Lipid-Based Drug Delivery Systems in Regenerative Medicine
Abstract
:1. Introduction
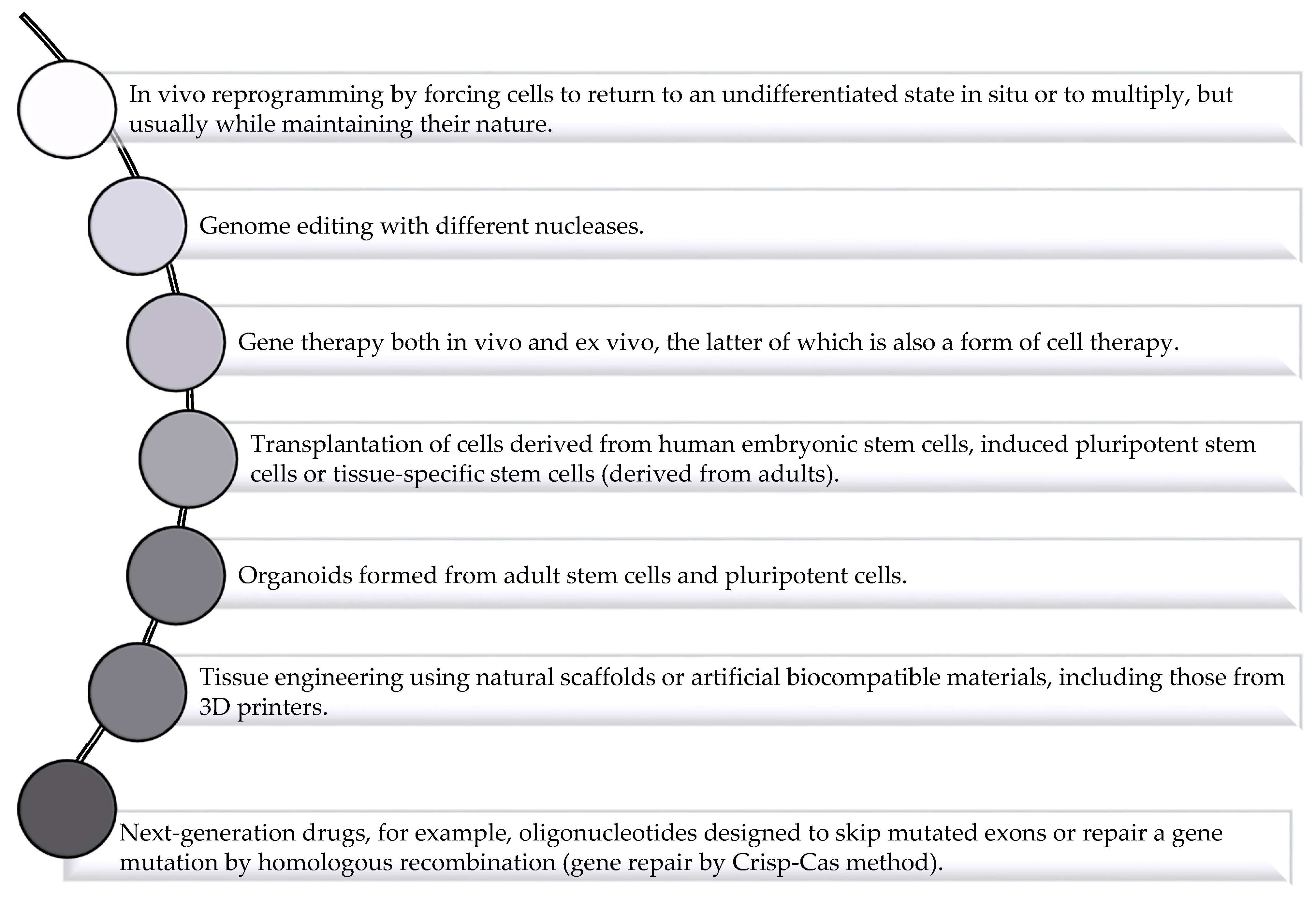
2. Application of Drug Delivery System to Solve Challenges Facing Regenerative Medicine
3. Materials Used in Regenerative Medicine
- The manufacturing process should be cost-effective and scalable to good manufacturing standards (GMP) [30].
| Types of Biomaterials | Fabrication Method | Properties | Applications | Reference |
|---|---|---|---|---|
| Chitosan/HA/bioglass Scaffolds | Freeze drying | Improved biomechanical properties and in-vitro biodegradability | Bone regenerations, implants | [45] |
| Silk fibroin (SF) and chitosan (CS) scaffolds | Freeze drying | The effect of different ratio in blend was optimized | Improved cartilage regeneration | [46] |
| SF/gelatin G, chondritin sulfate C, hyaluronic acid H scaffolds. | Freeze drying | High porosity, Enhanced proliferation and chondrogenic differentiation | Cartilage tissue engineering, bone marrow mesenchymal stem cells, BMSC | [47] |
| SF-polydopamine-E7 peptide functionalized scaffolds | Electrospun | Improved hydrophilicity, cellular proliferation and differentiation | BMSC, bone tissue engineering | [48] |
| SF/Graphene oxide functionalized by BMP-2 peptide | Electrospun | Coated scaffold improved biological properties and bone regeneration | BMSCs, sized bone defects | [49] |
| Fe3O4/Mesoporous bioactive glass/PCL | 3-D bioprinting | Sustained drug delivery with excellent magnetic heating ability, proliferation and mineralization of ECM | Local anticancer drug delivery | [50] |
| Tricalcium phosphate | 3-D printing | Ability to replicate cortico-cancellous alveolar bone architecture with dual layers including compact and porous structures | Bone tissue engineering | [51] |
| PLGA and Solid lipids (Softisan 154 and Witepsol H42) as porogen materials | Solid lipid templating | Easy control of architectural properties and scalable automated production, quick porogen extraction, avoids aqueous media and use of sophisticated equipments | Cartilage tissue engineering | [52] |
| Artificial ECM coated PLGA scaffolds | Solid lipid templating | Enabled suitable growth, proliferation and ECM metabolism of dermal fibroblasts for 14-days | 3-D substrate for Human dermal fibroblasts | [53] |
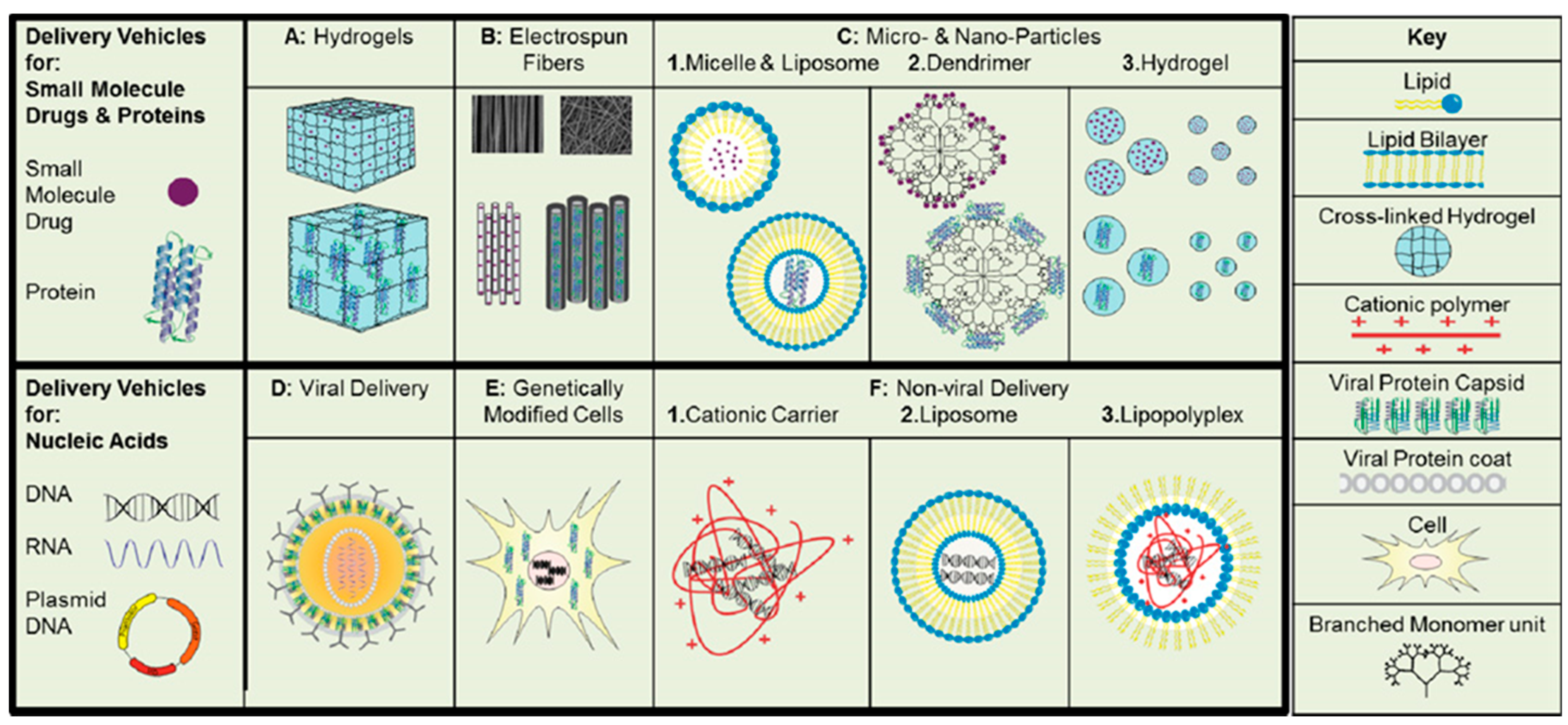
4. Lipids Used in Drug Delivery Systems
5. Lipid-Based Delivery Systems in Regenerative Medicine
5.1. Liposomes
5.1.1. Application of Liposomes in the Treatment of Spinal Cord Injury
5.1.2. Application of Liposomes in the Treatment of Neuron Damage
5.1.3. Application of Liposomes in the Treatment of Thrombosis
5.1.4. Application of Liposomes in the Treatment of Skin Wounds
Skin Repair for Burns
Skin Repair for Ultraviolet B (UVB) Radiation Damage
Skin Repair for Diabetic Ulcers
5.2. Lipid Nanoparticles
5.3. Lipid-Core Micelles
5.3.1. Micelles in Anti-Angiogenic Activity
5.3.2. Micelles in Bone Regeneration
5.3.3. Micelles in Myocardial Infarction
5.4. Colloids
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hasetine, W. A brave new medicine. A conversation with William Haseltine. Interview by Joe Flower. Health Forum. J. 1999, 42, 28–65. [Google Scholar] [PubMed]
- Chen, F.M.; Zhao, Y.M.; Jin, Y.; Shi, S. Prospects for translational regenerative medicine. Biotechnol. Adv. 2012, 30, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: Stem cells and regenerative medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef] [Green Version]
- Passweg, J.R.; Baldomero, H.; Bader, P.; Bonini, C.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Dufour, C.; Falkenburg, J.H.; Farge-Bancel, D.; et al. Hematopoietic SCT in Europe 2013: Recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow. Transplant. 2015, 50, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Marks, P.; Gottlieb, S. Balancing Safety and Innovation for Cell-Based Regenerative Medicine. N. Engl. J. Med. 2018, 378, 954–959. [Google Scholar] [CrossRef]
- Post, Y.; Clevers, H. Defining Adult Stem Cell Function at Its Simplest: The Ability to Replace Lost Cells through Mitosis. Cell Stem Cell 2019, 25, 174–183. [Google Scholar] [CrossRef]
- Rosenthal, N.; Badylak, S. Regenerative medicine: Today’s discoveries informing the future of medical practice. NPJ Regen Med. 2016, 1, 16007. [Google Scholar] [CrossRef] [Green Version]
- Couto, D.S.; Perez-Breva, L.; Cooney, C.L. Regenerative medicine: Learning from past examples. Tissue Eng. Part A 2012, 18, 2386–2393. [Google Scholar] [CrossRef]
- Rai, B.; Nurcombe, V.; Cool, S.M. Heparan sulfate-based treatments for regenerative medicine. Crit Rev. Eukaryot Gene Expr. 2011, 21, 1–12. [Google Scholar] [CrossRef]
- Brown, L.R. Commercial challenges of protein drug delivery. Expert Opin Drug Deliv. 2005, 2, 29–42. [Google Scholar] [CrossRef]
- Tabata, Y. Significance of biomaterials and drug delivery systems in tissue engineering. Connect. Tissue 2001, 33, 315–324. [Google Scholar]
- Geldenhuys, W.J.; Khayat, M.T.; Yun, J.; Nayeem, M.A. Drug Delivery and Nanoformulations for the Cardiovascular System. Res. Rev. Drug Deliv 2017, 1, 32–40. [Google Scholar] [PubMed]
- Tabata, Y. Current status of regenerative medical therapy based on drug delivery technology. Reprod. Biomed. Online 2008, 16, 70–80. [Google Scholar] [CrossRef]
- Newman, M.R.; Benoit, D.S. Local and targeted drug delivery for bone regeneration. Curr. Opin. Biotechnol. 2016, 40, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotman, S.G.; Grijpma, D.W.; Richards, R.G.; Moriarty, T.F.; Eglin, D.; Guillaume, O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J. Control. Release 2018, 269, 88–99. [Google Scholar] [CrossRef]
- Forman, M.B.; Ingram, D.A.; Murray, J.J. Role of perfluorochemical emulsions in the treatment of myocardial reperfusion injury. Am. Heart J. 1992, 124, 1347–1357. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H. Gene therapy for cartilage defects. J. Gene Med. 2005, 7, 1495–1509. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tabata, Y. Tissue engineering by modulated gene delivery. Adv. Drug Deliv. Rev. 2006, 58, 535–554. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, M.S.; Belluzo, M.S. Biodegradable polymers for bone tissue engineering. In Industrial Applications of Renewable Biomass Products; Springer: London, UK, 2017; pp. 47–74. [Google Scholar]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef] [Green Version]
- Wheelton, A.; Mace, J.; Khan, W.S.; Anand, S. Biomaterials and Fabrication to Optimise Scaffold Properties for Musculoskeletal Tissue Engineering. Curr. Stem Cell Res. 2016, 11, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Viji Chandran, S.; Arumugam, B.; Saravanan, S.; Devanand Venkatasubbu, G.; Selvamurugan, N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int. J. Biol Macromol 2018, 111, 953–958. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol Macromol 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Lei, Y.; Jeong, D.; Xiao, J.; Schaffer, D.V. Developing Defined and Scalable 3D Culture Systems for Culturing Human Pluripotent Stem Cells at High Densities. Cell. Mol. Bioeng. 2014, 7, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards ready-to-use 3-D scaffolds for regenerative medicine: Adhesion-based cryopreservation of human mesenchymal stem cells attached and spread within alginate-gelatin cryogel scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Jiang, X.; Hu, Q.; Zhang, C.; Wang, B. Rapid Fabrication of Ready-to-Use Gelatin Scaffolds with Prevascular Networks Using Alginate Hollow Fibers as Sacrificial Templates. ACS Biomater. Sci. Eng. 2020, 6, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, F.D.; Beidokhti, S.M.; Najaran, Z.T.; Saghi, S.S. Highly improved biological and mechanical features of bioglass-ceramic/gelatin composite scaffolds using a novel silica coverage. Ceram. Int. 2021, 47, 14048–14061. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Janoušková, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, E.; Ciraldo, F.E.; Boccaccini, A.R. Bioactive glass-ceramic scaffolds: Processing and properties. MRS Bull. 2017, 42, 226–232. [Google Scholar] [CrossRef]
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on current limits and potentialities of technologies for biomedical ceramic scaffolds production. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Akkineni, A.R.; Förster, Y.; Köhler, T.; Knaack, S.; Gelinsky, M.; Lode, A. Design and Fabrication of Complex Scaffolds for Bone Defect Healing: Combined 3D Plotting of a Calcium Phosphate Cement and a Growth Factor-Loaded Hydrogel. Ann. Biomed. Eng. 2017, 45, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Vishwanath, V.; Pramanik, K.; Biswas, A. Polymer edition. Optimization and evaluation of silk fibroin-chitosan freeze-dried porous scaffolds for cartilage tissue engineering application. Polym. Ed. 2016, 27, 657–674. [Google Scholar]
- Sawatjui, N.; Damrongrungruang, T.; Leeanansaksiri, W.; Jearanaikoon, P.; Hongeng, S.; Limpaiboon, T. Silk fibroin/gelatin–chondroitin sulfate–hyaluronic acid effectively enhances in vitro chondrogenesis of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2015, 52, 90–96. [Google Scholar] [CrossRef]
- Wu, J.; Cao, L.; Liu, Y.; Zheng, A.; Jiao, D.; Zeng, D.; Wang, X.; Kaplan, D.L.; Jiang, X. Functionalization of silk fibroin electrospun scaffolds via BMSC affinity peptide grafting through oxidative self-polymerization of dopamine for bone regeneration. ACS Appl. Mater. Interfaces 2019, 11, 8878–8895. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Almela, T.; Brook, I.M.; Khoshroo, K.; Rasoulianboroujeni, M.; Fahimipour, F.; Tahriri, M.; Dashtimoghadam, E.; El-Awa, A.; Tayebi, L.; Moharamzadeh, K.J.B. Simulation of cortico-cancellous bone structure by 3D printing of bilayer calcium phosphate-based scaffolds. Bioprinting 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Hacker, M.; Ringhofer, M.; Appel, B.; Neubauer, M.; Vogel, T.; Young, S.; Mikos, A.G.; Blunk, T.; Göpferich, A.; Schulz, M.B.J.B. Solid lipid templating of macroporous tissue engineering scaffolds. Biomaterials 2007, 28, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- van der Smissen, A.; Hoffmeister, P.G.; Friedrich, N.; Watarai, A.; Hacker, M.C.; Schulz-Siegmund, M.; Anderegg, U. Artificial extracellular matrices support cell growth and matrix synthesis of human dermal fibroblasts in macroporous 3D scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 1390–1402. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 801820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yao, W.; Wang, N.; Liu, C.; Zhou, H.; Chen, H.; Qiao, W. Synthesis and evaluation of mono- and multi-hydroxyl low toxicity pH-sensitive cationic lipids for drug delivery. Eur. J. Pharm. Sci. 2019, 133, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Saengkrit, N.; Saesoo, S.; Srinuanchai, W.; Phunpee, S.; Ruktanonchai, U.R. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf. B Biointerfaces 2014, 114, 349–356. [Google Scholar] [CrossRef]
- Gao, H.; Hui, K.M. Synthesis of a novel series of cationic lipids that can act as efficient gene delivery vehicles through systematic heterocyclic substitution of cholesterol derivatives. Gene 2001, 8, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Audouy, S.; Hoekstra, D. Cationic lipid-mediated transfection in vitro and in vivo (review). Mol. Membr. Biol. 2001, 18, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Almofti, M.R.; Harashima, H.; Shinohara, Y.; Almofti, A.; Baba, Y.; Kiwada, H. Cationic liposome-mediated gene delivery: Biophysical study and mechanism of internalization. Arch. Biochem. Biophys. 2003, 410, 246–253. [Google Scholar] [CrossRef]
- Li, S.; Tseng, W.C.; Stolz, D.B.; Wu, S.P.; Watkins, S.C.; Huang, L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: Implications for intravenous lipofection. Gene 1999, 6, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Eliyahu, H.; Joseph, A.; Schillemans, J.P.; Azzam, T.; Domb, A.J.; Barenholz, Y. Characterization and in vivo performance of dextran-spermine polyplexes and DOTAP/cholesterol lipoplexes administered locally and systemically. Biomaterials 2007, 28, 2339–2349. [Google Scholar] [CrossRef]
- Zhao, W.; Zhuang, S.; Qi, X.R. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011, 6, 3087–3098. [Google Scholar] [CrossRef] [Green Version]
- Obata, Y.; Tajima, S.; Takeoka, S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J. Control. Release 2010, 142, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Akaboshi, H.; Ogura, T.; Aikawa, T.; Kondo, T.; Tobori, N.; Yuasa, M. Preparation of pH-sensitive anionic liposomes designed for drug delivery system (DDS) application. J. Oleo Sci. 2015, 64, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuentz, M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov. Today Technol. 2012, 9, e71–e174. [Google Scholar] [CrossRef]
- Zhu, S.; Wonganan, P.; Lansakara, P.D.; O’Mary, H.L.; Li, Y.; Cui, Z. The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials 2013, 34, 2327–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaro, J.L. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J. 2015, 17, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Quach, T.; Hu, L.; Wahab, A.; Charman, W.N.; Stella, V.J.; Trevaskis, N.L.; Simpson, J.S.; Porter, C.J. Targeted delivery of a model immunomodulator to the lymphatic system: Comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J. Control. Release 2014, 177, 1–10. [Google Scholar] [CrossRef]
- Alexander, R.L.; Greene, B.T.; Torti, S.V.; Kucera, G.L. A novel phospholipid gemcitabine conjugate is able to bypass three drug-resistance mechanisms. Cancer Chemother Pharm. 2005, 56, 15–21. [Google Scholar] [CrossRef]
- Pedersen, P.J.; Christensen, M.S.; Ruysschaert, T.; Linderoth, L.; Andresen, T.L.; Melander, F.; Mouritsen, O.G.; Madsen, R.; Clausen, M.H. Synthesis and biophysical characterization of chlorambucil anticancer ether lipid prodrugs. J. Med. Chem. 2009, 52, 3408–3415. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F. Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, B.M. Chronic wound care guidelines issued. JAMA J. Am. Med. Assoc. 2007, 297, 938–939. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidences. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Li, J.; Liu, J.L.; Jiao, H.J.; Liu, B. Anti-Inflammation and JoInt. Lubrication Dual Effects of a Novel Hyaluronic Acid/Curcumin Nanomicelle Improve the Efficacy of Rheumatoid Arthritis Therapy. ACS Appl. Mater. Inter. 2018, 10, 23595–23604. [Google Scholar] [CrossRef]
- Todorovic, K.; Jovanovic, G.; Todorovic, A.; Mitic, A.; Stojiljkovic, N.; Ilic, S.; Stojanovic, N.; Stojnev, S. Effects of coenzyme Q10 encapsulated in nanoliposomes on wound healing processes after tooth extraction. J. Dent. Sci. 2018, 13, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Xu, Q.; Sun, H.; Shi, M.; Wang, D.; Guo, M.; Yu, J.; Zhao, C.; Feng, B. GHK-Cu-liposomes accelerate scald wound healing in mice by promoting cell proliferation and angiogenesis. Wound Repair Regen 2017, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Qi, X.; Lin, G.; Cui, F.; Li, F.; Wu, X. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci. Rep. 2016, 6, 29753. [Google Scholar] [CrossRef] [Green Version]
- Alwattar, J.K.; Chouaib, R.; Khalil, A.; Mehanna, M.M. A novel multifaceted approach for wound healing: Optimization and in vivo evaluation of spray dried tadalafil loaded pro-nanoliposomal powder. Int. J. Pharm. 2020, 587, 119647. [Google Scholar] [CrossRef]
- Sanad, R.A.; Abdel-Bar, H.M. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, D.; Li, J.; Wang, Y.; Guo, J.X.; Chen, Z.P.; Cai, B.C.; Yang, T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev. Ind. Pharm. 2013, 39, 197–204. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Degim, Z.; Celebi, N.; Alemdaroglu, C.; Deveci, M.; Ozturk, S.; Ozogul, C. Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int. Wound J. 2011, 8, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.Y.; Liu, L.L.; Xiang, Y.; Lu, Y.; Deng, L.F.; Zhang, H.B.; Santos, H.A.; Cui, W.G. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. 2019, 34. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Nejdl, L.; Kudr, J.; Ruttkay-Nedecky, B.; Jimenez, A.M.; Kopel, P.; Kremplova, M.; Masarik, M.; Stiborova, M.; Eckschlager, T.; et al. Fluorescence Characterization of Gold Modified Liposomes with Antisense N-myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide). Sensors 2016, 16, 290. [Google Scholar] [CrossRef] [Green Version]
- do Vale Ramos, R.C.; Alegrete, N. The role of pharmacotherapy in modifying the neurological status of patients with spinal and spinal cord injuries. Rev. Bras. Ortop. 2015, 50, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Bydon, M.; Lin, J.; Macki, M.; Gokaslan, Z.L.; Bydon, A. The current role of steroids in acute spinal cord injury. World Neurosurg. 2014, 82, 848–854. [Google Scholar] [CrossRef]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Xu, B.; Xue, W.W.; Yang, B.; Fan, Y.H.; Chen, B.; Xiao, Z.F.; Xue, X.Y.; Sun, Z.; Shu, M.Y.; et al. A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials 2020, 243, 119941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Zhang, H.Y.; Xu, H.L.; Zhao, Y.Z.; Li, Z.M.; Li, J.W.; Wang, H.L.; Zhuge, D.L.; Guo, X.; Xu, H.Z.; et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 2018, 8, 4429–4446. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.J.; Liu, Y.; Wang, H.J.; Ban, D.X.; Cheng, S.Z.; Ning, G.Z.; Wang, L.L.; Chang, J.; Feng, S.Q. New approach to treating spinal cord injury using PEG-TAT-modified, cyclosporine-A-loaded PLGA/polymeric liposomes. J. Drug Target. 2017, 25, 75–82. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Bejoy, J.; Song, L.; Wang, Z.; Sang, Q.X.; Zhou, Y.; Li, Y. Neuroprotective Activities of Heparin, Heparinase III, and Hyaluronic Acid on the Abeta42-Treated Forebrain Spheroids Derived from Human Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 2922–2933. [Google Scholar] [CrossRef]
- Yue, P.; Miao, W.; Gao, L.; Zhao, X.; Teng, J. Ultrasound-Triggered Effects of the Microbubbles Coupled to GDNF Plasmid-Loaded PEGylated Liposomes in a Rat Model of Parkinson’s Disease. Front. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.Y.; Barro, L.; Tsai, S.T.; Feng, T.W.; Wu, X.Y.; Chao, C.W.; Yu, R.S.; Chin, T.Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lee, Y.J. Rescuing cholinergic neurons from apoptotic degeneration by targeting of serotonin modulator-and apolipoprotein E-conjugated liposomes to the hippocampus. Int. J. Nanomed. 2016, 11, 6809–6824. [Google Scholar] [CrossRef] [Green Version]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Mikulik, R.; Masek, J.; Raska, M.; Turanek Knotigova, P.; Miller, A.D.; Turanek, J. Liposomal nanocarriers for plasminogen activators. J. Control. Release 2016, 227, 45–57. [Google Scholar] [CrossRef]
- Joo, H.; Wang, G.J.; George, M.G. Use of intravenous tissue plasminogen activator and hospital costs for patients with acute ischaemic stroke aged 18-64 years in the USA. Stroke Vasc. Neurol. 2016, 1, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, B.; Nayak, M.K.; Dash, D.; Agrawal, G.P.; Vyas, S.P. Development and characterization of highly selective target-sensitive liposomes for the delivery of streptokinase: In Vitro/In Vivo studies. Drug Deliv 2016, 23, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.P.; Li, C.L.; Zhou, D.Y.; Ding, C.; Jin, Y.Q.; Tian, Q.M.; Meng, X.Z.; Pu, K.F.; Zhu, Y.M. Cyclic RGD functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biomater. 2018, 70, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Matricardi, P.; Cencetti, C.; Peris, J.E.; Melis, V.; Carbone, C.; Escribano, E.; Zaru, M.; Fadda, A.M.; Manconi, M. Combination of argan oil and phospholipids for the development of an effective liposome-like formulation able to improve skin hydration and allantoin dermal delivery. Int. J. Pharm. 2016, 505, 204–211. [Google Scholar] [CrossRef]
- Li, F.; Shi, Y.J.; Liang, J.; Zhao, L. Curcumin-loaded chitosan nanoparticles promote diabetic wound healing via attenuating inflammation in a diabetic rat model. J. Biomater. Appl. 2019, 34, 476–486. [Google Scholar] [CrossRef]
- Hong, Y.J.; Pyo, C.G.; Kim, J.C. Liposomes incorporating hydrophobically modified silk fibroin: pH-dependent release. Int. J. Biol. Macromol. 2010, 47, 635–639. [Google Scholar] [CrossRef]
- Nunez, S.C.; Franca, C.M.; Silva, D.F.; Nogueira, G.E.; Prates, R.A.; Ribeiro, M.S. The influence of red laser irradiation timeline on burn healing in rats. Lasers Med. Sci. 2013, 28, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef] [Green Version]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Du, B.S.; Zhang, Z.Y.; Li, N. Madecassoside prevents A beta(25–35)-induced inflammatory responses and autophagy in neuronal cells through the class III PI3K/Beclin-1/Bcl-2 pathway. Int. Immunopharmacol. 2014, 20, 221–228. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Lu, Y.H.; Liu, D.H.; Li, C.C. Burn wound healing properties of asiaticoside and madecassoside. Exp. Med. 2016, 12, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Liu, M.F.; Wang, H.J.; Du, S. Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: Preparation optimization, in vitro dermal permeation, and in vivo bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.F.; Chen, W.C.; Zhang, X.Y.; Su, P.W.; Yue, F.; Zeng, S.Q.; Du, S. Improved surface adhesion and wound healing effect of madecassoside liposomes modified by temperature -responsive PEG-PCL-PEG copolymers. Eur. J. Pharm. Sci. 2020, 151. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Chen, P.P.; ZhuGe, D.L.; Zhu, Q.Y.; Jin, B.H.; Shen, B.X.; Xiao, J.; Zhao, Y.Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6, 1700344. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Chen, P.P.; Wang, L.F.; Tong, M.Q.; Ou, Z.H.; Zhao, Y.Z.; Xiao, J.; Fu, T.L.; Wei, X. Skin-permeable liposome improved stability and permeability of bFGF against skin of mice with deep second degree scald to promote hair follicle neogenesis through inhibition of scar formation. Colloids Surf. B Biointerfaces 2018, 172, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Herrling, T.; Jung, K.; Fuchs, J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol. Biomol Spectrosc 2006, 63, 840–845. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Afaq, F. Phytochemicals for prevention of solar ultraviolet radiation-induced damages. Photochem. Photobiol. 2008, 84, 489–500. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.E.; Choi, S.S.; Park, T.H. Protective Effects of Silkworm Hemolymph Extract and Its Fractions on UV-induced Photoaging. Biotechnol. Bioproc. E 2017, 22, 37–44. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.W.; Zeng, H.; Xie, X.Q.; Zang, G.X.; Ye, Y.C.; Tashiro, S.; Onodera, S.; Jiang, S.; Ikejima, T. p53-mediated autophagy adjustment is involved in the protection of silibinin against murine dermal inflammation and epidermal apoptosis induced by UVB irradiation. J. Asian Nat. Prod. Res. 2013, 15, 117–129. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Kumar, A.; Pant, M.C.; Singh, H.S.; Khandelwal, S. Assessment of the redox profile and oxidative DNA damage (8-OHdG) in squamous cell carcinoma of head and neck. J. Cancer Res. 2012, 8, 254–259. [Google Scholar]
- Recasens, M.; Garcia, M.; Del Rio, M.; Jorcano, J.L. Ultraviolet light induces epidermal disorganization and DNA damage in the skin-humanized mouse model; The efficacy of a broad-spectrum sunscreen to protect from histologic and molecular alterations. J. Am. Acad. Derm. 2006, 54, Ab189. [Google Scholar]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein Carbonylation. Antioxid Redox Sign 2010, 12, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Ai, Y.; Xu, X.; Zhu, S.; Zhang, B.; Tang, M.; Zhang, L.; He, T. Glabridin Liposome Ameliorating UVB-Induced Erythema and Lethery Skin by Suppressing Inflammatory Cytokine Production. J. Microbiol. Biotechnol. 2021, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Spanidi, E.; Karapetsas, A.; Voulgaridou, G.P.; Letsiou, S.; Aligiannis, N.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Mourtzinos, I.; Pappa, A.; et al. A New Controlled Release System for Propolis Polyphenols and Its Biochemical Activity for Skin Applications. Plants 2021, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Noha, A.; John, D. Diabetic foot disease: From the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J. Diabetes 2016, 7, 153. [Google Scholar]
- Naves, C.C.L.M. The Diabetic Foot: A Historical Overview and Gaps in Current Treatment. Adv. Wound Care 2016, 5, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model. Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Fukui, T.; Kawaguchi, A.T.; Takekoshi, S.; Miyasaka, M.; Sumiyoshi, H.; Tanaka, R. Liposome-Encapsulated Hemoglobin Accelerates Skin Wound Healing in Diabetic dB/dB Mice. Artif. Organs 2017, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Lee, S.W.; Pangeni, R.; Byun, Y.; Yoon, I.S.; Park, J.W. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater. 2017, 57, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Wang, A.X.; Liu, X.; Meisgen, F.; Grunler, J.; Botusan, I.R.; Narayanan, S.; Erikci, E.; Li, X.; Blomqvist, L.; et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Investig. 2015, 125, 3008–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Zhu, H.; Rodriguez, V.M. 1—Nanomaterials, nanofillers, and nanocomposites: Types and properties. In Health and Environmental Safety of Nanomaterials; Njuguna, J., Pielichowski, K., Zhu, H., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 3–27. [Google Scholar] [CrossRef]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured lipid carriers (NLC): The second generation of solid lipid nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef]
- Vairo, C.; Collantes, M.; Quincoces, G.; Villullas, S.; Peñuelas, I.; Pastor, M.; Gil, A.G.; Gainza, E.; Hernandez, R.M.; Igartua, M.; et al. Preclinical safety of topically administered nanostructured lipid carriers (NLC) for wound healing application: Biodistribution and toxicity studies. Int. J. Pharm. 2019, 569, 118484. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; D’Autilia, F.; Rossi, S.; Ferrari, F.; Grisoli, P.; Sorrenti, M.; Catenacci, L.; Del Fante, C.; Perotti, C.; et al. Wound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairing. Eur. J. Pharm. Biopharm. 2013, 84, 84–90. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Mannucci, B.; Grisoli, P.; Vigani, B.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2018, 13, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augusto Oshiro, J.; Rillo Sato, M.; Rocha Scardueli, C.; Jose Pimentel Lopes de Oliveira, G.; Paiva Abucafy, M.; Chorilli, M. Bioactive molecule-loaded drug delivery systems to optimize bone tissue repair. Curr. Protein Pept. Sci. 2017, 18, 850–863. [Google Scholar]
- Cao, Y. Future options of anti-angiogenic cancer therapy. Chin. J. Cancer 2016, 35, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; García, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Phillips, J.E.; Gersbach, C.A.; Wojtowicz, A.M.; García, A.J. Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J. Cell Sci. 2006, 119, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Hamidouche, Z.; Haÿ, E.; Vaudin, P.; Charbord, P.; Schüle, R.; Marie, P.J.; Fromigué, O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2 expression. FASEB J. 2008, 22, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Chen, H.-X.; Xue, Y.; Li, D.-M.; Wan, X.-C.; Ge, R.; Li, J.-C. Osteoblastogenic effects of dexamethasone through upregulation of TAZ expression in rat mesenchymal stem cells. J. Steroid Biochem. Mol. Biol. 2009, 116, 86–92. [Google Scholar] [CrossRef]
- Santo, V.E.; Ratanavaraporn, J.; Sato, K.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Tabata, Y. Cell engineering by the internalization of bioinstructive micelles for enhanced bone regeneration. Nanomedicine 2015, 10, 1707–1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
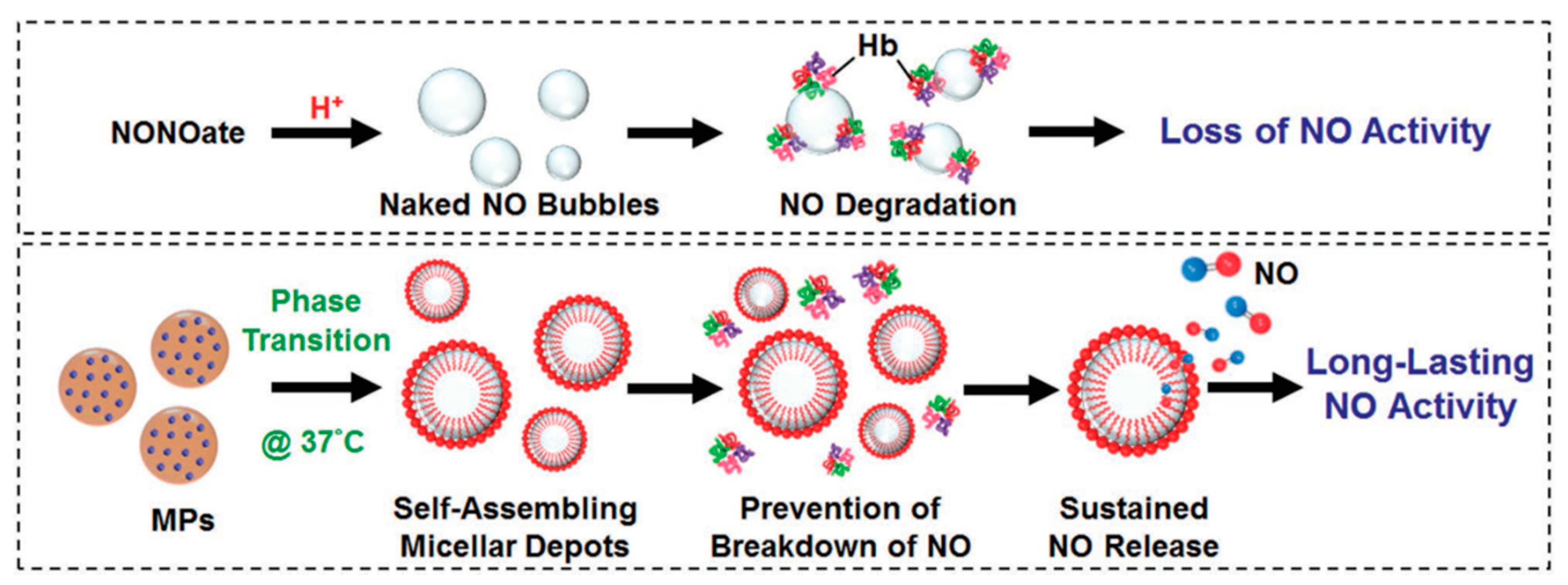
- Lin, Y.J.; Chen, C.C.; Chi, N.W.; Nguyen, T.; Lu, H.Y.; Nguyen, D.; Lai, P.L.; Sung, H.W. In Situ Self-Assembling Micellar Depots that Can Actively Trap and Passively Release NO with Long-Lasting Activity to Reverse Osteoporosis. Adv. Mater. 2018, 30, 1705605. [Google Scholar] [CrossRef]
- Phatharajaree, W.; Phrommintikul, A.; Chattipakorn, N. Matrix metalloproteinases and myocardial infarction. Can. J. Cardiol. 2007, 23, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Matsumura, S.-i.; Iwanaga, S.; Mochizuki, S.; Okamoto, H.; Ogawa, S.; Okada, Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J. Clin. Investig. 2005, 115, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, J.; Sievers, R.; Motion, J.M.; Kivimäe, S.; Fang, Q.; Lee, R.J. Delivery of lipid micelles into infarcted myocardium using a lipid-linked matrix metalloproteinase targeting peptide. Mol. Pharm. 2015, 12, 1150–1157. [Google Scholar] [CrossRef]
- Wang, J.; Seo, M.J.; Deci, M.B.; Weil, B.R.; Canty, J.M.; Nguyen, J. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int. J. Nanomed. 2018, 13, 6441. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-Q.; Wu, J.-Y.; Xiang, D.-X.; Luo, S.-L.; Hu, X.-B.; Tang, T.-T.; Sun, T.-L.; Liu, X.-Y. Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int. J. Nanomed. 2019, 14, 8345. [Google Scholar] [CrossRef] [Green Version]
- Townsend, J.M.; Dennis, S.C.; Whitlow, J.; Feng, Y.; Wang, J.; Andrews, B.; Nudo, R.J.; Detamore, M.S.; Berkland, C.J. Colloidal Gels with Extracellular Matrix Particles and Growth Factors for Bone Regeneration in Critical Size Rat Calvarial Defects. AAPS J. 2017, 19, 703–711. [Google Scholar] [CrossRef]
- Diba, M.; Camargo, W.A.; Brindisi, M.; Farbod, K.; Klymov, A.; Schmidt, S.; Harrington, M.J.; Draghi, L.; Boccaccini, A.R.; Jansen, J.A. Composite colloidal gels made of bisphosphonate-functionalized gelatin and bioactive glass particles for regeneration of osteoporotic bone defects. Adv. Funct. Mater. 2017, 27, 1703438. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, Z.; Jamal, S.; Detamore, M.S.; Berkland, C. Hybrid hydroxyapatite nanoparticle colloidal gels are injectable fillers for bone tissue engineering. Tissue Eng. Part A 2013, 19, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Roux, R.; Ladavière, C.; Montembault, A.; Delair, T. Particle assemblies: Toward new tools for regenerative medicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, J.; Kim, J.P.; Shen, H.; Yang, F.; Zhang, Q.; Xu, M.; Bi, W.; Wang, X.; Yang, J.; et al. Ionic Colloidal Molding as a Biomimetic Scaffolding Strategy for Uniform Bone Tissue Regeneration. Adv. Mater. 2017, 29, 1605546. [Google Scholar] [CrossRef] [PubMed]
- Eskinazi-Budge, A.; Manickavasagam, D.; Czech, T.; Novak, K.; Kunzler, J.; Oyewumi, M.O. Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. Drug Dev. Ind. Pharm. 2018, 44, 1583–1590. [Google Scholar] [CrossRef]
- Del Castillo-Santaella, T.; Ortega-Oller, I.; Padial-Molina, M.; O’Valle, F.; Galindo-Moreno, P.; Jódar-Reyes, A.B.; Peula-García, J.M. Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics 2019, 11, 388. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci 2018, 516, 57–66. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Khan, M.M.; Parveen, F.; Torchilin, V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials 2021, 14, 5371. https://doi.org/10.3390/ma14185371
Filipczak N, Yalamarty SSK, Li X, Khan MM, Parveen F, Torchilin V. Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials. 2021; 14(18):5371. https://doi.org/10.3390/ma14185371
Chicago/Turabian StyleFilipczak, Nina, Satya Siva Kishan Yalamarty, Xiang Li, Muhammad Muzamil Khan, Farzana Parveen, and Vladimir Torchilin. 2021. "Lipid-Based Drug Delivery Systems in Regenerative Medicine" Materials 14, no. 18: 5371. https://doi.org/10.3390/ma14185371
APA StyleFilipczak, N., Yalamarty, S. S. K., Li, X., Khan, M. M., Parveen, F., & Torchilin, V. (2021). Lipid-Based Drug Delivery Systems in Regenerative Medicine. Materials, 14(18), 5371. https://doi.org/10.3390/ma14185371







