Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Plant Residue with Iron Sulfates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Thermodynamic Calculation
2.3. Experimental Procedure
3. Results
3.1. ZPR Characterization
3.2. Sulfating of Pure Ferrites
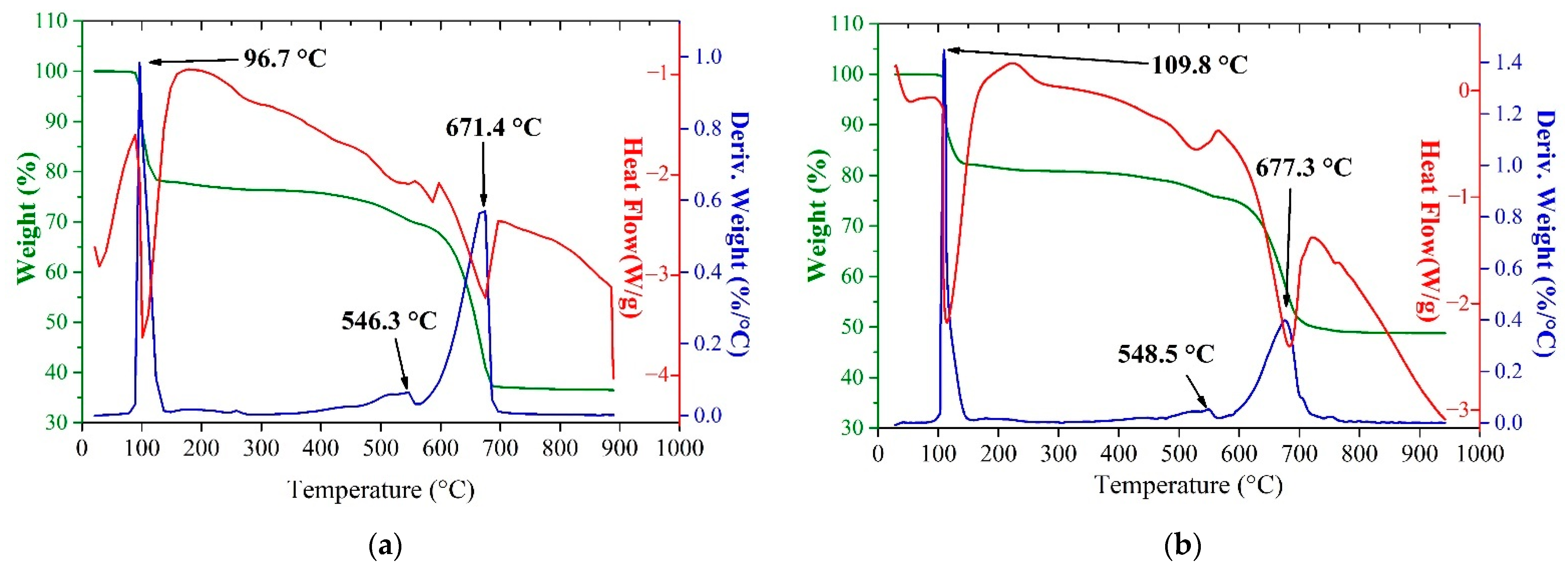
3.2.1. STA Analysis
3.2.2. Roasting Experiments
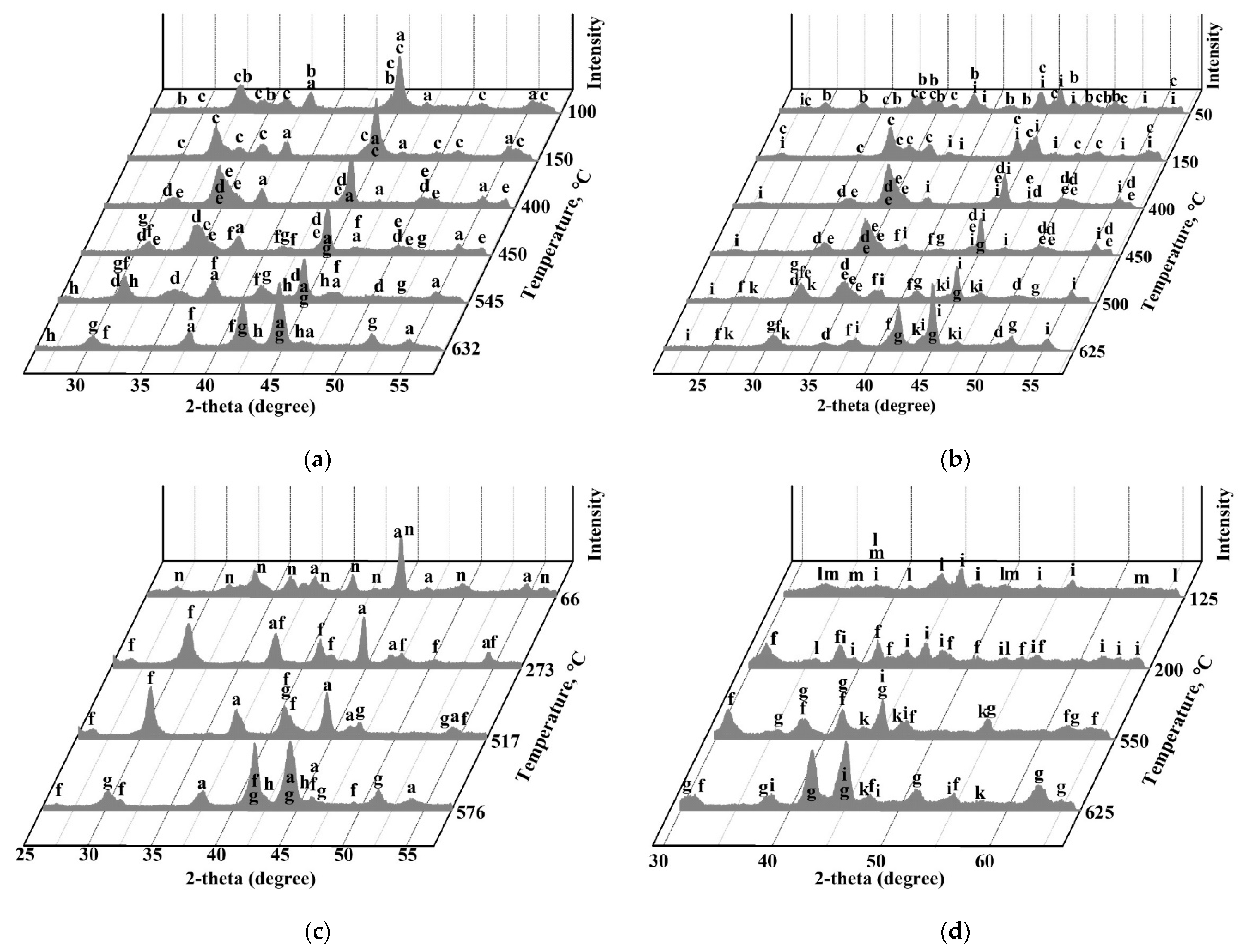
3.2.3. TRXRD Experiments
FeSO4∙7H2O → FeSO4∙4H2O → FeSO4∙H2O → Fe(OH)SO4 → Fe2(SO4)3
Fe2O(SO4)2
3.3. Sulfating of ZPR
3.3.1. Thermodynamic Calculation
3.3.2. Influence of Roasting Temperature on the Behavior of Valuable Elements in ZPR
3.3.3. Influence of Iron Sulfate Amount on the Behavior of Valuable Elements in ZPR
3.3.4. Kinetics of ZPR Sulfating Roasting
3.3.5. Characterization of Roasted and Water-Leached ZPR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
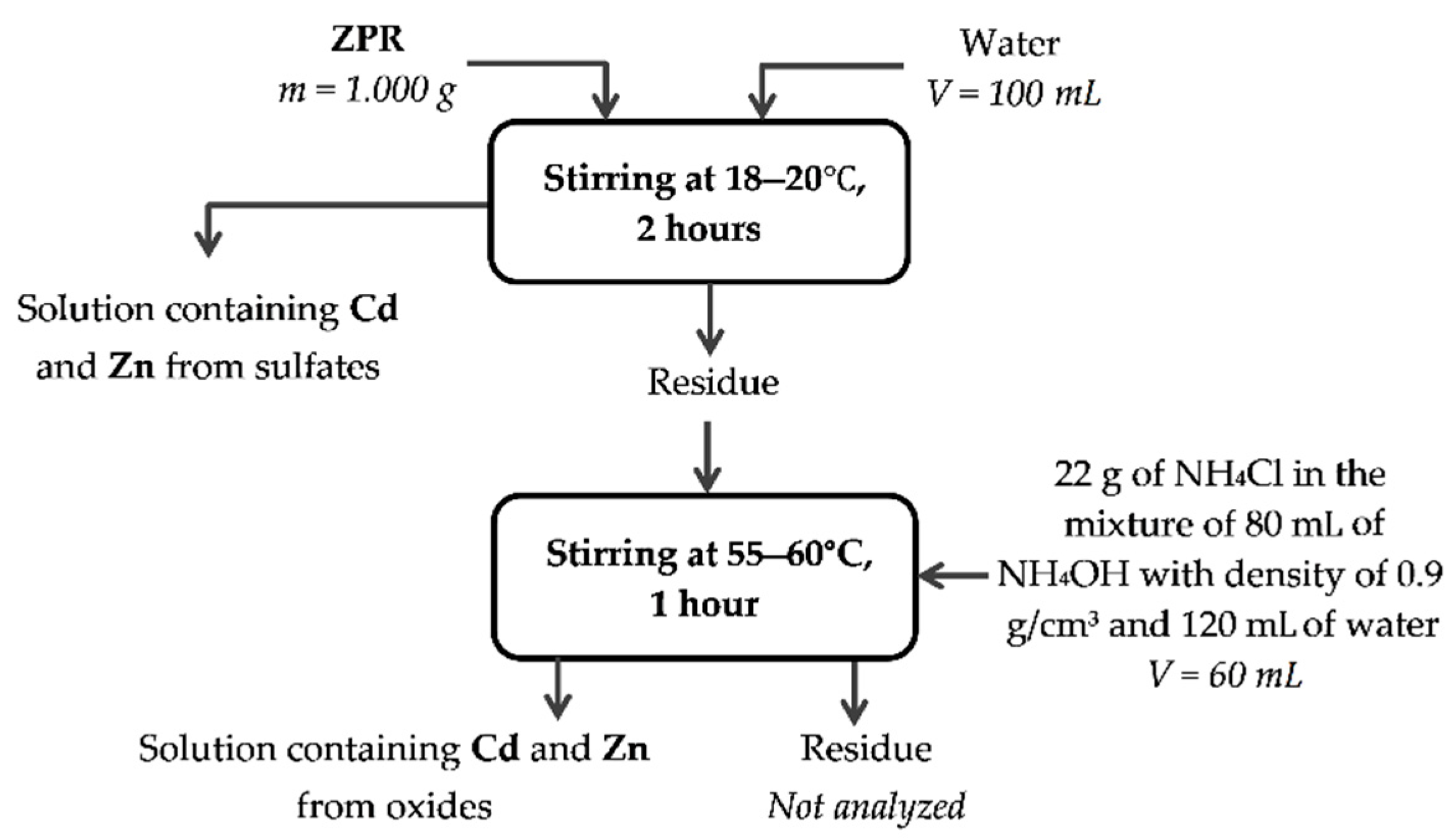
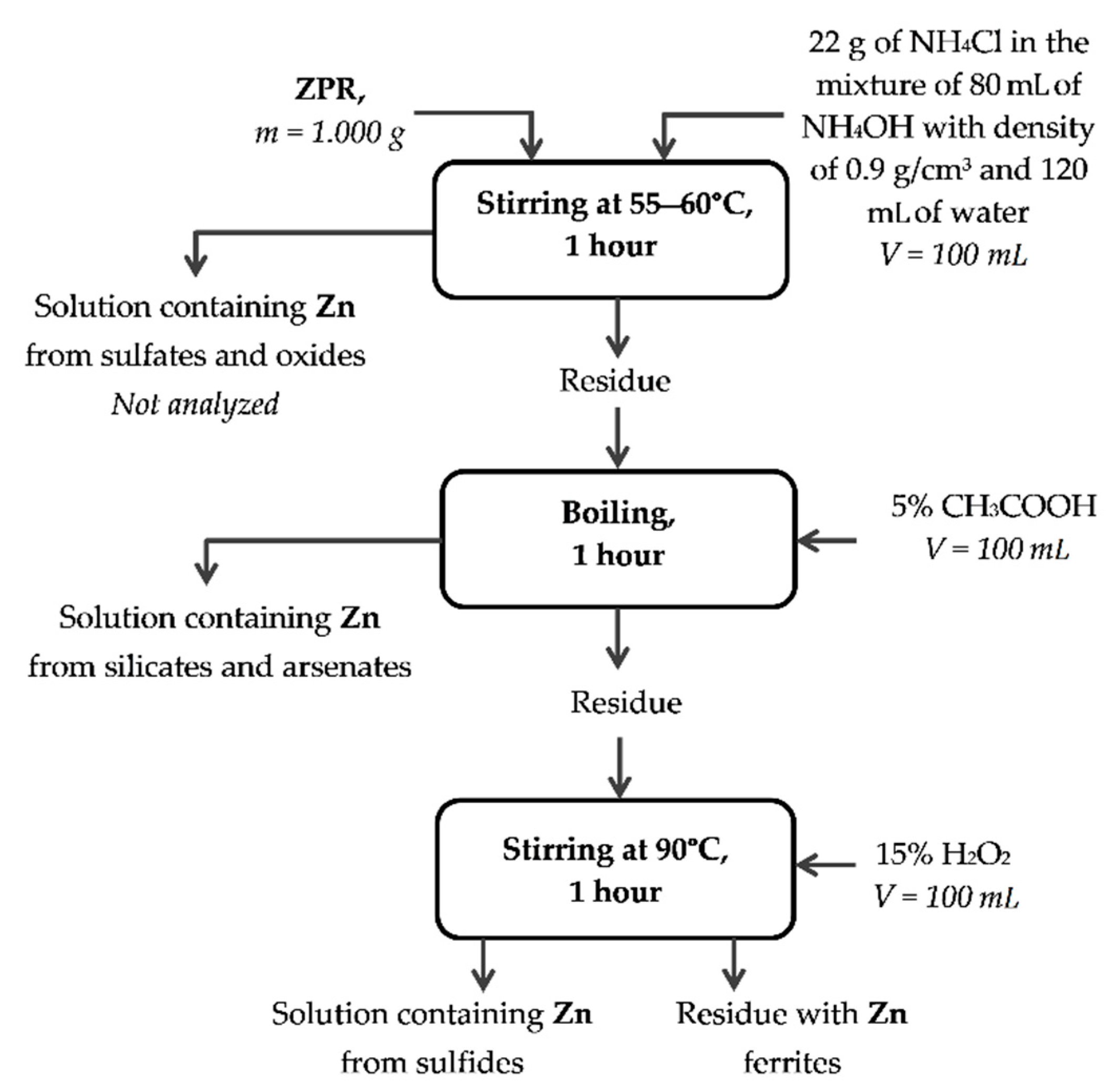


Appendix B
| Sample | Phase | δ | Δ = 2ε | Γexp | Heff | S |
|---|---|---|---|---|---|---|
| mm/s | mm/s | mm/s | kOe | % | ||
| ZPR roasted without additions at 600 °C | α-Fe2O3 | 0.36 | −0.20 | 0.22 | 515.8 | 5.5 |
| ZnFe2O4 | 0.33 | 0.42 | 0.36 | - | 94.5 | |
| ZPR roasted with addition of 48% Fe2(SO4)3 at 600 °C, then additionally calcined at 900 °C | ZnFe2O4 | 0.33 | 0.43 | 0.36 | - | 100 |
| ZPR roasted with addition of 48% Fe2(SO4)3 at 600 °C, water-leached, then additionally calcined at 900 °C | α-Fe2O3 | 0.37 | −0.21 | 0.29 | 514.5 | 75.2 |
| ZnFe2O4 | 0.34 | 0.41 | 0.32 | - | 24.8 |

References
- Wills, B.A.; Finch, J.A. Appendix I—Metallic Ore Minerals. In Wills’ Mineral Processing Technology. An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2016; pp. 463–474. [Google Scholar] [CrossRef]
- Mitra, S. Depletion, technology, and productivity growth in the metallic minerals industry. Miner. Econ. 2018, 32, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Sahu, K.; Pandey, B.D. Solid waste management in non-ferrous industries in India. Resour. Conserv. Recycl. 2004, 42, 99–120. [Google Scholar] [CrossRef]
- Vodyanitskii, Y.N.; Minkina, T.M.; Kubrin, S.; Pankratov, D.A.; Fedorenko, A.G. Common and rare iron, sulfur, and zinc minerals in technogenically contaminated hydromorphic soil from Southern Russia. Environ. Geochem. Health 2019, 42, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Razi, M.K.; Beuscher, M.; Chagnes, A. Recovery of Metal Values from Ni-Cd Cake Waste Residue of an Iranian Zinc Plant by Hydrometallurgical Route. Metals 2020, 10, 655. [Google Scholar] [CrossRef]
- Sinclair, R.J. The Extractive Metallurgy of Zinc, 1st ed.; Australasian Institute of Mining and Metallurgy: Carlton Victoria, Australia, 2005. [Google Scholar]
- Monhemius, A. The iron elephant: A brief history of hydrometallurgists’ struggles with element no.26. CIM J. 2017, 8. [Google Scholar] [CrossRef]
- Vignes, A. Extractive Metallurgy 2: Metallurgical Reaction Processes, 1st ed.; Wiley-ISTE: London, UK, 2011; pp. 69–70. [Google Scholar]
- Kozlov, P.A. The Waelz process; Ore and Metals Publishing House: Moscow, Russia, 2003; pp. 96–101. [Google Scholar]
- Stoychev, S.; Minchev, E.; Kyurkchiev, A.; Radonov, G. Technologies for Treatment of Zinc-Containing Waste from Metallurgy in KCM AD. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 799–809. [Google Scholar]
- Rüşen, A.; Topçu, M.A. Investigation of zinc extraction from different leach residues by acid leaching. Int. J. Environ. Sci. Technol. 2017, 15, 69–80. [Google Scholar] [CrossRef]
- Xing, P.; Ma, B.-Z.; Zeng, P.; Wang, C.-Y.; Wang, L.; Zhang, Y.-L.; Chen, Y.-Q.; Wang, S.; Wang, Q.-Y. Deep cleaning of a metallurgical zinc leaching residue and recovery of valuable metals. Int. J. Miner. Met. Mater. 2017, 24, 1217–1227. [Google Scholar] [CrossRef]
- Xie, T.F.; Sun, C.Y.; Li, G.J.; Luo, Y.G.; Zheng, X.M.; Ma, A.Y. Zinc Extraction from Industrial Waste Residue by Conventional Acid Leaching. In Characterization of Minerals, Metals, and Materials; Li, J., Zhang, M.M., Li, B.W., Monteiro, S.M., Ikhmayies, S., Kalay, Y.E., Hwang, J.-Y., Escobedo-Diaz, J.P., Carpenter, J.S., Brown, A.D., et al., Eds.; Springer: Cham, Switzerland, 2021; pp. 121–129. [Google Scholar]
- Vahidi, E.; Rashchi, F.; Moradkhani, D. Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA. Miner. Eng. 2008, 22, 204–206. [Google Scholar] [CrossRef]
- Ashtari, P.; Pourghahramani, P. Selective mechanochemical alkaline leaching of zinc from zinc plant residue. Hydrometallurgy 2015, 156, 165–172. [Google Scholar] [CrossRef]
- Huang, Y.; Geng, Y.; Han, G.; Cao, Y.; Peng, W.; Zhu, X.; Zhang, T.-A.; Dou, Z. A perspective of stepwise utilization of hazardous zinc plant purification residue based on selective alkaline leaching of zinc. J. Hazard. Mater. 2020, 389, 122090. [Google Scholar] [CrossRef]
- Liu, W.; Sun, S.; Zhang, L.; Jahanshahi, S.; Yang, J. Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner. Eng. 2012, 39, 213–218. [Google Scholar] [CrossRef]
- Guo, Z.-H.; Pan, F.-K.; Xiao, X.-Y.; Zhang, L.; Jiang, K.-Q. Optimization of brine leaching of metals from hydrometallurgical residue. Trans. Nonferrous Met. Soc. China 2010, 20, 2000–2005. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Niu, L.; Zhang, W.; Zhang, T.-A. High purity metal lead recovery from zinc direct leaching residue via chloride leaching and direct electrolysis. Sep. Purif. Technol. 2021, 263, 118329. [Google Scholar] [CrossRef]
- Palden, T.; Regadio, M.; Onghena, B.; Binnemans, K. Selective Metal Recovery from Jarosite Residue by Leaching with Acid-Equilibrated Ionic Liquids and Precipitation-Stripping. ACS Sustain. Chem. Eng. 2019, 7, 4239–4246. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Machiels, L.; Onghena, B.; Spooren, J.; Binnemans, K. Selective recovery of zinc from goethite residue in the zinc industry using deep-eutectic solvents. RSC Adv. 2020, 10, 7328–7335. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Chai, L.-Y.; Peng, B.; Li, M.; Liu, W.; Peng, N.; Hou, D.-K. Reduction Roasting of High Iron-Bearing Zinc Calcine under a CO-CO2 Gas: An Investigation of the Chemical and Mineralogical Transformations. JOM 2013, 65, 1589–1596. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Qin, W.; Peng, B.; Yang, K.; Zheng, Y. Recovery of zinc and iron from high iron-bearing zinc calcine by selective reduction roasting. J. Ind. Eng. Chem. 2014, 22, 272–279. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.-F.; Wang, S.; Chen, F.; Tan, Y.-J.; Zheng, F.-Q.; Yang, L.-Z. Characteristics of the reduction behavior of zinc ferrite and ammonia leaching after roasting. Int. J. Miner. Met. Mater. 2020, 27, 26–36. [Google Scholar] [CrossRef]
- Kashyap, V.; Taylor, P. Selective Extraction of Zinc from Zinc Ferrite. Min. Met. Explor. 2020, 38, 27–36. [Google Scholar] [CrossRef]
- Zheng, Y.-X.; Lv, J.-F.; Liu, W.; Qin, W.-Q.; Wen, S.-M. An innovative technology for recovery of zinc, lead and silver from zinc leaching residue. Physicochem. Probl. Miner. Process. 2016, 52. [Google Scholar] [CrossRef]
- Min, X.-B.; Jiang, G.-H.; Wang, Y.-Y.; Zhou, B.-S.; Xue, K.; Ke, Y.; Xu, Q.-J.; Wang, J.-W.; Ren, H.-C. Sulfidation roasting of zinc leaching residue with pyrite for recovery of zinc and iron. J. Central South Univ. 2020, 27, 1186–1196. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H.; Murland, A.L. Roasting of La Oroya Zinc Ferrite with Na2CO3. Met. Mater. Trans. A 2007, 38, 781–791. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H.; Murland, A.L. Use of Secondary Additives to Control the Dissolution of Iron during Na2CO3 Roasting of La Oroya Zinc Ferrite. Met. Mater. Trans. A 2007, 38, 793–808. [Google Scholar] [CrossRef]
- Holloway, P.C.; Etsell, T.H. Recovery of zinc, gallium and indium from La Oroya zinc ferrite using Na2CO3 roasting. Miner. Process. Extr. Metall. 2012, 117, 137–146. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Extraction of zinc from zinc ferrites by fusion with caustic soda. Miner. Eng. 2000, 13, 1417–1421. [Google Scholar] [CrossRef]
- Wang, H.B.; Zheng, C.Z.; Qin, S.C. Study of a Novel Chloride Volatilization Process for the Treatment of Jarosite Residue. In Proceedings of the PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 835–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Li, X. Zinc recovery from franklinite by sulphation roasting. Hydrometallurgy 2011, 109, 211–214. [Google Scholar] [CrossRef]
- Güler, E.; Seyrankaya, A.; Cöcen, İ. Effect of Sulfation Roasting on Metal Extraction from Çinkur Zinc Leach Residue. J. Ore Dress. 1999, 10, 1–10. [Google Scholar]
- Turan, M.D.; Altundoğan, H.S.; Tümen, F. Recovery of zinc and lead from zinc plant residue. Hydrometallurgy 2004, 75, 169–176. [Google Scholar] [CrossRef]
- Wang, R.-X.; Yang, Y.-D.; Liu, C.-X.; Zhou, J.; Fang, Z.; Yan, K.; Tian, L.; Xu, Z.-F. Recovery of lead and silver from zinc acid-leaching residue via a sulfation roasting and oxygen-rich chlorination leaching method. J. Central South Univ. 2020, 27, 3567–3580. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Peng, B.; Min, X.; Hu, M.; Peng, N.; Yuang, Y.; Lei, J. Study on separating of zinc and iron from zinc leaching residues by roasting with ammonium sulphate. Hydrometallurgy 2015, 158, 42–48. [Google Scholar] [CrossRef]
- Fekete, F.; Lázár, K.; Keszler, A.M.; Jánosity, A.; Zhibin, L.; Szilágyi, I.M.; Kótai, L. Recycling the industrial waste ZnFe2O4 from hot-dip galvanization sludge. J. Therm. Anal. Calorim. 2018, 134, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.C.S.; Pereira, D.D.; Mendes, F.R.P.; Araujo, M.F.L. A New Route for Treating Neutral Leaching Residue. In Proceedings of the PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 827–833. [Google Scholar] [CrossRef]
- Li, Y.-C.; Zhuo, S.-N.; Peng, B.; Min, X.-B.; Liu, H.; Ke, Y. Comprehensive recycling of zinc and iron from smelting waste containing zinc ferrite by oriented transformation with SO2. J. Clean. Prod. 2020, 263, 121468. [Google Scholar] [CrossRef]
- De Oliveira, C.C.S.; Pereira, D.D. Simulation of an Alternative Direct Leaching Process for High Iron Content Zinc Concentrates. In Proceedings of the PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 405–415. [Google Scholar] [CrossRef]
- Hu, M.; Peng, B.; Chai, L.-Y.; Li, Y.-C.; Peng, N.; Yuan, Y.-Z.; Chen, N. High-Zinc Recovery from Residues by Sulfate Roasting and Water Leaching. JOM 2015, 67, 2005–2012. [Google Scholar] [CrossRef]
- Jiang, G.-M.; Peng, B.; Liang, Y.-J.; Chai, L.-Y.; Wang, Q.-W.; Li, Q.-Z.; Hu, M. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1180–1187. [Google Scholar] [CrossRef]
- Altundoǧan, H.; Tümen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Nadirov, R.K. Recovery of Valuable Metals from Copper Smelter Slag by Sulfation Roasting. Trans. Indian Inst. Met. 2018, 72, 603–607. [Google Scholar] [CrossRef]
- Grudinsky, I.P.; Podjelnikova, E.S.; Dyubanov, V.G. Research on the Process of Sulphatizing Roasting of Copper Slag Flotation Tailings Using Iron Sulphates. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 459. [Google Scholar] [CrossRef]
- Grudinsky, I.P.; Zhiltsova, E.E.; Grigorieva, D.D.; Dyubanov, V.G. Experimental Study of the Sulphatizing Roasting of Flotation Tailings from Copper Slag Processing Using Iron Sulfates. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 666, p. 022046. [Google Scholar] [CrossRef]
- Pickles, C.A.; Marzoughi, O. Thermodynamic Investigation of the Sulphation Roasting of Electric Arc Furnace Dust. Minerals 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Filippova, N.A. The Phase Analysis of Ores and Their Processing Products, 2nd ed.; Himiya: Moscow, Russia, 1975. [Google Scholar]
- Talanov, V.; Shabelskaya, N.; Golovina, A. Method of copper ferrite preparation. Russia Patent RU 2451638 C2, 25 August 2010. [Google Scholar]
- Match! In Software for Phase Analysis Using Powder Diffraction; Version 3.11; Crystal Impact: Bonn, Germany, 2021.
- HSC Chemistry. Software for Chemical Reaction and Equilibrium Calculation; Version 9.9; Outotec: Pori, Finland, 2019. [Google Scholar]
- Kovalev, D.Y.; Ponomarev, V.I. Time-Resolved X-Ray Diffraction in SHS Research and Related Areas: An Overview. Int. J. Self-Propag. High-Temp. Synth. 2019, 28, 114–123. [Google Scholar] [CrossRef]
- Pankratov, D.A. Mössbauer study of oxo derivatives of iron in the Fe2O3-Na2O2 system. Inorg. Mater. 2013, 50, 82–89. [Google Scholar] [CrossRef]
- König, U.; Bertaut, E.; Gros, Y.; Mitrikov, M.; Chol, G. Models of the magnetic structure of zinc ferrite. Solid State Commun. 1970, 8, 759–764. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Wang, J.; Zhou, Q.; Zhou, X.; Kunkel, H.; Williams, G. Site preference of Fe in nanoparticles of ZnFe2O4. J. Magn. Magn. Mater. 2004, 268, 332–339. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K.K.; Guérault, H.; Greneche, J.-M. Magnetic properties of nanostructured ferrimagnetic zinc ferrite. J. Phys. Condens. Matter 2000, 12, 7795–7805. [Google Scholar] [CrossRef]
- Sato, T.; Haneda, K.; Seki, M.; Iijima, T. Morphology and magnetic properties of ultrafine ZnFe2O4 particles. Appl. Phys. A 1990, 50, 13–16. [Google Scholar] [CrossRef]
- Evans, B.J.; Hafner, S.S.; Weber, H.P. Electric Field Gradients at 57Fe in ZnFe2O4 and CdFe2O4. J. Chem. Phys. 1971, 55, 5282–5288. [Google Scholar] [CrossRef] [Green Version]
- Varshney, D.; Verma, K.; Kumar, A. Structural and vibrational properties of ZnxMn1−xFe2O4 (x = 0.0, 0.25, 0.50, 0.75, 1.0) mixed ferrites. Mater. Chem. Phys. 2011, 131, 413–419. [Google Scholar] [CrossRef]
- Waerenborgh, J.C.; Figueiredo, M.O.; Cabral, J.; Pereira, L. Temperature and Composition Dependence of the Cation Distribution in Synthetic ZnFeyAl2-yO4 (0 ≤ y ≤ 1) Spinels. J. Solid State Chem. 1994, 111, 300–309. [Google Scholar] [CrossRef]
- Toledo, J.; Valenzuela, M.; Bosch, P.; Armendáriz, H.; Montoya, A.; Nava, N.; Vázquez, A. Effect of AI3+ introduction into hydrothermally prepared ZnFe2O4. Appl. Catal. A Gen. 2000, 198, 235–245. [Google Scholar] [CrossRef]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive Smelting of Neutralized Red Mud for Iron Recovery and Produced Pig Iron for Heat-Resistant Castings. Metals 2019, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Nininger, R.C.; Schroeer, D. Mössbauer studies of the morin transition in bulk and microcrystalline α-Fe2O3. J. Phys. Chem. Solids 1978, 39, 137–144. [Google Scholar] [CrossRef]
- Grudinsky, P. TG-DSC raw data and plots for the mixtures containing ferrites (MFe2O4, where M = Zn, Cu or Zn0.5Cu0.5) and iron sulfates (Fe2(SO4)3∙9H2O or FeSO4∙7H2O). Mendeley data; Version 1. 2021. [Google Scholar] [CrossRef]
- Pelovski, Y.; Petkova, V.; Nikolov, S. Study of the mechanism of the thermochemical decomposition of ferrous sulphate monohydrate. Thermochim. Acta 1996, 274, 273–280. [Google Scholar] [CrossRef]
- Swami, M.S.R.; Prasad, T.P. Thermal analysis of iron(II) sulphate heptahydrate in air. J. Therm. Anal. Calorim. 1980, 19, 297–304. [Google Scholar] [CrossRef]
- Saini, A.; Kótai, L.; Sajo, I.E.; Szilagyi, I.M.; Lázár, K.; May, Z.; Fazekas, P.; Gács, I.; Sharma, V.; Banerji, K.K. Solid phase sulphatizing of zinc ferrite spinel with iron sulphates as an environmental friendly way for recovering zinc. Eur. Chem. Bull. 2012, 1, 7–13. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; Macdonald, R.J.C.; Lamarche, R.E. Solubility of silver sulfate in acidified ferric sulfate solutions. J. Chem. Eng. Data 1975, 20, 55–58. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) Physical constants of inorganic compounds. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; pp. 58–61. [Google Scholar]
- Liu, Q.; Tan, J.; Liu, C.Q.; Yin, Z.L.; Chen, Q.Y.; Liao, Z.; Xie, F.C.; Zhang, P.M. Thermodynamic study of metal sulfate de-composition process in bath smelting. Chin. J. Nonferrous Met. 2014, 24, 1629–1636. [Google Scholar]
- Fedorov, P.P.; Proidakova, V.Y.; Kuznetsov, S.; Voronov, V. Phase equilibria in systems of gallium sulfate with lithium or sodium sulfate. Russ. J. Inorg. Chem. 2017, 62, 1508–1513. [Google Scholar] [CrossRef]
- Stern, K.H. Thermal Decomposition of Inorganic Salts with Oxyanions, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 85. [Google Scholar]
- Guseva, A.F.; Neyman, A.Y.; Animitsa, I.E. Solid Phase Reactions during the Production and Exploitation of Inorganic Materials; Ural State University: Yekaterinburg, Russia, 2005; pp. 18–21. [Google Scholar]
- Rostovshchikova, T.; Smirnov, V.V.; Tsodikov, M.V.; Bukhtenko, O.V.; Maksimov, Y.V.; Kiseleva, O.I.; Pankratov, D. Catalytic conversions of chloroolefins over iron oxide nanoparticles 1. Isomerization of dichlorobutenes in the presence of iron oxide nanopaticles immobilized on silicas with different structures. Russ. Chem. Bull. 2005, 54, 1418–1424. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Korobov, M.S.; Pankratov, D.A.; Yurkov, G.; Gubin, S.P. Catalytic conversions of chloroolefins over iron oxide nanoparticles 2. Isomerization of dichlorobutenes over iron oxide nanoparticles stabilized on the surface of ultradispersed poly(tetrafluoroethylene). Russ. Chem. Bull. 2005, 54, 1425–1432. [Google Scholar] [CrossRef]
- Choi, H.; Seo, J.Y.; Kim, J.; Kim, C.S.; Uhm, Y.R.; Sun, G.M. Standard Iron Oxides and M$ddot{o}$ssbauer Spectroscopy. New Phys. Sae Mulli 2020, 70, 212–219. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.; Townsend, H. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M.; Krivtsov, G.G. Fe3—δO4 Nanoparticles Synthesized in the Presence of Natural Polyelectrolytes. Crystallogr. Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Goya, G.; Rechenberg, H. Superparamagnetic transition and local disorder in CuFe2O4 nanoparticles. Nanostruct. Mater. 1998, 10, 1001–1011. [Google Scholar] [CrossRef] [Green Version]
- Amir, M.; Gungunes, H.; Slimani, Y.; Tashkandi, N.; El Sayed, H.S.; Aldakheel, F.; Sertkol, M.; Sözeri, H.; Manikandan, A.; Ercan, I.; et al. Mössbauer Studies and Magnetic Properties of Cubic CuFe2O4 Nanoparticles. J. Supercond. Nov. Magn. 2018, 32, 557–564. [Google Scholar] [CrossRef]
- Chatterjee, B.K.; Bhattacharjee, K.; Dey, A.; Ghosh, C.K.; Chattopadhyay, K.K. Influence of spherical assembly of copper ferrite nanoparticles on magnetic properties: Orientation of magnetic easy axis. Dalton Trans. 2014, 43, 7930–7944. [Google Scholar] [CrossRef]
- Cross, W.B.; Affleck, L.; Kuznetsov, M.V.; Parkin, I.; Pankhurst, Q.A. Self-propagating high-temperature synthesis of ferrites MFe2O4 (M = Mg, Ba, Co, Ni, Cu, Zn); reactions in an external magnetic field. J. Mater. Chem. 1999, 9, 2545–2552. [Google Scholar] [CrossRef]
- Patil, V.; Kulkarni, R. Magnetic properties of Cu-Zn ferrite investigated by Mössbauer spectroscopy. Solid State Commun. 1979, 31, 551–555. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, R.T.A.; Shafi, M. Fluctuation in occupancy of Cu2+ ions in Zn- and Cd-substituted Cu-ferrites. J. Radioanal. Nucl. Chem. 2008, 277, 531–537. [Google Scholar] [CrossRef]
- Ata-Allah, S.; Hashhash, A. Jahn–Teller effect and superparamagnetism in zn substituted copper-gallate ferrite. J. Magn. Magn. Mater. 2006, 307, 191–197. [Google Scholar] [CrossRef]
- Modi, K.B.; Shah, S.; Kathad, C.R.; Dulera, S.V.; Jethvani, B.B.; Popat, M.V.; Lakhani, V.K.; Chandra, U. Study on Mössbauer Signature, Hyperfine Interaction Parameters and Removal of Delafossite Phase of Al3+-Modified Copper Ferrite. J. Supercond. Nov. Magn. 2014, 28, 1583–1588. [Google Scholar] [CrossRef]
- Van Alboom, A.; De Resende, V.G.; De Grave, E.; Gómez, J.A.M. Hyperfine interactions in szomolnokite (FeSO4·H2O). J. Mol. Struct. 2009, 924-926, 448–456. [Google Scholar] [CrossRef]
- Dékány, I.; Turi, L.; Homonnay, Z.; Vértes, A.; Burger, K. Preparation of nanosize FeS particles on SiO2 and clay mineral supports: SAXS and Mössbauer spectroscopic measurements. Colloids Surf. A Physicochem. Eng. Asp. 1996, 119, 195–203. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D.; Krausova, D.; Pikal, P. The Role of Intermediates in the Process of Red Ferric Pigment Manufacture from FeSO4⋅7H2O. Hyperfine Interact. 2002, 139/140, 437–445. [Google Scholar] [CrossRef]
- Ristic, M.; Music, S.; Orehovec, Z. Thermal decomposition of synthetic ammonium jarosite. J. Mol. Struct. 2005, 744-747, 295–300. [Google Scholar] [CrossRef]
- Haven, Y.; Noftle, R.E. The Mössbauer isomer shift in iron (III) sulfate. J. Chem. Phys. 1977, 67, 2825. [Google Scholar] [CrossRef]
- Majzlan, J.; Alpers, C.N.; Koch, C.B.; McCleskey, R.B.; Myneni, S.C.; Neil, J.M. Vibrational, X-ray absorption, and Mössbauer spectra of sulfate minerals from the weathered massive sulfide deposit at Iron Mountain, California. Chem. Geol. 2011, 284, 296–305. [Google Scholar] [CrossRef]
- Portov, A.B.; Ozerov, S.S.; Tsymbulov, L.B.; Mashyanov, A.K. Usage of sulfuric acid and iron sulfate solutions, copper, and nickel as binders in briquetting of an ore copper-nickel concentrate. Tsvetnye Met. 2016, 35–40. [Google Scholar] [CrossRef]
- Olson, E.S. Binder Modification and Development for Briquetting Steel Mill Residues; Report; University of North Dakota: Grand Forks, ND, USA, 1998; Available online: https://core.ac.uk/download/pdf/195150854.pdf (accessed on 24 June 2021).
- Kawatra, S.K.; Eisele, T.C.; Lewandowski, K.A.; Gurtler, J.A. Novel Binders and Methods for Agglomeration of Ore; Report; Michigan Technological University: Houghton, MI, USA, 2006. Available online: https://www.osti.gov/servlets/purl/902899 (accessed on 24 June 2021). [CrossRef] [Green Version]
- Shi, Y.; Fan, M. Reaction Kinetics for the Catalytic Oxidation of Sulfur Dioxide with Microscale and Nanoscale Iron Oxides. Ind. Eng. Chem. Res. 2007, 46, 80–86. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Chen, J.; Hu, H.; Chen, D.; Yao, H. The effect of minerals in coal ash on the formation of SO3 in flue gas. In Proceedings of the International Conference on Power Engineering (ICOPE), Yokohama, Japan, 30 November–4 December 2015; pp. 1–9. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Wu, H.; Yin, Y.; Chen, J. Heterogeneous Uptake and Oxidation of SO2 on Iron Oxides. J. Phys. Chem. C 2007, 111, 6077–6085. [Google Scholar] [CrossRef]
- Kolmachikhina, E.B.; Sviridov, A.; Naumov, K.D. Investigation into the Influence of Sodium Lignosulfonate, Anionic Surfactants, and Their Mixtures on the Copper Cementation Rate by Zinc. Russ. J. Non-Ferrous Met. 2020, 61, 488–493. [Google Scholar] [CrossRef]
- Chen, C.-S.; Shih, Y.-J.; Huang, Y.-H. Recovery of lead from smelting fly ash of waste lead-acid battery by leaching and electrowinning. Waste Manag. 2016, 52, 212–220. [Google Scholar] [CrossRef]
- Badanoiu, G.; Buzatu, T.; Ghica, V.G.; Buzatu, M.; Iacob, G.; Petrescu, I.M. Study of PbSO4 solubilisation in NaOH solution, for the treatment of oxide-sulphate pastes obtained from dismembered lead-acid batteries. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 209–218. [Google Scholar]
- Pankratov, D.A.; Grudinsky, P.I. Mossbauer spectra raw data for article “Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Leaching Residue with Iron Sulfates; Mendeley data” Version 1. Materials 2021. [Google Scholar] [CrossRef]



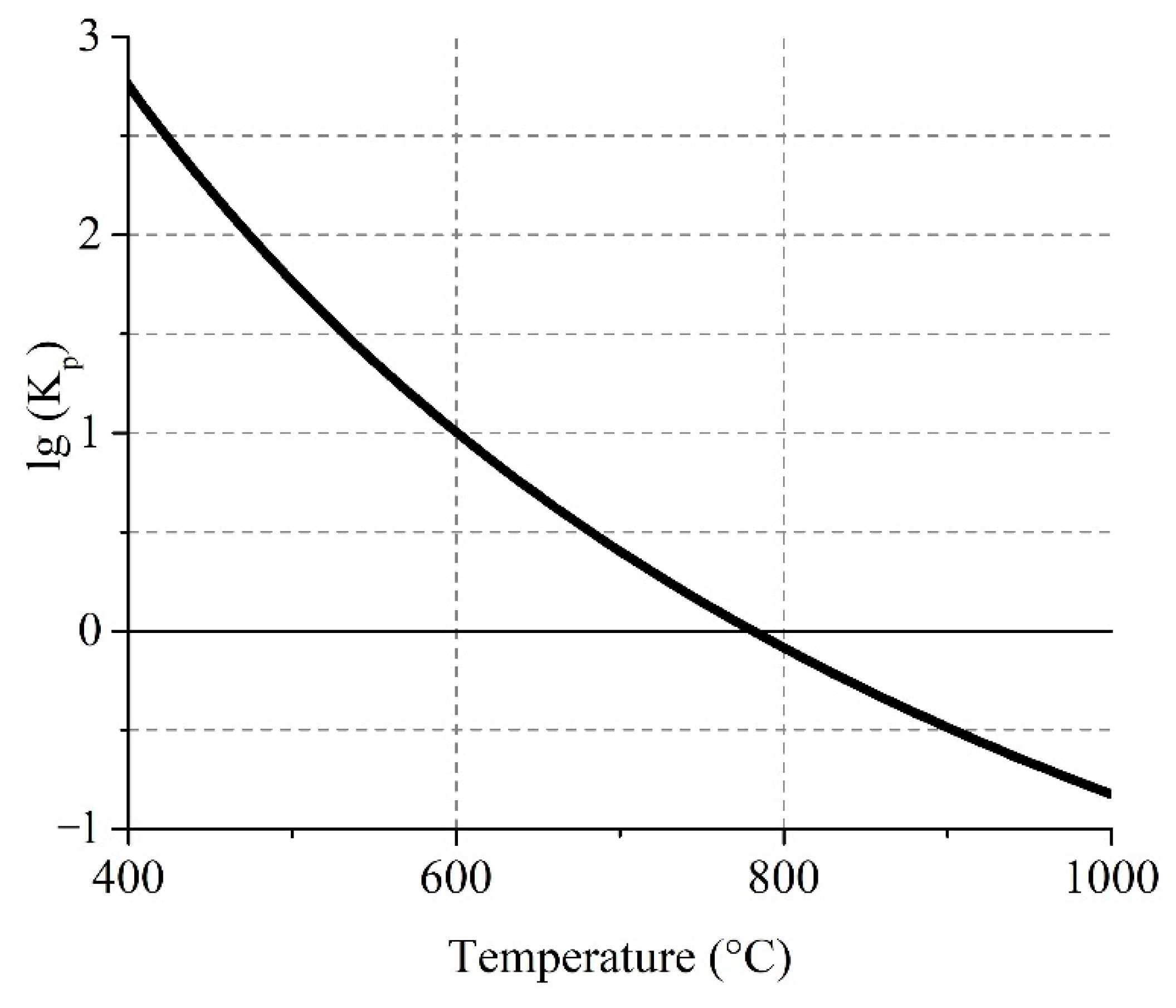
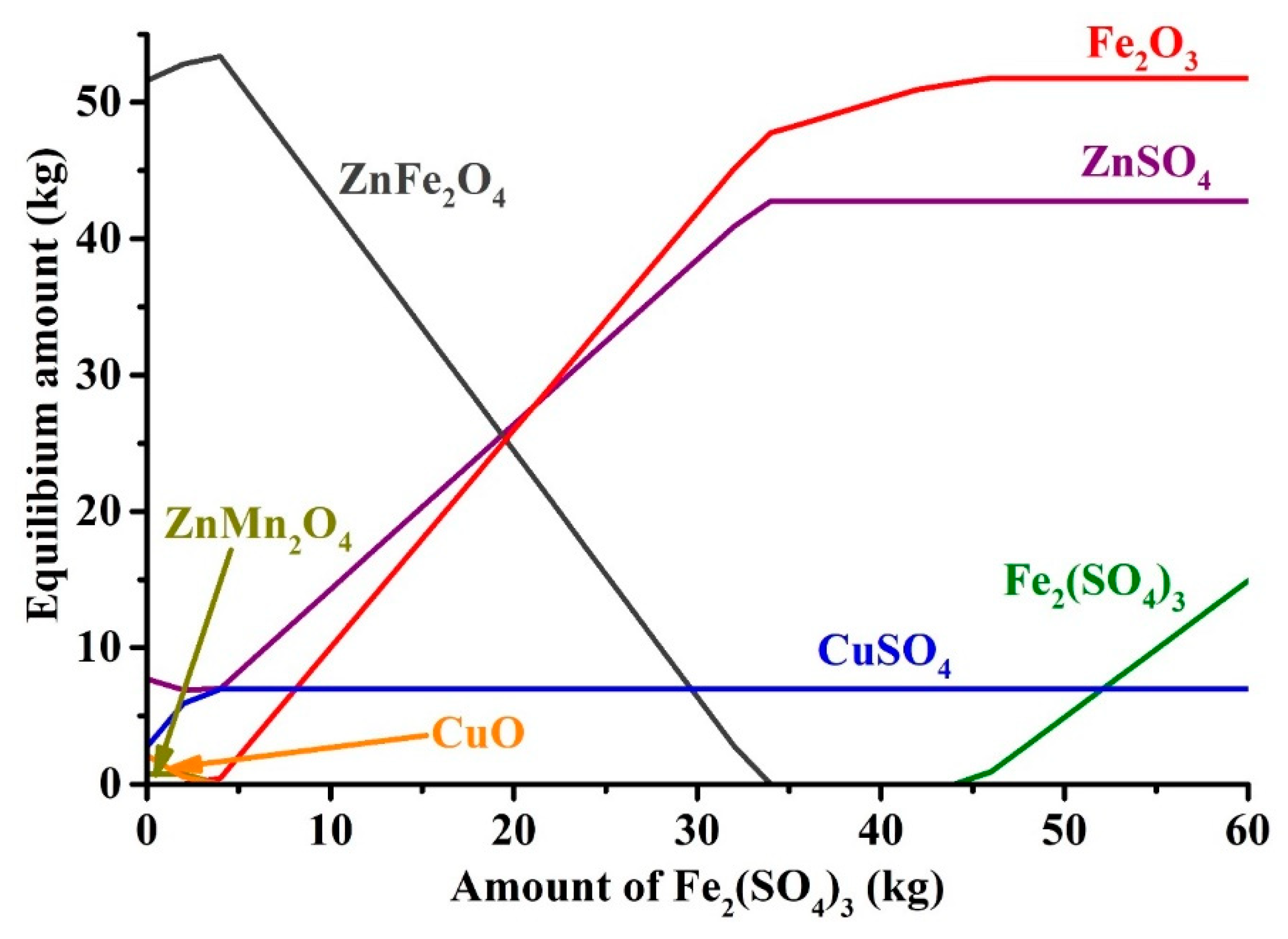
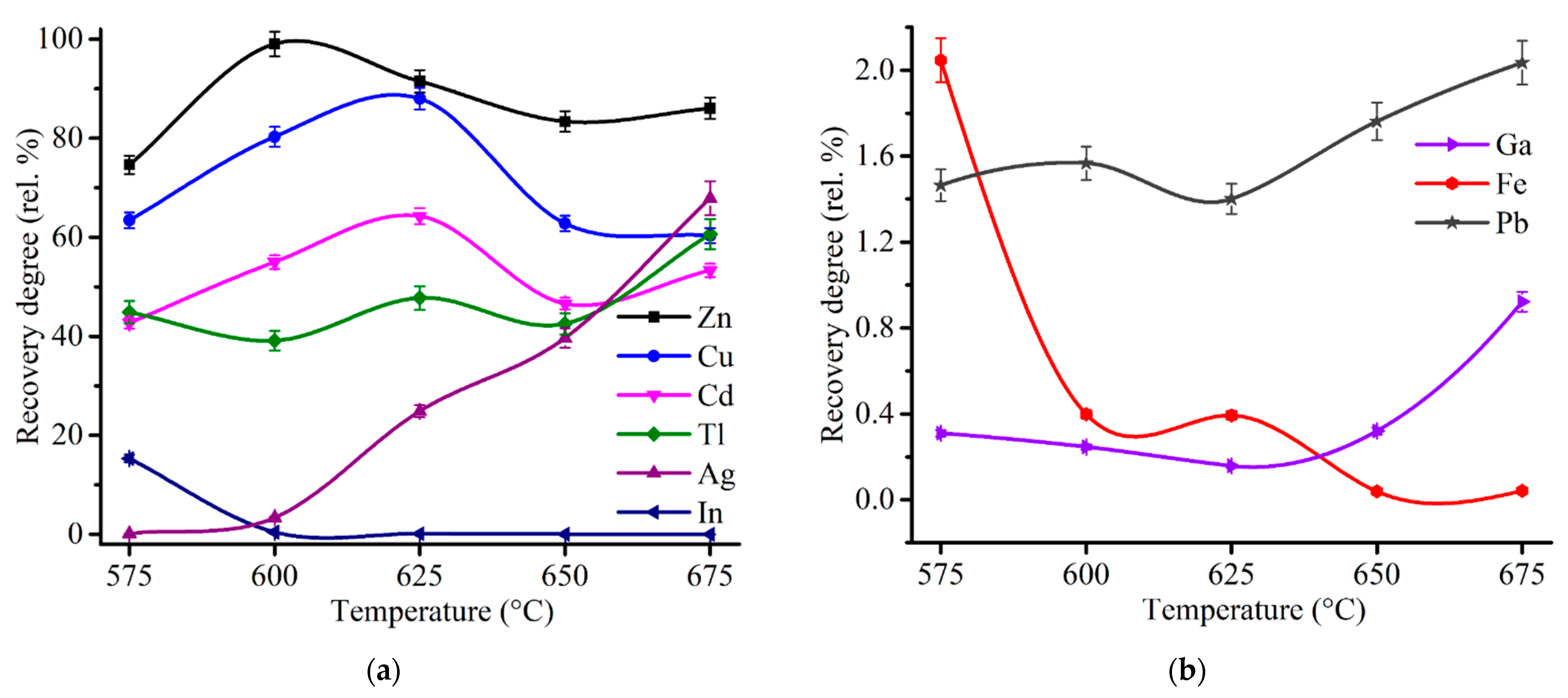







| Species | Content | Species | Content | Species | Content | Species | Content | Species | Content |
|---|---|---|---|---|---|---|---|---|---|
| ZnFe2O4 | 40.56 | CuFe2O4 | 5.13 | PbSO4 | 7.99 | H2O | 2.94 | Ca3Al2Si3O12 | 4.84 |
| ZnS | 1.34 | CuSO4∙5H2O | 4.68 | BaSO4 | 2.12 | CdO | 0.38 | SiO2 | 1.88 |
| ZnSO4∙6H2O | 10.86 | Cu2S | 0.18 | FeS | 2.25 | CdSO4 | 0.14 | Fe2O3 | 1.84 |
| ZnO | 2.67 | CuO | 0.08 | CaCO3 | 2 | CdS | 0.05 | As2O3 | 0.50 |
| Zn2SiO4 | 1.09 | CaSO4∙2H2O | 4.32 | MnS | 1.19 | MgO | 0.95 | CuFeS2 | 0.02 |
| Element | Zn | Cu | Cd | Fe | Pb | S | C | Al | Si | Mn | Ca | Mg | Ba | Sb | As | Ag | Ga | In | Tl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | wt.% | mg/kg | |||||||||||||||||
| content | 17.32 | 2.66 | 0.44 | 23.36 | 5.46 | 5.72 | 0.24 | 0.58 | 1.92 | 0.75 | 3.10 | 0.57 | 1.25 | 0.07 | 0.38 | 283 | 193 | 170 | 20 |
| Phase | Zn Distribution, Rel. % | Phase | Cu Distribution, Rel. % | Phase | Cd Distribution, Rel. % |
|---|---|---|---|---|---|
| Zn sulfates | 15.2 | Cu sulfates | 44.8 | Cd sulfates | 16.6 |
| Zn oxide | 12.4 | Cu oxides | 2.3 | Cd oxide + Cd silicates | 13.7 |
| Zn silicates + Zn arsenates | 3.7 | Cu2S + CuS + Cu5FeS4 | 5.5 | Cd sulfides | 0.8 |
| Zn sulfides | 5.2 | CuFeS2 | 0.3 | Cd ferrites | 37.9 |
| Zn ferrites | 63.5 | Cu ferrites | 47.1 | Cd low-soluble | 31 |
| Element | Ag | Ga | In | Tl |
|---|---|---|---|---|
| solubility in water, rel. % | not detected | 0.1 | not detected | 5.3 |
| Temperature of Spectra Collecting, K | 296 | 78 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subspectrum, # | Phase | δ | Δ = 2ε | Γexp | Heff | S | δ | Δ = 2ε | Γexp | Heff | S |
| mm/s | mm/s | mm/s | kOe | % | mm/s | mm/s | mm/s | kOe | % | ||
| 1 | α-Fe2O3 | 0.37 | -0.21 | 0.22 | 516.1 | 4.5 | 0.46 | 0.40 | 0.27 | 537.4 | 5.5 |
| 2 | Zn1−xAxFe2−yByO4 | 0.34 | 0.50 | 0.43 | - | 83 | 0.43 | 0.50 | 0.44 | - | 82 |
| 3 | ZnFe2O4 | 0.34 | 0.36 | 0.21 | - | 13 | 0.43 | 0.36 | 0.21 | - | 13 |
| Temperature, °C | Phase Composition of Roasted Samples |
|---|---|
| 550 | α-Fe2O3 MFe2O4 Fe2(SO4)3 MSO4∙nH2O (<5%) unidentified phases |
| 600 | α-Fe2O3 MxFe2O4 MSO4∙nH2O |
| 650 | α-Fe2O3 MxFe2O4 MSO4∙nH2O |
| 700 | α-Fe2O3 MxFe2O4 MSO4∙nH2O (<5%) |
| Model Name | Integral Form | Time Range, min | T, °C | R2 (Adjusted R-Squared) | T, °C | R2 (Adjusted R-Squared) | ||
|---|---|---|---|---|---|---|---|---|
| Zn | Cu | Zn | Cu | |||||
| Jander | [1 − (1 − x)1/3]2 | 0–12.5 | 600 | 0.7247 | 0.5797 | 650 | 0.8275 | 0.7160 |
| 12.5–90 | 600 | 0.3372 | - | 650 | 0.5000 | - | ||
| Hinstling- Brounstein | 1 − 2x/3 − (1 − x)2/3 | 0–12.5 | 600 | 0.7716 | 0.6484 | 650 | 0.8538 | 0.7563 |
| 12.5–90 | 600 | 0.4211 | - | 650 | 0.5693 | - | ||
| Shrinking sphere | 1 − (1 − x)1/3 | 0–12.5 | 600 | 0.9562 | 0.8599 | 650 | 0.9802 | 0.9569 |
| 12.5–90 | 600 | 0.6074 | - | 650 | 0.7959 | - | ||
| Erofeev-Avrami n = 2 | [−ln(1 − x)]1/2 | 0–12.5 | 600 | 0.9738 | 0.9906 | 650 | 0.9644 | 0.9741 |
| 12.5–90 | 600 | 0.8030 | - | 650 | 0.9691 | - | ||
| Erofeev-Avrami n = 3 | [−ln(1 − x)]1/3 | 0–12.5 | 600 | 0.8333 | 0.9139 | 650 | 0.8331 | 0.8361 |
| 12.5–90 | 600 | 0.9837 | - | 650 | 0.9653 | - | ||
| Erofeev-Avrami n = 4 | [−ln(1 − x)]1/4 | 0–12.5 | 600 | 0.6968 | 0.5004 | 650 | 0.7122 | 0.7138 |
| 12.5–90 | 600 | 0.9571 | - | 650 | 0.8578 | - | ||
| Chemical reaction of first order | −ln(1 − x) | 0–12.5 | 600 | 0.9077 | 0.7469 | 650 | 0.9578 | 0.9125 |
| 12.5–90 | 600 | 0.4351 | - | 650 | 0.6597 | - | ||
| Chemical reaction of second order | (1 − x)−1 − 1 | 0–12.5 | 600 | 0.7278 | 0.4778 | 650 | 0.8639 | 0.7578 |
| 12.5–90 | 600 | 0.2317 | - | 650 | 0.4316 | - | ||
| Temperature of Spectra Collecting, K | 296 | 78 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Subspectrum, # | Phase | δ | Δ = 2ε | Γexp | Heff | S | δ | Δ = 2ε | Γexp | Heff | S |
| mm/s | mm/s | mm/s | kOe | % | mm/s | mm/s | mm/s | kOe | % | |||
| Roasted | 1 | α-Fe2O3 | 0.37 | −0.18 | 0.32 | 513.3 | 21.8 | 0.48 | 0.33 | 0.33 | 539.6 | 18 |
| 2 | γ-Fe2O3 | 0.31 | −0.01 | 0.55 | 488.2 | 23 | 0.47 | 0.05 | 0.41 | 526.5 | 17 | |
| 3 | 0.32 | −0.08 | 1.17 | 441 | 17 | 0.41 | −0.03 | 0.67 | 508.3 | 29 | ||
| 4 | FeSO4∙H2O | 1.26 | 2.72 | 0.47 | - | 3.6 | 1.37 | 3.12 | 0.71 | - | 5.3 | |
| 5 | Fe2O(SO4)2∙xH2O | 0.40 | 0.60 | 0.63 | - | 21 | 0.50 | 0.61 | 0.57 | - | 21 | |
| 6 | ZnFe2O4 | 0.35 | 0.41 | 0.29 | - | 14 | 0.46 | 0.37 | 0.30 | - | 10 | |
| Leached | 1 | α-Fe2O3 | 0.37 | −0.19 | 0.32 | 513.9 | 31.4 | 0.48 | 0.38 | 0.30 | 539.7 | 27 |
| 2 | γ-Fe2O3 | 0.33 | −0.03 | 0.72 | 486.8 | 26 | 0.47 | 0.02 | 0.53 | 527.2 | 30 | |
| 3 | 0.34 | −0.03 | 1.77 | 438 | 19 | 0.41 | 0.01 | 0.74 | 505 | 24 | ||
| 6 | ZnFe2O4 | 0.35 | 0.44 | 0.35 | - | 11.3 | 0.44 | 0.41 | 0.32 | - | 9 | |
| 7 | Fe+3Oh | 0.33 | - | 1.77 | - | 11.9 | 0.44 | - | 2.0 | - | 11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudinsky, P.; Pankratov, D.; Kovalev, D.; Grigoreva, D.; Dyubanov, V. Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Plant Residue with Iron Sulfates. Materials 2021, 14, 5020. https://doi.org/10.3390/ma14175020
Grudinsky P, Pankratov D, Kovalev D, Grigoreva D, Dyubanov V. Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Plant Residue with Iron Sulfates. Materials. 2021; 14(17):5020. https://doi.org/10.3390/ma14175020
Chicago/Turabian StyleGrudinsky, Pavel, Denis Pankratov, Dmitry Kovalev, Darya Grigoreva, and Valery Dyubanov. 2021. "Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Plant Residue with Iron Sulfates" Materials 14, no. 17: 5020. https://doi.org/10.3390/ma14175020
APA StyleGrudinsky, P., Pankratov, D., Kovalev, D., Grigoreva, D., & Dyubanov, V. (2021). Comprehensive Study on the Mechanism of Sulfating Roasting of Zinc Plant Residue with Iron Sulfates. Materials, 14(17), 5020. https://doi.org/10.3390/ma14175020








