A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water
Abstract
:1. Introduction
2. Physicochemical Properties of Zeolites and Mesoporous Silica Materials
2.1. Zeolites
2.2. Mesoporous Silica Materials (MCM-41 and SBA-15)
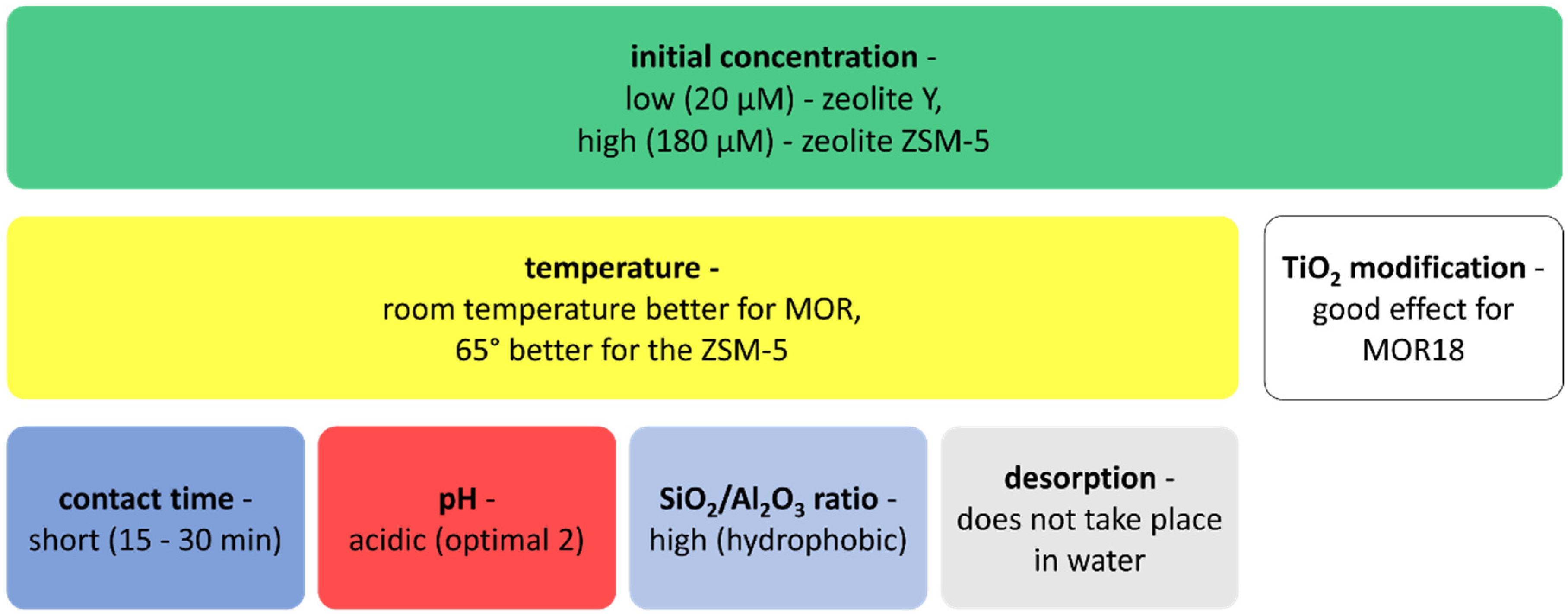
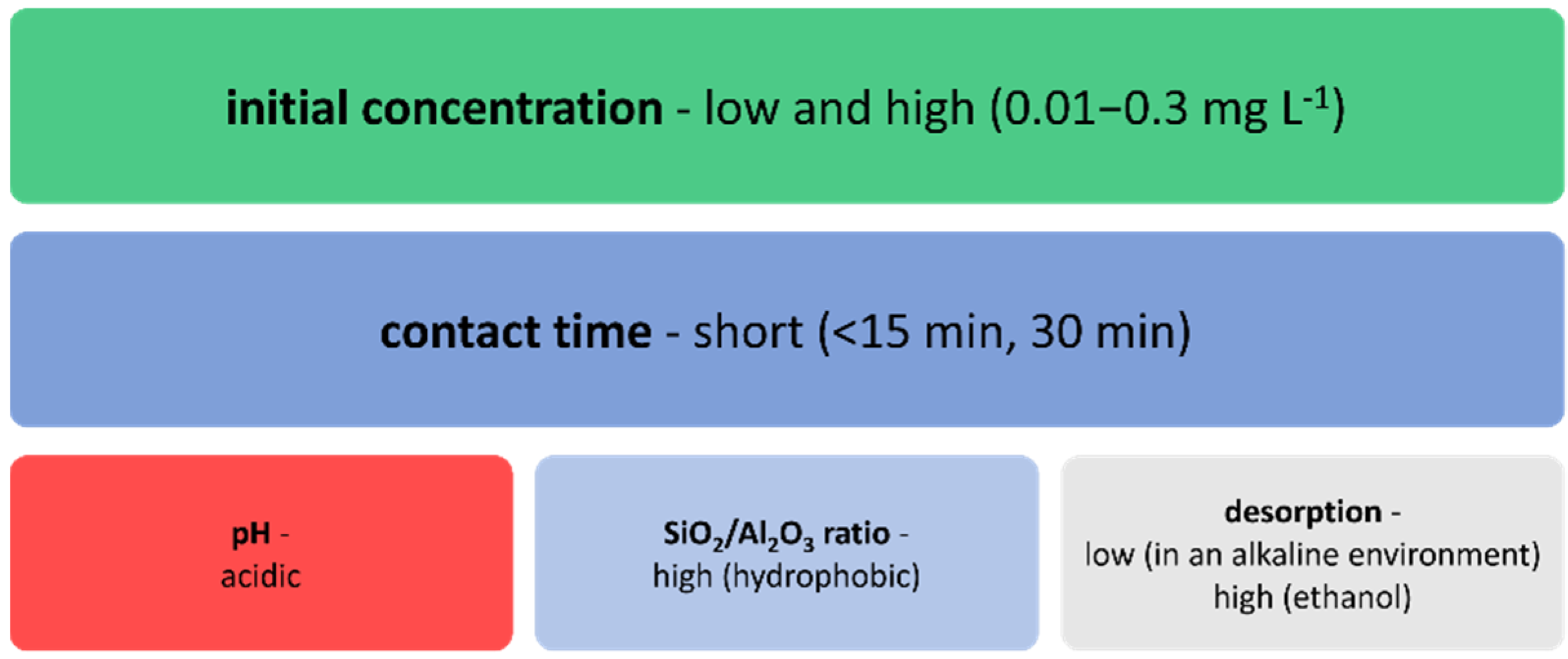
3. Factors Affecting the Adsorption of NSAIDs and Antibiotics by Zeolites and Mesoporous Silica Materials
3.1. Characteristics of the Selected NSAIDs and Antibiotics
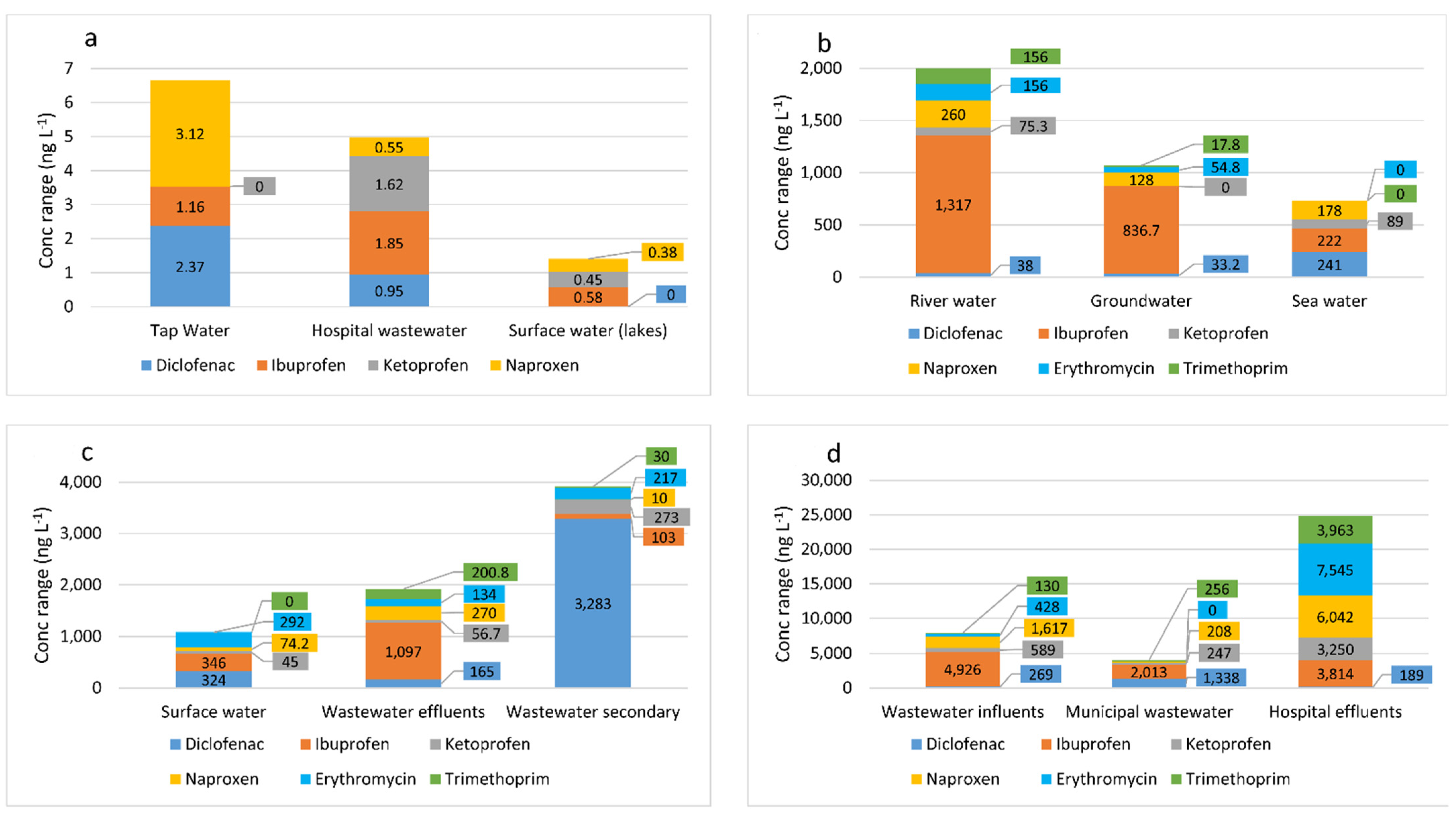
3.2. Methods of Extraction and Determination of NSAIDs and Antibiotics in the Aquatic Environment
4. Potential Applications of Zeolites and Mesoporous Silica Materials in Water Treatment—Discussion
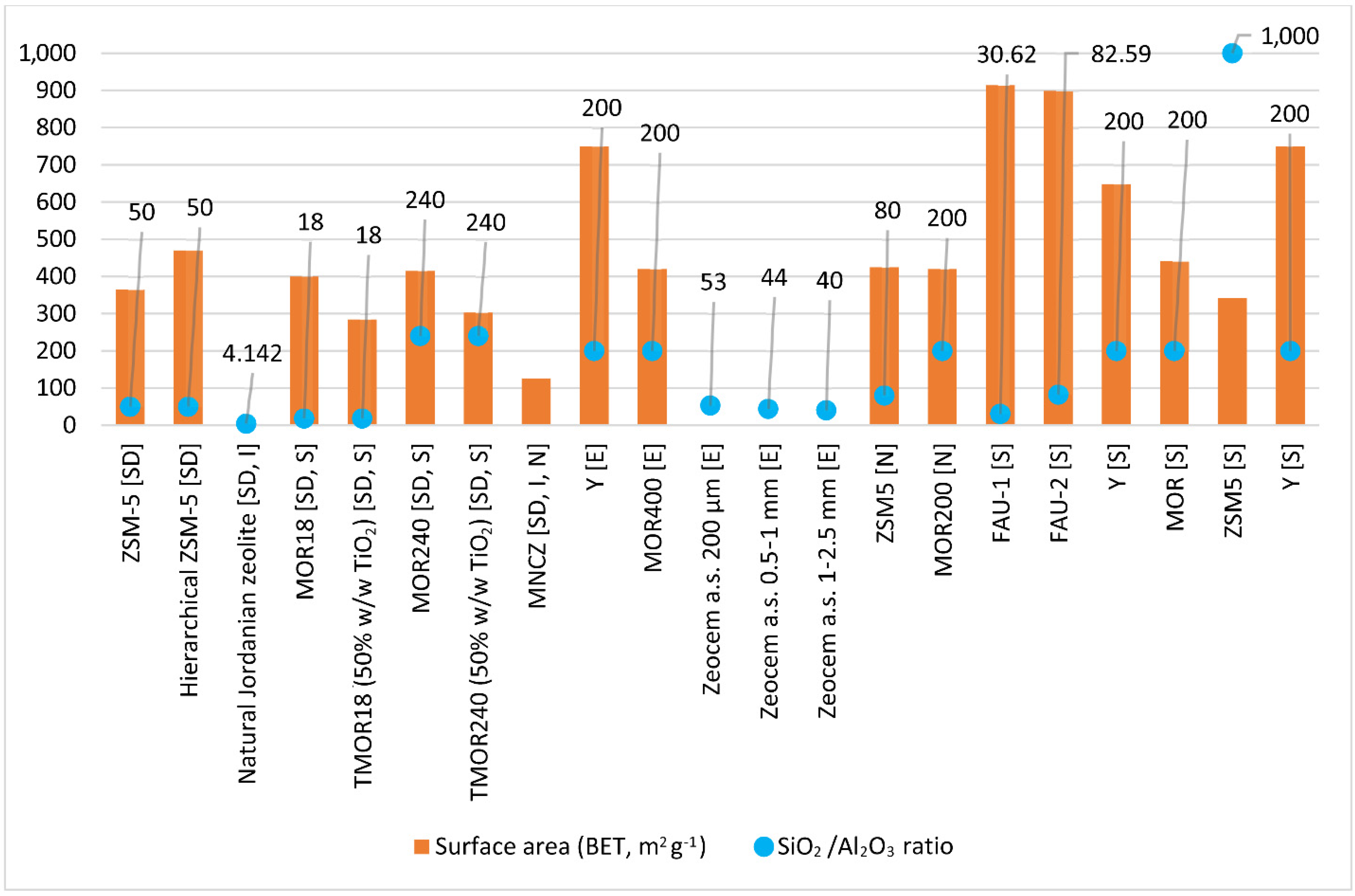
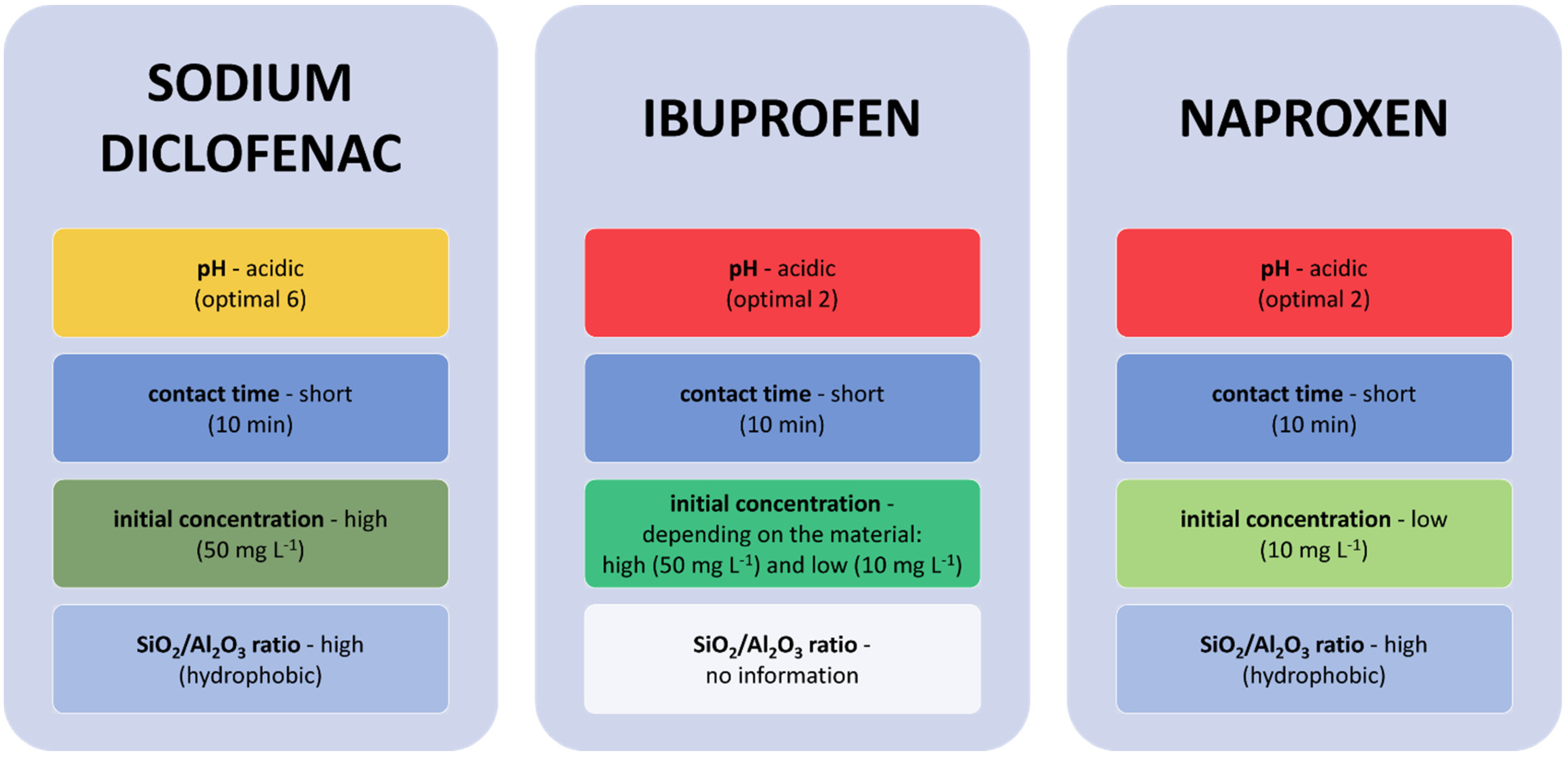
4.1. Adsorption of Selected NSAIDs (Sodium Diclofenac, Ibuprofen, and Naproxen) and Selected Antibiotics (Erythromycin and Sulfamethoxazole) on Zeolites
4.2. Adsorption of Selected NSAIDs (Diclofenac, Ibuprofen, and Ketoprofen) and Selected Antibiotics (Sulfamethoxazole, Tetracycline, and Trimethoprim) on MCM-41 and SBA-15
5. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; García Maria Ángeles, F.; Prados-Joya, G.; Perez, R.O. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Gómez-Pacheco, C.V.; Sanchez-Polo, M.; Peñalver, J.J.L.; Perez, R.O. Tetracycline removal from water by adsorption/bioadsorption on activated carbons and sludge-derived adsorbents. J. Environ. Manag. 2013, 131, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. Antimicrobial agents. Curr. Opin. Chem. Biol. 1997, 1, 169–175. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment- A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Alba, A.R.F.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Schafhauser, B.H.; Kristofco, L.; de Oliveira, C.M.R.; Brooks, B.W. Global review and analysis of erythromycin in the environment: Occurrence, bioaccumulation and antibiotic resistance hazards. Environ. Pollut. 2018, 238, 440–451. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Der Beek, T.A.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Larsson, D.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of Pharmaceuticals in Sewage Treatment Plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of Pharmaceuticals during Drinking Water Treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of Endocrine-Disruptor, Pharmaceutical, and Personal Care Product Chemicals during Simulated Drinking Water Treatment Processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhuka, V.; Dube, S.; Nindi, M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-OrbitrapTM MS. Emerg. Contam. 2020, 6, 250–258. [Google Scholar] [CrossRef]
- WHO. WHO Model Lists of Essential Medicines; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Yang, S.; Carlson, K. Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Res. 2003, 37, 4645–4656. [Google Scholar] [CrossRef]
- Kulkarni, P.; Olson, N.D.; Raspanti, G.A.; Goldstein, R.E.R.; Gibbs, S.G.; Sapkota, A.; Sapkota, A.R. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. Int. J. Environ. Res. Public Health 2017, 14, 668. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.-Q.; Wang, R.; Feng, L.; Zhang, L.-Q. Determination of selected pharmaceuticals in tap water and drinking water treatment plant by high-performance liquid chromatography-triple quadrupole mass spectrometer in Beijing, China. Environ. Sci. Pollut. Res. 2015, 22, 1854–1867. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Ta, T.T. A Preliminary Study on the Occurrence of Pharmaceutically Active Compounds in Hospital Wastewater and Surface Water in Hanoi, Vietnam. CLEAN Soil Air Water 2014, 42, 267–275. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total. Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Lolić, A.; Paíga, P.; Santos, L.H.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: Occurrence and environmental risk. Sci. Total. Environ. 2015, 508, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovic, M.; Skrbic, B.; Živančev, J.; Climent, L.F.; Barceló, D. Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole–linear ion trap in different types of water in Serbia. Sci. Total. Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef]
- Santos, L.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total. Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallenborn, R.; Fick, J.; Lindberg, R.; Moe, M.; Nielsen, K.M.; Tysklind, M.; Vasskog, T. Pharmaceutical Residues in Northern European Environments: Consequences and Perspectives. In Pharmaceuticals in the Environment; Springer: Berlin/Heidelberg, Germany, 2008; pp. 61–74. [Google Scholar]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemisty. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Römbke, J.; Meller, M.; Ternes, T.A. Environmental Fate of Pharmaceuticals in Water/Sediment Systems. Environ. Sci. Technol. 2005, 39, 5209–5218. [Google Scholar] [CrossRef]
- Li, W. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Lapworth, D.; Baran, N.; Stuart, M.; Ward, R. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Gin, K.Y.-H.; Lin, A.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total. Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; Siegrist, H. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barceló, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Bound, J.P.; Voulvoulis, N. Household Disposal of Pharmaceuticals as a Pathway for Aquatic Contamination in the United Kingdom. Environ. Health Perspect. 2005, 113, 1705–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, C.; Johansson, A.K.; Alvan, G.; Bergman, K.; Kühler, T. Are pharmaceuticals potent environmental pollutants?: Part I: Environmental risk assessments of selected active pharmaceutical ingredients. Sci. Total Environ. 2006, 364, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of antibiotics in the aquatic environment. Sci. Total. Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Huschek, G.; Hansen, P.D.; Maurer, H.H.; Krengel, D.; Kayser, A. Environmental risk assesssment of medicinal products for human use according to European Commission recommendations. Environ. Toxicol. 2004, 19, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Chaudhry, M.J.I.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Scheil, V.; Schwaiger, J. Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio). Anal. Bioanal. Chem. 2007, 387, 1405–1416. [Google Scholar] [CrossRef]
- Liu, F.; Ying, G.-G.; Tao, R.; Zhao, J.-L.; Yang, J.-F.; Zhao, L.-F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar] [CrossRef]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total. Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef]
- Gebhardt, W.; Schröder, H.F. Liquid chromatography–(tandem) mass spectrometry for the follow-up of the elimination of persistent pharmaceuticals during wastewater treatment applying biological wastewater treatment and advanced oxidation. J. Chromatogr. A 2007, 1160, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Khraisheh, M.A.; Ahmad, M.N.; Allen, S. Adsorption behaviour of methylene blue onto Jordanian diatomite: A kinetic study. J. Hazard. Mater. 2009, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Brown, J.M.; Allen, S.J. The application of kudzu as a medium for the adsorption of heavy metals from dilute aqueous wastestreams. Bioresour. Technol. 2001, 78, 195–201. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of basic dyes from aqueous solution onto activated carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G. Adsorption of Methylene Blue onto activated carbon produced from steam activated bituminous coal: A study of equilibrium adsorption isotherm. Chem. Eng. J. 2006, 124, 103–110. [Google Scholar] [CrossRef]
- Vieno, N.M.; Härkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of Pharmaceuticals in River Water and Their Elimination in a Pilot-Scale Drinking Water Treatment Plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masters, A.F.; Maschmeyer, T. Zeolites—From curiosity to cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Valdés, M.G.; Pérez-Cordoves, A.; Díaz-García, M. Zeolites and zeolite-based materials in analytical chemistry. TrAC Trends Anal. Chem. 2006, 25, 24–30. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M.; Franus, M. Synthesis and characterization of zeolites prepared from industrial fly ash. Environ. Monit. Assess. 2014, 186, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Franus, M.; Wdowin, M.; Bandura, L.; Franus, W. Removal of environmental pollutions using zeolites from fly ash: A review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Niwa, M.; Katada, N.; Okumura, K. Introduction to Zeolite Science and Catalysis. In Superconductivity; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; Volume 141, pp. 1–8. [Google Scholar]
- Li, Y.; Yu, J. New Stories of Zeolite Structures: Their Descriptions, Determinations, Predictions, and Evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Burton, A. Recent trends in the synthesis of high-silica zeolites. Catal. Rev. 2018, 60, 132–175. [Google Scholar] [CrossRef]
- Burton, A.W.; Zones, S.I.; Elomari, S. The chemistry of phase selectivity in the synthesis of high-silica zeolites. Curr. Opin. Colloid Interface Sci. 2005, 10, 211–219. [Google Scholar] [CrossRef]
- Maesen, T. The zeolite scene—An overview. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2007; Volume 168, pp. 1–12. ISBN 0444530630. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; Available online: https://www.elsevier.com/books/atlas-of-zeolite-framework-types/baerlocher/978-0-444-53064-6 (accessed on 24 September 2020).
- Foster, M.; Rivin, I.; Treacy, M.; Friedrichs, O.D. A geometric solution to the largest-free-sphere problem in zeolite frameworks. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Meier, W.M.; Baerlocher, C. Zeolite type frameworks: Connectivities, configurations and conformations. In Structures and Structure Determination; Springer: Berlin/Heidelberg, Germany, 1999; pp. 141–161. [Google Scholar]
- Feng, S.-H.; Li, G.-H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar]
- Wang, Y.U.J.; Jia, D.E.A.N.; Rui-Juan, S.U.N.; Hao-Wen, Z.H.U.; Zhou, D.M. Adsorption and cosorption of tetracycline and copper(ll) on montmorillonite as affected by solution pH. Environ. Sci. Technol. 2008, 42, 3254–3259. [Google Scholar] [CrossRef]
- Miyake, M.; Tamura, C.; Matsuda, M. Resource Recovery of Waste Incineration Fly Ash: Synthesis of Zeolites A and P. J. Am. Ceram. Soc. 2004, 85, 1873–1875. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C. Effects of step-change of synthesis temperature on synthesis of zeolite 4A from coal fly ash. Microporous Mesoporous Mater. 2006, 88, 145–151. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, A. Effect of stirring on the dissolution of coal fly ash and synthesis of pure-form Na-A and -X zeolites by two-step process. Adv. Powder Technol. 2009, 20, 473–479. [Google Scholar] [CrossRef]
- Nascimento, M.; Soares, P.S.M.; Souza, V.P. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- Ma, W.; Brown, P.W.; Komarneni, S. Characterization and cation exchange properties of zeolite synthesized from fly ashes. J. Mater. Res. 1998, 13, 3–7. [Google Scholar] [CrossRef]
- Steenbruggen, G.; Hollman, G. The synthesis of zeolites from fly ash and the properties of the zeolite products. J. Geochem. Explor. 1998, 62, 305–309. [Google Scholar] [CrossRef]
- Park, M.; Choi, C.L.; Lim, W.T.; Kim, M.C.; Choi, J.; Heo, N.H. Molten-salt method for the synthesis of zeolitic materials: I. Zeolite formation in alkaline molten-salt system. Microporous Mesoporous Mater. 2000, 37, 81–89. [Google Scholar] [CrossRef]
- Woolard, C.D.; Petrus, K.; van der Horst, M. The use of a modified fly ash as an adsorbent for lead. Water SA 2000, 26, 531–536. [Google Scholar]
- Querol, X.; Moreno, N.; Umana, J.; Juan, R.; Hernandez, S.; Pereira, C.F.; Ayora, C.; Janssen, M.; Garcia-Martinez, J.; Linares-Solano, A.; et al. Application of zeolitic material synthesised from fly ash to the decontamination of waste water and flue gas. J. Chem. Technol. Biotechnol. 2002, 77, 292–298. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Plana, F.; Andres, J.M.; Janssen, M.; Nugteren, H. Pure zeolite synthesis from silica extracted from coal fly ashes. J. Chem. Technol. Biotechnol. 2002, 77, 274–279. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Bialecka, B. Hydrothermal synthesis of zeolites from Polish coal fly ash. Pol. J. Environ. Stud. 2005, 14, 713–719. [Google Scholar]
- Derkowski, A.; Franus, W.; Beran, E.; Czímerová, A. Properties and potential applications of zeolitic materials produced from fly ash using simple method of synthesis. Powder Technol. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Balfour, G.; Gitari, W.M.; Hums, E. Synthesis of hydroxy sodalite from coal fly ash using waste industrial brine solution. J. Environ. Sci. Health Part A 2011, 46, 1699–1707. [Google Scholar] [CrossRef]
- Srinivasan, A.; Grutzeck, M.W. The Adsorption of SO2by Zeolites Synthesized from Fly Ash. Environ. Sci. Technol. 1999, 33, 1464–1469. [Google Scholar] [CrossRef]
- Sakthivel, T.; Reid, D.L.; Goldstein, I.; Hench, L.; Seal, S. Hydrophobic High Surface Area Zeolites Derived from Fly Ash for Oil Spill Remediation. Environ. Sci. Technol. 2013, 47, 5843–5850. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M. Wykorzystanie popiołów lotnych klasy F do produkcji materiału zeolitowego na skalę półtechniczną. Polityka Energ. 2011, 14, 79–91. [Google Scholar]
- Cordoves, A.; Valdés, M.; Fernández, J.C.; Luis, G.; García-Calzón, J.; García, M.E. Characterization of the binding site affinity distribution of a surfactant-modified clinoptilolite. Microporous Mesoporous Mater. 2008, 109, 33–48. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. ChemInform Abstract: A Review on Synthesis, Characterization and Industrial Applications of Flyash Zeolites. TrAC Trends Anal. Chem. 2012, 33, 65–132. [Google Scholar] [CrossRef]
- Martucci, A.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Dondi, F.; Alberti, A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Microporous Mesoporous Mater. 2012, 148, 174–183. [Google Scholar] [CrossRef]
- de Ridder, D.; Verberk, J.; Heijman, B.; Amy, G.; van Dijk, J. Zeolites for nitrosamine and pharmaceutical removal from demineralised and surface water: Mechanisms and efficacy. Sep. Purif. Technol. 2012, 89, 71–77. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Göltner, C.G.; Henke, S.; Weissenberger, M.C.; Antonietti, M. Mesoporous Silica from Lyotropic Liquid Crystal Polymer Templates. Angew. Chem. Int. Ed. 1998, 37, 613–616. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Schwanke, A.J.; Balzer, R.; Pergher, S. Microporous and mesoporous materials from natural and inexpensive sources. In Handbook of Ecomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 5, pp. 3379–3399. ISBN 9783319682556. [Google Scholar]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Feng, P.; Gier, T.E.; Sieger, P.; Leon, R.; Petroff, P.M.; Schüth, F.; Stucky, G.D. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, X.; Cui, X. A two-step route to synthesis of small-pored and thick-walled SBA-16-type mesoporous silica under mildly acidic conditions. J. Colloid Interface Sci. 2007, 307, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guan, X.; Song, J.; Di, Y.; Zhang, D.; Ge, X.; Zhao, L.; Xiao, F.-S. Highly efficient synthesis of ordered mesoporous silica materials with controllable microporosity using surfactant mixtures as templates. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 194–202. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Gao, P.; Liang, Z.; Zhao, Z.; Wang, W.; Yang, C.; Hu, B.; Cui, F. Enhanced adsorption of steroid estrogens by one-pot synthesized phenyl-modified mesoporous silica: Dependence on phenyl-organosilane precursors and pH condition. Chemosphere 2019, 234, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Zhang, Z.; Pang, X.; Yu, X. Highly selective luminescent sensing of Cu2+ in aqueous solution based on a Eu(III)-centered periodic mesoporous organosilicas hybrid. Mater. Des. 2019, 172, 107712. [Google Scholar] [CrossRef]
- Luoa, G.; Li, Y.; Wang, A.; Lin, Q.; Zhang, G.; Wang, C. Dummy molecularly imprinted mesoporous silicates for selective adsorption of 2-naphthol. Open Chem. 2015, 13, 756–762. [Google Scholar] [CrossRef]
- Miao, Y.; Sun, X.; Lv, J.; Yan, G. -Phosphorescent Mesoporous Surface Imprinting Microspheres: Preparation and Application for Transferrin Recognition from Biological Fluids. ACS Appl. Mater. Interfaces 2018, 11, 2264–2272. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process; Springer: Dordrecht, The Netherlands, 2012; pp. 15–37. [Google Scholar]
- Banerjee, S.; Barman, S.; Halder, G. Sorptive elucidation of rice husk ash derived synthetic zeolite towards deionization of coalmine waste water: A comparative study. Groundw. Sustain. Dev. 2017, 5, 137–151. [Google Scholar] [CrossRef]
- Chakraborty, P.; Show, S.; Banerjee, S.; Halder, G. Mechanistic insight into sorptive elimination of ibuprofen employing bi-directional activated biochar from sugarcane bagasse: Performance evaluation and cost estimation. J. Environ. Chem. Eng. 2018, 6, 5287–5300. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Martínez, J.M.M. Operation costs of the solar photo-catalytic degradation of pharmaceuticals in water: A mini-review. Chemosphere 2018, 211, 482–488. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total. Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, I. Water Treatment by Adsorption Columns: Evaluation at Ground Level. Sep. Purif. Rev. 2013, 43, 175–205. [Google Scholar] [CrossRef]
- Monteil, H.; Péchaud, Y.; Oturan, N.; Oturan, M.A. A review on efficiency and cost effectiveness of electro- and bio-electro-Fenton processes: Application to the treatment of pharmaceutical pollutants in water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Wood, T.P.; Du Preez, C.; Steenkamp, A.; Duvenage, C.; Rohwer, E.R. Database-driven screening of South African surface water and the targeted detection of pharmaceuticals using liquid chromatography—High resolution mass spectrometry. Environ. Pollut. 2017, 230, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Veras, T.B.; de Paiva, A.L.R.; Duarte, M.M.M.B.; Napoleão, D.C.; Cabral, J.J.D.S.P. Analysis of the presence of anti-inflammatories drugs in surface water: A case study in Beberibe river—PE, Brazil. Chemosphere 2019, 222, 961–969. [Google Scholar] [CrossRef]
- Tramèr, M.R.; Moore, R.A.; Reynolds, D.J.M.; McQuay, H.J. Quantitative estimation of rare adverse events which follow a biological progression: A new model applied to chronic NSAID use. Pain 2000, 85, 169–182. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Chaiamnuay, S.; Allison, J.J.; Curtis, J.R. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs. Am. J. Health Pharm. 2006, 63, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Sliwowski, Z.; Pajdo, R.; Drozdowicz, D.; Ptak, A.; Hahn, E.G. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc. Res. Tech. 2001, 53, 343–353. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical Metabolites in The Environment: Analytical Challenges And Ecological Risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Lienert, J.; Güdel, K.; Escher, B.I. Screening Method for Ecotoxicological Hazard Assessment of 42 Pharmaceuticals Considering Human Metabolism and Excretory Routes. Environ. Sci. Technol. 2007, 41, 4471–4478. [Google Scholar] [CrossRef]
- Davies, N.M.; Andersen, K.E. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin. Pharmacokinet. 1997, 33, 184–213. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An Overview of Clinical Pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–161. [Google Scholar] [CrossRef]
- Gaskell, H.; Derry, S.; Wiffen, P.J.; Moore, R.A. Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2017, 5, CD007355. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Naproxen up to Date: A Review of its Pharmacological Properties and Therapeutic Efficacy and Use in Rheumatic Diseases and Pain States. Drugs 1979, 18, 241–277. [Google Scholar] [CrossRef]
- Bowalgaha, K.; Elliot, D.J.; MacKenzie, P.I.; Knights, K.M.; Swedmark, S.; Miners, J.O. S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): Role of UGT2B7 in the elimination of naproxen. Br. J. Clin. Pharmacol. 2005, 60, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Colburn, W.A.; Di Santo, A.R.; Gibaldi, M. Pharmacokinetics of Erythromycin on Repetitive Dosing. J. Clin. Pharmacol. 1977, 17, 592–600. [Google Scholar] [CrossRef]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017, 27, 164–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Ven, A.J.; Mantel, M.A.; Vree, T.B.; Koopmans, P.P.; Van Der Meer, J.W. Formation and elimination of sulphamethoxazole hydroxylamine after oral administration of sulphamethoxazole. Br. J. Clin. Pharmacol. 1994, 38, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Michałek, K.; Lechowicz, M.; Pastuszczak, M.; Wojas-Pelc, A. The use of trimethoprim and sulfamethoxazole (TMP-SMX) in dermatology. Folia Med. Crac. 2015, 55, 35–41. [Google Scholar]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, J.L.; Leeder, J.S.E.; Van Haandel, L.; Pearce, R.E. In Vitro Hepatic Oxidative Biotransformation of Trimethoprim. Drug Metab. Dispos. 2015, 43, 1372–1380. [Google Scholar] [CrossRef] [Green Version]
- America, N.I. of H. (NIH): U.S. of PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 25 September 2020).
- Ling, Y.; Klemes, M.J.; Xiao, L.; Alsbaiee, A.; Dichtel, W.R.; Helbling, D.E. Benchmarking Micropollutant Removal by Activated Carbon and Porous β-Cyclodextrin Polymers under Environmentally Relevant Scenarios. Environ. Sci. Technol. 2017, 51, 7590–7598. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Tysklind, M.; Larsson, D.J. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 2010, 58, 516–523. [Google Scholar] [CrossRef]
- Schmidt, S.; Hoffmann, H.; Garbe, L.-A.; Schneider, R.J. Liquid chromatography–tandem mass spectrometry detection of diclofenac and related compounds in water samples. J. Chromatogr. A 2018, 1538, 112–116. [Google Scholar] [CrossRef]
- Vanderford, B.J.; Snyder, S. Analysis of Pharmaceuticals in Water by Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry. Environ. Sci. Technol. 2006, 40, 7312–7320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palli, L.; Spina, F.; Varese, G.C.; Vincenzi, M.; Aragno, M.; Arcangeli, G.; Mucci, N.; Santianni, D.; Caffaz, S.; Gori, R. Occurrence of selected pharmaceuticals in wastewater treatment plants of Tuscany: An effect-based approach to evaluate the potential environmental impact. Int. J. Hyg. Environ. Health 2019, 222, 717–725. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Pacheco-Juárez, J.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. A Survey of the Presence of Pharmaceutical Residues in Wastewaters. Evaluation of Their Removal using Conventional and Natural Treatment Procedures. Molecules 2020, 25, 1639. [Google Scholar] [CrossRef] [Green Version]
- Landová, P.; Vávrová, M. A new method for macrolide antibiotics determination in wastewater from three different wastewater treatment plants. Acta Chim. Slovaca 2017, 10, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Semreen, M.H.; Shanableh, A.; Semerjian, L.; Alniss, H.; Mousa, M.; Bai, X.; Acharya, K. Simultaneous Determination of Pharmaceuticals by Solid-phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry: A Case Study from Sharjah Sewage Treatment Plant. Molecules 2019, 24, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jank, L.; Hoff, R.; Da Costa, F.J.; Pizzolato, T.M. Simultaneous determination of eight antibiotics from distinct classes in surface and wastewater samples by solid-phase extraction and high-performance liquid chromatography–electrospray ionisation mass spectrometry. Int. J. Environ. Anal. Chem. 2014, 94, 1013–1037. [Google Scholar] [CrossRef]
- Opris, O.; Soran, M.-L.; Coman, V.; Copaciu, F.; Ristoiu, D. Determination of some frequently used antibiotics in waste waters using solid phase extraction followed by high performance liquid chromatography with diode array and mass spectrometry detection. Open Chem. 2013, 11, 1343–1351. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, H.-D.; Chung, E.G.; Kim, Y. Determination of 18 veterinary antibiotics in environmental water using high-performance liquid chromatography-q-orbitrap combined with on-line solid-phase extraction. J. Chromatogr. B 2018, 1084, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rac, V.; Rakić, V.; Stošić, D.; Pavlović, V.; Bosnar, S.; Auroux, A. Enhanced accessibility of active sites in hierarchical ZSM-5 zeolite for removal of pharmaceutically active substances: Adsorption and microcalorimetric study. Arab. J. Chem. 2020, 13, 1945–1954. [Google Scholar] [CrossRef]
- Attia, T.M.S.; Hu, X.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F.; Daana, M.; Khamis, M.; Karaman, R.; Khoury, H.; Qurie, M. Removal of Selected Pharmaceuticals from Aqueous Solutions Using Natural Jordanian Zeolite. Arab. J. Sci. Eng. 2019, 44, 209–215. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 photocatalyst for degradation of organic compounds in water and air supported on highly hydrophobic FAU zeolite: Structural, sorptive, and photocatalytic studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Duke, M.C.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.; da Costa, J.C.D. Seawater desalination performance of MFI type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- An, Y.; De Ridder, D.J.; Zhao, C.; Schoutteten, K.; Bussche, J.V.; Zheng, H.; Chen, G.; Vanhaecke, L. Adsorption and photocatalytic degradation of pharmaceuticals and pesticides by carbon doped-TiO2 coated on zeolites under solar light irradiation. Water Sci. Technol. 2016, 73, 2868–2881. [Google Scholar] [CrossRef] [Green Version]
- Attia, T.M.S.; Hu, X.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Exp. Nanosci. 2012, 9, 551–560. [Google Scholar] [CrossRef]
- Szabová, P.; Plekancová, M.; Gróf, N.; Bodík, I. Slovak natural zeolites as a suitable medium for antibiotics elimination from wastewater. Acta Chim. Slovaca 2019, 12, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Blasioli, S.; Martucci, A.; Paul, G.; Gigli, L.; Cossi, M.; Johnston, C.T.; Marchese, L.; Braschi, I. Removal of sulfamethoxazole sulfonamide antibiotic from water by high silica zeolites: A study of the involved host–guest interactions by a combined structural, spectroscopic, and computational approach. J. Colloid Interface Sci. 2014, 419, 148–159. [Google Scholar] [CrossRef]
- Braschi, I.; Martucci, A.; Blasioli, S.; Mzini, L.L.; Ciavatta, C.; Cossi, M. Effect of humic monomers on the adsorption of sulfamethoxazole sulfonamide antibiotic into a high silica zeolite Y: An interdisciplinary study. Chemosphere 2016, 155, 444–452. [Google Scholar] [CrossRef]
- Wang, C.-F.; Li, J.-S.; Wang, L.-J.; Sun, X.-Y. Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J. Hazard. Mater. 2008, 155, 58–64. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Bui, T.X.; Pham, V.H.; Le, S.T.; Choi, H. Adsorption of pharmaceuticals onto trimethylsilylated mesoporous SBA-15. J. Hazard. Mater. 2013, 254–255, 345–353. [Google Scholar] [CrossRef]
- Liu, M.; Hou, L.-A.; Yu, S.; Xi, B.; Zhao, Y.; Xia, X. MCM-41 impregnated with A zeolite precursor: Synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chem. Eng. J. 2013, 223, 678–687. [Google Scholar] [CrossRef] [Green Version]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.; Meyer, M.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, J.; Lapworth, D.; Nkhuwa, D.; Stuart, M.; Gooddy, D.; Bell, R.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2015, 72, 51–63. [Google Scholar] [CrossRef] [Green Version]


| Frame-Work Type | Ring Number and Pore Opening Size [73] | Framework Density [73] | Accessible Area Maximum [74] | Maximum Diameter of a Sphere [74] | |
|---|---|---|---|---|---|
| (Å × Å) | (Å × Å) | (T-Atoms per 1000 Å) | (m2 g−1) | (Å) | |
| FAU | 12 ring 7.4 × 7.4 | - | 12.7 | 1211.42 | 11.24 |
| MOR | 12 ring 6.5 × 7.4 | 8 ring 2.6 × 5.7 | 17.2 | 1010.22 | 6.70 |
| MFI | 10 ring 5.1 × 5.5 | 10 ring 5.3 × 5.6 | 17.9 | 834.41 | 6.36 |
| Type of Zeolite | Conditions of Synthesis | NaOH/Fly Ash Ratio | Reference | ||
|---|---|---|---|---|---|
| NaOH [M] | T [°C] | t [h] | |||
| Na-A | 0.5–3.5 | 60 | 10–48 | 0.5–3.5 | [78] |
| 2.0 | 100 | 2 | 0.8 | [79] | |
| 2.2 | 85 | 12 | 0.23 | [80] | |
| 2.0–5.0 | 100–150 | 0.5–6 | 0.5–1.6 | [81] | |
| Na-P1 | 2.8–5.0 | 25 | 48 | 0.28–0.5 | [82] |
| 2.0 | 90–150 | 12 | - | [83] | |
| 3.0 | 103 | 12 | 0.5 | [84] | |
| 1.0–3.0 | 90 | 21 | 0.4–1.2 | [85] | |
| 0.5–5.0 | 150–200 | 3–48 | - | [86] | |
| 3.0 | 125 | 8 | - | [87] | |
| 0.4–0.5 | 120 | 3–24 | 0.08–0.64 | [88] | |
| 3.0 | 125 | 9 | 0.96 | [86] | |
| 1.16 | 80–320 | 6 | 0.28 | [89] | |
| 1.0 | 105 | 24 | 0.8 | [90] | |
| - | 100 | 12–48 | 1.0 | [91] | |
| Na-X | 3.0 | 90 | 24–72 | 0.3 | [92] |
| 3.0 | 75 | 24 | 2.4 | [90] | |
| - | 10 | 120 | - | [93] | |
| 3.0 | 75 | 24 | 0.33 | [65] | |
| Common Name | Diclofenac | Ibuprofen | Ketoprofen | Naproxen |
|---|---|---|---|---|
| CAS Number | 15307-86-5 | 15687-27-1 | 22071-15-4 | 22204-53-1 |
| Molecular weight | 296.15 | 206.28 | 254.28 | 230.26 |
| pKa a | 4.15 | 4.91 | 4.45 | 4.15 |
| log Kow a | 4.51 | 3.97 | 3.12 | 3.18 |
| CEC b (ng L−1) | 4560 | 194,711 | 48,978 | 827,999 |
| Classification group | acetic acid derivatives | propionic acid derivatives | propionic acid derivatives | propionic acid derivatives |
| Therapeutic use/mechanism of action | NSAID/ non-selective COX inhibitor | NSAID/ non-selective COX inhibitor | NSAID/ non-selective COX inhibitor | NSAID/ non-selective COX inhibitor |
| Half-lives, hours | 2 | 1.2–2 | 1.1–4 | 12–17 |
| Metabolite | hydroxy metabolites, glucuronic acid, sulfate, and taurine | hydroxylated and carboxylated derivatives | glucuronide metabolite | desmethylnaproxen, glucuronide metabolit |
| Reference | [133] | [134,135] | [136] | [137,138] |
| Common Name | Erythromycin | Sulphamethoxazole | Tetracycline | Trimethoprim |
|---|---|---|---|---|
| CAS Number | 114-07-8 | 723-46-6 | 60-54-8 | 738-70-5 |
| Molecular weight | 733.93 | 253.28 | 444.44 | 290.32 |
| pKa a | 8.88 | 1.6 5.7 | 3.30 | 7.12 |
| log Kow a | 3.06 | 0.89 | 1.37 | 0.91 |
| CEC b (ng L−1) | - | 9.8 × 107 | 6.7 × 107 | 3.3 × 106 |
| Classification group | macrolide antibiotic | sulfonamides | tetracyclines | anisoles |
| Therapeutic Use/mechanism of action | Antibiotic/ bacteriostatic | Antibiotic/ bacteriostatic | Antibiotic/ bacteriostatic | Antibiotic/ bactericidal |
| Half-lives, hours | 2–3.5 | 10 | 6–12 | 8–10 |
| Metabolite | N-desmethylerythromycin | hydroxysulfamethoxazole, acetylsulfamethoxazole ulfamethoxazole N4-hydroxylamine, sulfamethoxazole N-glucuronide | not metabolized | demethylated 3′- and 4′-metabolite |
| Reference | [139,140] | [141,142] | [143] | [142,144] |
| Common Name | Extraction Technique/Sorbent | Determination Method | Level (µg L−1) | Reference |
|---|---|---|---|---|
| Diclofenac | SPE/polymer | LC-MS/MS | 2.0–6.30 0.91–1.90 0.18–2.60 | [148] |
| SPE/hydrophilic-lipophilic polymer | LC-MS | 0.116 | [149] | |
| SPE/hydrophilic-lipophilic polymer | LC-MS | 0.113–4.882 | [150] | |
| Ibuprofen | SPE/ hydrophilic-lipophilic polymer | LC-MS/MS | 27.30 | [151] |
| Ketoprofen | SPE/ hydrophilic-lipophilic polymer | LC-MS | 0.031–3.511 | [150] |
| Naproxen | SPE/ hydrophilic-lipophilic polymer | LC-MS | 22.50 | [149] |
| SPE/ hydrophilic-lipophilic polymer | LC-MS/MS | 19.90 | [151] | |
| Erythromycin | SPE/ hydrophilic-lipophilic polymer | LC-MS | 0.509–0.149 | [152] |
| SPE/ hydrophilic-lipophilic polymer | LC-MS/MS | 0.785 | [153] | |
| Sulphamethoxazole | SPE/polymer | LC-MS | 0.376–0.572 | [154] |
| SPE/ hydrophilic-lipophilic polymer | LC-MS | 2.060 | [149] | |
| SPE/ hydrophilic-lipophilic polymer | LC-MS/MS | 0.024 | [153] | |
| Tetracycline | SPE /hydrophilic-lipophilic polymer | LC-MS | 146.0 | [155] |
| Trimethoprim | SPE /hydrophilic-lipophilic polymer | LC-MS/MS | 0.007 | [156] |
| SPE/ polymer | LC-MS | 0.27–0.94 | [154] | |
| SPE /hydrophilic-lipophilic polymer | LC-MS | 1.140 | [149] |
| Adsorbate | Framework Type of Zeolite (Si/Al Ratio) | Dose (g L−1) | Contact Time (h) | T (°C) | Concentrations | pH | Reference |
|---|---|---|---|---|---|---|---|
| Sodium diclofenac | zeolite ZSM-5 hierarchical ZSM-5 | 0.05 | 1.0 | 29.85 | 10–1000 µM | - | [157] |
| Sodium diclofenac | natural Jordanian zeolite (intermediate silica) | 2.0 | 1.2 | - | 10.0; 20.0; 40.0; 50.0 × 104 µg L−1 | 6.0 | [159] |
| Sodium diclofenac | TMOR18 (50% w/w TiO2) TMOR240 (50% w/w TiO2) | 0.7 | - | - | 100 μg L−1 | - | [162] |
| Sodium diclofenac | MNCZ | 0.05 | - | 30 ± 1 | 100 µg L−1 | 2.0–9.0 | [158] |
| Erythromycin | zeolite Y | - | - | - | 0–5 × 103 µg L−1 | - | [98] |
| Erythromycin | Zeocem a.s. 200 μm Zeocem a.s. 0.5–1 mm Zeocem a.s. 1–2.5 mm | 0.05 | 0.5 | - | 0.016 µg L−1 0.037 µg L−1 | pH = 6.85 pH = 7.01 | [164] |
| Ibuprofen | natural Jordanian zeolite (Intermediate silica) | 1.0 | 1.2 | - | 10.0; 20.0; 40.0; 50.0 × 104 µg L−1 | 2.0 | [159] |
| Ibuprofen Naproxen | MNCZ | 0.05 | - | 30 ± 1 | 100 µg L−1 | 2.0–9.0 | [158] |
| Naproxen | ZSM5 MOR200 | 0.05 | - | - | 2 µg L−1 | 6.0 | [99] |
| Sulfametoksazole | FAU-1 FAU-2 | 0.50 | 2.0 | - | 1.0 × 105 μg L−1 | 6.5 | [62] |
| Sulfamethoxazole | zeolite Y MOR ZSM5 | 0.50 | 24.0 | 21 21 65 | 30 µM | - | [165] |
| Sulphamethoxazole | TMOR18 (50% w/w TiO2) TMOR240 (50% w/w TiO2) | 0.70 | - | - | 100 μg L−1 | - | [162] |
| Sulphamethoxazole | zeolite Y | 0.50 | 1.0 | - | 50 µM | 5–8 | [166] |
| Adsorbent | Adsorbate | pH |
Temp (°C) |
Conc. Range (mg L−1) |
Surface Area (m2 g−1) |
Freundlich Sorption Capacity (KF) |
Langmuir Sorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| MCM-41 | Diclofenac | 7.0 | 25 | 0.04−0.3 | 755 | 0.05 | 0.11 | [168] |
| SBA-15 | Diclofenac | 7.0 | 25 | 0.04−0.3 | 890 | 0.11 | 0.13 | [168] |
| SBA-15 | Diclofenac | 5.0 | 25 | 0.01−0.3 | 737 | 0.72 | 0.34 | [55] |
| SBA-15 | Ibuprofen | 5.0 | 25 | 0.01−0.3 | 737 | 1.50 | 0.41 | [55] |
| SBA-15 | Ketoprofen | 5.0 | 25 | 0.01−0.3 | 737 | 1.09 | 0.28 | [55] |
| AMCM-41 | Tetracycline | 7.0 | 30 | 300 | 485 | 368.58 | 415.10 | [170] |
| AMCM-41 | Tetracycline | 7.0 | 40 | 300 | 485 | 364.21 | 417.50 | [170] |
| AMCM-41 | Tetracycline | 7.0 | 50 | 300 | 485 | 362.15 | 419.30 | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grela, A.; Kuc, J.; Bajda, T. A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials 2021, 14, 4994. https://doi.org/10.3390/ma14174994
Grela A, Kuc J, Bajda T. A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials. 2021; 14(17):4994. https://doi.org/10.3390/ma14174994
Chicago/Turabian StyleGrela, Agnieszka, Joanna Kuc, and Tomasz Bajda. 2021. "A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water" Materials 14, no. 17: 4994. https://doi.org/10.3390/ma14174994
APA StyleGrela, A., Kuc, J., & Bajda, T. (2021). A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials, 14(17), 4994. https://doi.org/10.3390/ma14174994