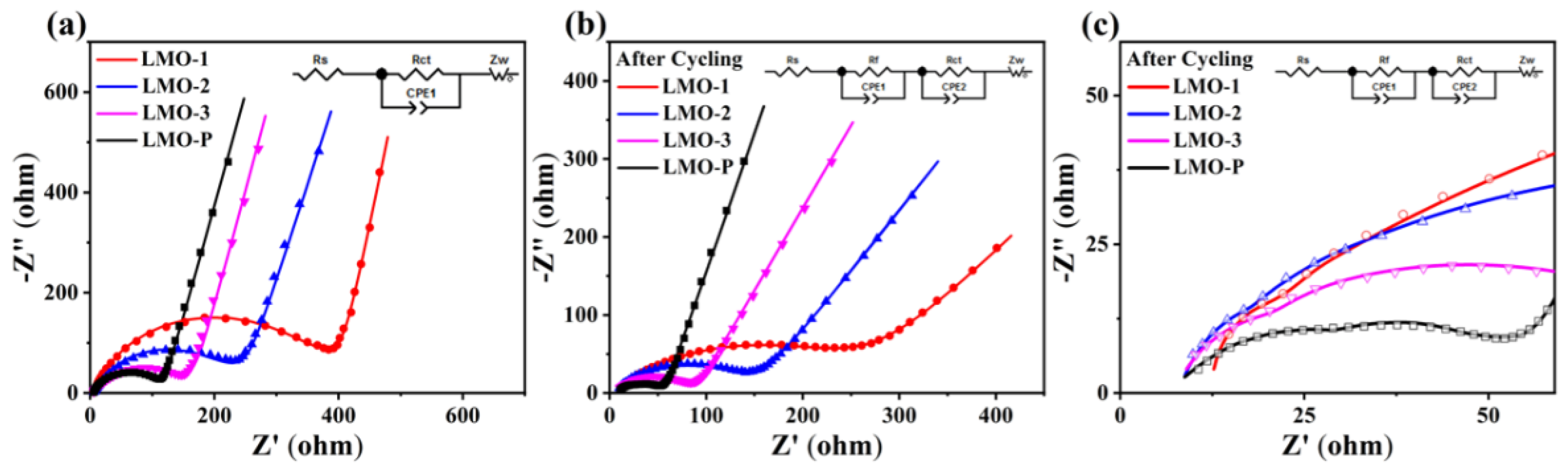
| Sample | Li/Mn exp | Li/Mn theoretical | Mn Valance exp | x Values in xLi2MnO3·(1 − x)LiMnO2 |
|---|---|---|---|---|
| o-LiMnO2 | 1 | 1 | 3 | 0 |
| Li2MnO3 | 2 | 2 | 4 | 1 |
| LMO-1 | 1.114 | \ | 3.114 | 0.114 |
| LMO-2 | 1.072 | \ | 3.072 | 0.072 |
| LMO-3 | 1.045 | \ | 3.045 | 0.045 |
| LMO-P | 1.009 | \ | 3.009 | 0.009 |
| Sample | Mn3+ | Mn4+ | x-Values in xLi2MnO3·(1 − x)LiMnO2 |
|---|---|---|---|
| LMO-1 | 88.8% | 11.2% | 0.112 |
| LMO-2 | 92.9% | 7.1% | 0.071 |
| LMO-3 | 95.3% | 4.7% | 0.047 |
| LMO-P | 99.3% | 0.7% | 0.007 |
| Samples | As Prepared | After Cycling | |||
|---|---|---|---|---|---|
| Rs (Ω) | Rct (Ω) | Rs (Ω) | Rf (Ω) | Rct (Ω) | |
| LMO-1 | 5.56 | 384.19 | 12.71 | 23.53 | 237.61 |
| LMO-2 | 5.88 | 274.01 | 8.80 | 24.88 | 134.10 |
| LMO-3 | 5.52 | 146.73 | 8.99 | 27.53 | 64.07 |
| LMO-P | 5.38 | 112.42 | 8.75 | 29.41 | 30.44 |
| Product | Method | Voltage Range | Synthesis Condition | Current Density (mA g−1) | Maximum/Selected Cycle Discharge Capacity (mAh g−1) | Reference |
|---|---|---|---|---|---|---|
| 0.23Li2MnO3·0.77LiMnO2 | Solid state | 2.0−4.5 V | 750 °C/20 h | 20 | 218/218 (30th) | [11] |
| 0.61Li2MnO3·0.39LiMnO2 | Sol–gel | 2.0−4.8 V | 600 °C/3 h 900 °C/12 h | 10 | 177/167 (30th) | [12] |
| 0.44Li2MnO3·0.56LiMnO2 | Hydrothermal + solid state + pyrolysis reduction | 2.0−4.8 V | 200 °C/2 h 450 °C/10 h 500 °C/15 h 340 °C/4 h | 30 | 270/200 (30th) | [13] |
| H0.46Li1.54MnO3 | Hydrothermal | 2.0−4.8 V | 180 °C/48 h | 200 | 208/120 (20th) | [14] |
| LiMnO2−Li2MnO3 | Hydrothermal | 2.0−4.5 V | 200 °C/72 h | 10 | 192/182 (5th) | [51] |
| o-LiMnO2 | Hydrothermal | 2.0−4.5 V | 160 °C/12 h | 20 | 173/162 (20th) | [52] |
| m-LiMnO2 Mixed m/o-LiMnO2 o-LiMnO2 | Hydrothermal | 2.0−4.5 V | 180 °C/4 h 180 °C/8 h 220 °C/8 h | 20 | 219.8/94.5 (50th) 198.8/112.5 (50th) 180.0/106.8 (50th) | [31] |
| o-LiMnO2 nanorods | Solid state | 2.0−4.25 V | 750 °C/10 h | 20 | 178.6/165.3 (40th) | [53] |
| Mesoporous o-LiMnO2 | Solid state | 2.0−4.4 V | 600 °C/3 h | 20 | 191.5/162.6 (50th) | [54] |
| o-LiMnO2 | Dynamic hydrothermal | 2.0−4.5 V | 200 °C/3 h | 30 | 166/145 (50th) | [28] |
| 0.072Li2MnO3·0.928LiMnO2 | Dynamic hydrothermal | 2.0−4.8 V | 200 °C/5 h | 30 | 198.4/190.5 (50th) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-B.; Hu, H.-S.; Lin, W.; Xu, Q.-H.; Gong, J.-D.; Chai, W.-K.; Shen, C.-Q. Electrochemically Inert Li2MnO3: The Key to Improving the Cycling Stability of Li-Rich Manganese Oxide Used in Lithium-Ion Batteries. Materials 2021, 14, 4751. https://doi.org/10.3390/ma14164751
Wang L-B, Hu H-S, Lin W, Xu Q-H, Gong J-D, Chai W-K, Shen C-Q. Electrochemically Inert Li2MnO3: The Key to Improving the Cycling Stability of Li-Rich Manganese Oxide Used in Lithium-Ion Batteries. Materials. 2021; 14(16):4751. https://doi.org/10.3390/ma14164751
Chicago/Turabian StyleWang, Lian-Bang, He-Shan Hu, Wei Lin, Qing-Hong Xu, Jia-Dong Gong, Wen-Kui Chai, and Chao-Qi Shen. 2021. "Electrochemically Inert Li2MnO3: The Key to Improving the Cycling Stability of Li-Rich Manganese Oxide Used in Lithium-Ion Batteries" Materials 14, no. 16: 4751. https://doi.org/10.3390/ma14164751
APA StyleWang, L.-B., Hu, H.-S., Lin, W., Xu, Q.-H., Gong, J.-D., Chai, W.-K., & Shen, C.-Q. (2021). Electrochemically Inert Li2MnO3: The Key to Improving the Cycling Stability of Li-Rich Manganese Oxide Used in Lithium-Ion Batteries. Materials, 14(16), 4751. https://doi.org/10.3390/ma14164751





