Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Maghemite and Maghemite-TiO2 Nanoparticles
2.2. Structural Measurements
2.3. Magnetic Characterization
2.4. Heating Efficiency
3. Results and Discussion
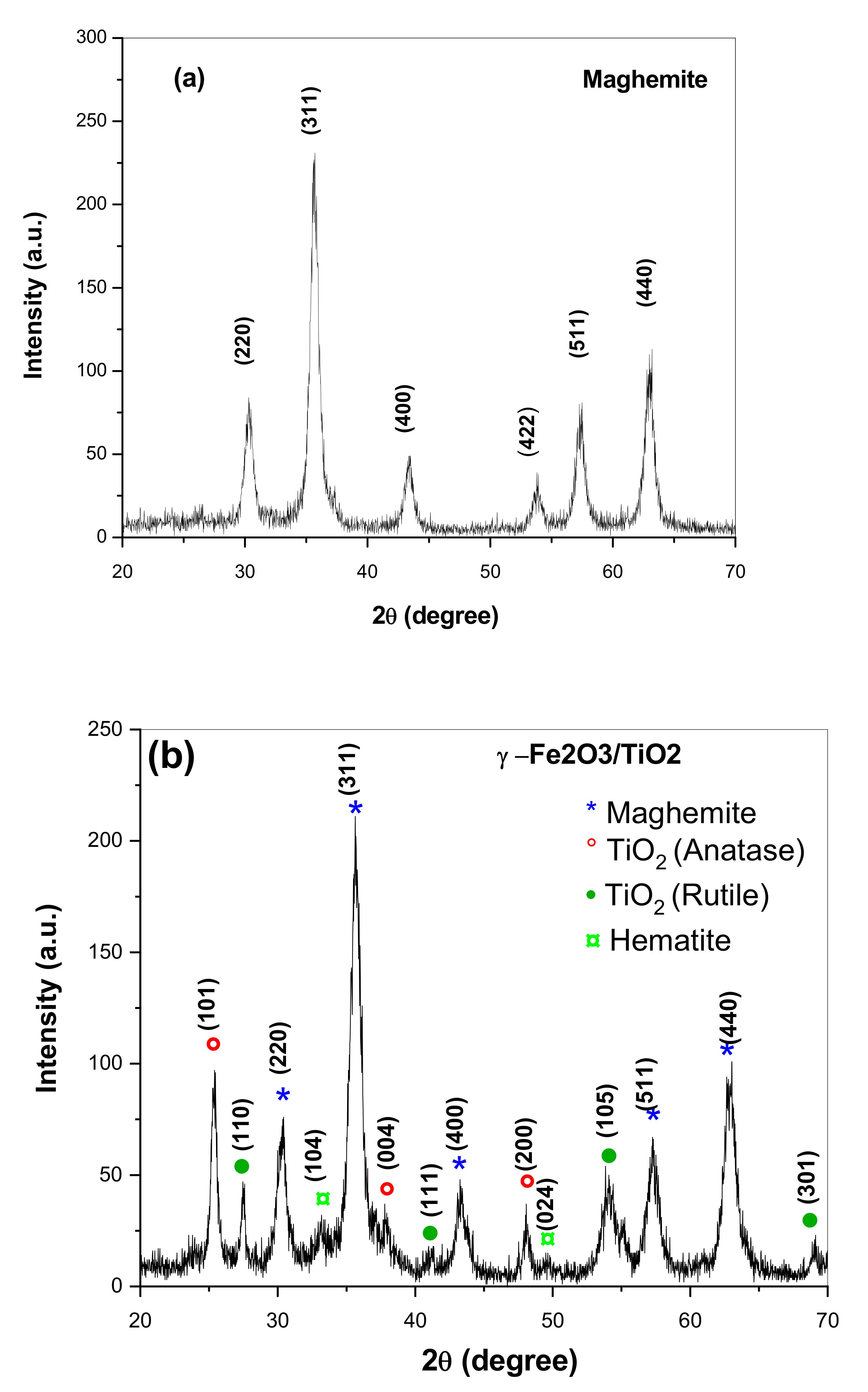
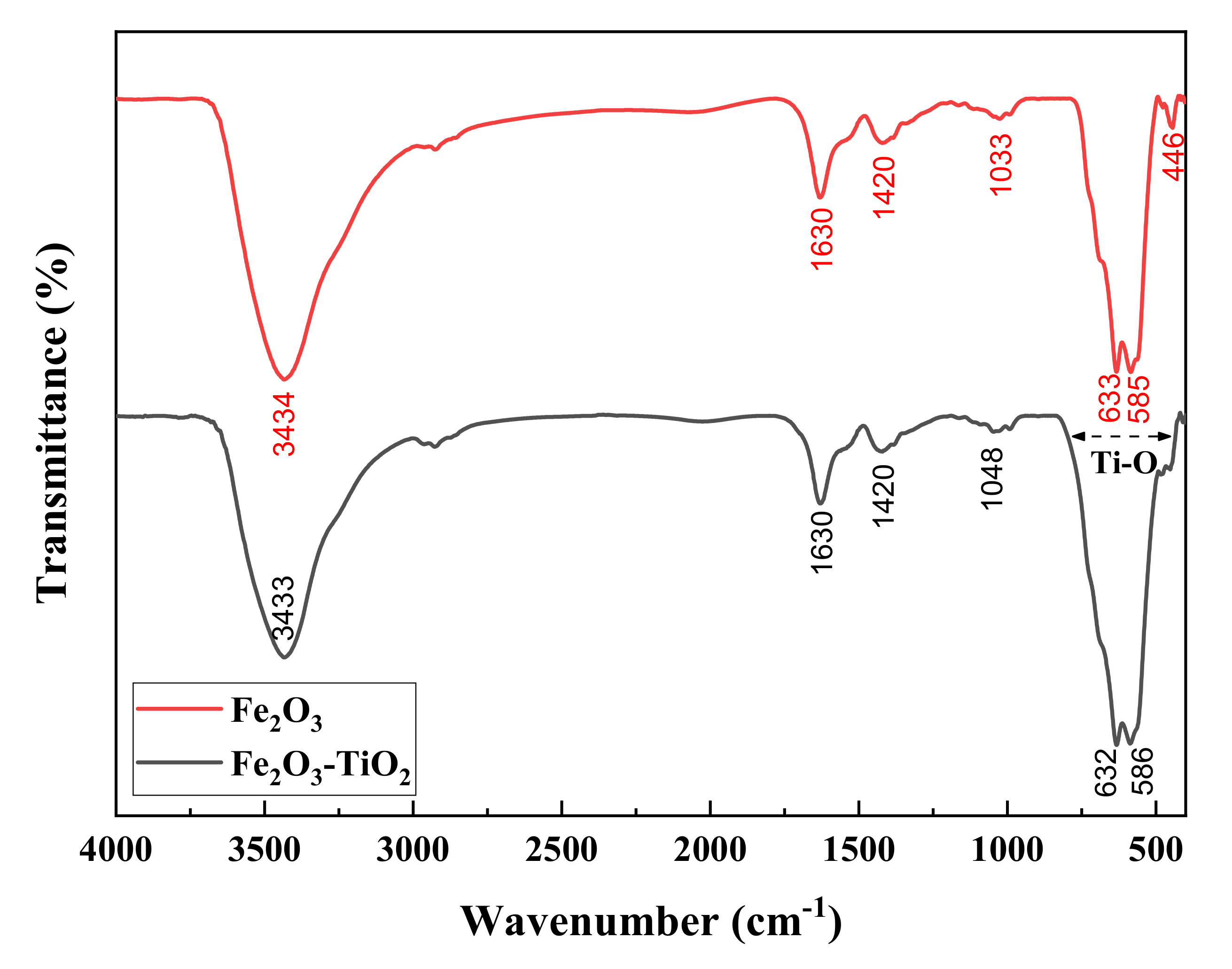
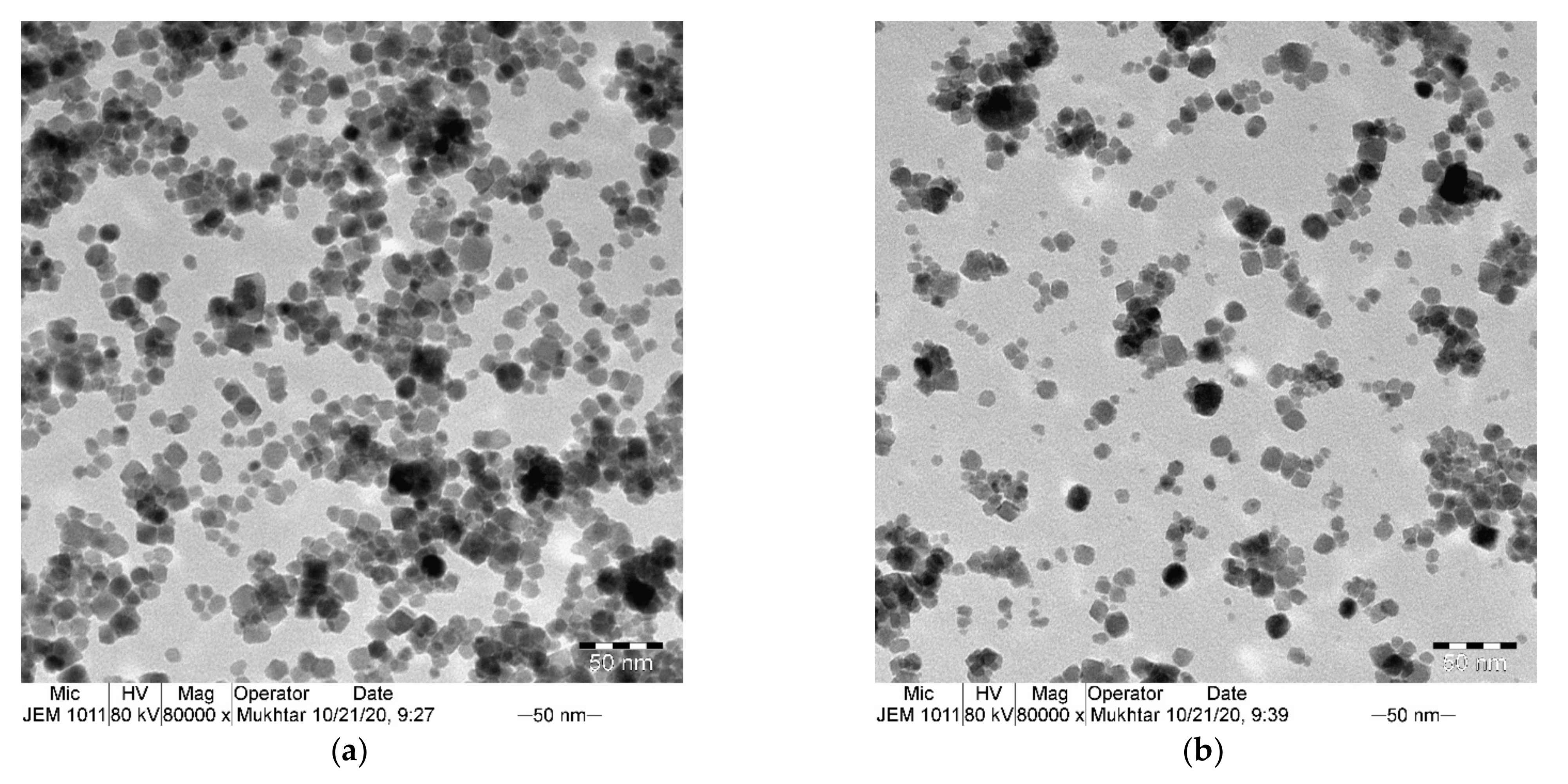
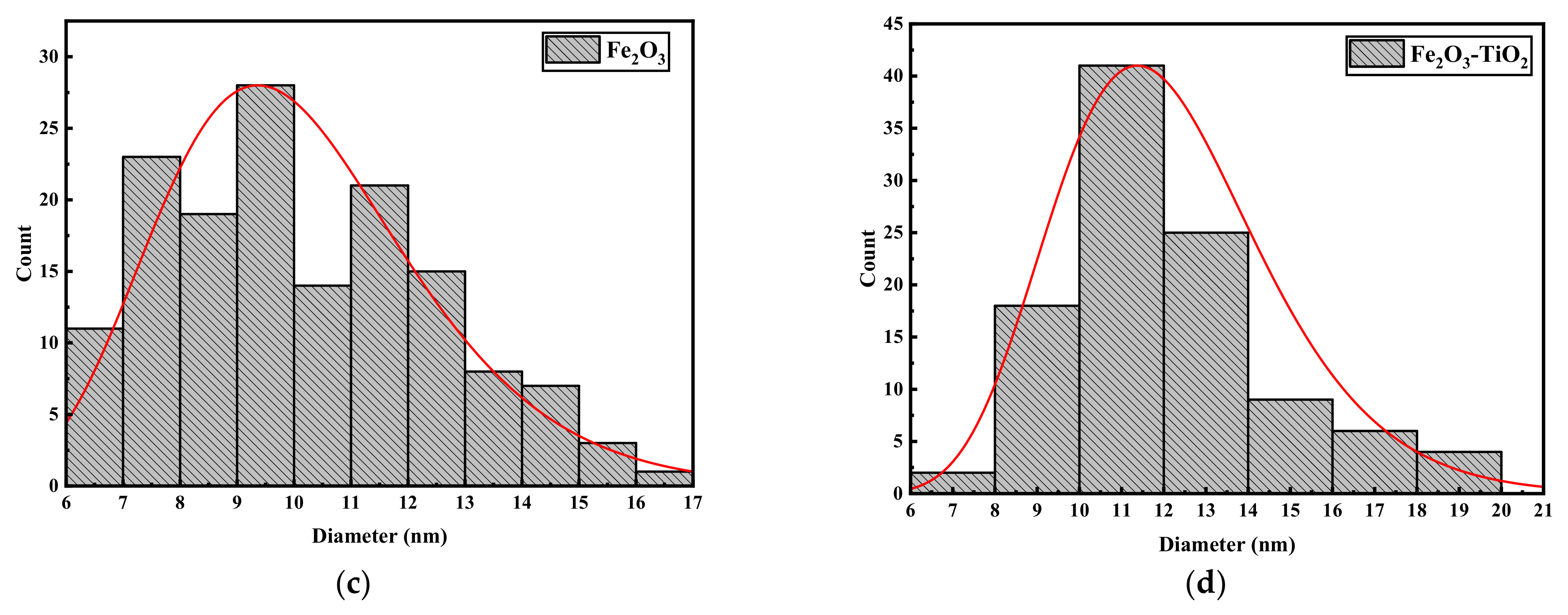
3.1. Structural Properties
3.2. Magnetic Characterization
3.2.1. SQUID Measurements
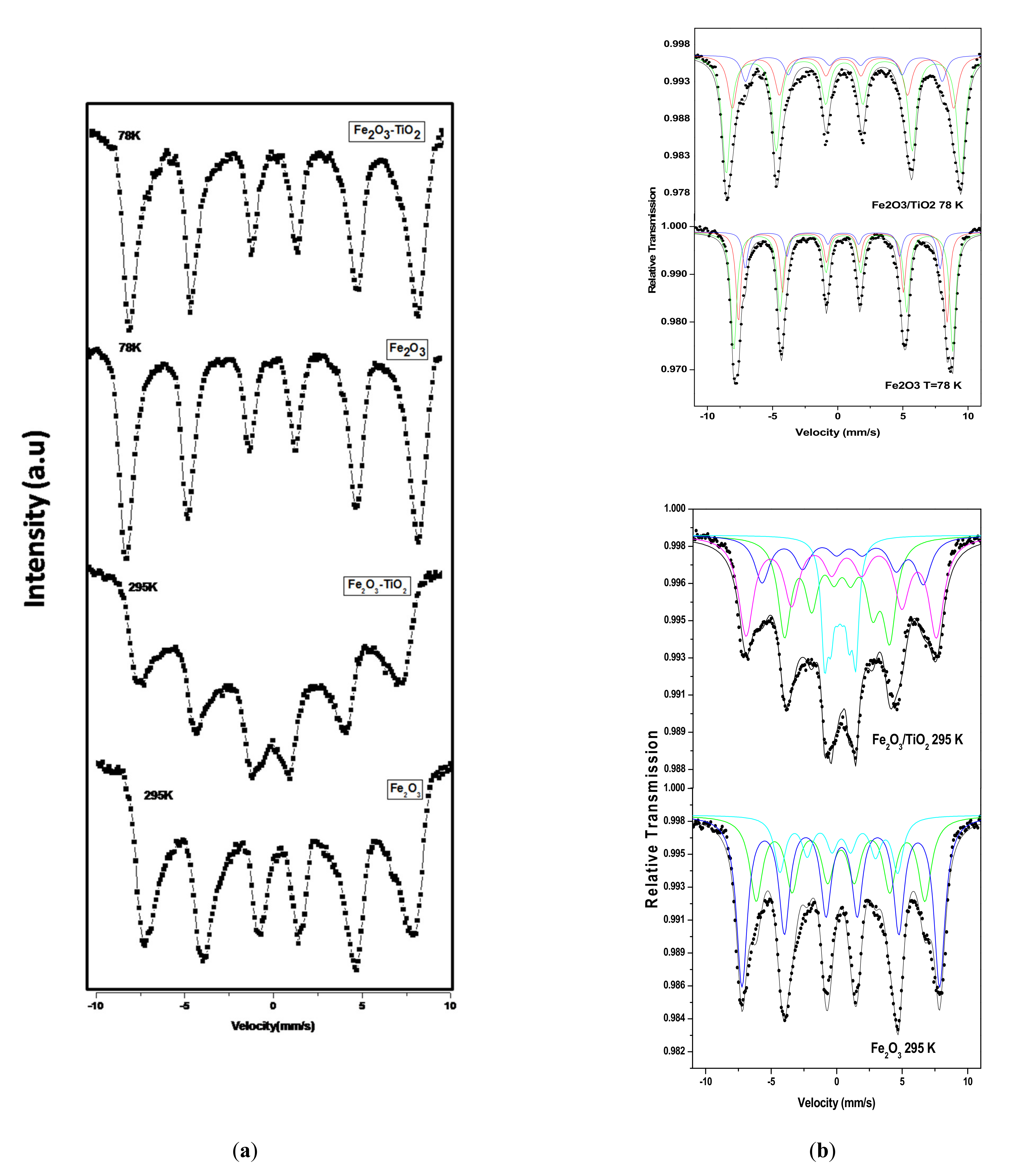
3.2.2. Mössbauer Spectroscopy
3.3. Heating Efficiency Measurements
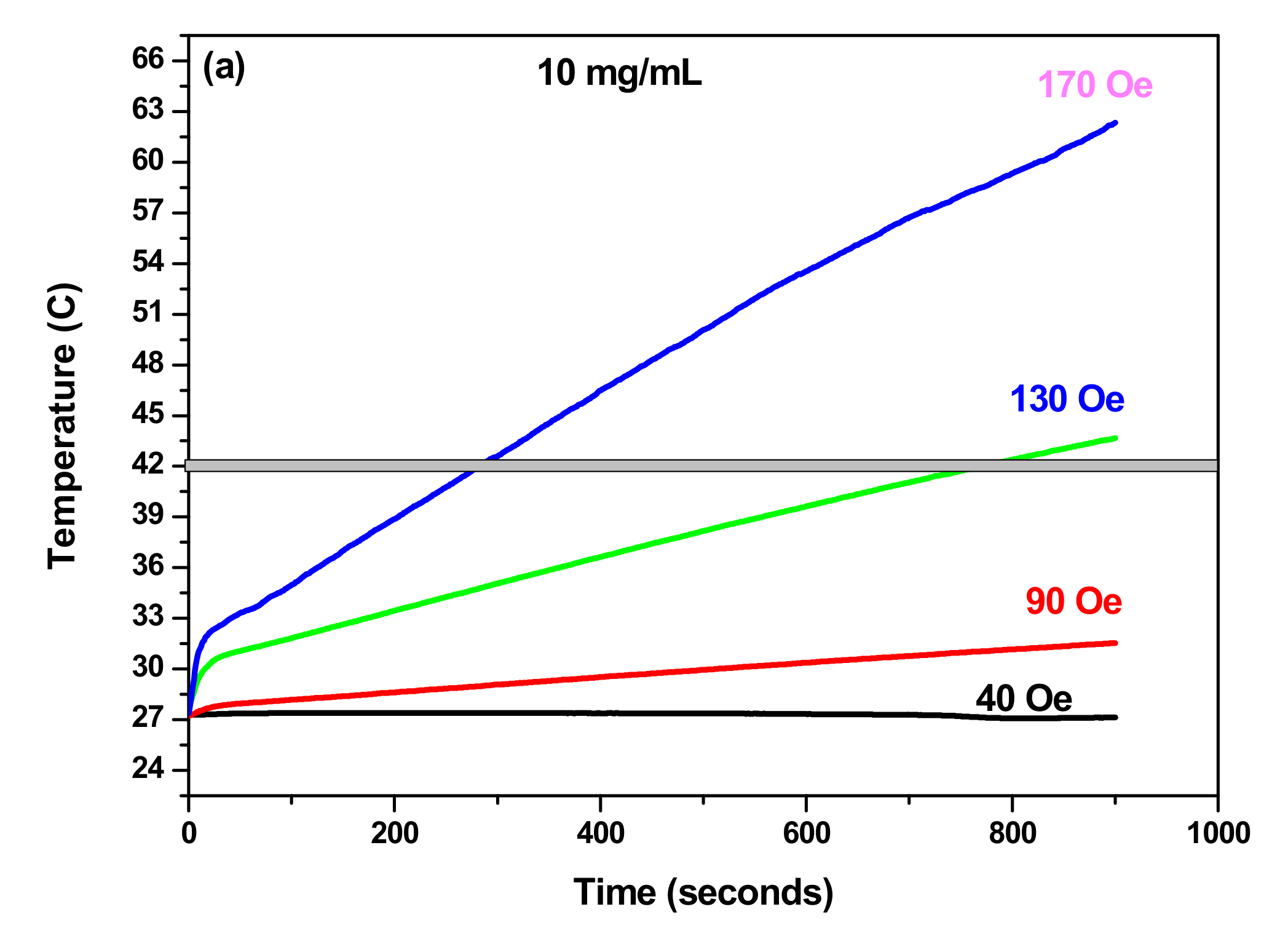
3.3.1. SAR as Function of Concentration
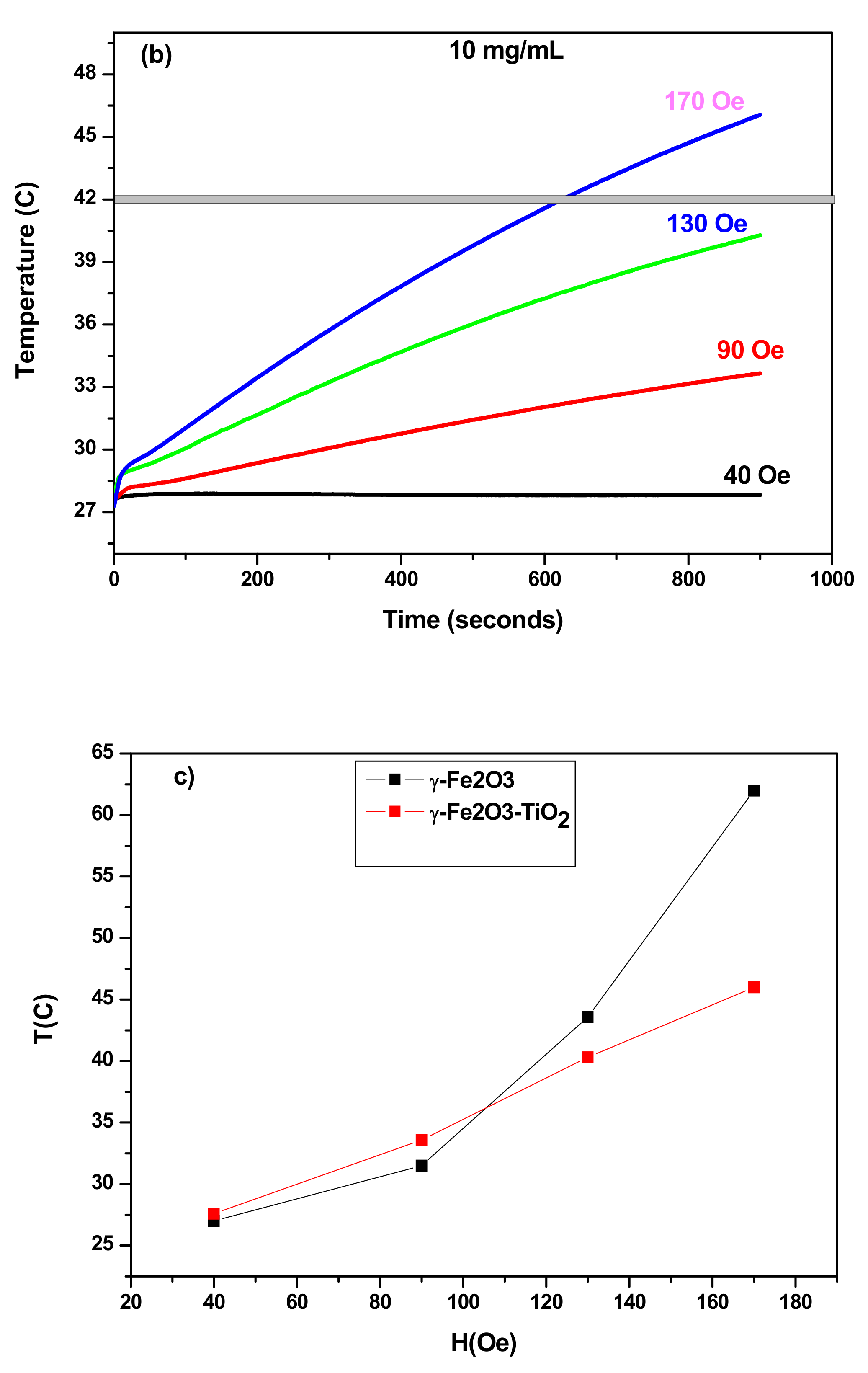
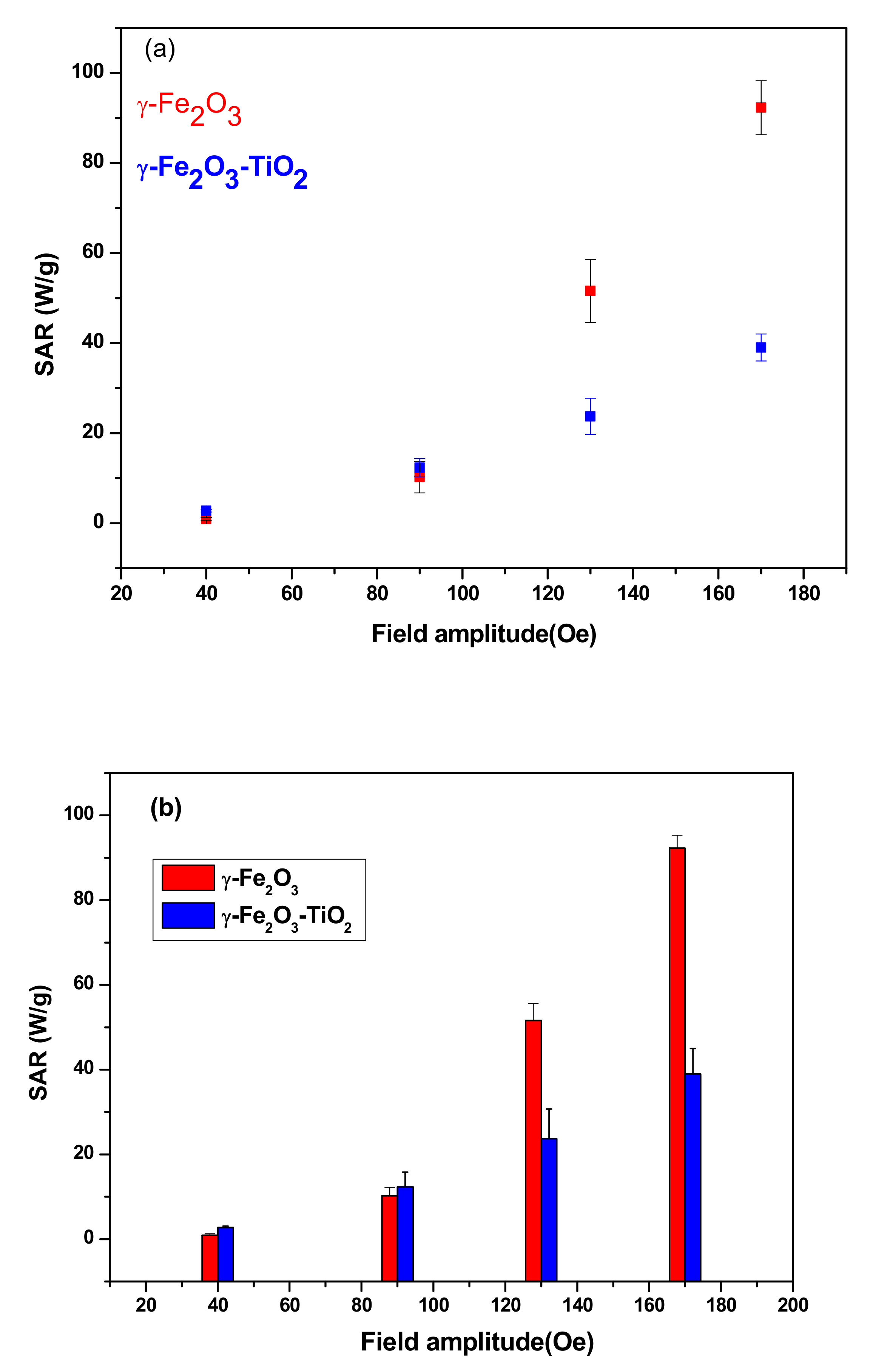
3.3.2. SAR as Function of Field Amplitude
3.3.3. Comparison of Heating Ability
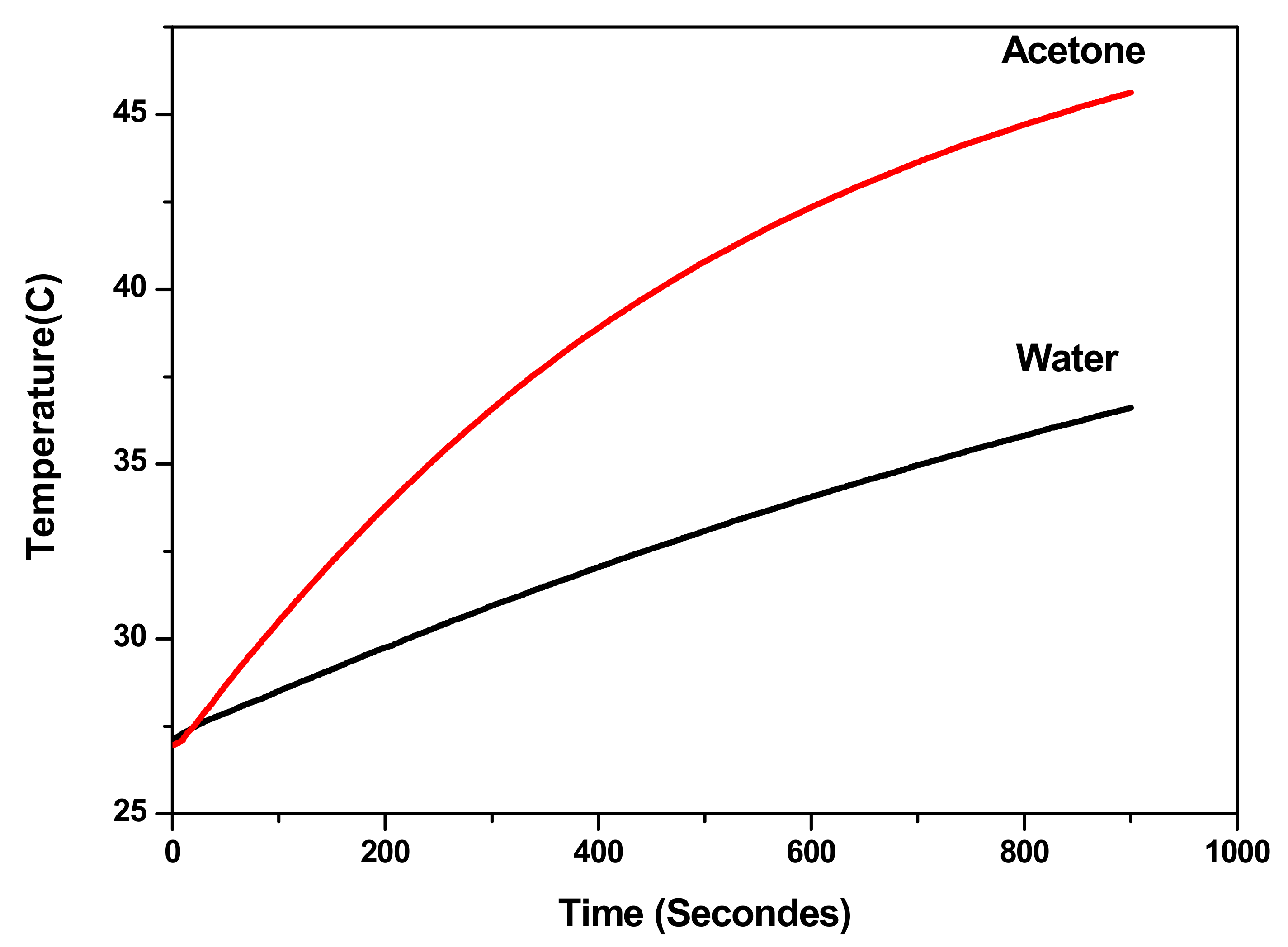
3.3.4. Mechanism of Heating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef]
- Khot, V.; Pawar, S. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, M.J.; Ahn, C.-H.; Kim, H.; Chung, I.J.; Jung, S.; Kim, Y.-H.; Youn, H.; Chung, J.W. The intratumoral administration of ferucarbotran conjugated with doxorubicin improved therapeutic effect by magnetic hyperthermia combined with pharmacotherapy in a hepatocellular carcinoma model. J. Exp. Clin. Cancer Res. 2014, 33, 57. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Sakamoto, W.; Yogo, T.; Ishimura, K. Magnetically Responsive Smart Nanoparticles for Cancer Treatment with a Combination of Magnetic Hyperthermia and Remote-Control Drug Release. Theranostics 2014, 4, 834–844. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, J.E.; Jin, S.M.; Lee, J.H.; Lee, I.S.; Yang, I.; Kim, J.-S.; Kim, S.K.; Cho, M.-H.; et al. Designed Fabrication of Multifunctional Magnetic Gold Nanoshells and Their Application to Magnetic Resonance Imaging and Photothermal Therapy. Angew. Chem. 2006, 118, 7918–7922. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- Yang, T.; Tang, Y.; Liu, L.; Lv, X.; Wang, Q.; Ke, H.; Deng, Y.; Yang, H.; Yang, X.; Liu, G.; et al. Size-Dependent Ag2S Nanodots for Second Near-Infrared Fluorescence/Photoacoustics Imaging and Simultaneous Photothermal Therapy. ACS Nano 2017, 11, 1848–1857. [Google Scholar] [CrossRef]
- Kong, A.; Wang, H.; Li, J.; Shan, Y. Preparation of super paramagnetic crystalline mesoporous γ-Fe2O3 with high surface. Mater. Lett. 2008, 62, 943–945. [Google Scholar] [CrossRef]
- Kazeminezhad, I.; Mosivand, S. Phase Transition of Electrooxidized Fe3O4to γ and α-Fe2O3Nanoparticles Using Sintering Treatment. Acta Phys. Pol. A 2014, 125, 1210–1214. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature Sol-gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K.; Ali, R.; Al-Zahrani, H.; Trivilegio, T.; Alanazi, A.H.; Khan, A.L.; Boudjelal, M.; AlKushi, A. Preparation of iron oxide mesoporous magnetic microparticles as novel multidrug carriers for synergistic anticancer therapy and deep tumor penetration. Sci. Rep. 2019, 9, 9481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmenares, J.C.; Ouyang, W.; Ojeda, M.; Kuna, E.; Chernyayeva, O.; Lisovytskiy, D.; De, S.; Luque, R.; Balu, A.M. Mild ultrasound-assisted synthesis of TiO 2 supported on magnetic nanocomposites for selective photo-oxidation of benzyl alcohol. Appl. Catal. B Environ. 2016, 183, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Meng, G.; Wu, J.; Li, D.; Liu, Z. Study on enhanced photocatalytic activity of magnetically recoverable Fe3O4@C@TiO2 nanocomposites with core–shell nanostructure. Opt. Mater. 2015, 46, 52–58. [Google Scholar] [CrossRef]
- Li, W.; Wu, H. Sodium citrate functionalized reusable Fe3O4@TiO2 photocatalyst for water purification. Chem. Phys. Lett. 2017, 686, 178–182. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Wojciechowska, A.; Grzechulska-Damszel, J.; Narkiewicz, U.; Śniadecki, Z.; Idzikowski, B. Effective processes of phenol degradation on Fe3O4–TiO2 nanostructured magnetic photocatalyst. J. Phys. Chem. Solids 2019, 136, 109178. [Google Scholar] [CrossRef]
- Islam, S.; Kusumoto, Y.; Mamun, A.A.; Horie, Y. Photocatalytic and AC magnetic-field induced enhanced cytotoxicity of Fe3O4–TiO2 core-shell nanocomposites against HeLa cells. Catal. Commun. 2011, 16, 39–44. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Jiang, W.; Huang, Y.; Zhang, W.; Liang, G.; Yang, S. Porous γ-Fe2O3 microspheres decorated with TiO2 nanocrystals as highly recyclable photocatalysts and potential highly efficient magnetic hyperthermia agents. J. Mater. Sci. Mater. Electron. 2018, 29, 20856–20865. [Google Scholar] [CrossRef]
- Hilger, I.; Frühauf, K.; Andrä, W.; Hiergeist, R.; Hergt, R.; Kaiser, W.A. Heating Potential of Iron Oxides for Therapeutic Purposes in Interventional Radiology. Acad. Radiol. 2002, 9, 198–202. [Google Scholar] [CrossRef]
- Lemine, O.; Madkhali, N.; Hjiri, M.; All, N.A.; Aida, M. Comparative heating efficiency of hematite (α-Fe2O3) and nickel ferrite nanoparticles for magnetic hyperthermia application. Ceram. Int. 2020, 46, 28821–28827. [Google Scholar] [CrossRef]
- Kita, E.; Hashimoto, S.; Kayano, T.; Minagawa, M.; Yanagihara, H.; Kishimoto, M.; Yamada, K.; Oda, T.; Ohkohchi, N.; Takagi, T.; et al. Heating characteristics of ferromagnetic iron oxide nanoparticles for magnetic hyperthermia. J. Appl. Phys. 2010, 107, 09B321. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.D.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Lemine, O.; Omri, K.; Iglesias, M.; Velasco, V.; Crespo, P.; de la Presa, P.; El Mir, L.; Bouzid, H.; Yousif, A.; Al-Hajry, A. γ-Fe2O3 by Sol-gel with large nanoparticles size for magnetic hyperthermia application. J. Alloy Compd. 2014, 607, 125–131. [Google Scholar] [CrossRef]
- Thorat, N.D.; Lemine, O.; Bohara, R.; Omri, K.; El Mir, L.; Tofail, S.A.M. Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys. Chem. Chem. Phys. 2016, 18, 21331–21339. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [Green Version]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin, Germany, 1912; pp. 387–409. [Google Scholar]
- Masuda, Y.; Kato, K. Synthesis and phase transformation of TiO2 nano-crystals in aqueous solutions. J. Ceram. Soc. Jpn. 2009, 117, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.J.; Watson, G.W. A Density Functional Theory + U Study of Oxygen Vacancy Formation at the (110), (100), (101), and (001) Surfaces of Rutile TiO2. J. Phys. Chem. C 2009, 113, 7322–7328. [Google Scholar] [CrossRef]
- Larumbe, S.; Gomez-Polo, C.; Pérez-Landazábal, J.; Pastor, J.M. Effect of a SiO2coating on the magnetic properties of Fe3O4nanoparticles. J. Physics Condens. Matter 2012, 24, 266007. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Geng, B.; Wang, M.; Sun, X. Photocatalytic performance and magnetic separation of TiO2-functionalized γ-Fe2O3, Fe, and Fe/Fe2O3 magnetic particles. J. Alloy Compd. 2017, 700, 113–121. [Google Scholar] [CrossRef]
- Hernando, A.; Vázquez, M.; Kulik, T.; Prados, C. Analysis of the dependence of spin-spin correlations on the thermal treatment of nanocrystalline materials. Phys. Rev. B 1995, 51, 3581–3586. [Google Scholar] [CrossRef]
- Brown, J.W.F. Theory of the Approach to Magnetic Saturation. Phys. Rev. 1940, 58, 736–743. [Google Scholar] [CrossRef]
- Komogortsev, S.; Iskhakov, R. Law of approach to magnetic saturation in nanocrystalline and amorphous ferromagnets with improved transition behavior between power-law regimes. J. Magn. Magn. Mater. 2017, 440, 213–216. [Google Scholar] [CrossRef]
- Craik, D.J. Magnetic Oxides; John Wiley & Sons: London, UK; New York, NY, USA, 1975. [Google Scholar]
- Lyubutin, I.; Starchikov, S.; Bukreeva, T.; Lysenko, I.; Sulyanov, S.; Korotkov, N.; Rumyantseva, S.; Marchenko, I.; Funtov, K.; Vasiliev, A. In situ synthesis and characterization of magnetic nanoparticles in shells of biodegradable polyelectrolyte microcapsules. Mater. Sci. Eng. C 2014, 45, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Marco, J.F.; Morales, M.D.P.; Serna, C.J. Effect of Nature and Particle Size on Properties of Uniform Magnetite and Maghemite Nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Gutiérrez, L.; del Mercato, L.L.; Herranz, F.; Chubykalo-Fesenko, O.; Veintemillas-Verdaguer, S.; Parak, W.J.; Morales, M.P.; González, J.M.; Hernando, A.; et al. Magnetic Capsules for NMR Imaging: Effect of Magnetic Nanoparticles Spatial Distribution and Aggregation. J. Phys. Chem. C 2011, 115, 6257–6264. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ortega, F.; Fernández, B.L.C.; Delgado, A.V.; Iglesias, G.R. Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics 2019, 11, 517. [Google Scholar] [CrossRef] [Green Version]
- Hadadian, Y.; Ramos, A.P.; Pavan, T.Z. Role of zinc substitution in magnetic hyperthermia properties of magnetite nanoparticles: Interplay between intrinsic properties and dipolar interactions. Sci. Rep. 2019, 9, 18048. [Google Scholar] [CrossRef] [Green Version]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509–1513. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.I.; Patel, V.; Ogrady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Chung, S.H.; Hoffmann, A.; Bader, S.D.; Liu, C.; Kay, B.K.; Makowski, L.; Chen, L. Biological sensors based on Brownian relaxation of magnetic nanoparticles. Appl. Phys. Lett. 2004, 85, 2971–2973. [Google Scholar] [CrossRef]

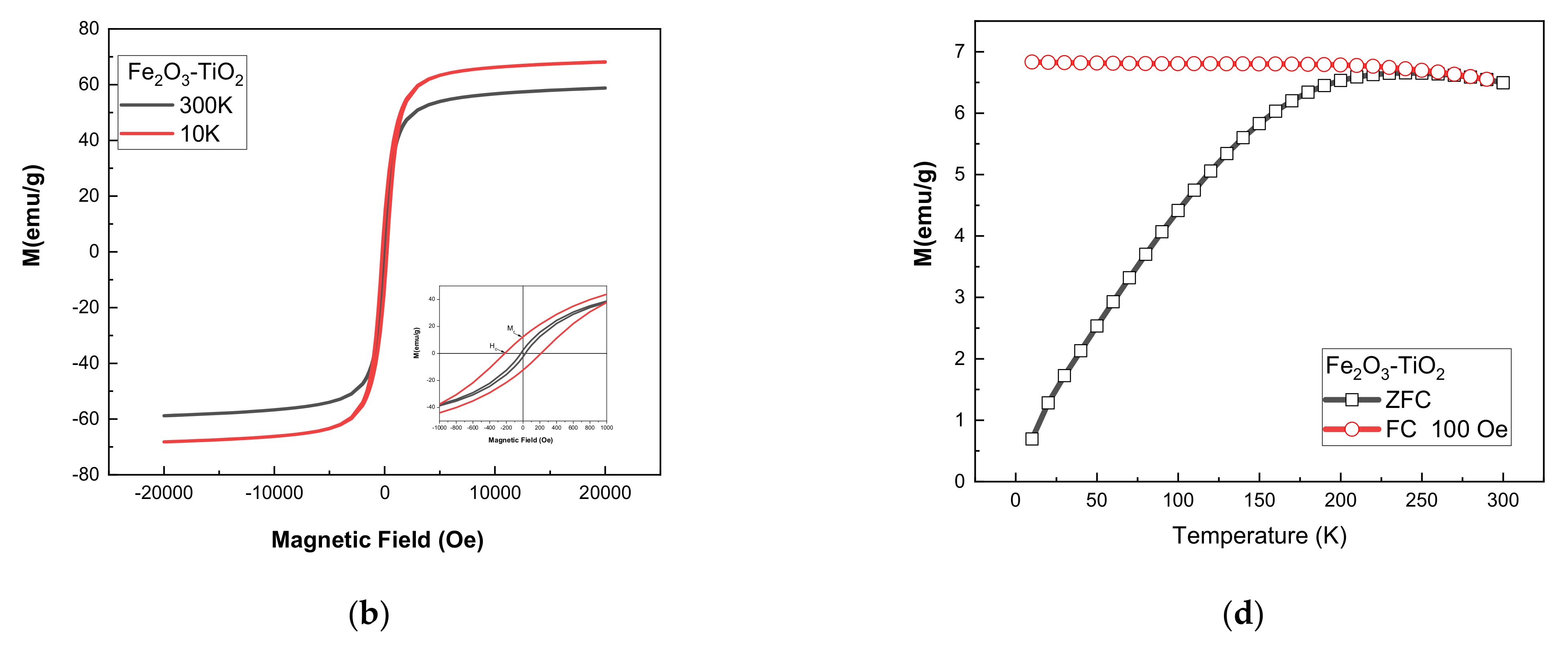


| Samples | Ms (emu/g) | Mr (emu/g) | Mr/Ms | Hc (Oe) | Keff (erg/cm3) | D (nm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 K | 300 K | 10 K | 300 K | 10 K | 300 K | 10 K | 300 K | 10 K | 300 K | ||
| Fe2O3 | 97.76 | 84.55 | 17.51 | 2.69 | 0.179 | 0.032 | 252.17 | 23.26 | 6.44 × 103 | 5.68 × 104 | 8.5 |
| Fe2O3-TiO2 | 68.11 | 58.77 | 12.25 | 3.11 | 0.18 | 0.053 | 217.51 | 27 | 3.51 × 103 | 3.43 × 103 | 11 |
| Samples | Component | Isomer Shifts δ ± (0.01) mm/s | Quadrupole Shifts ε ± (0.002) mm/s | Magnetic Hyperfine Field Bhf (±0.1) T | Area A (±1) % | ||||
| 78 K | 295 K | 78 K | 295 K | 78 K | 295 K | 78 K | 295 K | ||
| Fe2O3 | A | 0.40 | 0.31 | −0.007 | −0.021 | 49.7 | 40.0 | 34 | 33 |
| B | 0.43 | 0.34 | −0.004 | −0.029 | 52.1 | 46.7 | 52 | 51 | |
| C | 0.40 | 0.26 | −0.024 | −0.078 | 46.4 | 27.8 | 14 | 16 | |
| Fe2O3/TiO2 | A | 0.37 | 0.70 | −0.013 | −0.253 | 47.9 | 38.2 | 32 | 17 |
| B | 0.43 | 0.54 | −0.020 | −0.211 | 50.9 | 45.1 | 55 | 36 | |
| C | 0.46 | 0.21 | −0.045 | −0.191 | 42.6 | 24.9 | 13 | 30 | |
| D | − | 0.24 | − | −0.009 | − | 7.4 | − | 17 | |
| ILP (nH m2/kg) | SAR (W/g) | Time Needed to Reach Hyperthermia Temperature 42 °C (min) | Maximum Temperature (°C) | Concentration | Samples |
|---|---|---|---|---|---|
| 1.57 | 92.3 | 4.8 | 62 | 10 mg/mL | γ-Fe2O3 |
| 0.64 | 39 | 10.5 | 46 | γ-Fe2O3-TiO2 | |
| 0.73 | 44.46 | 3 | 73 | 20 mg/mL | γ-Fe2O3 |
| 0.67 | 41.15 | 4.6 | 57.5 | γ-Fe2O3-TiO2 |
| Magnetic Nanoparticles | Synthesis Method | Frequency (kHz) | Field (Oe) | Medium | ILP (nHm2/kg) | Ref |
|---|---|---|---|---|---|---|
| γ-Fe2O3 | Modified Sol-gel | 332.8 | 170 | distilled water | 1.57 | This work |
| γ-Fe2O3 | Modified Sol-gel | 523 | 100 | distilled water | 1.3 | [25] |
| γ-Fe2O3 -TiO2 | Modified Sol-gel | 332.8 | 170 | distilled water | 0.64 | This work |
| γ-Fe2O3@TiO2 | Hydrothermal | 55 | 86.7 | Physiological saline | − | [20] |
| Fe2O3 | Hydrothermal | 200 | 251.3 | distilled water | 1.12 | [40] |
| Zn 0.1Fe0.9Fe2O4 | coprecipitation method | 339 | 92 | distilled water | 5.4 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemine, O.M.; Madkhali, N.; Alshammari, M.; Algessair, S.; Gismelseed, A.; El Mir, L.; Hjiri, M.; Yousif, A.A.; El-Boubbou, K. Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency. Materials 2021, 14, 5691. https://doi.org/10.3390/ma14195691
Lemine OM, Madkhali N, Alshammari M, Algessair S, Gismelseed A, El Mir L, Hjiri M, Yousif AA, El-Boubbou K. Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency. Materials. 2021; 14(19):5691. https://doi.org/10.3390/ma14195691
Chicago/Turabian StyleLemine, O. M., Nawal Madkhali, Marzook Alshammari, Saja Algessair, Abbasher Gismelseed, Lassad El Mir, Moktar Hjiri, Ali A. Yousif, and Kheireddine El-Boubbou. 2021. "Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency" Materials 14, no. 19: 5691. https://doi.org/10.3390/ma14195691
APA StyleLemine, O. M., Madkhali, N., Alshammari, M., Algessair, S., Gismelseed, A., El Mir, L., Hjiri, M., Yousif, A. A., & El-Boubbou, K. (2021). Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency. Materials, 14(19), 5691. https://doi.org/10.3390/ma14195691







