Enhanced Thermoelectric Performance by Surface Engineering in SnTe-PbS Nanocomposites
Abstract
:1. Introduction
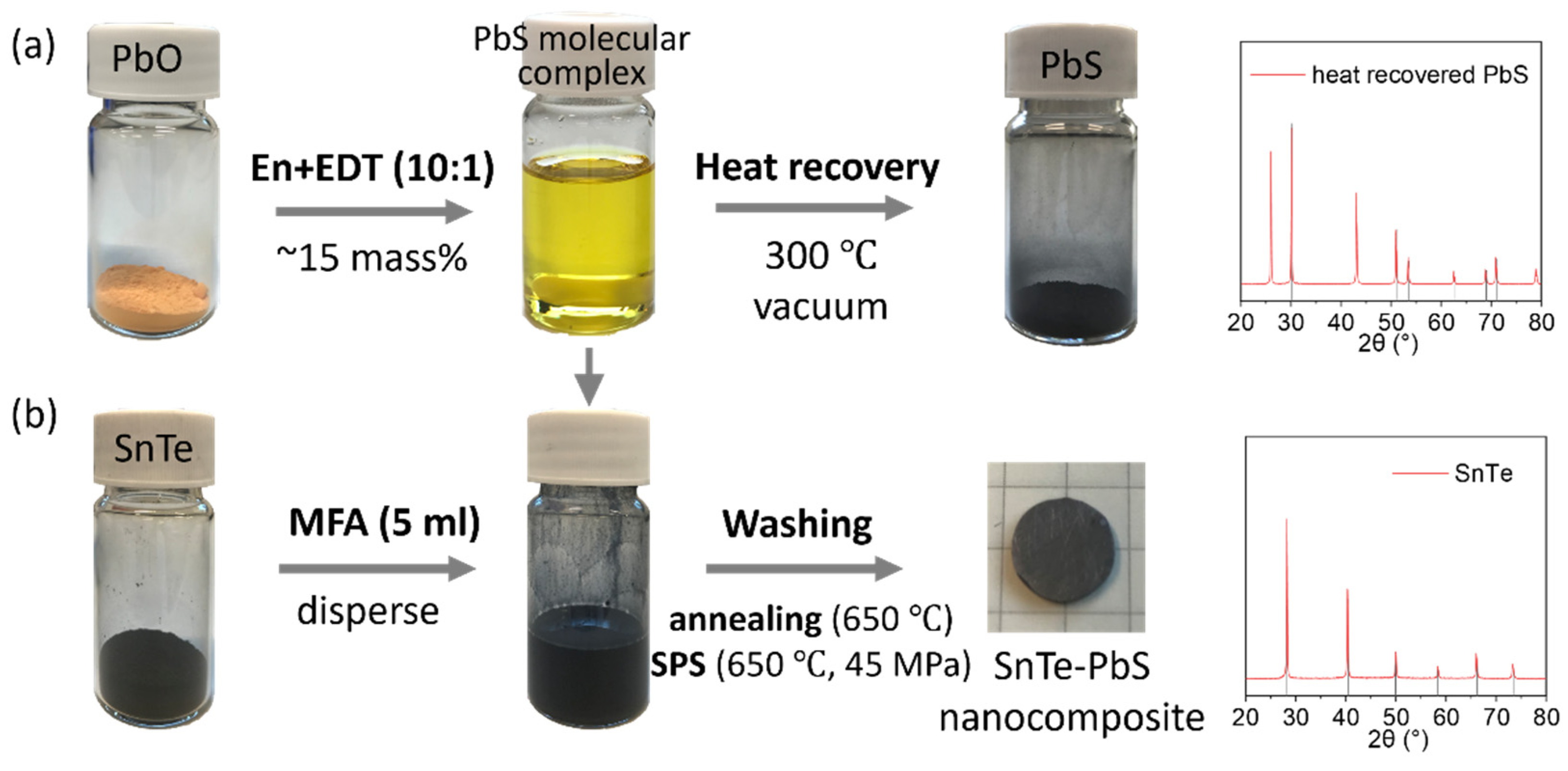
2. Materials and Methods
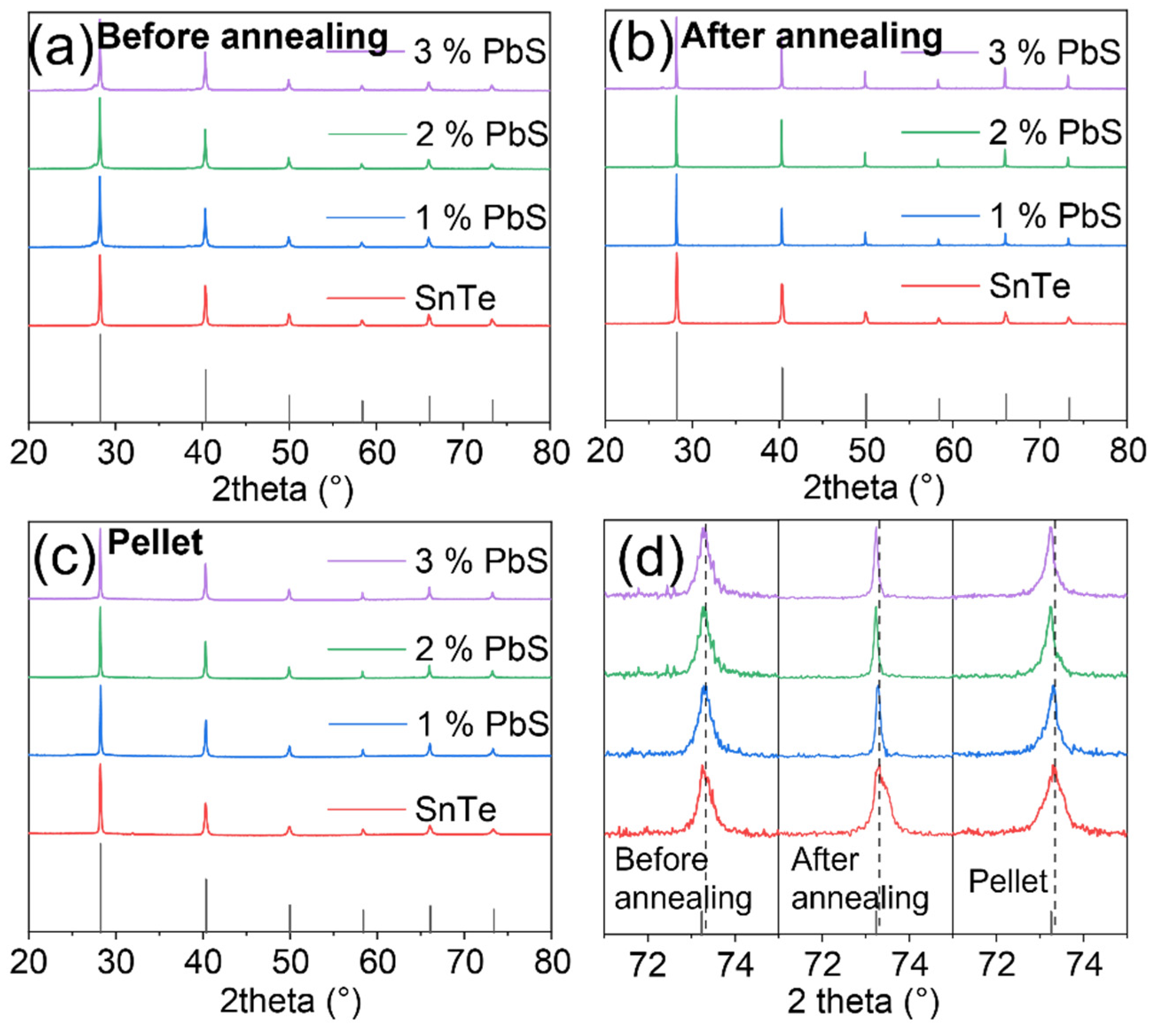
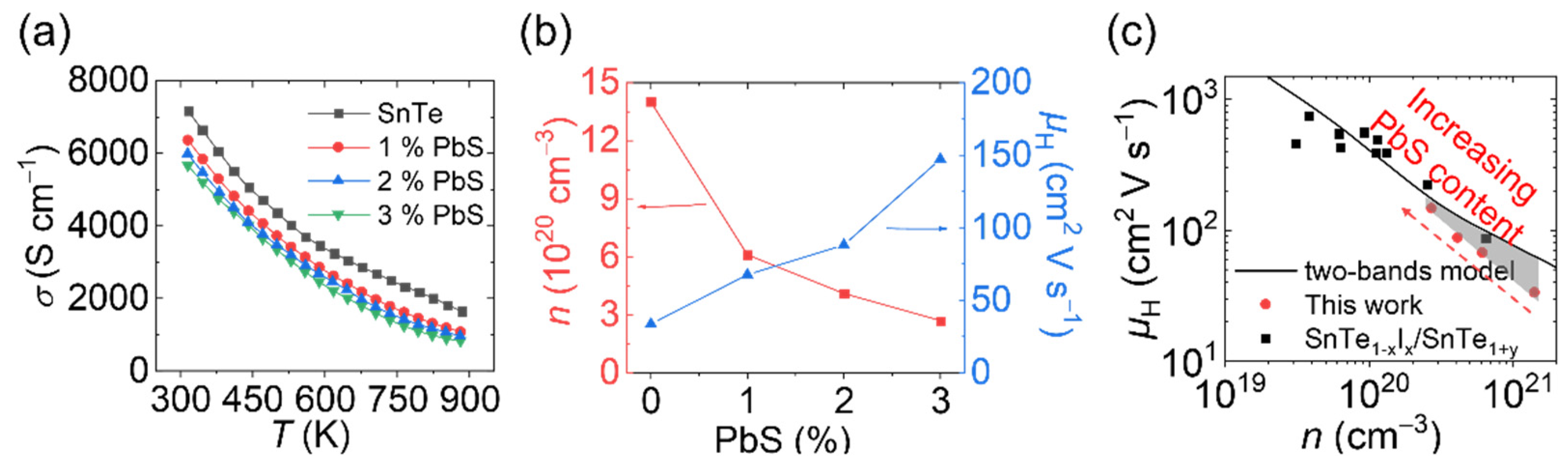
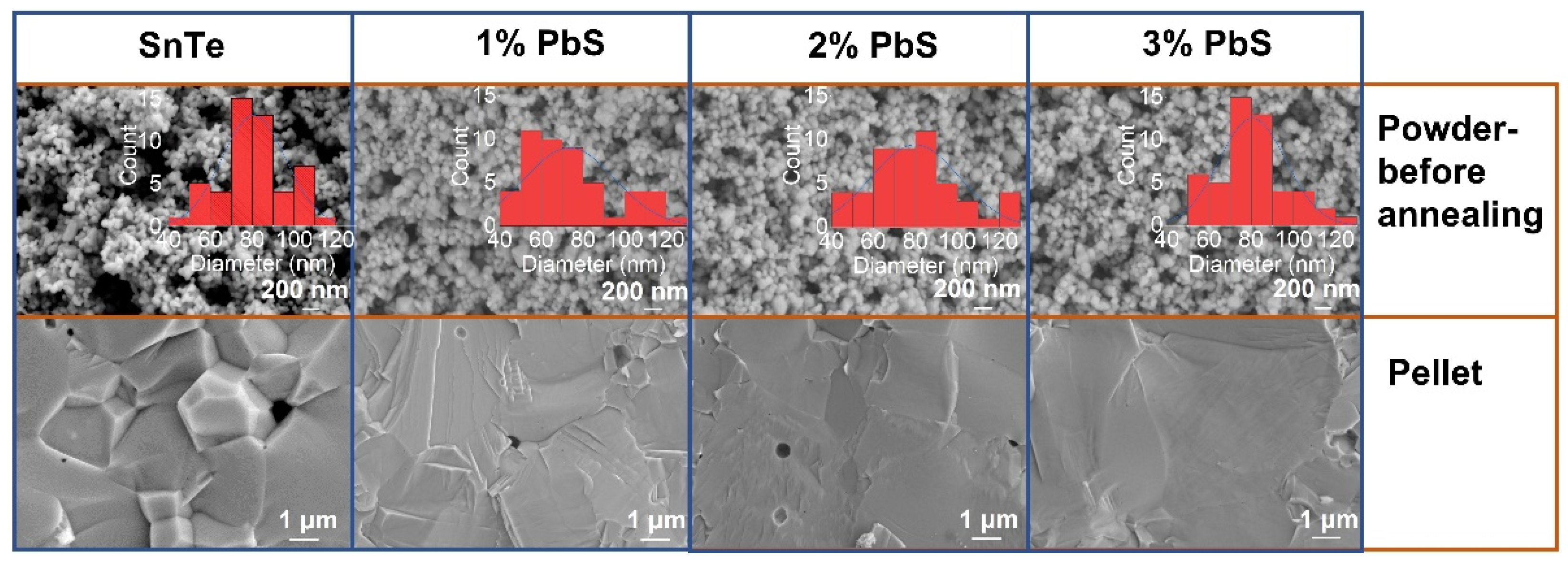
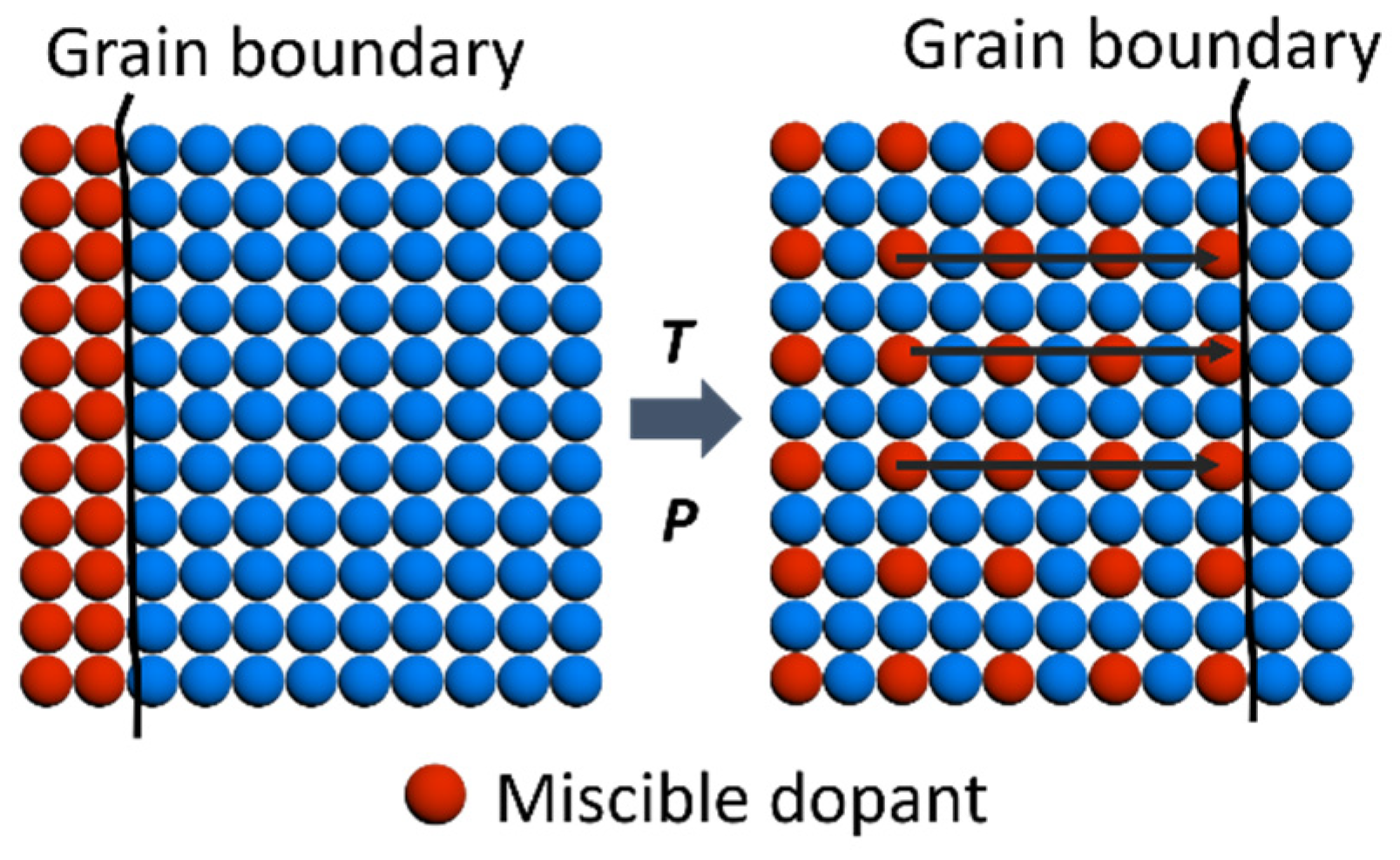
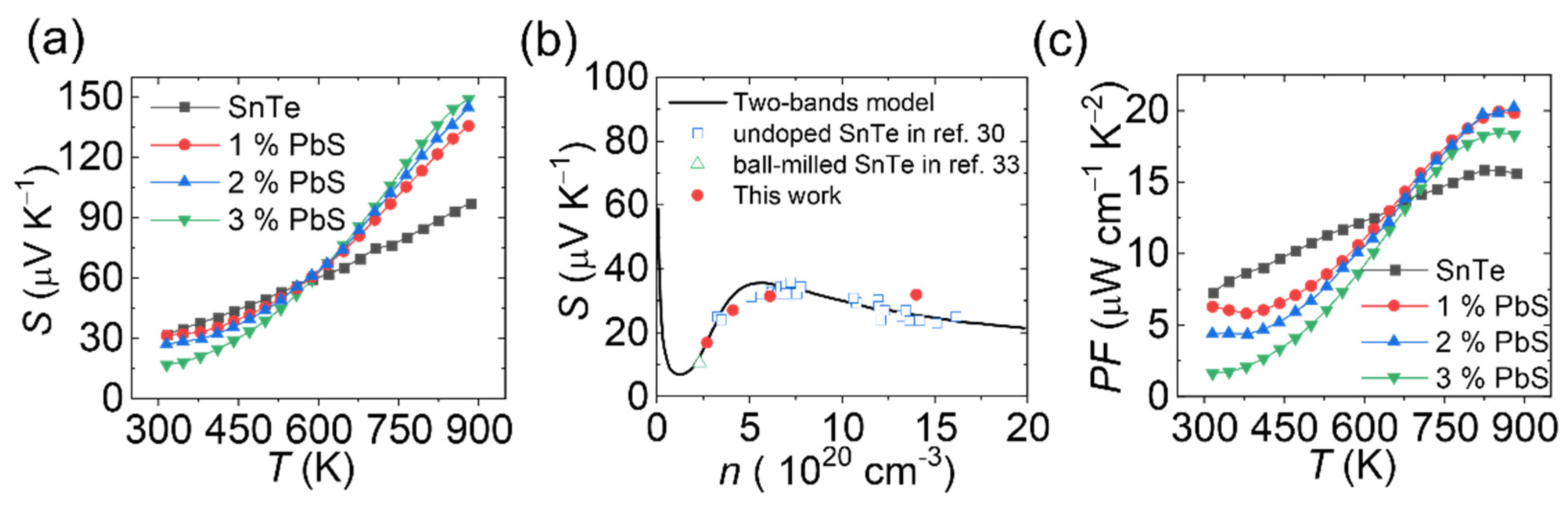
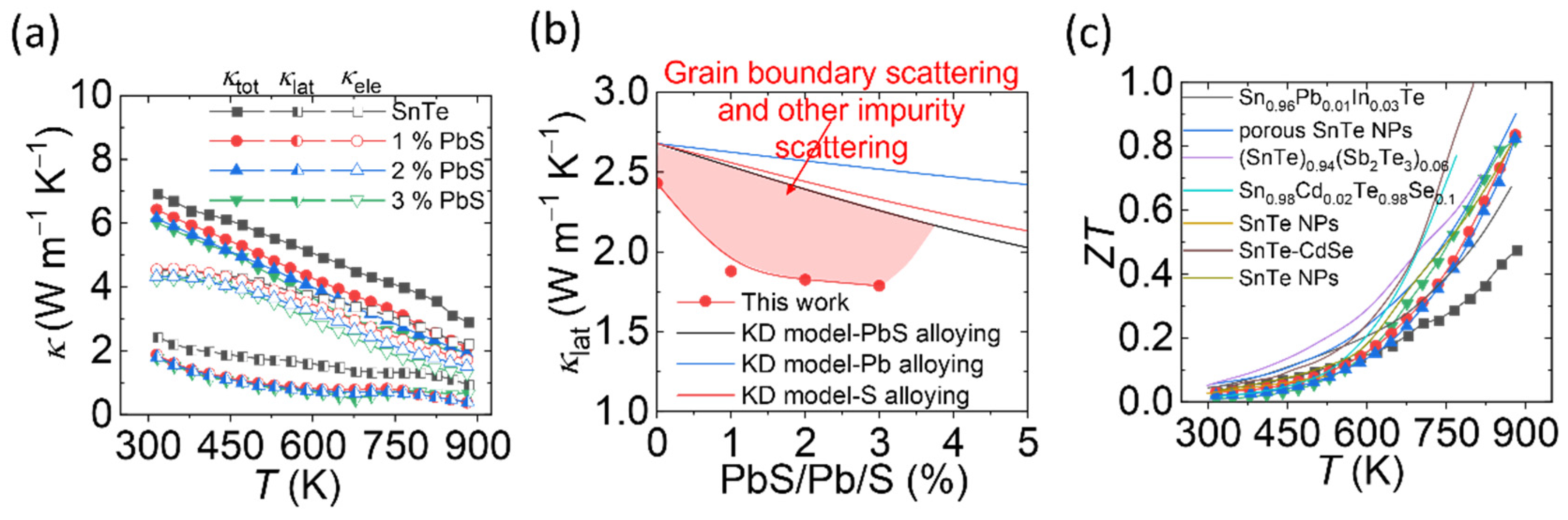
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibáñez, M.; Luo, Z.; Genç, A.; Piveteau, L.; Ortega, S.; Cadavid, D.; Dobrozhan, O.; Liu, Y.; Nachtegaal, M.; Zebarjadi, M.; et al. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016, 7, 10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, L.D. Thermoelectric materials: Energy conversion between heat and electricity. J. Materiomics 2015, 1, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Hu, J.; Zhang, S.; Deng, M.; Han, C.-G.; Liu, Y. New trends, strategies and opportunities in thermoelectric materials: A perspective. Mater. Today Phys. 2017, 1, 50–60. [Google Scholar] [CrossRef]
- Yu, X. Seeking new, highly effective thermoelectrics. Science 2020, 367, 1196–1197. [Google Scholar]
- Pang, H.; Qiu, Y.; Wang, D.; Qin, Y.; Huang, R.; Yang, Z.; Zhang, X.; Zhao, L.D. Realizing N-type SnTe Thermoelectrics with Competitive Performance through Suppressing Sn Vacancies. J. Am. Chem. Soc. 2021, 143, 8538–8542. [Google Scholar] [CrossRef]
- Moshwan, R.; Yang, L.; Zou, J.; Chen, Z.G. Eco-Friendly SnTe Thermoelectric Materials: Progress and Future Challenges. Adv. Funct. Mater. 2017, 27, 1703278. [Google Scholar] [CrossRef]
- Liu, W.; Tan, X.; Yin, K.; Liu, H.; Tang, X.; Shi, J.; Zhang, Q.; Uher, C. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si1-xSnx solid solutions. Phys. Rev. Lett. 2012, 108, 166601. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.D.; Dravid, V.P.; Kanatzidis, M.G. The panoscopic approach to high performance thermoelectrics. Energy Environ. Sci. 2014, 7, 251–268. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Chang, C.; Zhao, L.D. Anharmoncity and low thermal conductivity in thermoelectrics. Mater. Today Phys. 2018, 4, 50–57. [Google Scholar] [CrossRef]
- Jana, M.K.; Biswas, K. Crystalline Solids with Intrinsically Low Lattice Thermal Conductivity for Thermoelectric Energy Conversion. ACS Energy Lett. 2018, 3, 1315–1324. [Google Scholar] [CrossRef]
- Ibáñez, M.; Genç, A.; Hasler, R.; Liu, Y.; Dobrozhan, O.; Nazarenko, O.; De La Mata, M.; Arbiol, J.; Cabot, A.; Kovalenko, M.V. Tuning transport properties in thermoelectric nanocomposites through inorganic ligands and heterostructured building blocks. ACS Nano 2019, 13, 6572–6580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibañez, M.; Hasler, R.; Genc, A.; Liu, Y.; Kuster, B.; Schuster, M.; Dobrozhan, O.; Cadavid, D.; Arbiol, J.; Cabot, A.; et al. Ligand-mediated band engineering in bottom-up assembled SnTe nanocomposites for thermoelectric energy conversion. J. Am. Chem. Soc. 2019, 141, 8025–8029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Lim, K.H.; Ibáñez, M.; Ortega, S.; Li, M.; David, J.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; et al. High Thermoelectric Performance in Crystallographically Textured n-Type Bi2Te3–xSex Produced from Asymmetric Colloidal Nanocrystals. ACS Nano 2018, 12, 7174–7184. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Ortega, S.; Ibáñez, M.; Lim, K.H.; Grau-Carbonell, A.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; Kovalenko, M.V.; et al. Crystallographically Textured Nanomaterials Produced from the Liquid Phase Sintering of BixSb2–xTe3 Nanocrystal Building Blocks. Nano Lett. 2018, 18, 2557–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcabrini, M.; Genç, A.; Liu, Y.; Kleinhanns, T.; Lee, S.; Dirin, D.N.; Akkerman, Q.A.; Kovalenko, M.V.; Arbiol, J.; Ibáñez, M. Exploiting the Lability of Metal Halide Perovskites for Doping Semiconductor Nanocomposites. ACS Energy Lett. 2021, 6, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Shenoy, U.S.; Anand, S.; Waghmare, U.V.; Biswas, K. Mg alloying in SnTe facilitates valence band convergence and optimizes thermoelectric properties. Chem. Mater. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Al Rahal Al Orabi, R.; Mecholsky, N.A.; Hwang, J.; Kim, W.; Rhyee, J.-S.; Wee, D.; Fornari, M. Band Degeneracy, Low Thermal Conductivity, and High Thermoelectric Figure of Merit in SnTe–CaTe Alloys. Chem. Mater. 2015, 28, 376–384. [Google Scholar] [CrossRef]
- Ju, H.; Kim, J. Anion-exchanged porous SnTe nanosheets for ultra-low thermal conductivity and high-performance thermoelectrics. Chem. Eng. J. 2020, 402, 126274. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, X.; Zhang, F.; Shi, Q.; Tang, M.; Ang, R. Routes for advancing SnTe thermoelectrics. J. Mater. Chem. A 2020, 8, 16790–16813. [Google Scholar] [CrossRef]
- Han, G.; Zhang, R.; Popuri, S.R.; Greer, H.F.; Reece, M.J.; Bos, J.-W.W.G.; Zhou, W.; Knox, A.R.; Gregory, D.H. Large-scale surfactant-free synthesis of p-Type SnTe nanoparticles for thermoelectric applications. Materials 2017, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, C.L.; Webber, D.H.; Schueller, E.C.; Brutchey, R.L. Solution-Phase Conversion of Bulk Metal Oxides to Metal Chalcogenides Using a Simple Thiol-Amine Solvent Mixture. Angew. Chem. Int. Ed. 2015, 54, 8378–8381. [Google Scholar] [CrossRef]
- Wang, N.; West, D.; Liu, J.; Li, J.; Yan, Q.; Gu, B.-L.; Zhang, S.B.; Duan, W. Microscopic origin of the p-type conductivity of the topological crystalline insulator SnTe and the effect of Pb alloying. Phys. Rev. B 2014, 89, 045142. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, X.; Wang, D.; Huang, R.; Yang, Z.; Zhang, X.; Qiu, Y.; Zhao, L.-D. Realizing ranged performance in SnTe through integrating bands convergence and DOS distortion. J. Materiomics 2021. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.D.; Shi, F.; Doak, J.W.; Lo, S.H.; Sun, H.; Wolverton, C.; Dravid, V.P.; Uher, C.; Kanatzidis, M.G. High thermoelectric performance of p-type SnTe via a synergistic band engineering and nanostructuring approach. J. Am. Chem. Soc. 2014, 136, 7006–7017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gibbs, Z.M.; Wang, H.; Han, Y.; Xin, C.; Li, L.; Snyder, G.J. Optimization of thermoelectric efficiency in SnTe: The case for the light band. Phys. Chem. Chem. Phys. 2014, 16, 20741–20748. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, G.S. Grain boundary energy anisotropy: A review. J. Mater. Sci. 2011, 46, 5881–5895. [Google Scholar] [CrossRef] [Green Version]
- Voyiadjis, G.Z. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications; CRC Press: Boca Raton, FL, USA, 2000; Volume 126, p. 687. [Google Scholar]
- Hillert, M.; Purdy, G.R. Chemically induced grain boundary migration. Acta Metall. 1978, 26, 333–340. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Cahn, J.W. Mechanism for diffusion induced grain boundary migration. Acta Metall. 1981, 29, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Fournelle, R.A. On the thermodynamic driving force for diffusion-induced grain boundary migration, discontinuous precipitation and liquid film migration in binary alloys. Mater. Sci. Eng. A 1991, 138, 133–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, B.; Lan, Y.; Lukas, K.; Liu, W.; Esfarjani, K.; Opeil, C.; Broido, D.; Chen, G.; Ren, Z. High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc. Natl. Acad. Sci. USA 2013, 110, 13261–13266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, Y.; Pei, Y.; Chen, Y.; Yuan, B.; Zhang, S.; Deng, Y.; Gong, S.; He, J.; Zhao, L.D. Enhancing thermoelectric performance of SnTe via nanostructuring particle size. J. Alloys Compd. 2017, 709, 575–580. [Google Scholar] [CrossRef]
- Gayner, C.; Amouyal, Y. Energy Filtering of Charge Carriers: Current Trends, Challenges, and Prospects for Thermoelectric Materials. Adv. Funct. Mater. 2020, 30, 1–17. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Wu, H.; Yin, M.; Pei, Y.; Gong, S.; Huang, L.; Pennycook, S.J.; He, J.; Zhao, L.D. Simultaneously enhancing the power factor and reducing the thermal conductivity of SnTe via introducing its analogues. Energy Environ. Sci. 2017, 10, 2420–2431. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131–6140. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Hao, S.; Hanus, R.C.; Zhang, X.; Anand, S.; Bailey, T.P.; Rettie, A.J.E.; Su, X.; Uher, C.; Dravid, V.P.; et al. High Thermoelectric Performance in SnTe-AgSbTe2 Alloys from Lattice Softening, Giant Phonon-Vacancy Scattering, and Valence Band Convergence. ACS Energy Lett. 2018, 3, 705–712. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Ibáñez, M. Enhanced Thermoelectric Performance by Surface Engineering in SnTe-PbS Nanocomposites. Materials 2021, 14, 5416. https://doi.org/10.3390/ma14185416
Chang C, Ibáñez M. Enhanced Thermoelectric Performance by Surface Engineering in SnTe-PbS Nanocomposites. Materials. 2021; 14(18):5416. https://doi.org/10.3390/ma14185416
Chicago/Turabian StyleChang, Cheng, and Maria Ibáñez. 2021. "Enhanced Thermoelectric Performance by Surface Engineering in SnTe-PbS Nanocomposites" Materials 14, no. 18: 5416. https://doi.org/10.3390/ma14185416
APA StyleChang, C., & Ibáñez, M. (2021). Enhanced Thermoelectric Performance by Surface Engineering in SnTe-PbS Nanocomposites. Materials, 14(18), 5416. https://doi.org/10.3390/ma14185416






