Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Material Preparation
2.2.1. Synthesis of BAG
2.2.2. Dentin Adhesive Preparation
2.3. Micro-Tensile Bond Strength (μTBS) Test
2.4. FE-SEM of the Adhesive Surface
2.5. FE-SEM of Dentin Surface
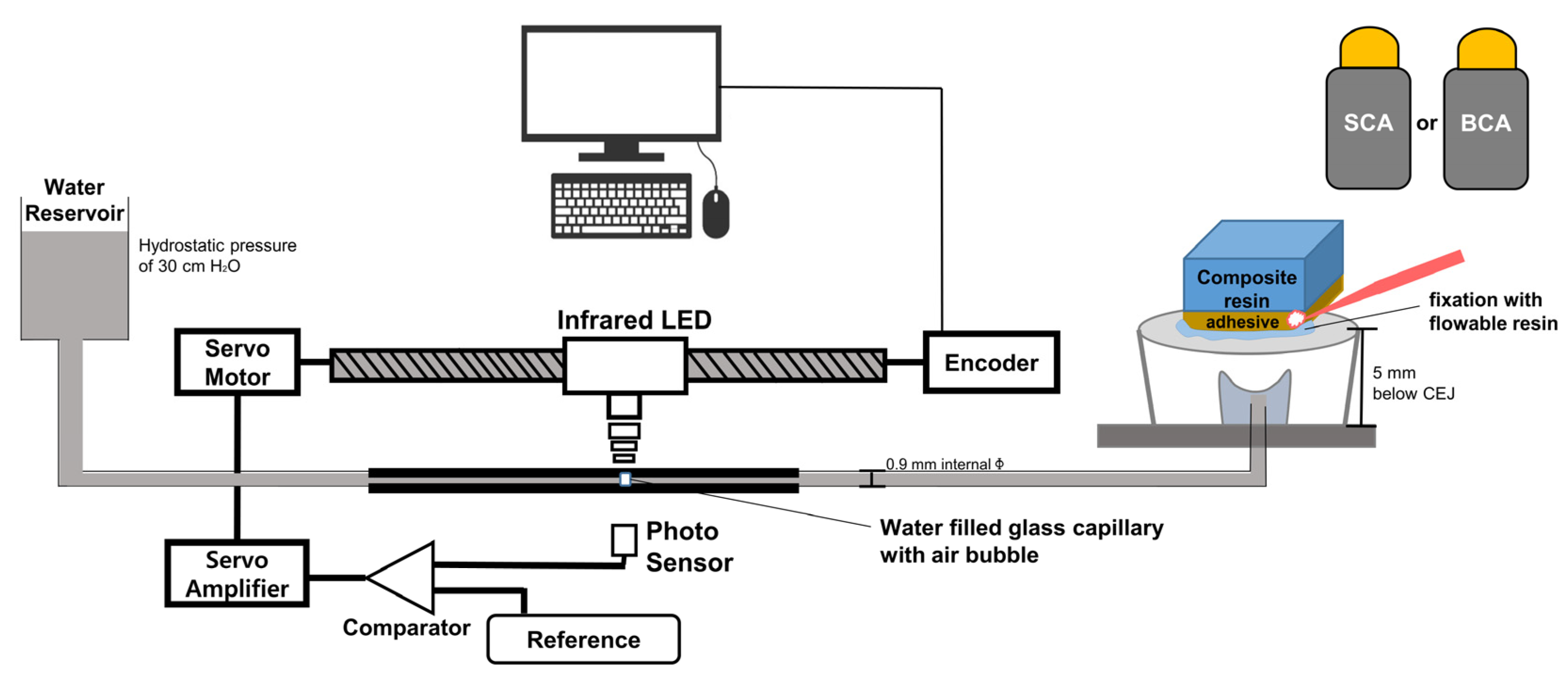
2.6. Dentinal Fluid Flow (DFF) Rate Measurement
2.7. Confocal Raman Spectroscopy
2.8. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength (μTBS) Test
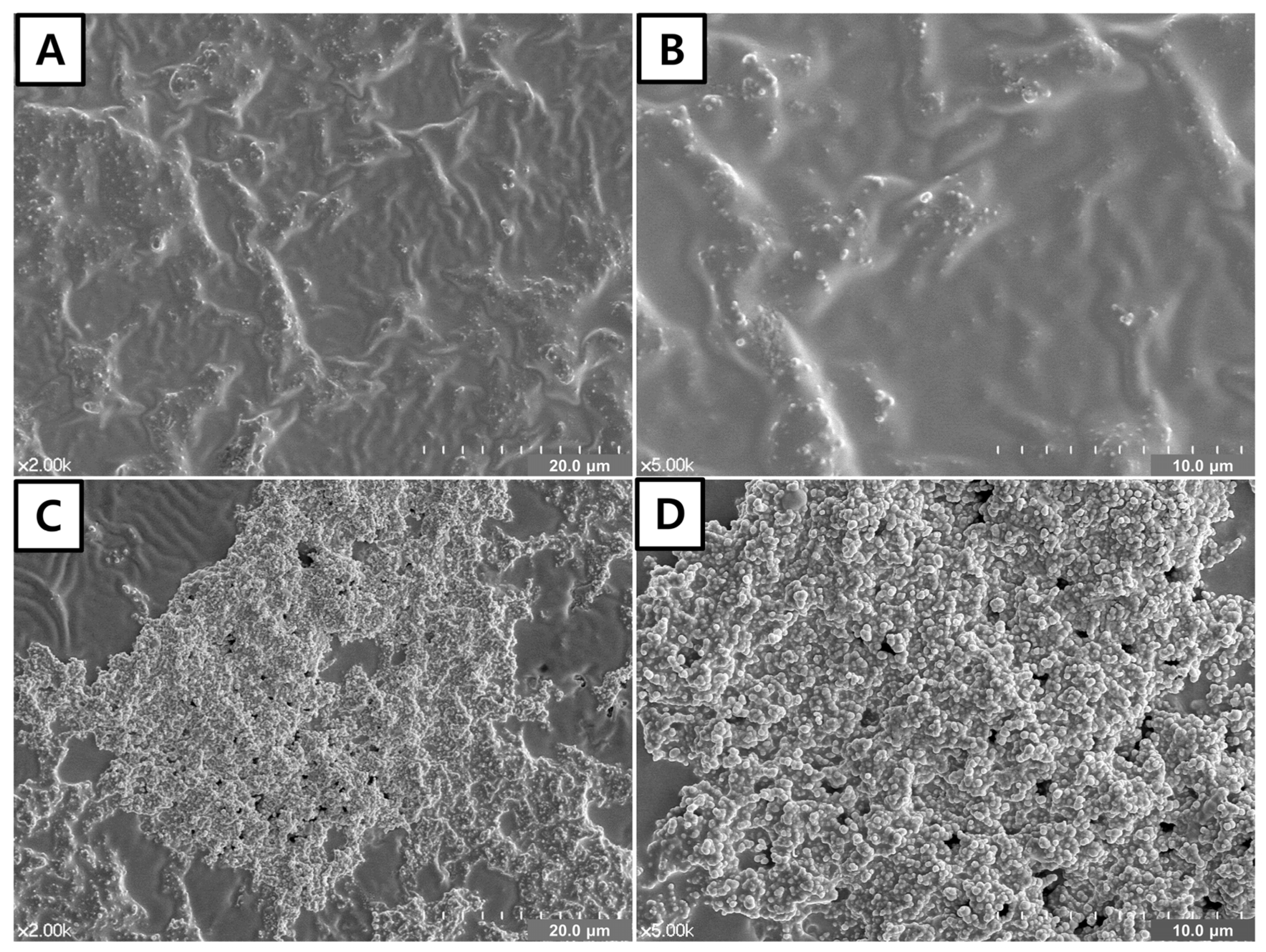
3.2. FE-SEM of the Adhesive Surface
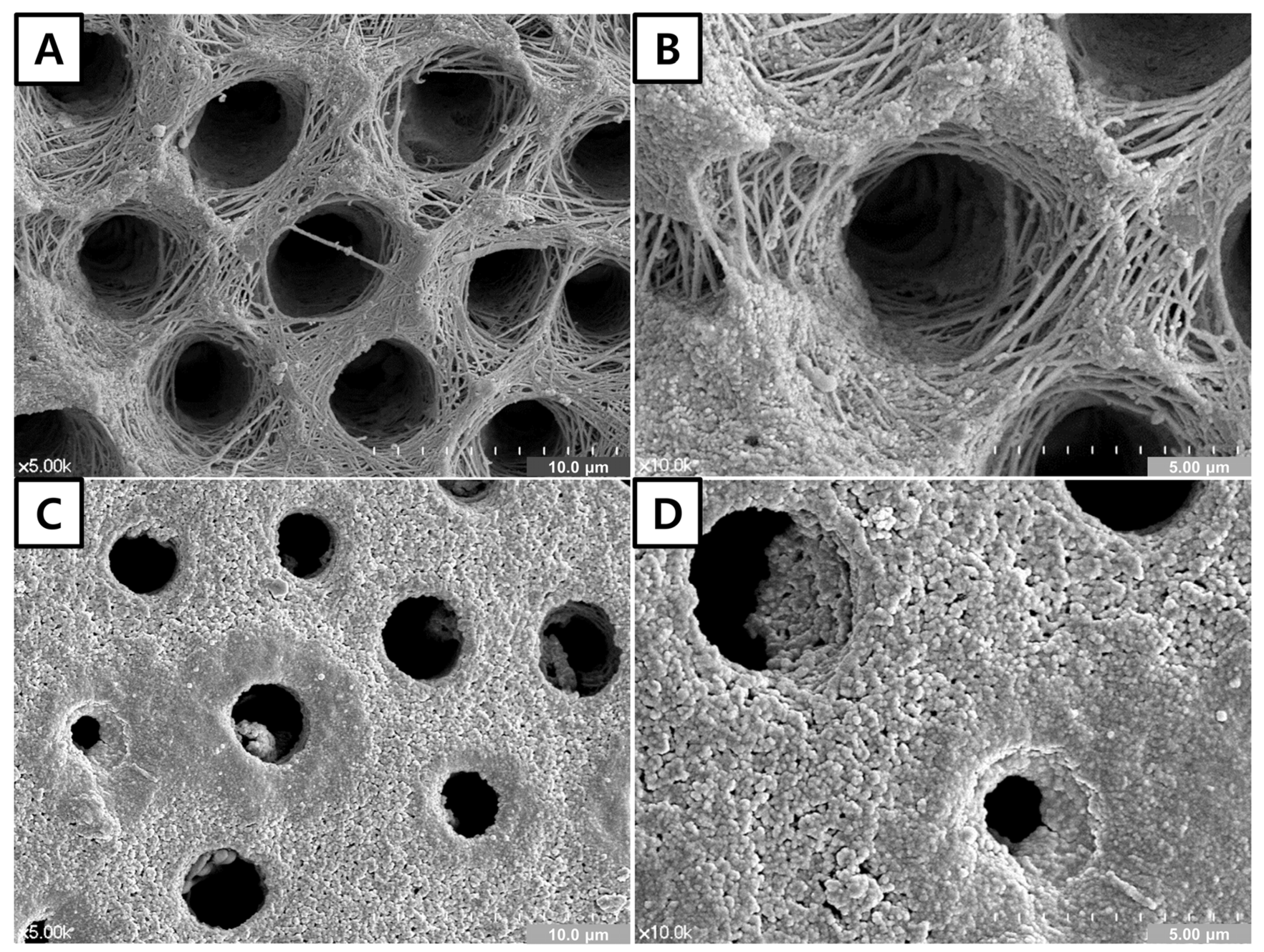
3.3. FE-SEM of the Dentin Surface
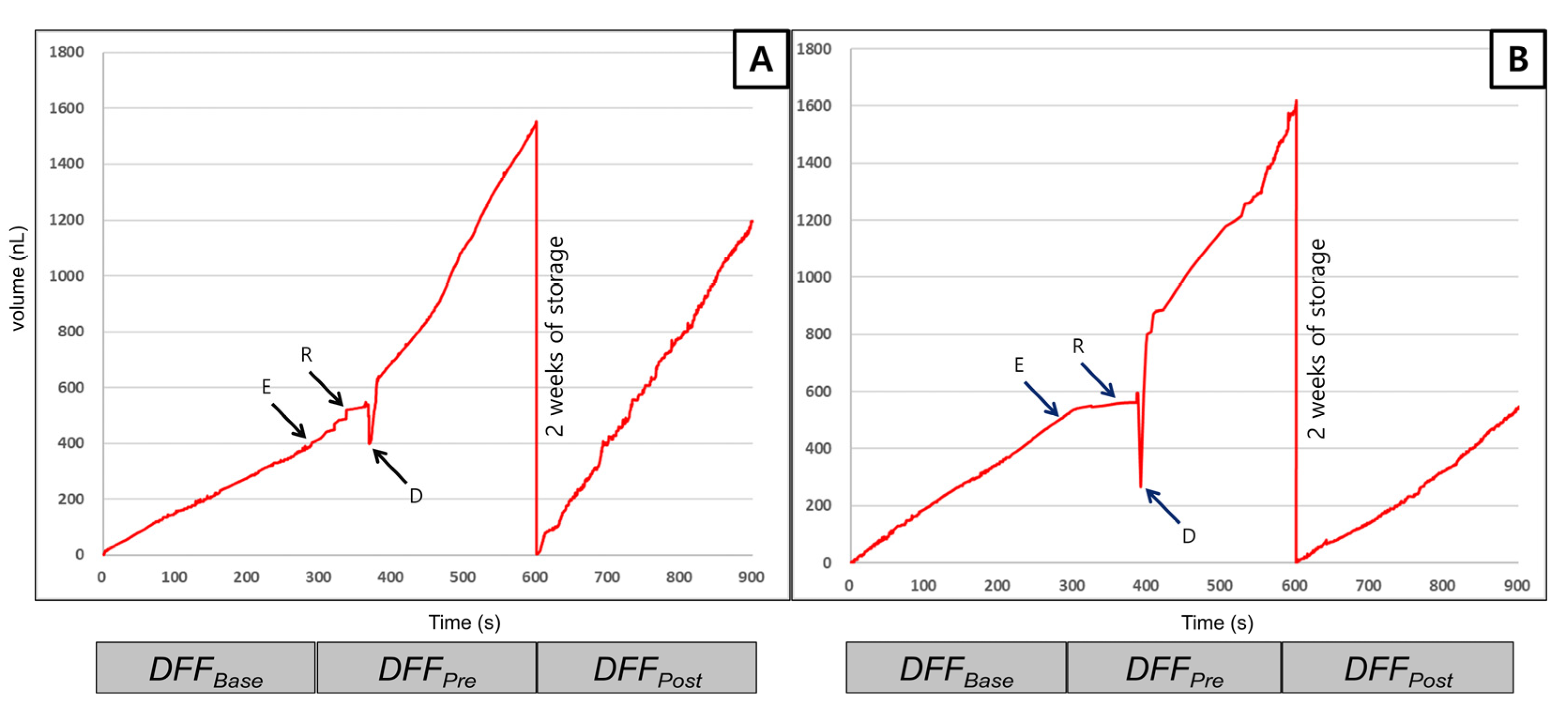
3.4. Dentinal Fluid Flow (DFF) Rate Measurement
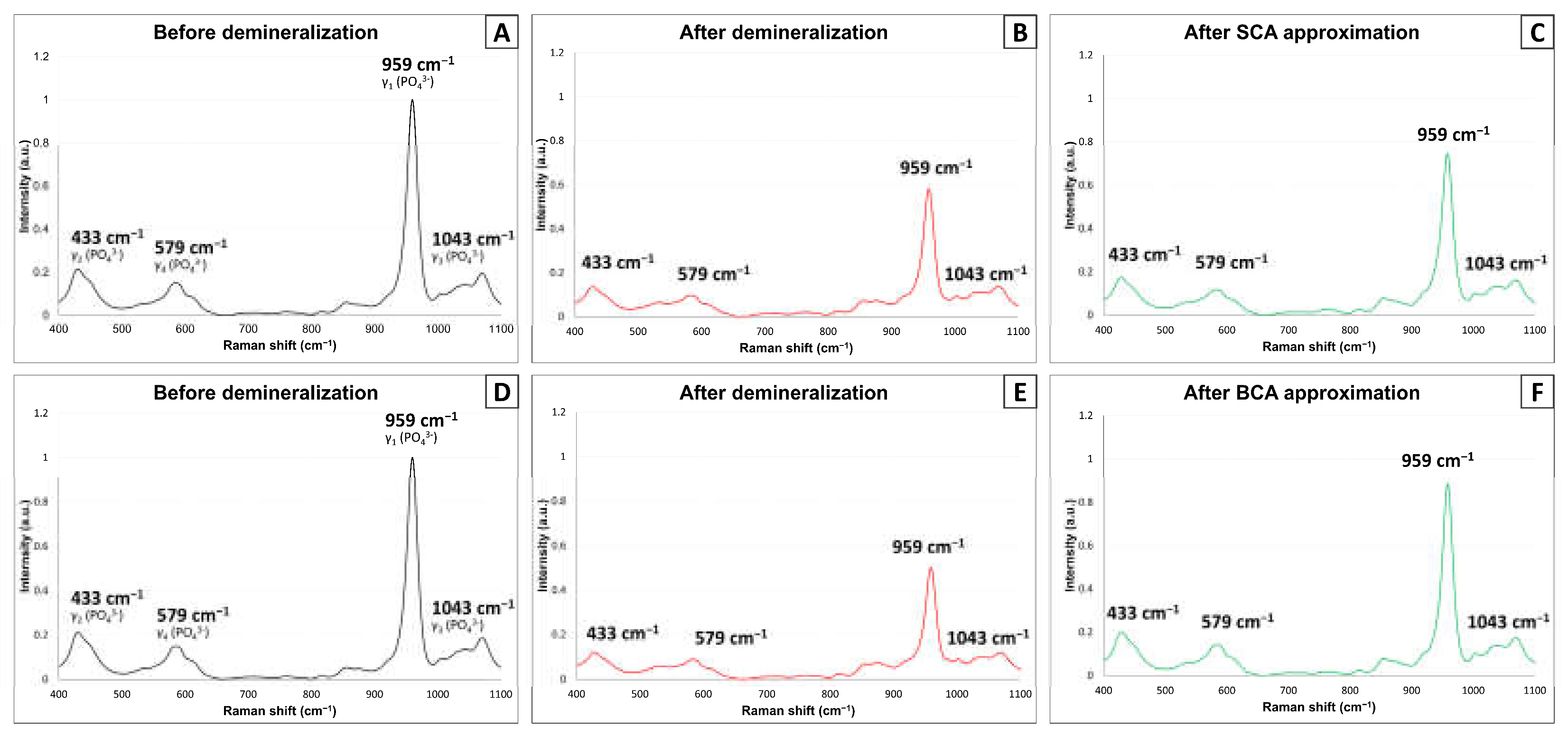
3.5. Confocal Raman Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawes, C. What is the critical pH and why does a tooth dissolve in acid? J. Can. Dent. Assoc. 2003, 69, 722–724. [Google Scholar] [PubMed]
- Tay, F.R.; Pashley, D.H.; Yiu, C.; Cheong, C.; Hashimoto, M.; Itou, K.; Yoshiyama, M.; King, N.M. Nanoleakage types and potential implications: Evidence from unfilled and filled adhesives with the same resin composition. Am. J. Dent. 2004, 17, 182–190. [Google Scholar] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; di Lenarda, R.; de Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.P.; Moda, M.D.; Suzuki, T.Y.; Godas, A.G.; Sundfeld, R.H.; Briso, A.L.; Santos, P.H. Effect of Fluoride-Releasing Adhesive Systems on the Mechanical Properties of Eroded Dentin. Braz. Dent. J. 2016, 27, 153–159. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Shen, H.; Suh, B.I. Bioactive dental restorative materials: A review. Am. J. Dent. 2013, 26, 219–227. [Google Scholar] [PubMed]
- Comba, A.; Maravic, T.; Valente, L.; Girlando, M.; Cunha, S.R.; Checchi, V.; Salgarello, S.; Tay, F.R.; Scotti, N.; Breschi, L.; et al. Effect of benzalkonium chloride on dentin bond strength and endogenous enzymatic activity. J. Dent. 2019, 85, 25–32. [Google Scholar] [CrossRef]
- Maravic, T.; Comba, A.; Cunha, S.R.; Angeloni, V.; Cadenaro, M.; Visinitini, E.; Navarra, C.O.; Salgarello, S.; Breschi, L.; Mazzoni, A. Long-term bond strength and endogenous enzymatic activity of a chlorhexidine-containing commercially available adhesive. J. Dent. 2019, 84, 60–66. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; de Stefano, E.D.; Prati, C. Biomimetic remineralization of human dentin using promising innovative calcium-silicate hybrid “smart” materials. Dent. Mater. 2011, 27, 1055–1069. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Sauro, S.; Watson, T.F.; Toledano, M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J. Endod. 2012, 38, 1227–1232. [Google Scholar] [CrossRef]
- Larsson, P.; Howell, D.; Pita, J.; Blanco, L. Aspiration and characterization of predentin fluid in developing rat teeth by means of a micropuncture and micro-analytical technique. J. Dent. Res. 1988, 67, 870–875. [Google Scholar] [CrossRef]
- Lundgren, T.; Nannmark, U.; Linde, A. Calcium ion activity and pH in the odontoblast-predentin region: Ion-selective microelectrode measurements. Calcif. Tissue Int. 1992, 50, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.A. Reaction Patterns in Human Teeth; CRC-Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Pashley, D.H.; Carvalho, R. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef]
- Rosales-Leal, J.I.; de la Torre-Moreno, F.J.; Bravo, M. Effect of pulp pressure on the micropermeability and sealing ability of etch & rinse and self-etching adhesives. Oper. Dent. 2007, 32, 242–250. [Google Scholar] [PubMed]
- Sauro, S.; Thompson, I.; Watson, T.F. Effects of common dental materials used in preventive or operative dentistry on dentin permeability and remineralization. Oper. Dent. 2011, 36, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Banomyong, D.; Kanchanasantikul, P.; Wong, R.H. Effects of casein phosphopeptide–amorphous calcium phosphate remineralizing paste and 8% arginine desensitizing paste on dentin permeability. J. Investig. Clin. Dent. 2013, 4, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioactive ceramics. Ann. N. Y. Acad. Sci. 1988, 523, 54–71. [Google Scholar] [CrossRef]
- Bakry, A.S.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. Cytotoxicity of 45S5 bioglass paste used for dentine hypersensitivity treatment. J. Dent. 2011, 39, 599–603. [Google Scholar] [CrossRef]
- Bae, W.J.; Min, K.S.; Kim, J.J.; Kim, J.J.; Kim, H.W.; Kim, E.C. Odontogenic responses of human dental pulp cells to collagen/nanobioactive glass nanocomposites. Dent. Mater. 2012, 28, 1271–1279. [Google Scholar] [CrossRef]
- Salehi, S.; Davis, H.B.; Ferracane, J.L.; Mitchell, J.C. Sol-gel-derived bioactive glasses demonstrate antimicrobial effects on common oral bacteria. Am. J. Dent. 2015, 28, 111–115. [Google Scholar] [PubMed]
- Bakry, A.S.; Marghalani, H.Y.; Amin, O.A.; Tagami, J. The effect of a bioglass paste on enamel exposed to erosive challenge. J. Dent. 2014, 42, 1458–1463. [Google Scholar] [CrossRef]
- Deng, M.; Wen, H.L.; Dong, X.L.; Li, F.; Xu, X.; Li, H.; Li, J.Y.; Zhou, X.D. Effects of 45S5 bioglass on surface properties of dental enamel subjected to 35% hydrogen peroxide. Int. J. Oral. Sci. 2013, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef]
- Davis, H.B.; Gwinner, F.; Mitchell, J.C.; Ferracane, J.L. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent. Mater. 2014, 30, 1187–1194. [Google Scholar] [CrossRef]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Hyun, H.K.; Ferracane, J.L. Influence of biofilm formation on the optical properties of novel bioactive glass-containing composites. Dent. Mater. 2016, 32, 1144–1151. [Google Scholar] [CrossRef]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.S.; Roh, J.; Sauro, S.; Kim, H.W.; Lee, J.H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef]
- Kohda, N.; Iijima, M.; Kawaguchi, K.; Toshima, H.; Muguruma, T.; Endo, K.; Mizoguchi, I. Inhibition of enamel demineralization and bond-strength properties of bioactive glass containing 4-META/MMA-TBB-based resin adhesive. Eur. J. Oral Sci. 2015, 123, 202–207. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of Composites Containing Bioactive Glasses on Demineralized Dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef]
- Khvostenko, D.; Mitchell, J.C.; Hilton, T.J.; Ferracane, J.L.; Kruzic, J.J. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent. Mater. 2013, 29, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, H.E.; Lee, J.-E.; Park, I.-S.; Kim, H.-G.; Kwon, J.; Kim, D.-S. Effects of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 2021, 11, 1–10. [Google Scholar]
- Lee, J.H.; Kang, M.S.; Mahapatra, C.; Kim, H.W. Effect of Aminated Mesoporous Bioactive Glass Nanoparticles on the Differentiation of Dental Pulp Stem Cells. PLoS ONE 2016, 11, e0150727. [Google Scholar]
- Deng, D.; Yang, H.; Guo, J.; Chen, X.; Zhang, W.; Huang, C. Effects of different artificial ageing methods on the degradation of adhesive–dentine interfaces. J. Dent. 2014, 42, 1577–1585. [Google Scholar] [CrossRef]
- Tas, A.C. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 degrees C in synthetic body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar]
- Perdigao, J.; Lambrechts, P.; van Meerbeek, B.; Vanherle, G.; Lopes, A.L. Field emission SEM comparison of four postfixation drying techniques for human dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef]
- Ciucchi, B.; Bouillaguet, S.; Holz, J.; Pashley, D. Dentinal fluid dynamics in human teeth, in vivo. J. Endod. 1995, 21, 191–194. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.J.; Kim, D.S.; Lee, I.B. The evaluation of dentinal tubule occlusion by desensitizing agents: A real-time measurement of dentinal fluid flow rate and scanning electron microscopy. Oper. Dent. 2013, 38, 419–428. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.; McDonough, W.G. Chemistry of Silanes: Interfaces in Dental Polymers and Composites. J. Res. Natl. Inst. Stand Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, M.G.; Ferracane, J.L.; Davis, H.; Bae, H.E.; Choi, D.; Kim, D.S. Effect of bioactive glass-containing resin composite on dentin remineralization. J. Dent. 2018, 75, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-formation ability--predictor of “bioactivity”? Acta Biomater 2010, 6, 4181–4188. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Pashley, D.H.; Montanari, M.; Chersoni, S.; Carvalho, R.M.; Toledano, M.; Osorio, R.; Tay, F.R.; Prati, C. Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent. Mater. 2007, 23, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Marin, E.; Hiraishi, N.; Honma, T.; Boschetto, F.; Zanocco, M.; Zhu, W.; Adachi, T.; Kanamura, N.; Yamamoto, T.; Pezzotti, G.J. Raman spectroscopy for early detection and monitoring of dentin demineralization. Dent. Mater. 2020, 36, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bora, T.; Al Ghaithi, A.; Thukral, S.; Dutta, J. Raman spectroscopy detects changes in bone mineral quality and collagen cross-linkage in staphylococcus infected human bone. Sci. Rep. 2018, 8, 9417. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Zhu, W.; Boffelli, M.; Adachi, T.; Ichioka, H.; Yamamoto, T.; Marunaka, Y.; Kanamura, N. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: I, theoretical foundations. Anal. Bioanal. Chem. 2015, 407, 3325–3342. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Pezzotti, G.; Yamamoto, T.; Ichioka, H.; Boffelli, M.; Zhu, W.; Kanamura, N. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: II, application to decayed human teeth. Anal. Bioanal. Chem. 2015, 407, 3343–3356. [Google Scholar] [CrossRef]
| Material | Content (wt.%) | |
|---|---|---|
| SCA | BCA | |
| UDMA (Diurethane dimethacrylate) | 42.8 | 42.8 |
| HEMA (Ethylene glycol methacrylate) | 12.2 | 12.2 |
| CQ (Camphorquinone) | 0.5 | 0.5 |
| EDMAB (ethyl-4-dimethylamino benzoate) | 1.0 | 1.0 |
| BHT (2,6-di-tert-butyl-4-methylphenol) | 0.25 | 0.25 |
| TP (2,2′-(P-tolylimino)-diethanol) | 0.25 | 0.25 |
| Ethanol | 40.0 | 40.0 |
| BAG 85S | - | 3.0 |
| Silica | 3.0 | - |
| Storage Solution | Composition (Amount, g/L) |
|---|---|
| 27 mM HCO3− Tris SBF | NaCl (6.547) NaHCO3 (2.268) KCl (0.373) Na2HPO4∙2H2O (0.178) MgCl2∙6H2O (0.305) CaCl2∙2H2O (0.368) Na2SO4 (0.071) (CH2OH)3CNH2 (6.057) |
| Adhesive | Immediate | Aged |
|---|---|---|
| SCA | 29.66 ± 7.71 a | 18.91 ± 8.63 b |
| BCA | 32.47 ± 11.56 a | 21.68 ± 6.93 b |
| Adhesive | DFF Rate (nL/s) | |||
|---|---|---|---|---|
| (%) | ||||
| SCA | 1.38 ± 0.65 A | 3.68 ± 0.44 B | 3.91 ± 0.58 C | + 6.54 a |
| BCA | 1.79 ± 0.26 A | 3.61 ± 0.14 B | 1.80 ± 0.12 C | −50.10 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Jang, J.-H.; Woo, S.U.; Choi, K.-K.; Kim, S.-Y.; Ferracane, J.L.; Lee, J.-H.; Choi, D.; Choi, S.; Kim, S.; et al. Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin. Materials 2021, 14, 5423. https://doi.org/10.3390/ma14185423
Kim H-J, Jang J-H, Woo SU, Choi K-K, Kim S-Y, Ferracane JL, Lee J-H, Choi D, Choi S, Kim S, et al. Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin. Materials. 2021; 14(18):5423. https://doi.org/10.3390/ma14185423
Chicago/Turabian StyleKim, Hyun-Jung, Ji-Hyun Jang, Sang Uk Woo, Kyoung-Kyu Choi, Sun-Young Kim, Jack L. Ferracane, Jung-Hwan Lee, Dongseok Choi, Samjin Choi, Soogeun Kim, and et al. 2021. "Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin" Materials 14, no. 18: 5423. https://doi.org/10.3390/ma14185423
APA StyleKim, H.-J., Jang, J.-H., Woo, S. U., Choi, K.-K., Kim, S.-Y., Ferracane, J. L., Lee, J.-H., Choi, D., Choi, S., Kim, S., Bang, A., & Kim, D.-S. (2021). Effect of Novel Bioactive Glass-Containing Dentin Adhesive on the Permeability of Demineralized Dentin. Materials, 14(18), 5423. https://doi.org/10.3390/ma14185423











