Green Composite Sensor for Monitoring Hydroxychloroquine in Different Water Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Cork-Graphite Sensor
2.3. Electrochemical Measurements
3. Results
3.1. Effects of Supporting Electrolyte
3.2. Effects of Scan Rate
3.3. DPV Analytical Curve
3.4. Determination of HCQ in Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO; IFRC; Unicef. Key Messages and Actions for Prevention and Control in Schools. Key Messag. Actions COVID-19 Prev. Control Sch. 2020, 1, 13. [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard; WTO: Geneva, Switzerland, 2021.
- Dagens, A.; Sigfrid, L.; Cai, E.; Lipworth, S.; Cheung, V.; Harris, E.; Bannister, P.; Rigby, I.; Horby, P. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: Rapid review. BMJ 2020, 369. [Google Scholar] [CrossRef]
- Fox, R. Anti-malarial drugs: Possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus 1996, 5, 219–234. [Google Scholar] [CrossRef]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An analysis of the spatio-temporal occurrence of anthelmintic veterinary drug residues in groundwater. Sci. Total Environ. 2021, 769, 144804. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.H.; Muir, D.C.G. Identifying new persistent and bioaccumulative organics among chemicals in commerce. III: Byproducts, impurities, and transformation products. Environ. Sci. Technol. 2013, 47, 5259–5266. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Boeing, W.J.; Borgomeo, E.; Xu, Y.; Weng, Y. Linking reservoir ecosystems research to the sustainable development goals. Sci. Total Environ. 2021, 781, 146769. [Google Scholar] [CrossRef] [PubMed]
- Dabić, D.; Babić, S.; Škorić, I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere 2019, 230, 268–277. [Google Scholar] [CrossRef]
- Bensalah, N.; Midassi, S.; Ahmad, M.I.; Bedoui, A. Degradation of hydroxychloroquine by electrochemical advanced oxidation processes. Chem. Eng. J. 2020, 402, 126279. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yu, S.; Hong, Y.W.; Lin, Q.Y.; Li, H.B. Pharmaceutical residues in tidal surface sediments of three rivers in southeastern China at detectable and measurable levels. Environ. Sci. Pollut. Res. 2013, 20, 8391–8403. [Google Scholar] [CrossRef] [PubMed]
- Arguelho, M.L.P.M.; Andrade, J.F.; Stradiotto, N.R. Electrochemical study of hydroxychloroquine and its determination in plaquenil by differential pulse voltammetry. J. Pharm. Biomed. Anal. 2003, 32, 269–275. [Google Scholar] [CrossRef]
- United States Pharmacopoeia; United States Pharmacopeial Convention: Rockville, MD, USA, 2007; p. 2311.
- British Pharmacopoeia; British Pharmacopoeia Commission, The Stationery Office: London, UK, 2008; p. 1099.
- Deroco, P.B.; Fatibello-Filho, O.; Arduini, F.; Moscone, D. Effect of Different Carbon Blacks on the Simultaneous Electroanalysis of Drugs as Water Contaminants Based on Screen-printed Sensors. Electro Anal. 2019, 31, 2145–2154. [Google Scholar] [CrossRef]
- Henrique, J.M.M.; Monteiro, M.K.S.; Cardozo, J.C.; Martínez-Huitle, C.A.; da Silva, D.R.; dos Santos, E.V. Integrated-electrochemical approaches powered by photovoltaic energy for detecting and treating paracetamol in water. J. Electroanal. Chem. 2020, 876, 114734. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Da Silva, D.R.; Quiroz, M.A.; Vilar, V.J.P.; Martínez-Huitle, C.A.; Dos Santos, E.V. Applicability of cork as novel modifiers to develop electrochemical sensor for caffeine determination. Materials 2021, 14, 37. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Paiva, S.S.M.; da Silva, D.R.; Vilar, V.J.P.; Martínez-Huitle, C.A.; dos Santos, E.V. Novel cork-graphite electrochemical sensor for voltammetric determination of caffeine. J. Electroanal. Chem. 2019, 839, 283–289. [Google Scholar] [CrossRef]
- Lourenção, B.C.; Medeiros, R.A.; Rocha-Filho, R.C.; Mazo, L.H.; Fatibello-Filho, O. Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta 2009, 78, 748–752. [Google Scholar] [CrossRef]
- Katseli, V.; Economou, A.; Kokkinos, C. A novel all-3D-printed cell-on-a-chip device as a useful electroanalytical tool: Application to the simultaneous voltammetric determination of caffeine and paracetamol. Talanta 2020, 208, 120388. [Google Scholar] [CrossRef]
- Silva, K.N.O.; Paiva, S.S.M.; Souza, F.L.; Silva, D.R.; Martínez-Huitle, C.A.; Santos, E.V. Applicability of electrochemical technologies for removing and monitoring Pb2+ from soil and water. J. Electroanal. Chem. 2018, 816, 171–178. [Google Scholar] [CrossRef]
- Zuliani, A.; Cano, M.; Calsolaro, F.; Puente Santiago, A.R.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Berlier, G.; Cravotto, G.; Martina, K.; Luque, R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels 2021, 5, 720–731. [Google Scholar] [CrossRef]
- Cova, C.M.; Zuliani, A.; Puente Santiago, A.R.; Caballero, A.; Muñoz-Batista, M.J.; Luque, R. Microwave-assisted preparation of Ag/Ag2S carbon hybrid structures from pig bristles as efficient HER catalysts. J. Mater. Chem. A 2018, 6, 21516–21523. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Santos, E.C.M.M.; Silva, D.R.; Martínez-Huitle, C.A.; dos Santos, E.V. Simultaneous determination of paracetamol and caffeine in pharmaceutical formulations and synthetic urine using cork-modified graphite electrodes. J. Solid State Electrochem 2020, 24, 1789–1800. [Google Scholar] [CrossRef]
- Silva, I.B.; de Araújo, D.M.; Vocciante, M.; Ferro, S.; Martínez-Huitle, C.A.; Dos Santos, E.V. Electrochemical determination of lead using a composite sensor obtained from low-cost green materials: Graphite/cork. Appl. Sci. 2021, 11, 2355. [Google Scholar] [CrossRef]
- Gil, L. New cork-based materials and applications. Materials 2015, 8, 625–637. [Google Scholar] [CrossRef] [Green Version]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Sabino, M.A.; Fernandas, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.P.; Bordado, J.C. Cork—a renewable raw material: Forecast of industrial potential and development priorities. Front. Mater. 2015, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.S.; Porto, P.S.S.; Pintor, A.M.A.; Ruphuy, G.; Costa, M.F.; Boaventura, R.A.R.; Vilar, V.J.P. New insights on the removal of mineral oil from oil-in-water emulsions using cork by-products: Effect of salt and surfactants content. Chem. Eng. J. 2016, 285, 709–717. [Google Scholar] [CrossRef]
- Liwka-Kaszyńska, M.S.; Kot-Wasik, A.; Namieśnik, J. Preservation and Storage of Water Samples. Crit. Rev. Environ. Sci. Technol. 2003, 33, 31–44. [Google Scholar] [CrossRef]
- Deroco, P.B.; Vicentini, F.C.; Oliveira, G.G.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-wave voltammetric determination of hydroxychloroquine in pharmaceutical and synthetic urine samples using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2014, 719, 19–23. [Google Scholar] [CrossRef]
- Jakubowska, M. Signal Processing in Electrochemistry. Electroanalysis 2011, 23, 553–572. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous determination of salbutamol and propranolol in biological fluid samples using an electrochemical sensor based on functionalized-graphene, ionic liquid and silver nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; ISBN 9780387331508. [Google Scholar]
- Currie, L.A. Nomenclature in Evaluation of Analytical Methods Including Detection and Quantification Capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Danzer, K.; Currie, L.A. Guideline for calibration in analytical chemistry—Part 1. Fundamentals and single component calibration. Pure Appl. Chem. 1998, 70, 993–1014. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. About estimating the limit of detection of heteroscedastic analytical systems. Anal. Chim. Acta. 2009, 655, 30–37. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. ELECTROCHEMICAL METHODS Fundamentals and Applications, Bard, A.J., Faulkner, L.R., Eds.; 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Mater Mahnashi, H.; Mahmoud, A.M.; Saad Alkahtani, A.; El-Wekil, M.M. Simultaneous electrochemical detection of azithromycin and hydroxychloroquine based on VS2 QDs embedded N, S @graphene aerogel/cCNTs 3D nanostructure. Microchem. J. 2021, 163, 105925. [Google Scholar] [CrossRef]
- Khoobi, A.; Mehdi Ghoreishi, S.; Behpour, M. Sensitive and selective determination of hydroxychloroquine in the presence of uric acid using a new nanostructure self-assembled monolayer modified electrode: Optimization by multivariate data analysis. Analyst 2014, 139, 4064–4072. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Attaran, A.M.; Amin, A.M.; Khoobi, A. Multiwall carbon nanotube-modified electrode as a nanosensor for electrochemical studies and stripping voltammetric determination of an antimalarial drug. RSC Adv. 2015, 5, 14407–14415. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Khoobi, A.; Salavati-Niasari, M. Electrochemical study of a self-assembled monolayer of N,N′-bis[(E)-(1-pyridyl) methylidene]-1,3-propanediamine formed on glassy carbon electrode: Preparation, characterization and application. Anal. Methods 2013, 5, 6727–6733. [Google Scholar] [CrossRef]
- Khoobi, A.; Ghoreishi, S.M.; Behpour, M.; Shaterian, M.; Salavati-Niasari, M. Design and evaluation of a highly sensitive nanostructure-based surface modification of glassy carbon electrode for electrochemical studies of hydroxychloroquine in the presence of acetaminophen. Colloids Surf. B Biointerfaces 2014, 123, 648–656. [Google Scholar] [CrossRef]
- Khalil, M.M.; El-aziz, G.M.A.; Ashry, A. Potentiometric sensors based on hydroxychloroquine-phosphotungstate ion-pair and β-cyclodextrin ionophore for improved determination of hydroxychloroquine sulfate. J. Iran. Chem. Soc. 2018, 15, 2411–2421. [Google Scholar] [CrossRef]
- George, J.M.; Mathew, B. Cyclodextrin-mediated gold nanoparticles as multisensing probe for the selective detection of hydroxychloroquine drug. Korean J. Chem. Eng. 2021, 38, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A. Environment-Friendly Electrochemical Processes. Materials 2021, 14, 1548. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemistry Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
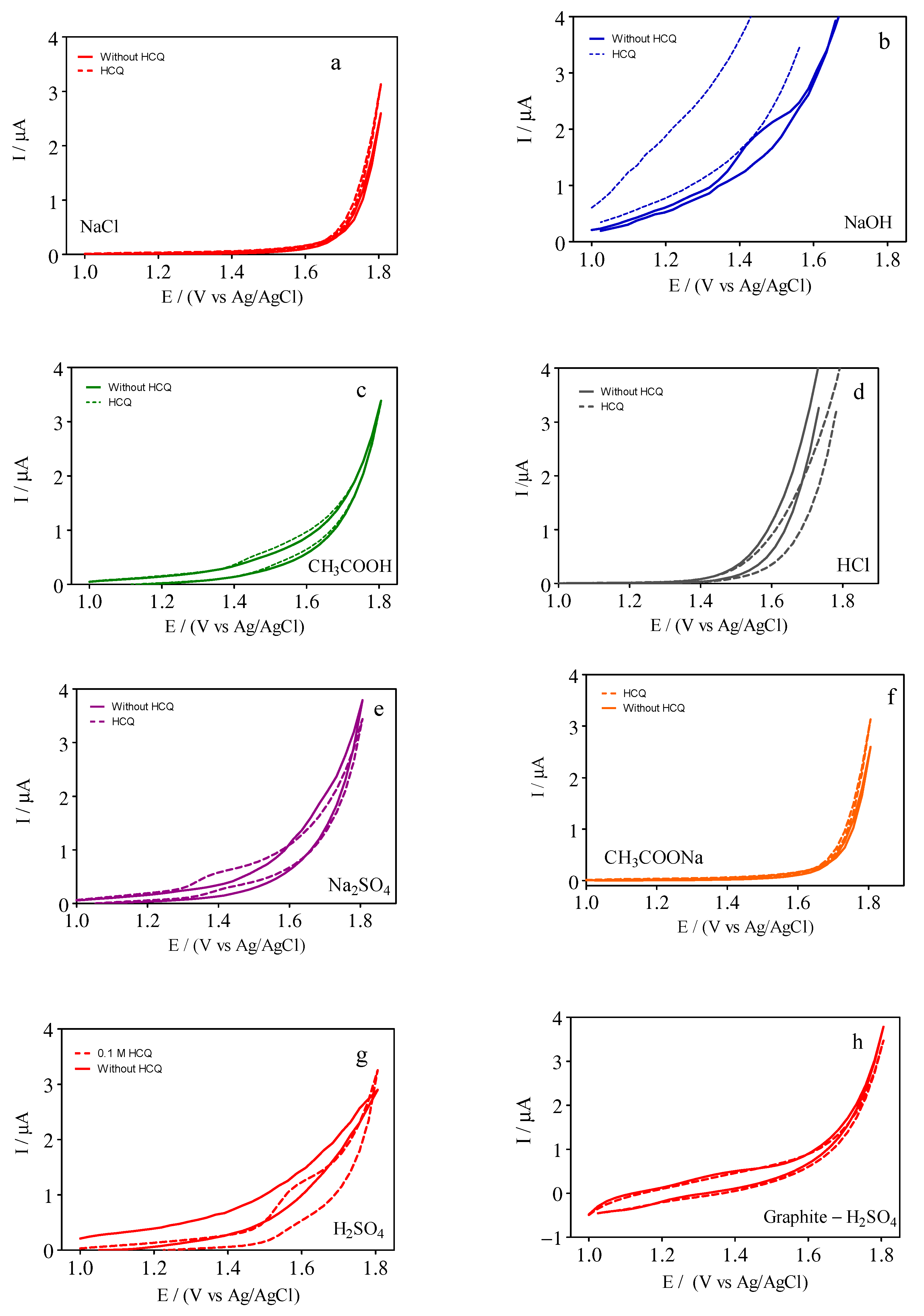
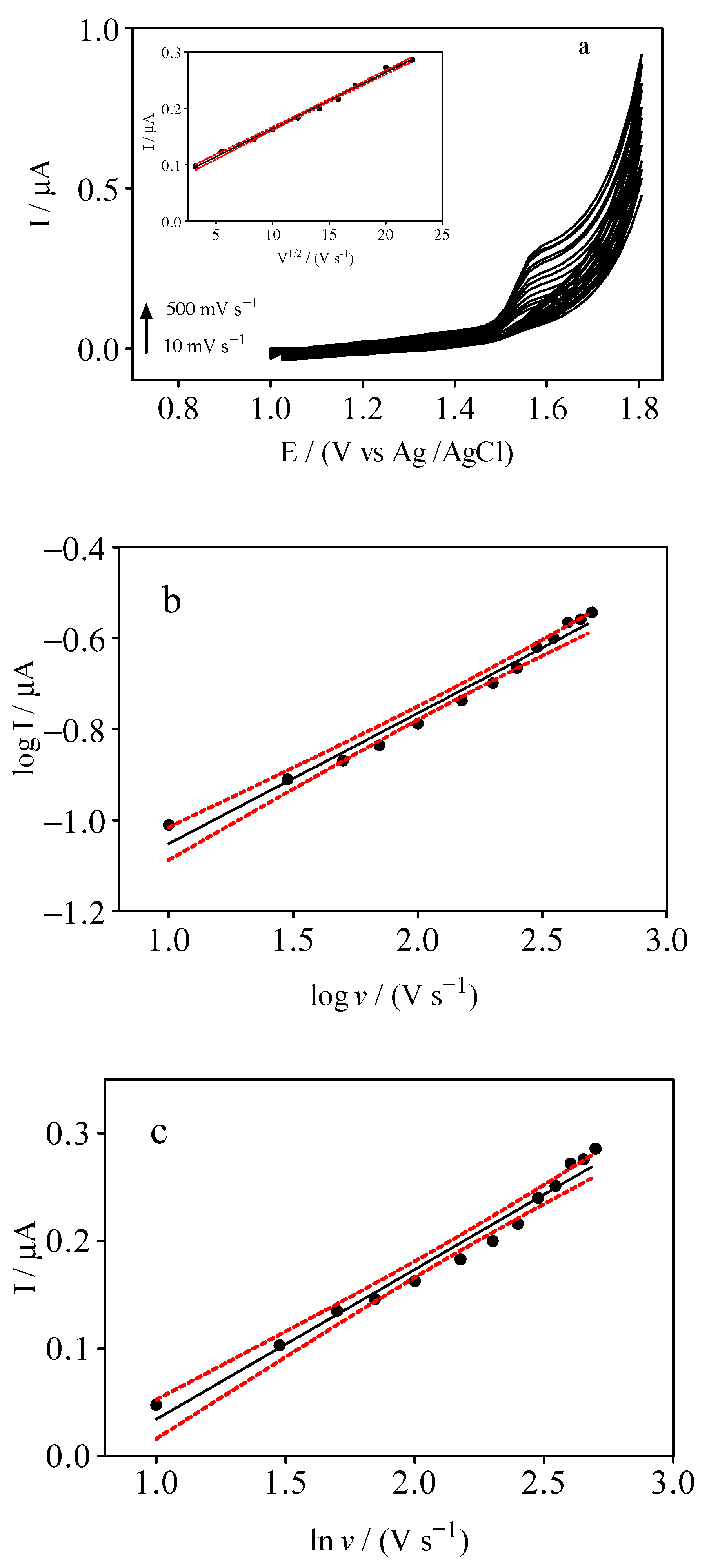
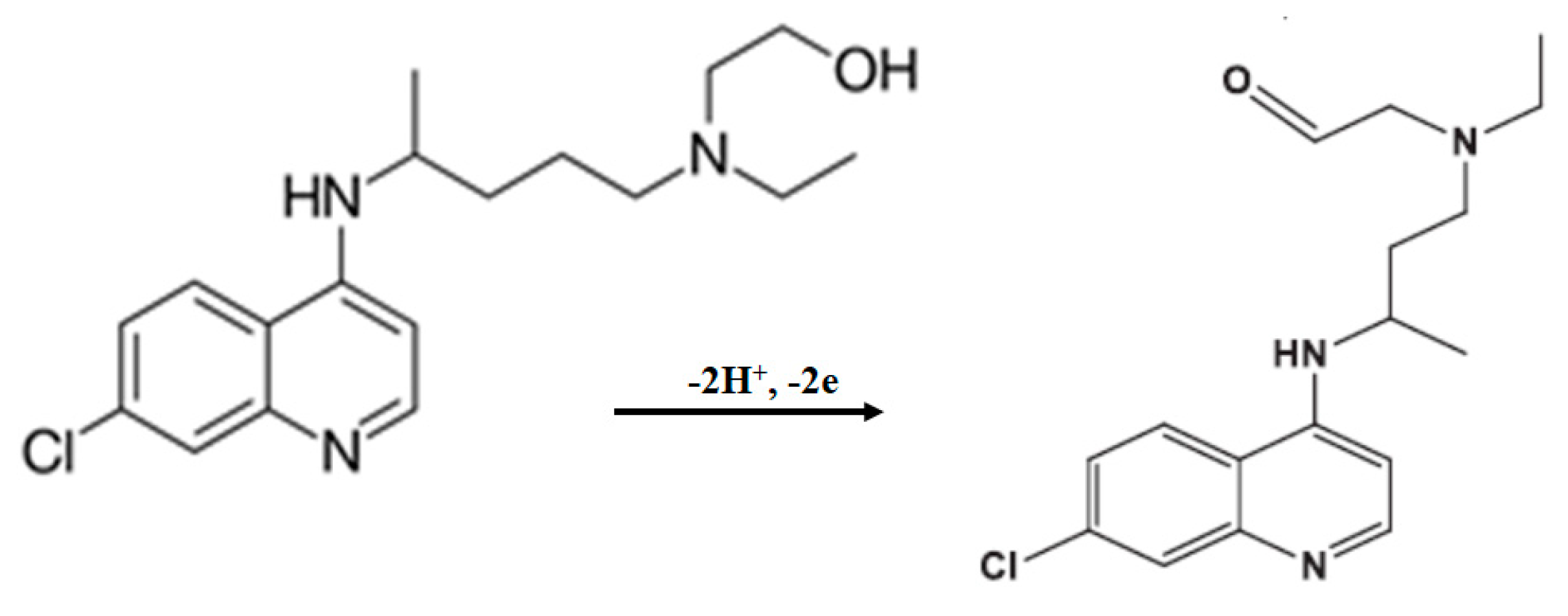
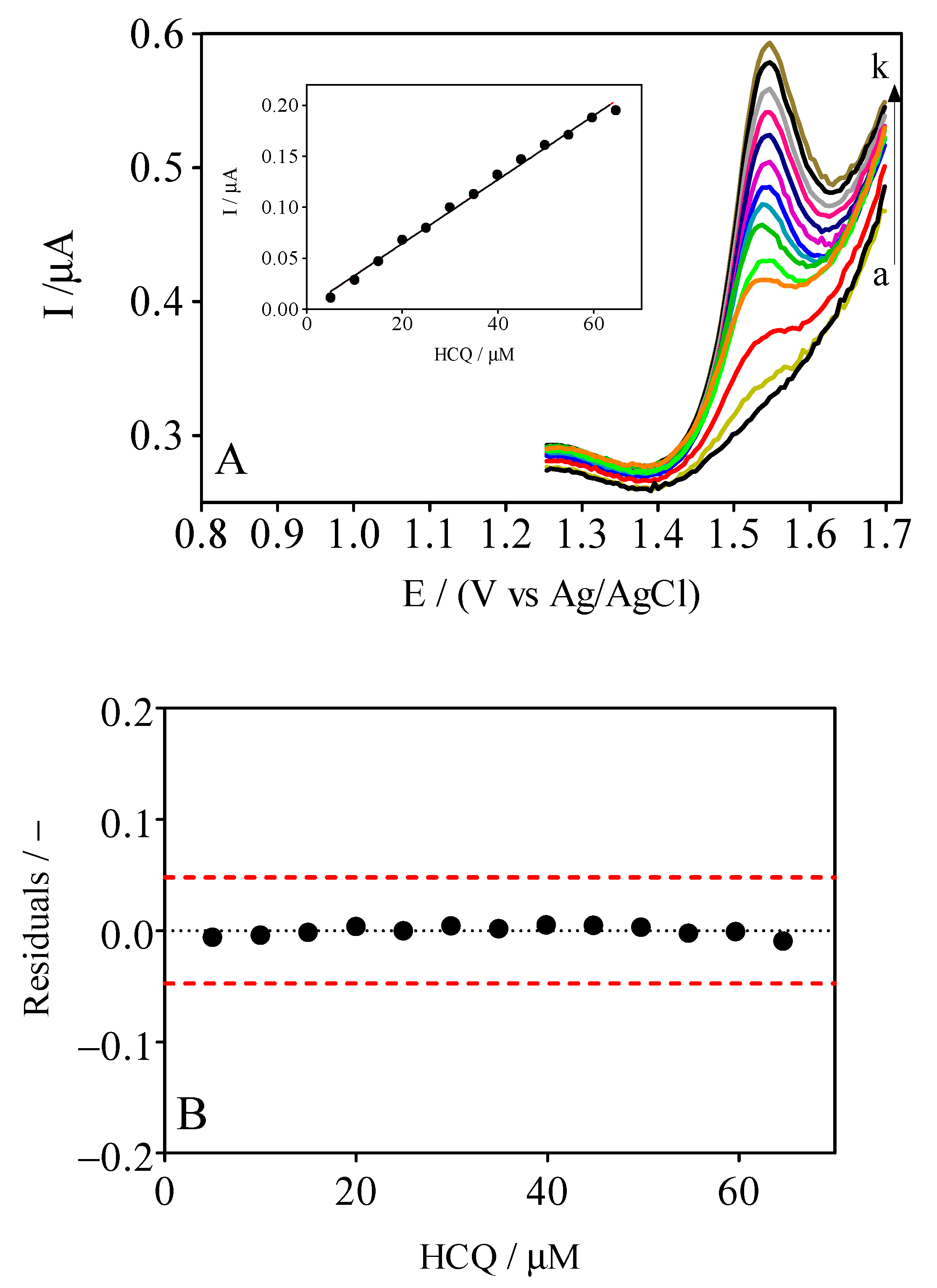

| Electrolyte | Ep/mV | ip/µA | ip/µA mm−2 |
|---|---|---|---|
| NaCl | - | - | - |
| NaOH | +1.45 | 1.89 | 15.7 |
| CH3COOH | +1.45 | 0.33 | 0.28 |
| HCl | - | - | - |
| Na2SO4 | +1.40 | 0.058 | 0.48 |
| CH3COONa | - | - | |
| H2SO4 | +1.55 | 1.11 | 9.25 |
| Electrodes | Method | Electrolyte | Linear Range µM | LOD/µM | Ref. |
|---|---|---|---|---|---|
| GrRAC | DPV | 0.1 M H2SO4 | 5–65 | 1.05 | This work |
| 5 Modified carbon paste | Potentiometric titration | 0.01 M Sodium tetraphenylborate | 1–1000 | 0.78 0.46 | [45] |
| Glassy carbon | DPV | B-R buffer (pH = 4.0) | 35–100 | 0.336 | [11] |
| 4 VS2 QDs | DPV | B.R. buffer (pH = 6.0) | 0.84–22.5 | 0.277 | [40] |
| 6 BDD | SWV | 0.1 M H2SO4 | 0.1–1.9 | 0.06 | [31] |
| 3 MWCNTs/CPE | AdSDPV | Phosphate buffer (pH = 8.0) | 0.57–100 | 0.006 | [42] |
| 1 GC-PMPD SAM | DPV | B–R buffer (pH = 8.0) | 0.05–12.8 12.3–111 | 0.00451 | [43] |
| 1 GC-PMPDA SAM | DPV | B-R buffer (pH = 8.0) | 0.09–10.2 10.2–98.2 | 0.00465 | [44] |
| Schiff’s base modified GCE | DPV | B.R. solution (pH = 6.0) | 0.007–11.9 | 0.00465 | [41] |
| 2 β-CD-AuNP | DPV | Phosphate buffer (pH = 6.0) | 0.01–0.05 | 0.00261 | [46] |
| Sample | Present Method | HCQ Added/µM | HCQ Found/µM | Recovery (%) |
|---|---|---|---|---|
| River | UV-vis | 69.5 | 83.67 ± 0.52 | 120.4 |
| DPV * | 69.5 | 108.0 ± 8.38 | 144.0 | |
| Lagoon | UV-vis | 69.5 | 84.67 ± 1.18 | 121.8 |
| DPV * | 69.5 | 76.20 ± 1.08 | 111.0 | |
| Tap water | UV-vis | 69.5 | 80.00 ± 0.67 | 110.0 |
| DPV * | 69.5 | 83.50 ± 0.35 | 120.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, D.M.; Paiva, S.d.S.M.; Henrique, J.M.M.; Martínez-Huitle, C.A.; Dos Santos, E.V. Green Composite Sensor for Monitoring Hydroxychloroquine in Different Water Matrix. Materials 2021, 14, 4990. https://doi.org/10.3390/ma14174990
de Araújo DM, Paiva SdSM, Henrique JMM, Martínez-Huitle CA, Dos Santos EV. Green Composite Sensor for Monitoring Hydroxychloroquine in Different Water Matrix. Materials. 2021; 14(17):4990. https://doi.org/10.3390/ma14174990
Chicago/Turabian Stylede Araújo, Danyelle M., Suelya da Silva M. Paiva, João Miller M. Henrique, Carlos A. Martínez-Huitle, and Elisama V. Dos Santos. 2021. "Green Composite Sensor for Monitoring Hydroxychloroquine in Different Water Matrix" Materials 14, no. 17: 4990. https://doi.org/10.3390/ma14174990







