Effect of Rare-Earth Ions on the Optical and PL Properties of Novel Borosilicate Glass Developed from Agricultural Waste
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
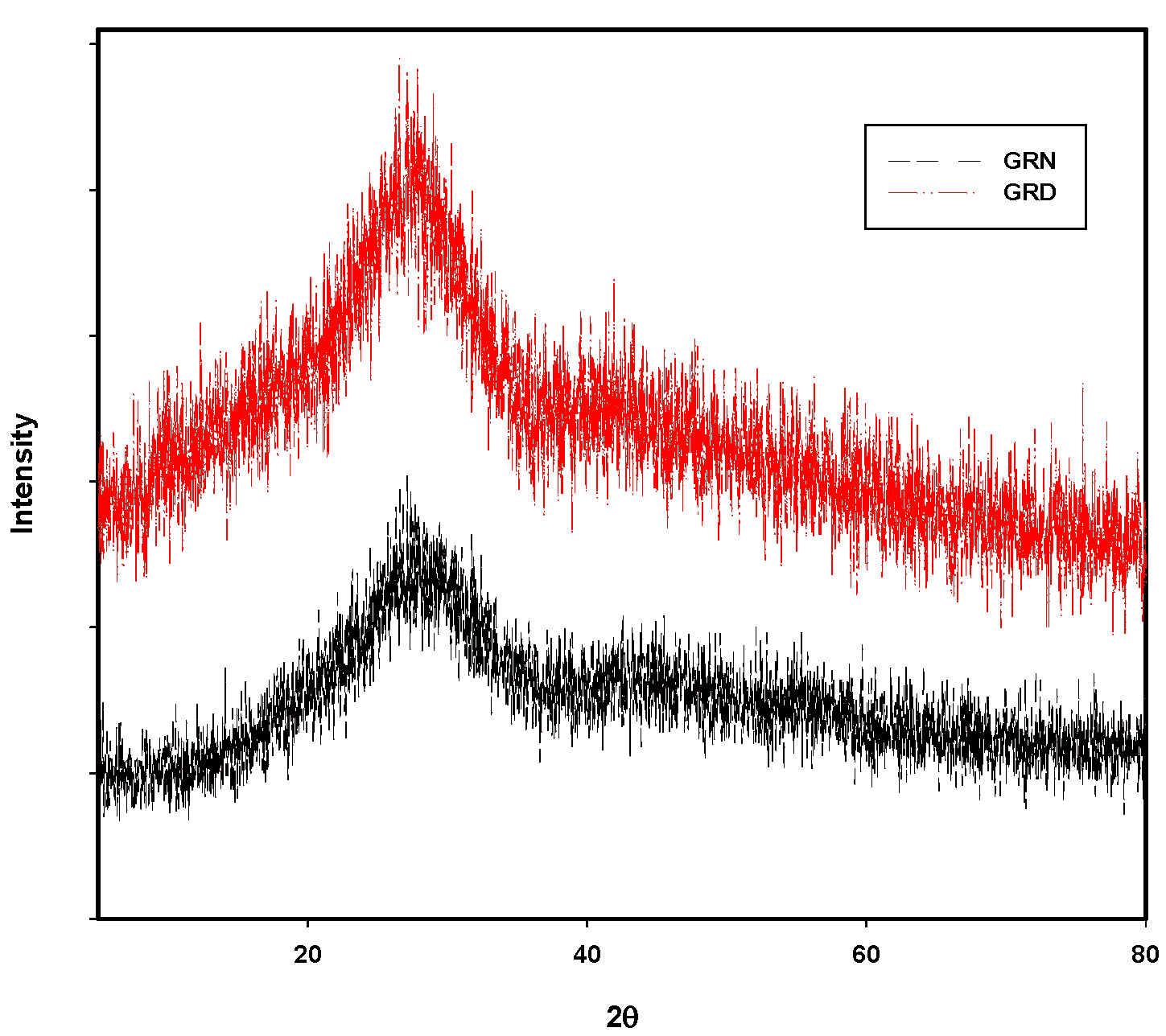
3.1. Structural Identification Technique [XRD, EDX, and SEM]
3.2. UV Transmittance Spectroscopy Analysis
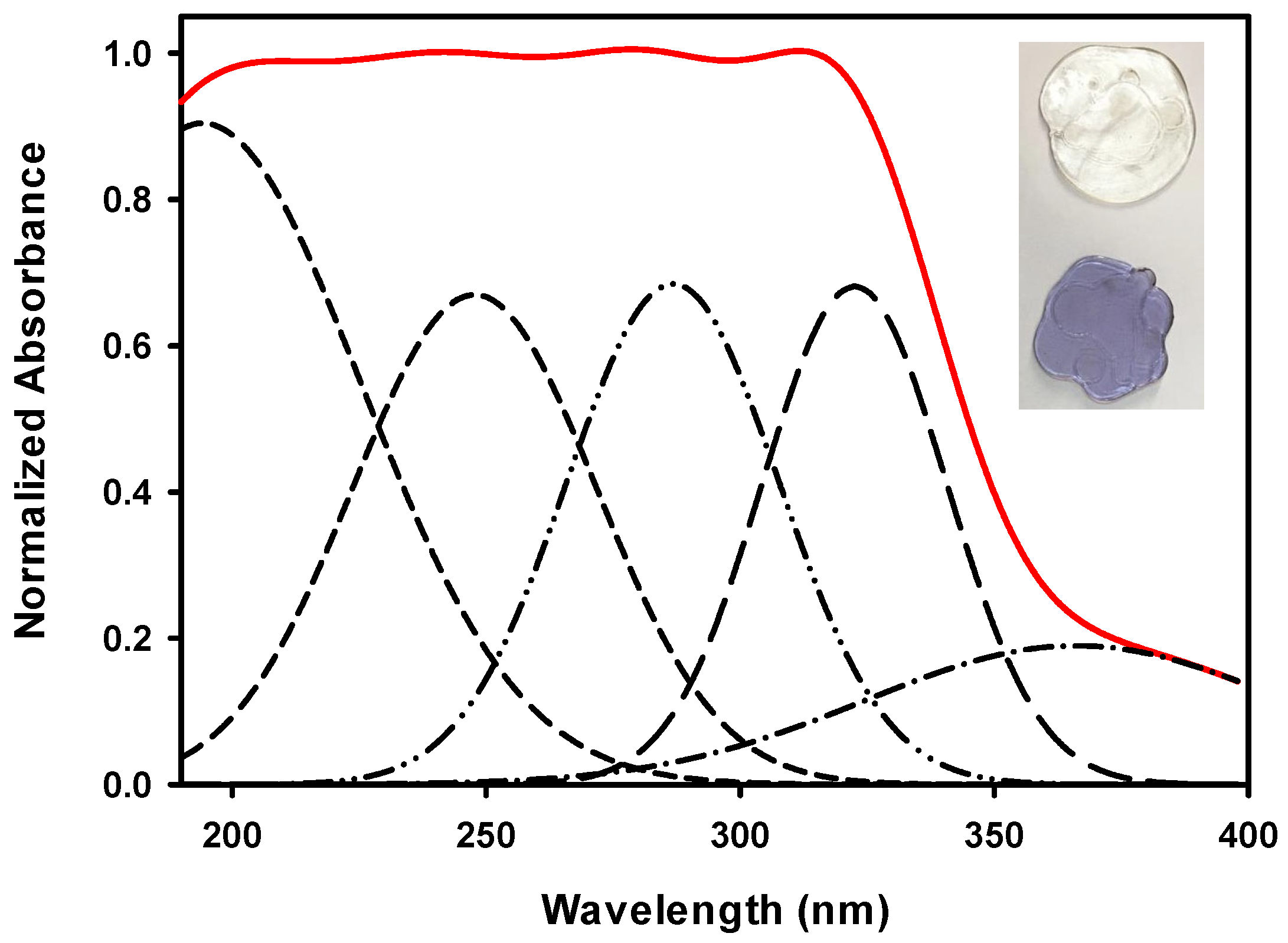
3.2.1. Deconvoluted Absorption Spectra of GR Sample
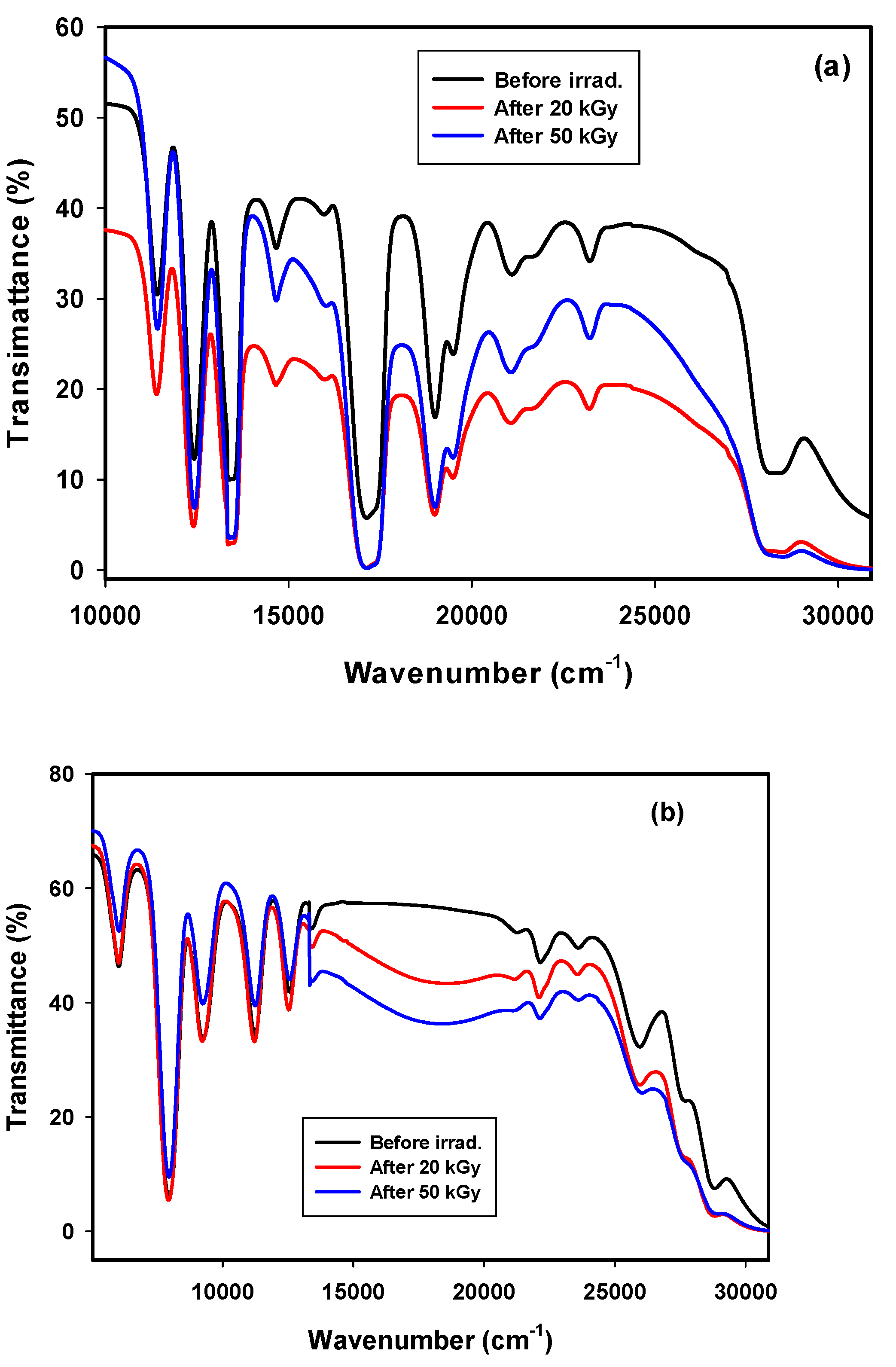
3.2.2. Transmittance Spectra of GRN
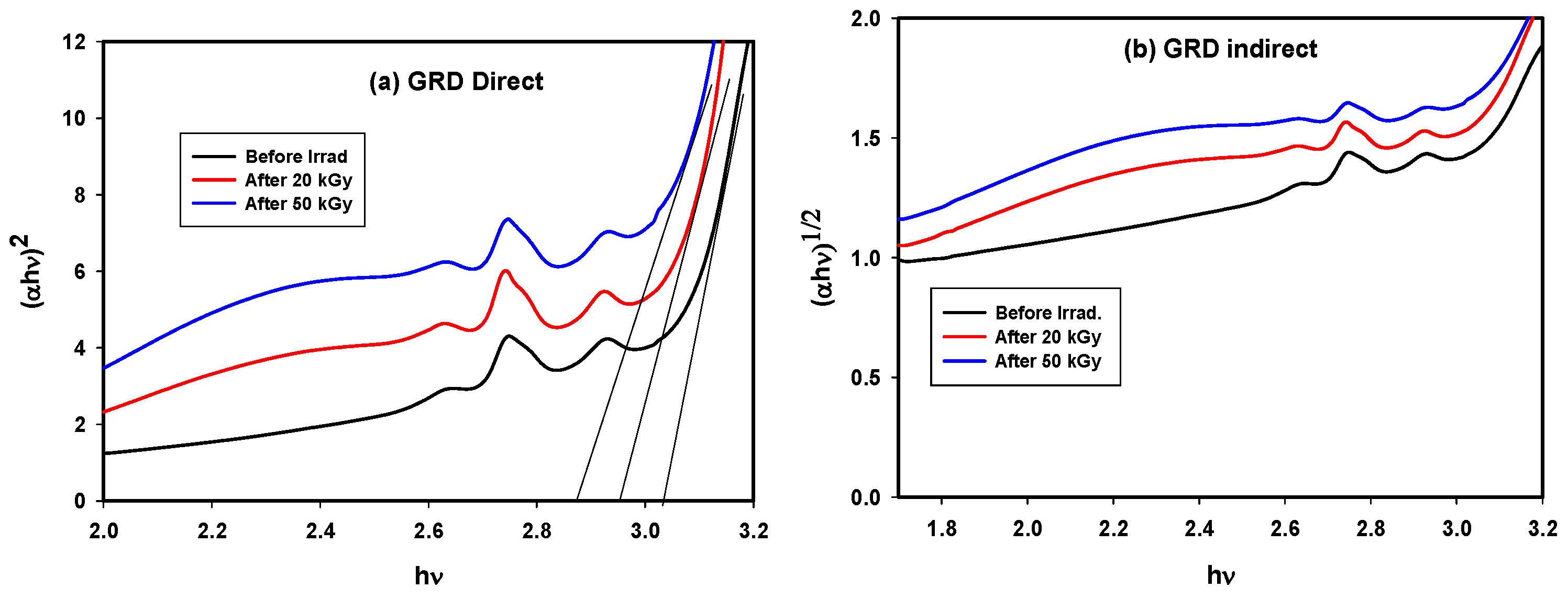
3.2.3. Transmittance Spectra of GRD
3.3. Photoluminescence
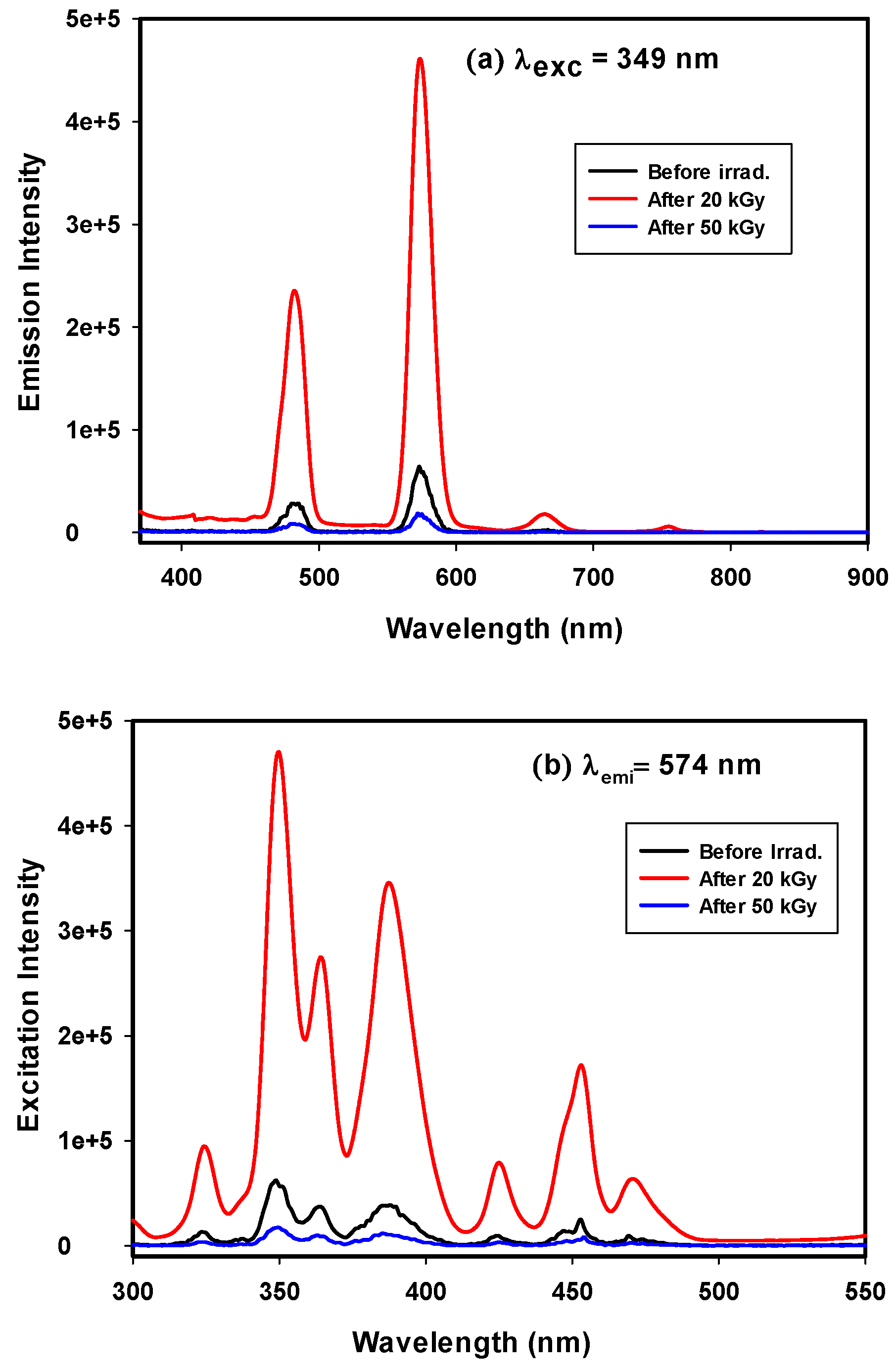
3.3.1. Photoluminescence of GRN
3.3.2. Photoluminescence of GRD
- Decay curve for GRD samples
- 2.
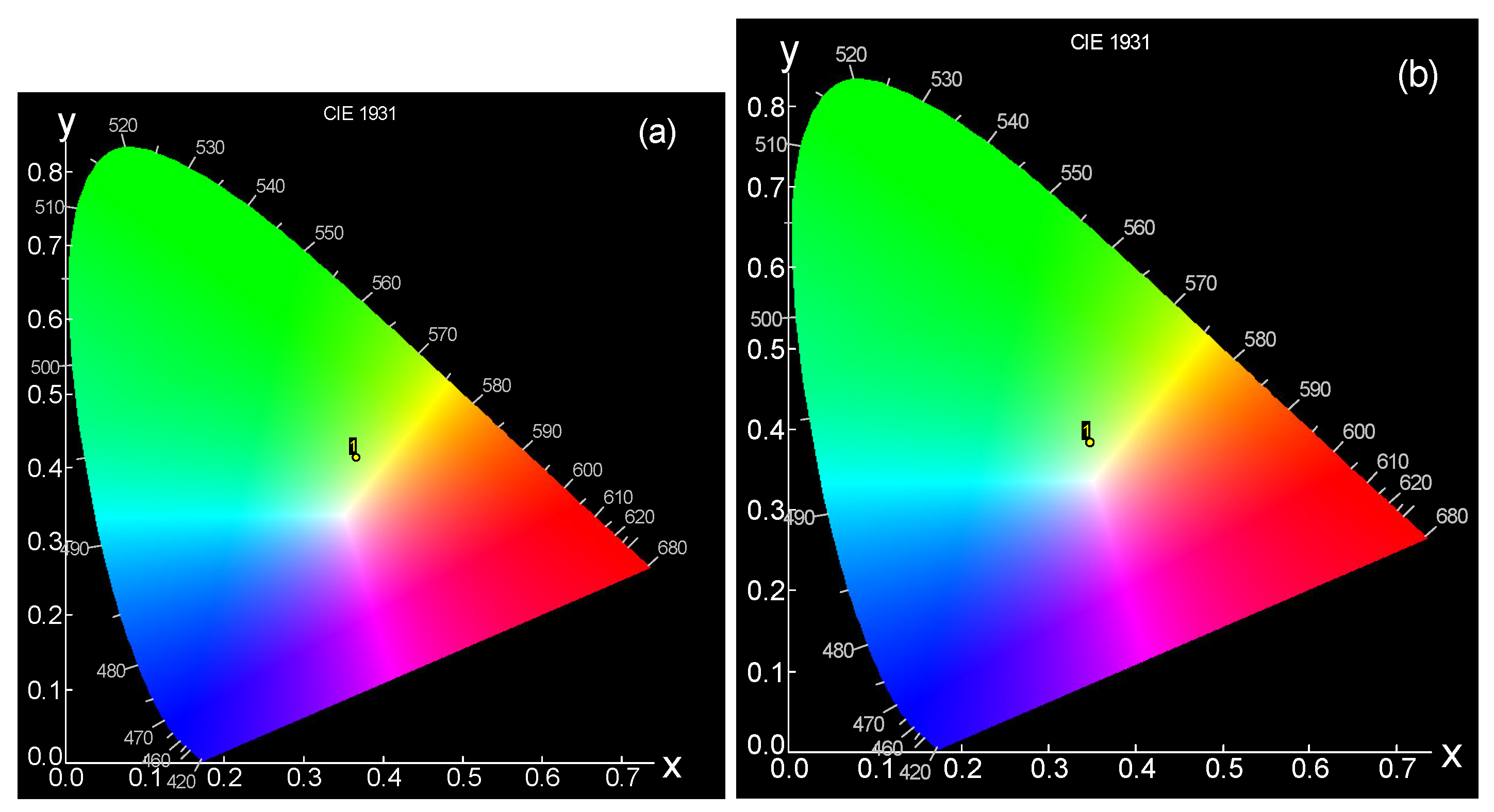
- CIE color chromaticity co-ordinates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuscharoen, S.; Ruengsri, S.; Kaewkhao, J. Development of Barium Borosilicate Glass Using Rice Husk Ash: Effect of BaO. Adv. Mater. Res. 2013, 770, 201–204. [Google Scholar] [CrossRef]
- Ruengsri, S.; Insiripong, S.; Sangwaranatee, N.; Kaewkhao, J. Development of barium borosilicate glasses for radiation shielding materials using rice husk ash as a silica source. Prog. Nucl. Energy 2015, 83, 99–104. [Google Scholar] [CrossRef]
- Umar, S.; Halimah, M.; Chan, K.; Latif, A. Physical, structural and optical properties of erbium doped rice husk silicate borotellurite (Er-doped RHSBT) glasses. J. Non-Crystalline Solids 2017, 472, 31–38. [Google Scholar] [CrossRef]
- Ugheoke, I.B.; Mamat, O. A critical assessment and new research directions of rice husk silica processing methods and properties. Maejo Int. J. Sci. Technol. 2012, 6, 430–448. [Google Scholar]
- Onojah, A.D.; Agbendeh, N.A.; Mbakaan, C. Rice husk ash refractory: The temperature dependent crystalline phase aspects. IJRRAS 2013, 151, 246–248. [Google Scholar]
- Jiang, J.; Shang, X.; Wang, H.; Xu, Y.-B.; Gao, Y.; Zhou, Q. Diagnostic value of contrast-enhanced ultrasound in thyroid nodules with calcification. Kaohsiung J. Med. Sci. 2015, 31, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sójka, L.; Tang, Z.; Furniss, D.; Sakr, H.; Oladeji, A.; Beres-Pawlik, E.; Dantanarayana, H.; Faber, E.; Seddon, A.B.; Benson, T.M.; et al. Broadband, mid-infrared emission from Pr3+doped GeAsGaSe chalcogenide fiber, optically clad. Opt. Mater. 2014, 36, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-Q.; Batentschuk, M.; Osvet, A.; Pinna, L.; Brabec, C.J. Rare-Earth Ion Doped Up-Conversion Materials for Photovoltaic Applications. Adv. Mater. 2011, 23, 2675–2680. [Google Scholar] [CrossRef]
- Jha, K.; Jayasimhadri, M. Spectroscopic investigation on thermally stable Dy3+ doped zinc phosphate glasses for white light emitting diodes. J. Alloy. Compd. 2016, 688, 833–840. [Google Scholar] [CrossRef]
- Adel, A.; Farag, M.; El-Okr, M.; Elrasasi, T.; El-Mansy, M. Preparation and Characterization of Phosphate Glasses Co-doped with Rare Earth Ions. Egypt. J. Chem. 2020, 63, 2–3. [Google Scholar] [CrossRef]
- El-Alaily, N.; Sallam, O.; Ezz-Eldin, F. Effect of gamma irradiation on some spectroscopic properties of phosphate glass containing samarium ions. J. Non-Crystalline Solids 2019, 523, 119604. [Google Scholar] [CrossRef]
- Kassab, L.R.P.; de Araujo, C.B. Linear and nonlinear optical properties of some tellurium oxide glasses. In Tecnological Advances in Tellurite Glasses: Properties, Processing and Applications; Rivera, V.A.G., Manzani, D., Eds.; Springer: Cham, Switzerland, 2017; Chapter 2; pp. 15–39. ISBN 978-3-319-53038-3. [Google Scholar] [CrossRef]
- Praveena, R.; Venkatramu, V.; Babu, P.; Jayasankar, C. Fluorescence spectroscopy of Sm3+ ions in P2O5–PbO–Nb2O5 glasses. Phys. B: Condens. Matter 2008, 403, 3527–3534. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Spectroscopic investigations of Nd3+ ions in B2O3–PbO–Al2O3–WO3 glasses. J. Mol. Struct. 2006, 792–793, 201–206. [Google Scholar] [CrossRef]
- Zaman, F.; Rooh, G.; Srisittipokakun, N.; Kim, H.; Kaewnuam, E.; Meejitpaisan, P.; Kaewkhao, J. Scintillation and luminescence characteristics of Ce3+doped in Li2O–Gd2O3–BaO–B2O3 scintillating glasses. Radiat. Phys. Chem. 2016, 130, 158–163. [Google Scholar] [CrossRef]
- Mohan, S.; Thind, K.S.; Sharma, G.; Gerward, L. Spectroscopic investigations of Nd3+ doped flouro- and chloro-borate glasses. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2008, 70, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Boonin, K.; Kaewkhao, J.; Ratana, T.; Limsuwan, P. Preparation and Properties of Bi2O3-B2O3-Nd2O3 Glass System. Procedia Eng. 2011, 8, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Sallam, O.; Alhodaib, A.; El Aal, S.A.; Ezz-Eldin, F. Influence of gamma ray on optical and structural properties of commercial glass enriched with copper oxide. Inorg. Chem. Commun. 2020, 124, 108388. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.; Kaewkhao, J. High transparency La2O3-CaO-B2O3-SiO2 glass for diagnosis x-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Ezz-Eldin, F.; Mahmoud, H.; Abdelaziz, T.D.; El-Alaily, N. Response of commercial window glass to gamma doses. Phys. B: Condens. Matter 2007, 403, 576–585. [Google Scholar] [CrossRef]
- Zamratul, M.; Zaidan, A.; Khamirul, A.; Nurzilla, M.; Halim, S. Formation, structural and optical characterization of neodymium doped-zinc soda lime silica based glass. Results Phys. 2016, 6, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, J.; Coelho, J.; Hungerford, G.; Hussain, N.S. Lasing transition (4F3/2→4I11/2) at 1.06μm in neodymium oxide doped lithium boro tellurite glass. Phys. B: Condens. Matter 2010, 405, 4696–4701. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth borate glasses doped with europium ions evidenced by FT-IR spectroscopy. J. Mater. Sci. Mater. Electron. 2007, 19, 424–428. [Google Scholar] [CrossRef]
- Algradee, M.A.; Sultan, M.; Samir, O.M.; Alwany, A.E.B. Electronic polarizability, optical basicity and interaction parameter for Nd2O3 doped lithium–zinc–phosphate glasses. Appl. Phys. A 2017, 123, 524. [Google Scholar] [CrossRef]
- Chimalawong, P.; Kirdsiri, K.; Kaewkhao, J.; Limsuwan, P. Investigation on the Physical and Optical Properties of Dy3+ Doped Soda-Lime-Silicate Glasses. Procedia Eng. 2012, 32, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Ajroud, M.; Haouari, M.; BEN Ouada, H.; Maaref, H.; Brenier, A.; Garapon, C. Investigation of the spectroscopic properties of Nd3+-doped phosphate glasses. J. Phys. Condens. Matter 2000, 12, 3181–3193. [Google Scholar] [CrossRef]
- Speghini, A.; Peruffo, M.; Casarin, M.; Ajò, D.; Bettinelli, M. Electronic spectroscopy of trivalent lanthanide ions in lead zinc borate glasses. J. Alloy Compd. 2000, 300–301, 174–179. [Google Scholar] [CrossRef]
- Chimalawong, P.; Kaewkhao, J.; Kedkaew, C.; Limsuwan, P. Optical and electronic polarizability investigation of Nd3+-doped soda-lime silicate glasses. J. Phys. Chem. Solids 2010, 71, 965–970. [Google Scholar] [CrossRef]
- Bulus, I.; Dalhatu, S.A.; Hussin, R.; Shamsuri, W.N.W.; Yamusa, Y.A. The role of dysprosium ions on the physical and optical properties of lithium-borosulfophosphate glasses. Int. J. Mod. Phys. B 2017, 31, 1750101. [Google Scholar] [CrossRef]
- Rajiv, A.; Reddy, M.S.; Uchil, J.; Reddy, C.N. Thermal and Optical properties of Sodium-Phospho-Zinc-Neodymium oxide glass system. J. Adv. Sci. Res. 2014, 5, 32–39. [Google Scholar]
- Peijzel, P.S.; Vergeer, P.; Meijerink, A.; Reid, M.F.; Boatner, L.; Burdick, G.W. 4fn−15d→4fn emission of Ce3+, Pr3+, Nd3+, Er3+, and Tm3+ in LiYF4 and YPO4. Phys. Rev. B. 2005, 71, 045116. [Google Scholar] [CrossRef]
- Bokare, A.; Pai, M.; Athawale, A.A. Surface modified Nd doped TiO2 nanoparticles as photocatalysts in UV and solar light irradiation. Sol. Energy 2013, 91, 111–119. [Google Scholar] [CrossRef]
- De Oliveira, M.A.S.; De Araújo, C.B.; Messaddeq, Y. Upconversion ultraviolet random lasing in Nd^3+ doped fluoroindate glass powder. Opt. Express 2011, 19, 5620–5626. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, L.; Upender, G.; Kuladeep, R.; Rao, D.N. Structural, thermal, optical properties and simulation of white light of titanium-tungstate-tellurite glasses doped with dysprosium. Mater. Res. Bull. 2014, 50, 424–431. [Google Scholar] [CrossRef]
- Kaur, S.; Arora, D.; Kumar, S.; Singh, G.; Mohan, S.; Kaur, P.; Kriti; Singh, D. Blue-yellow emission adjustability with aluminium incorporation for cool to warm white light generation in dysprosium doped borate glasses. J. Lumin. 2018, 202, 168–175. [Google Scholar] [CrossRef]
- Talewar, R.A.; Mahamuda, S.; Swapna, K.; Rao, A. Near UV based Dy3+ ions doped alkaline-earth chloro borate glasses for white LED’s and visible lasers. Opt. Laser Technol. 2019, 119, 105646. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zhang, J.-C.; Liu, X.-G.; Lin, L.-W. Enhanced luminescence of novel Ca3B2O6:Dy3+ phosphors by Li+-codoping for LED applications. Ceram. Int. 2012, 38, 1065–1070. [Google Scholar] [CrossRef]
- Pisarska, J. Optical properties of lead borate glasses containing Dy3+ions. J. Phys.: Condens. Matter 2009, 21, 285101. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, N.; Kumar, K.U.; Jayasankar, C. Dy3+-doped zinc fluorophosphate glasses for white luminescence applications. Spectrochim. Acta Part A 2013, 113, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Klimesz, B.; Ryba-Romanowski, W.; Lisiecki, R. Oxyfluorotellurite glasses doped by dysprosium ions. Thermal and optical properties. Opt. Mater. 2015, 42, 538–543. [Google Scholar] [CrossRef]
- Rayappan, I.A.; Maheshvaran, K.; Babu, S.S.; Marimuthu, K. Dysprosium doped lead fluoroborate glasses: Structural, optical, and thermal investigations. Phys. Status Solidi 2011, 209, 570–578. [Google Scholar] [CrossRef]
- McCamy, C.S. Correlated color temperature as an explicit function of chromaticity coordinates. Color Res. Appl. 1992, 17, 142–144. [Google Scholar] [CrossRef]
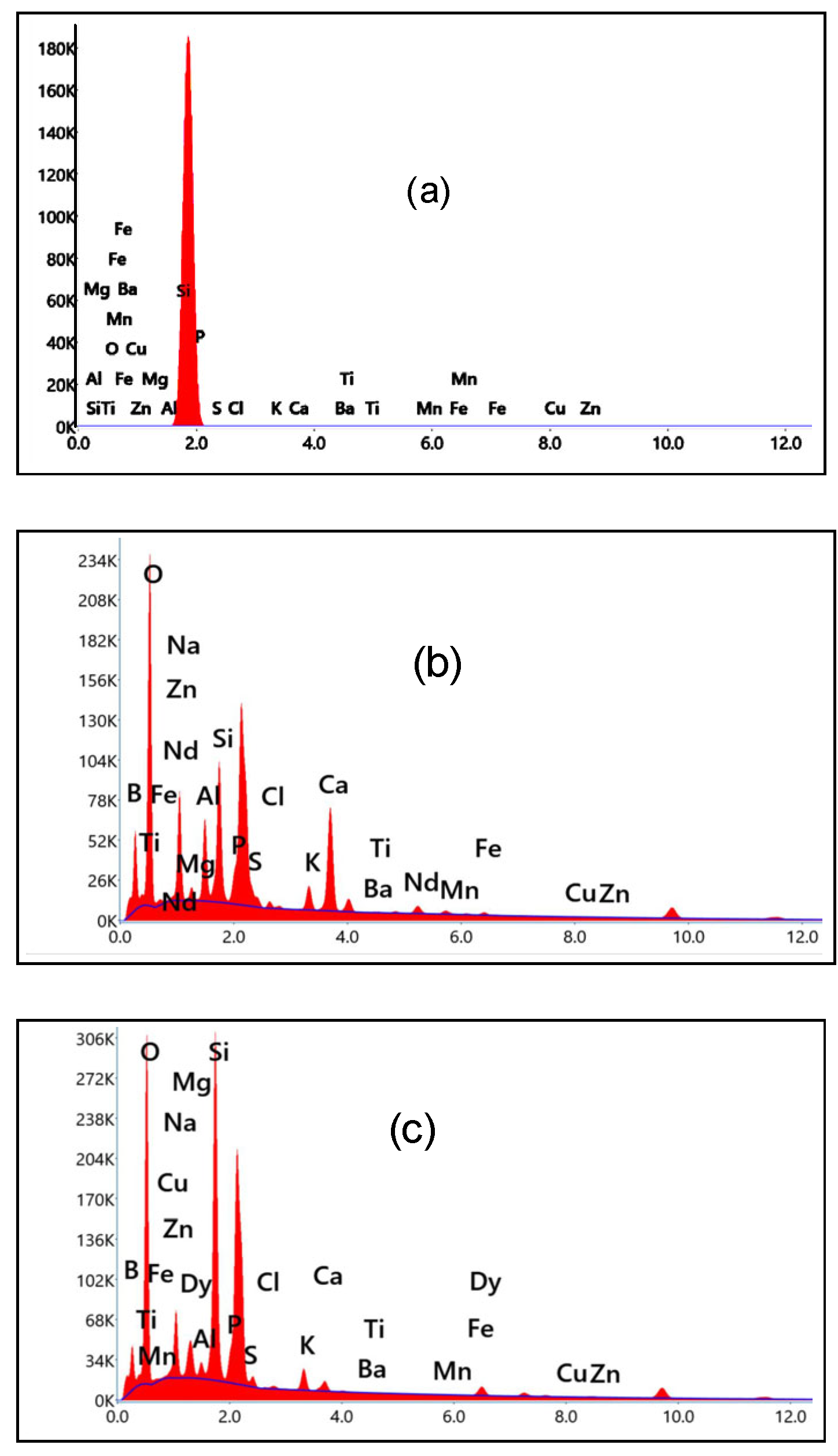
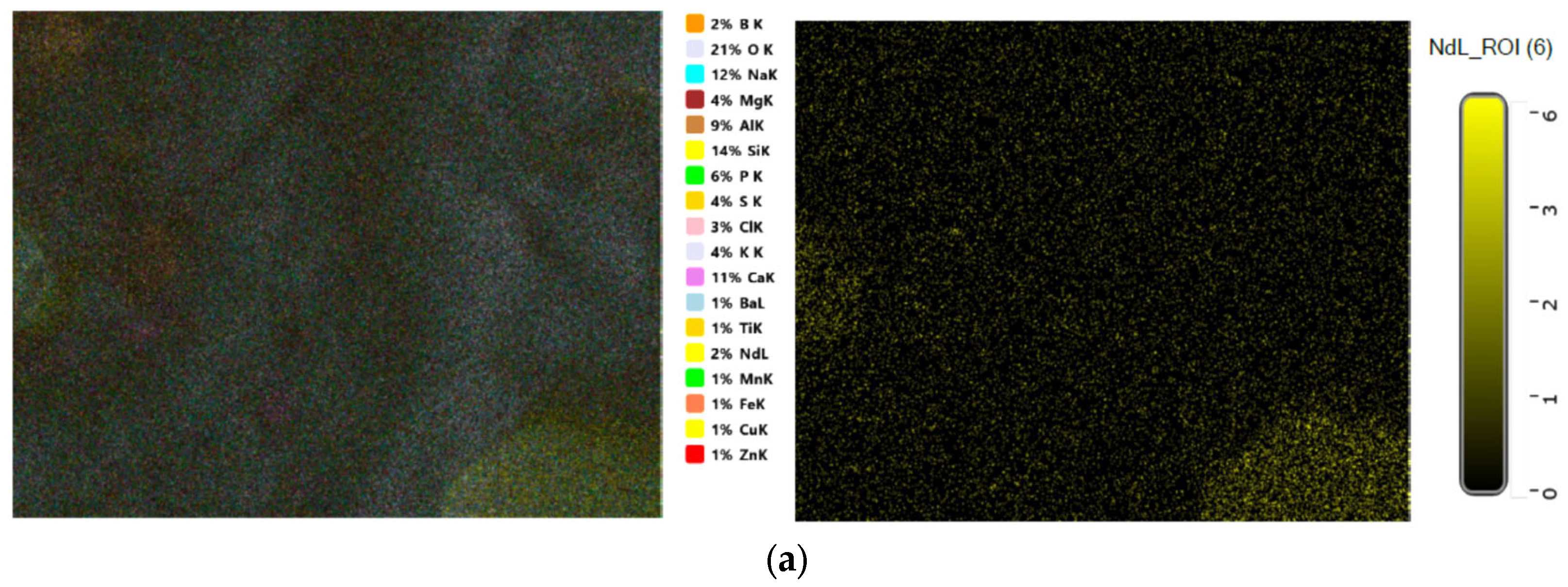
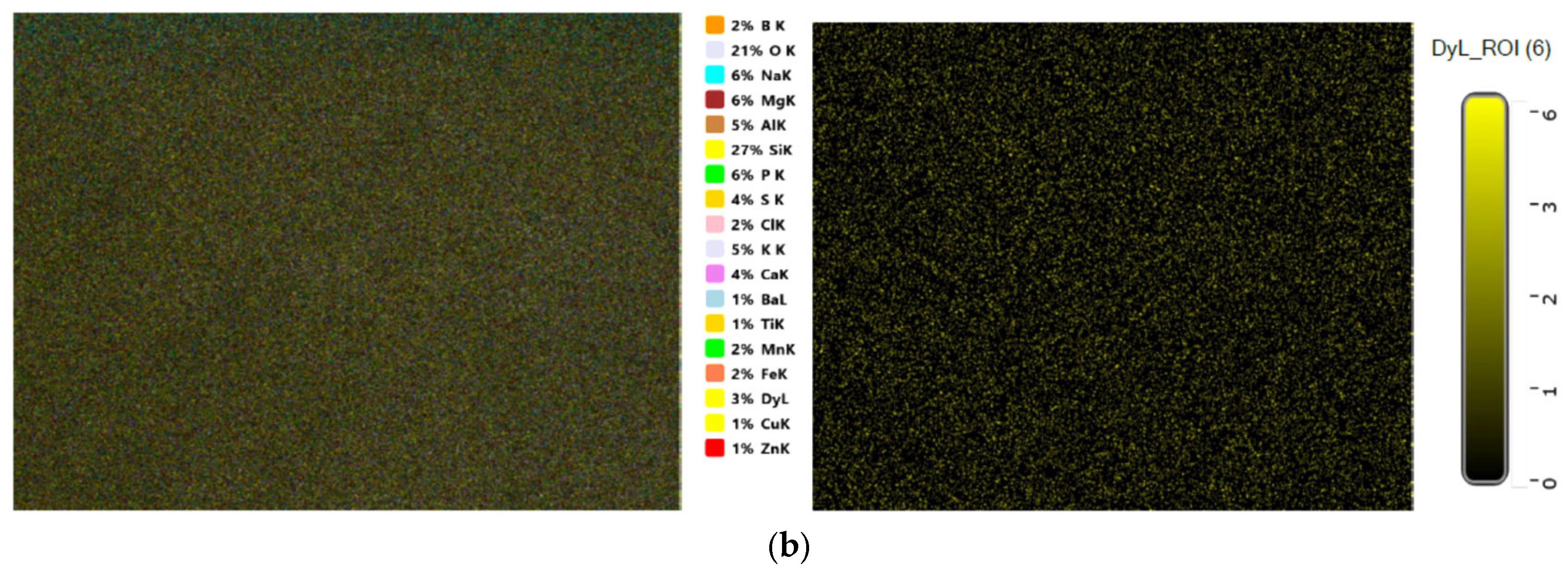



| Sample | Elements | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Mn | Fe | Cu | Zn | Ba | B | Nd | Dy | |
| RA | 0.14 | 0.5 | 0.4 | 0.4 | 87.0 | 6.5 | 0.01 | 0.05 | 0.19 | 0.04 | 0.01 | 0.32 | 1.89 | 0.43 | 0.49 | 0.13 | 1.5 | 0 | 0 |
| GRN | 36.2 | 6.87 | 0.67 | 0.9 | 21.01 | 5.02 | 4.93 | 0.43 | 2.35 | 1.9 | 0.02 | 0.11 | 1.99 | 0.33 | 0.82 | 0.35 | 11.5 | 4.59 | 0 |
| GRD | 34.4 | 5.83 | 1.05 | 0.83 | 18.55 | 5.13 | 5.53 | 0.14 | 2.58 | 1.46 | 0.03 | 0.13 | 1.34 | 0.31 | 0.86 | 0.13 | 12.6 | 0 | 9.09 |
| Sample | Direct Energy Gap | Indirect Energy Gap | ||||
|---|---|---|---|---|---|---|
| Before Irrad. | After Irrad. | Before Irrad. | After Irrad. | |||
| 20 kGy | 50 kGy | 20 kGy | 50 kGy | |||
| GRN | 3.52 | 3.49 | 3.42 | 3.03 | 3.022 | 3.00 |
| GRD | 3.03 | 2.97 | 2.87 | 2.90 | 2.81 | 2.78 |
| Sample | Y/b | Color Co-Ordination (x,y) | CCT | |
|---|---|---|---|---|
| X | Y | |||
| Before Irrad. | 2.269 | 0.370 | 0.415 | 4483.09 |
| After 20 kGy | 1.958 | 0.375 | 0.377 | 4157.73 |
| After 50 kGy | 2.115 | 0.365 | 0.398 | 4541.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhodaib, A.; Ibrahim, O.; Abd El All, S.; Ezzeldin, F. Effect of Rare-Earth Ions on the Optical and PL Properties of Novel Borosilicate Glass Developed from Agricultural Waste. Materials 2021, 14, 5607. https://doi.org/10.3390/ma14195607
Alhodaib A, Ibrahim O, Abd El All S, Ezzeldin F. Effect of Rare-Earth Ions on the Optical and PL Properties of Novel Borosilicate Glass Developed from Agricultural Waste. Materials. 2021; 14(19):5607. https://doi.org/10.3390/ma14195607
Chicago/Turabian StyleAlhodaib, Aiyeshah, Omnia Ibrahim, Suzy Abd El All, and Fatthy Ezzeldin. 2021. "Effect of Rare-Earth Ions on the Optical and PL Properties of Novel Borosilicate Glass Developed from Agricultural Waste" Materials 14, no. 19: 5607. https://doi.org/10.3390/ma14195607






