Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Procedures
2.3.1. COSMO-RS Studies
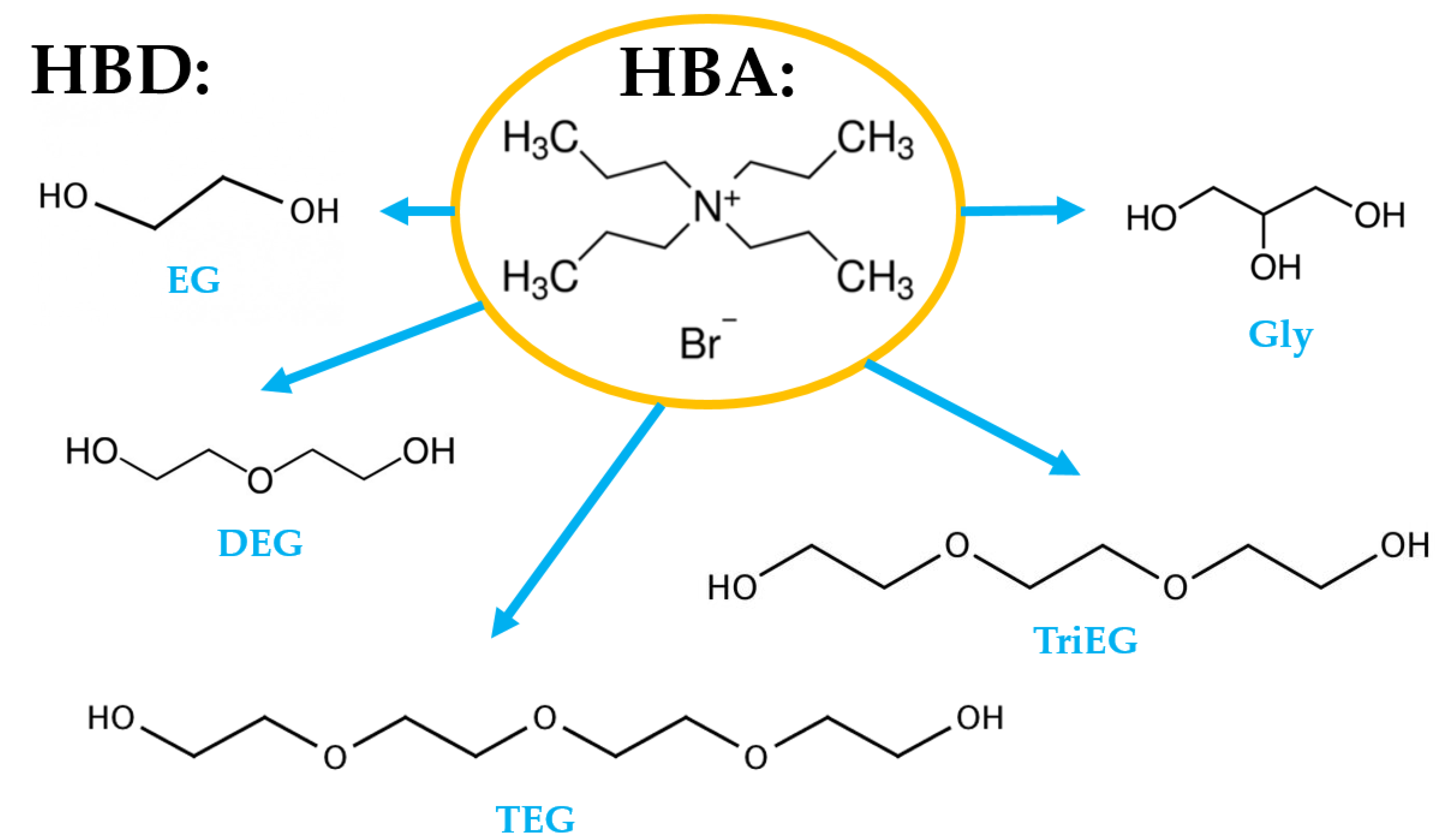
2.3.2. Preparation of DES
2.3.3. Preparation of Model Impurities and Biogas
2.3.4. Absorption Process
2.3.5. Regeneration of DES
2.3.6. Chromatographic Analysis
2.3.7. Physicochemical Properties of DES
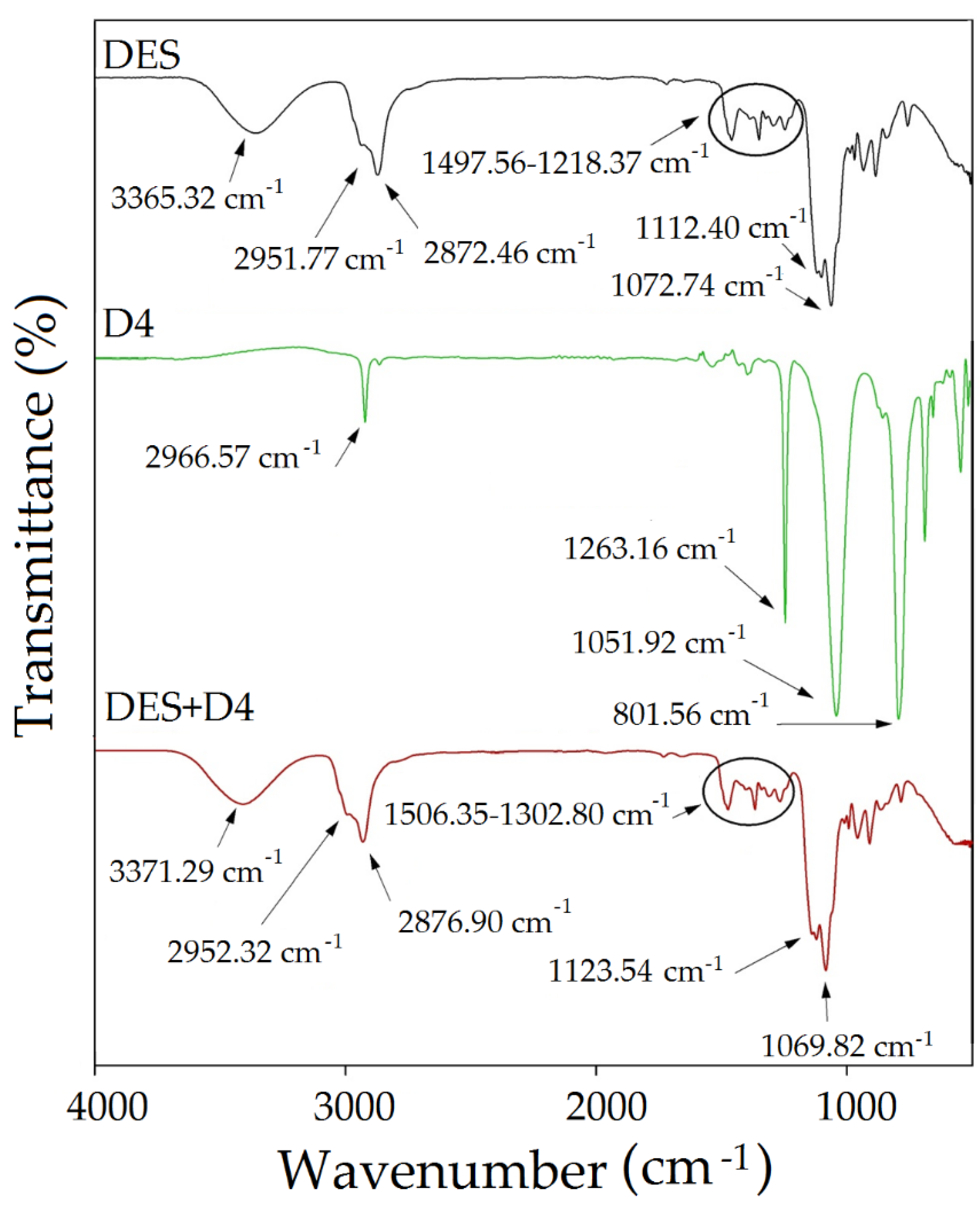
FT-IR Analysis
Viscosity and Density Measurements
Melting Point Measurements
3. Results and Discussion
3.1. COSMO-RS Molecular Simulation
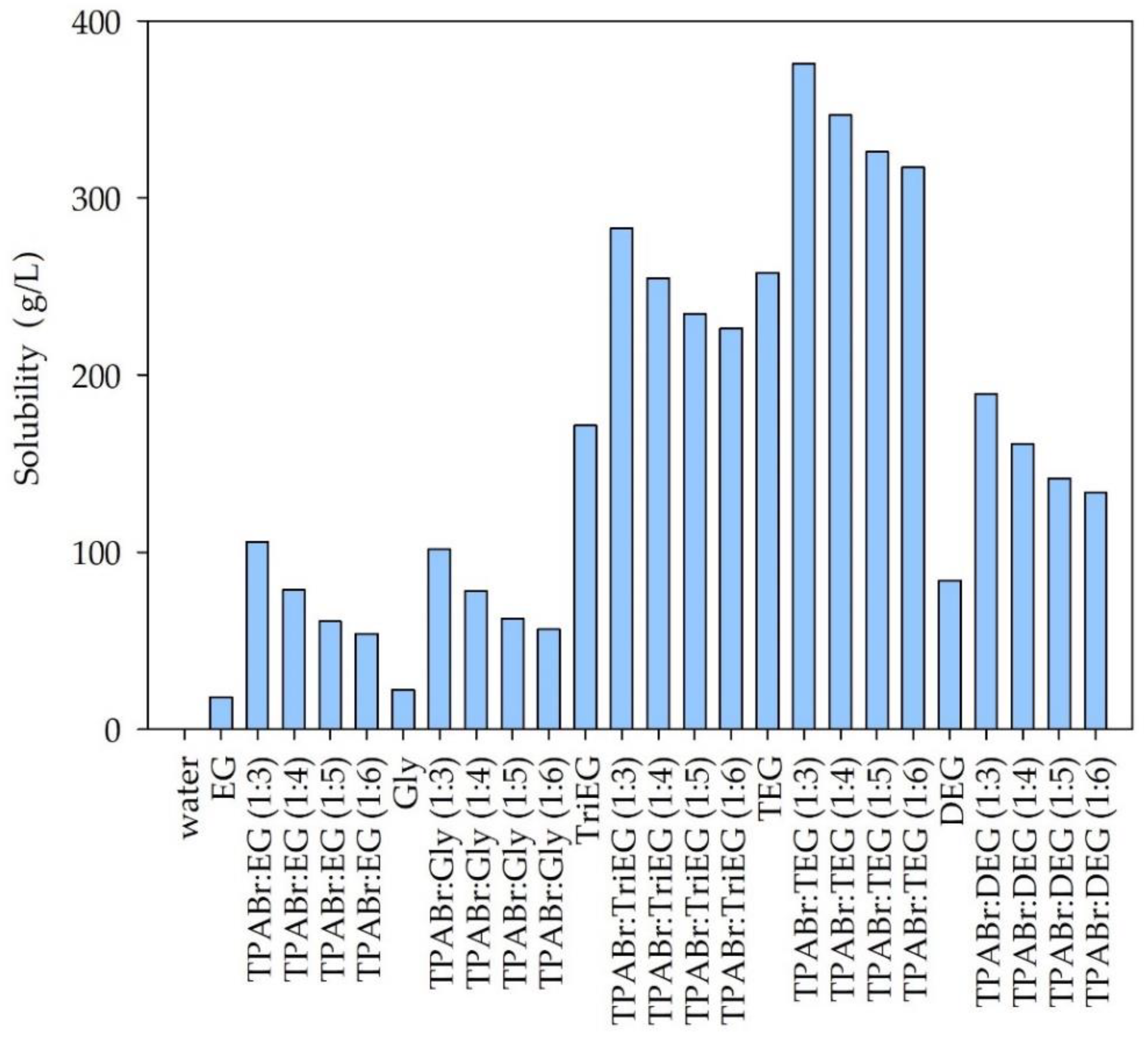
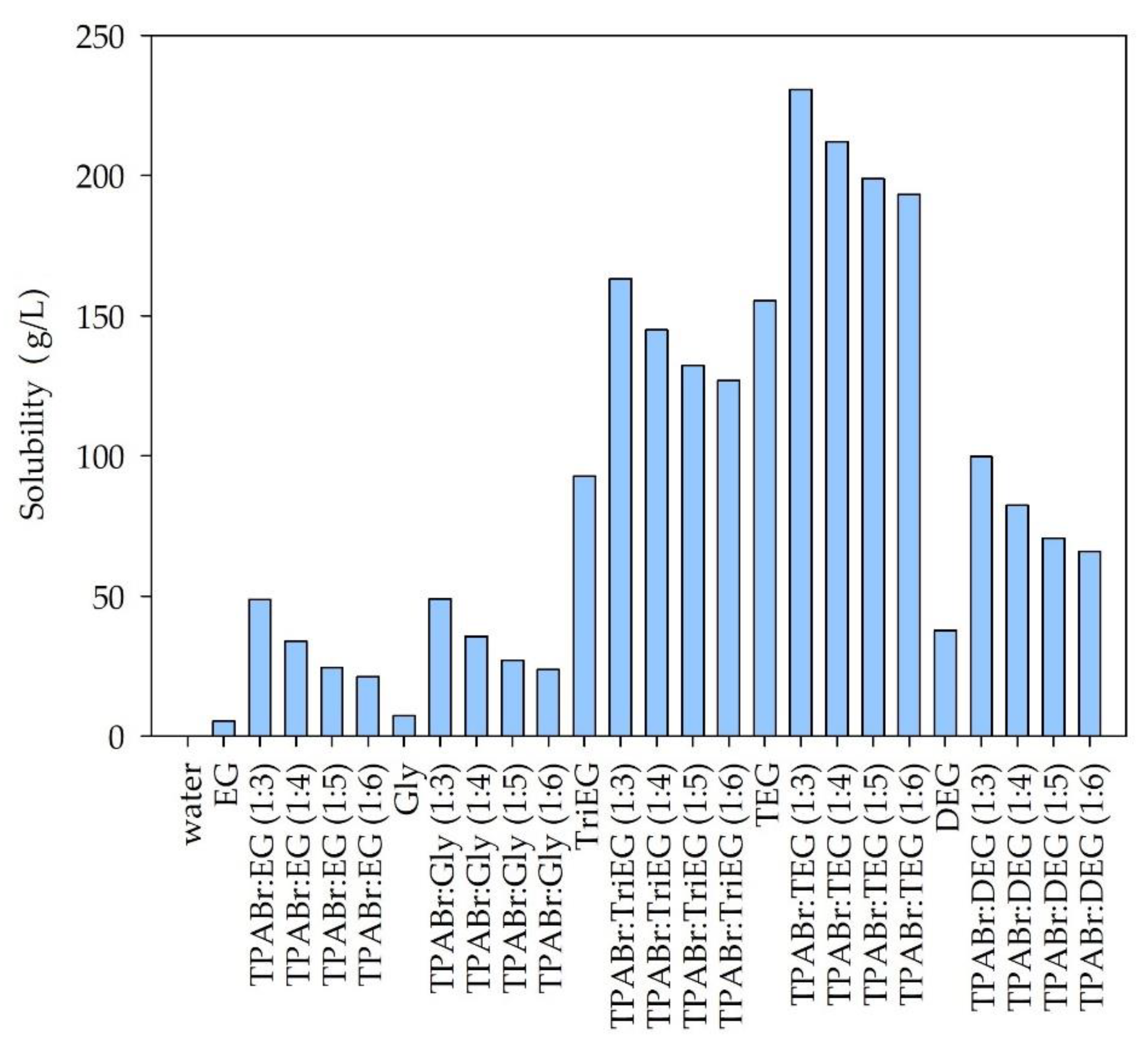
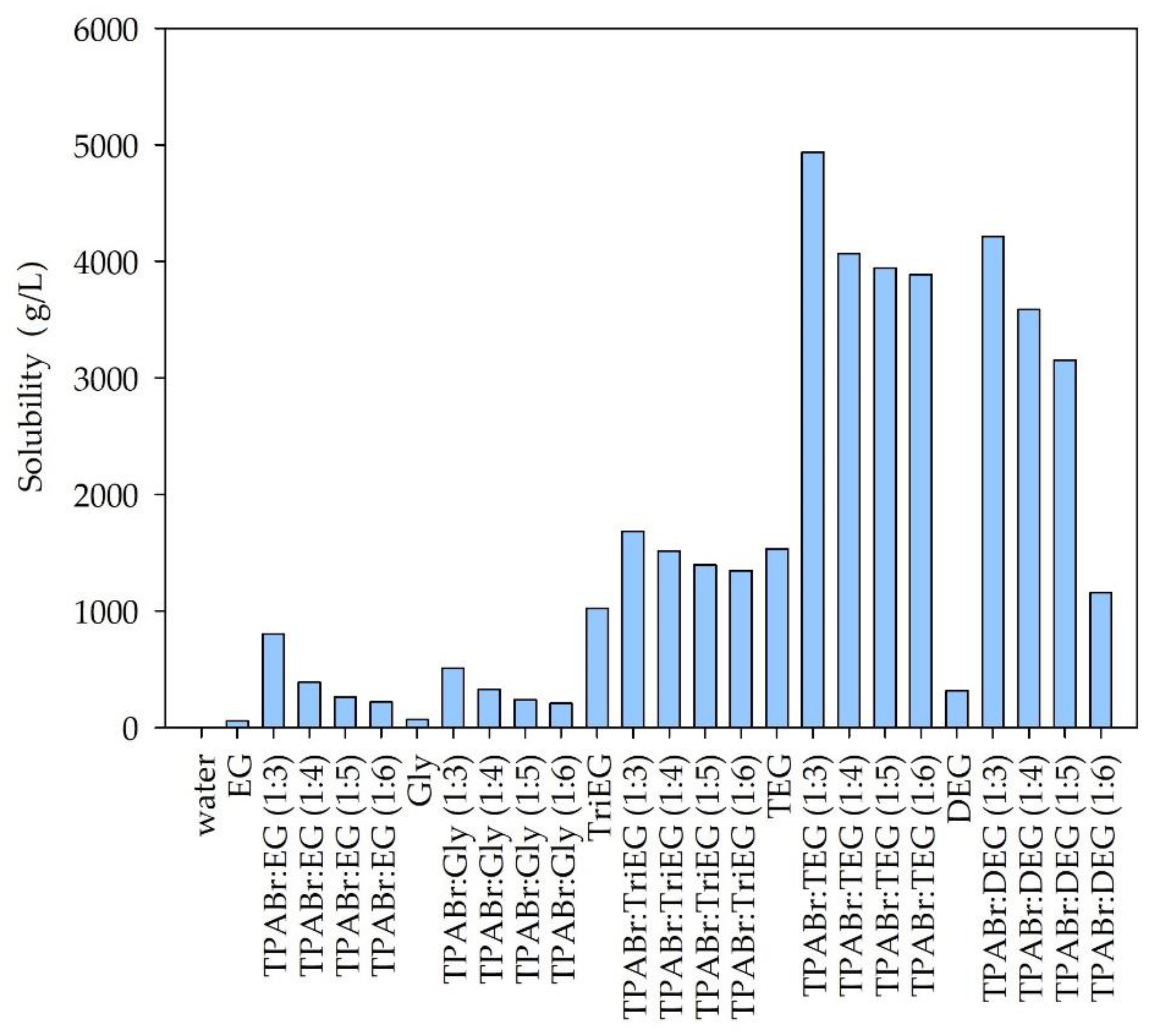
3.1.1. Solubility of Siloxanes in DESs—Preselection of DES
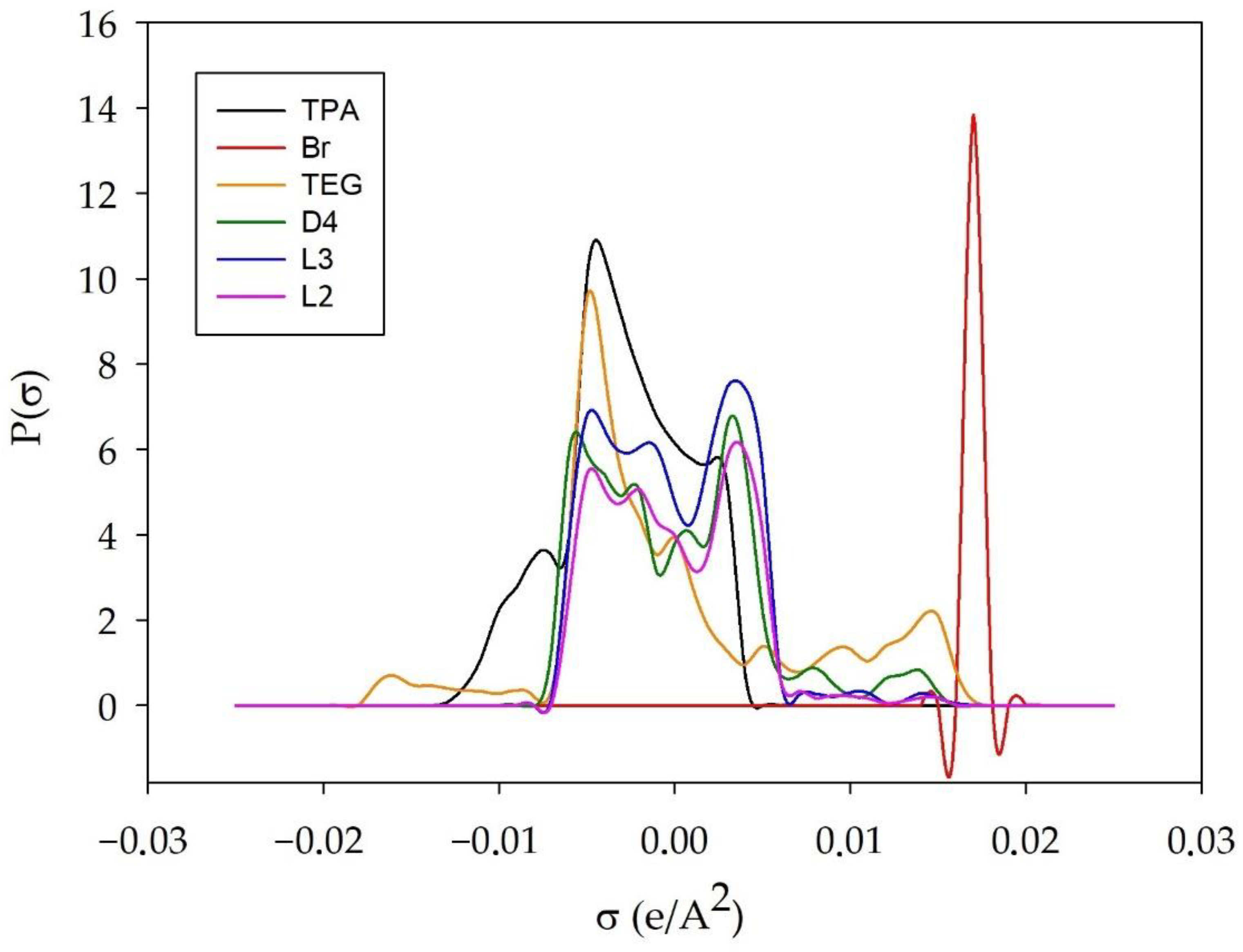
3.1.2. σ-Profile
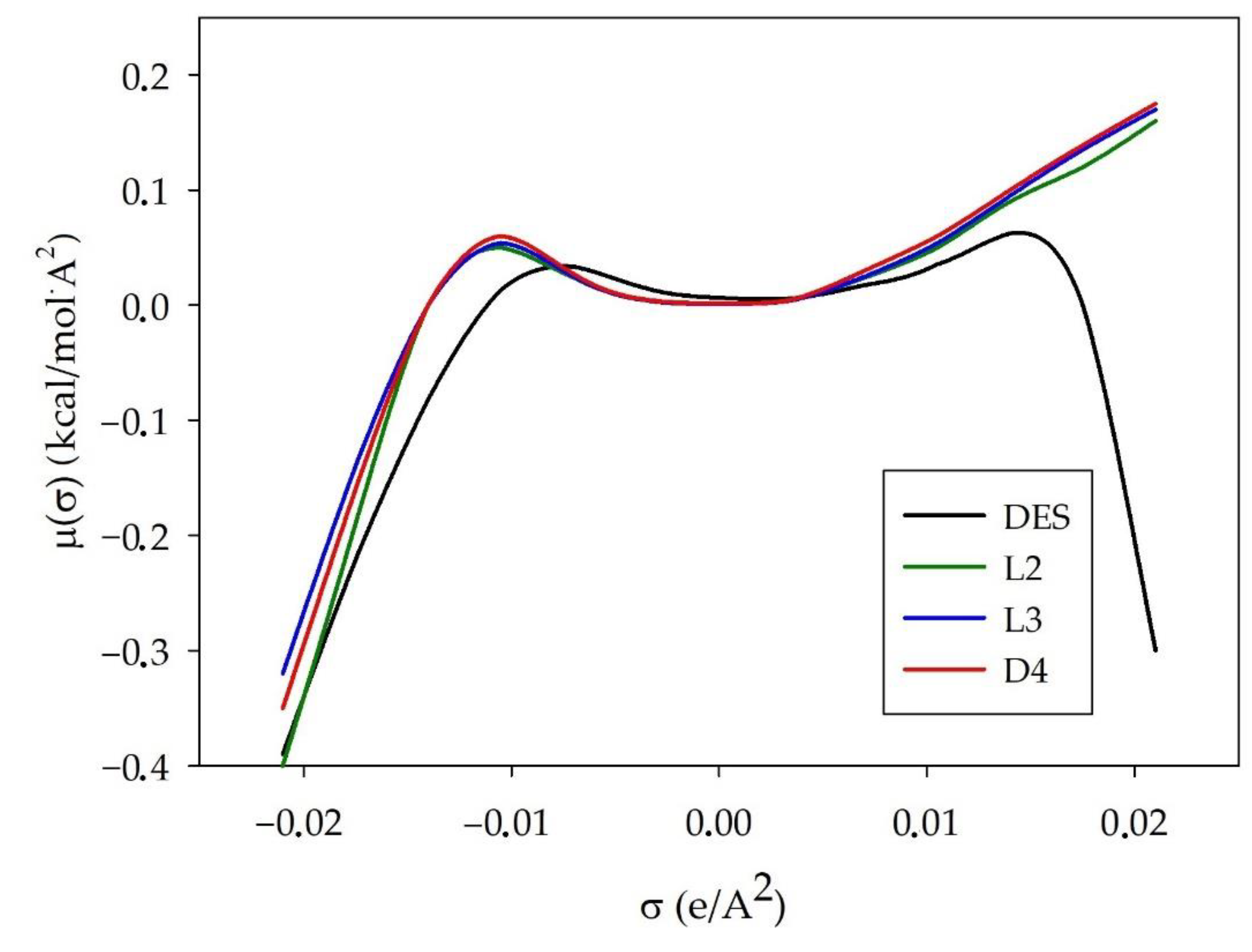
3.1.3. σ-Potential
3.2. Structural and Physicochemical Properties of DES
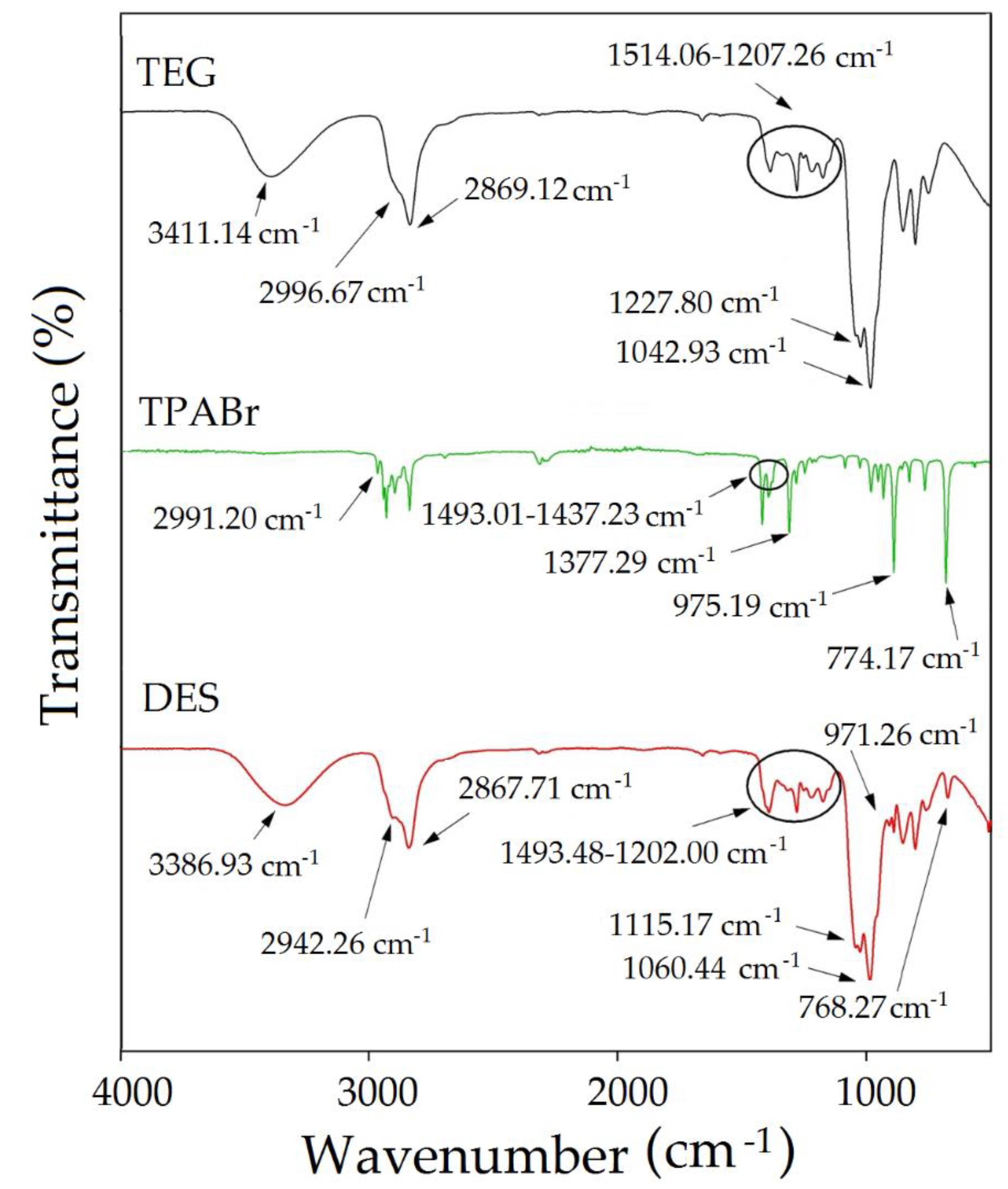
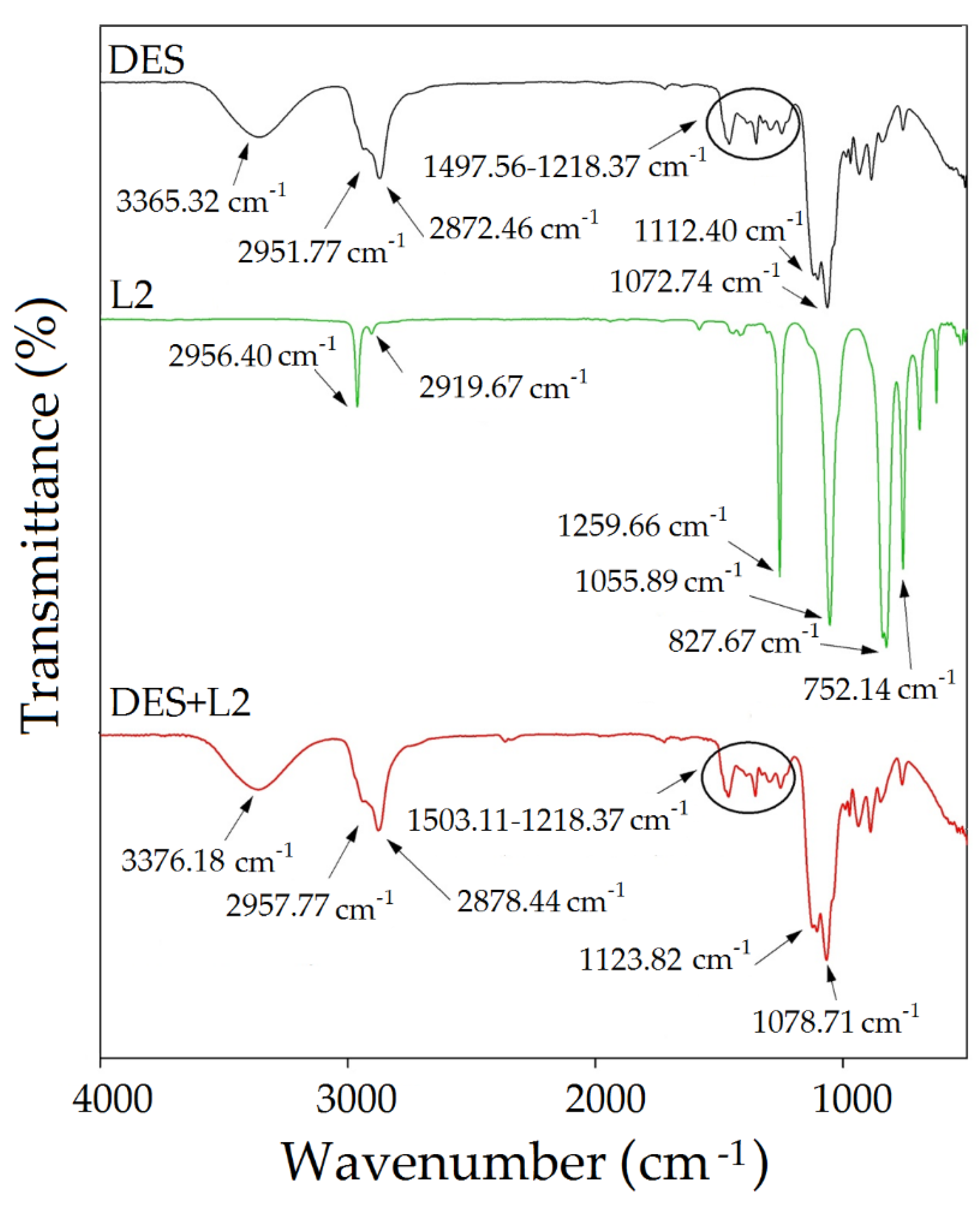
3.2.1. FT-IR Analysis
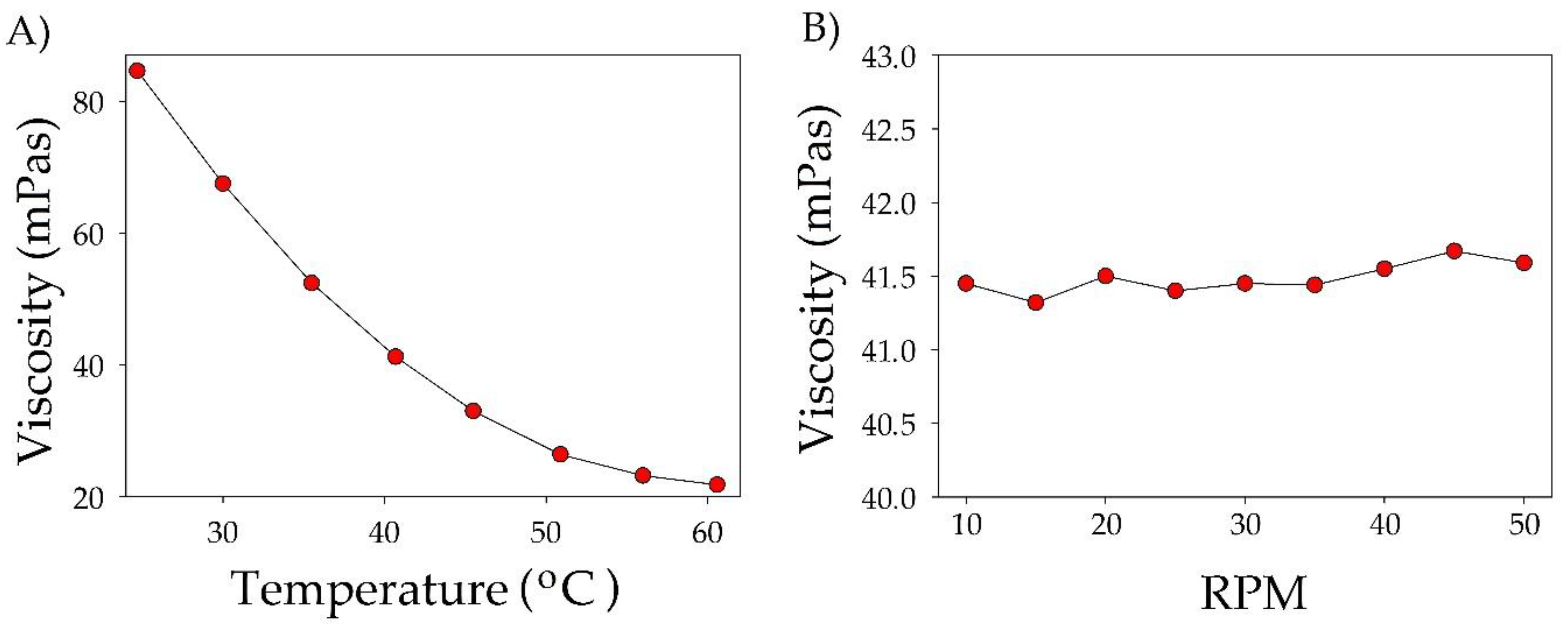
3.2.2. Viscosity and Density Measurements
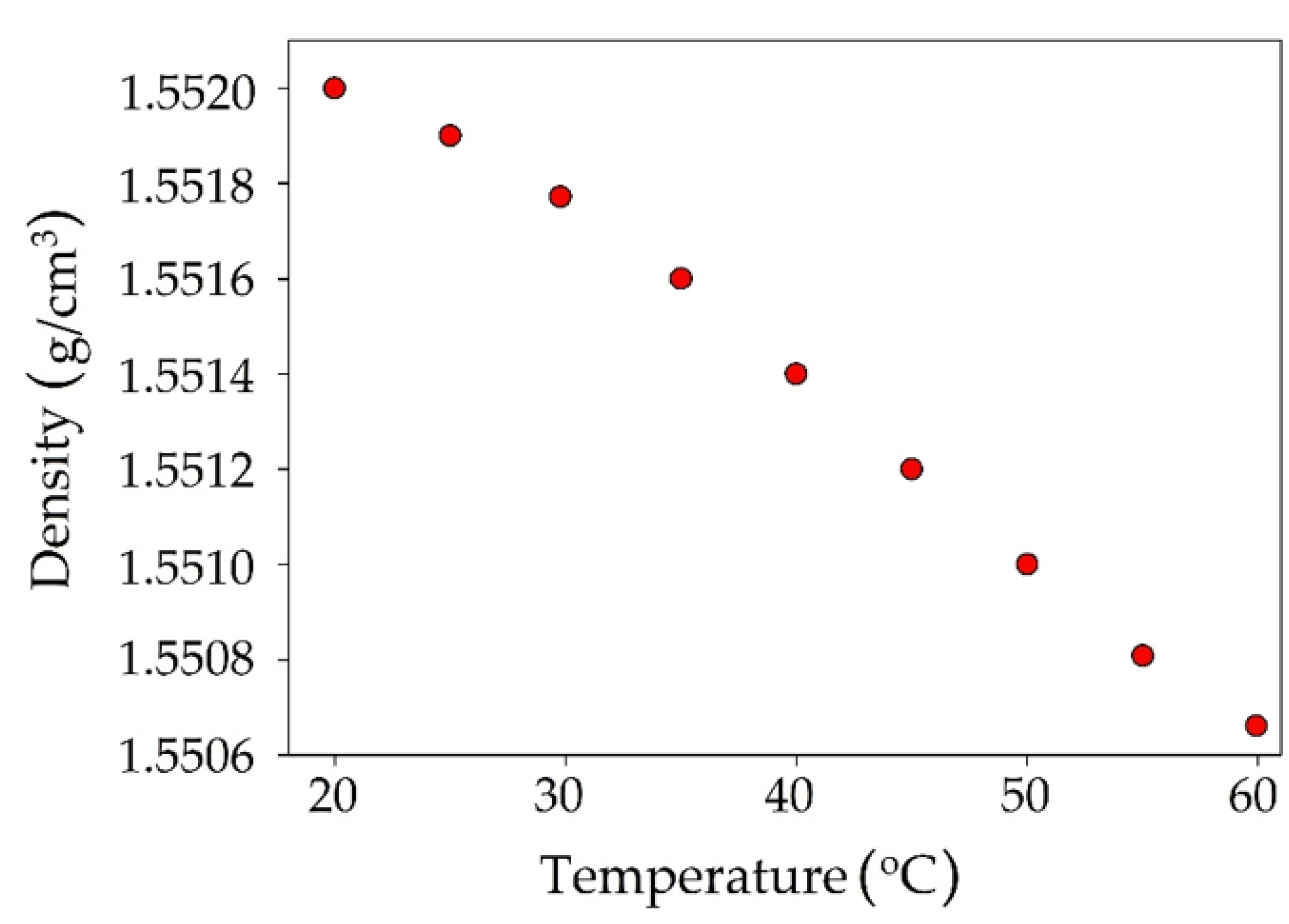
3.2.3. Melting Point Measurements
3.3. An Experimental Studies on Absorption of Siloxane Compounds
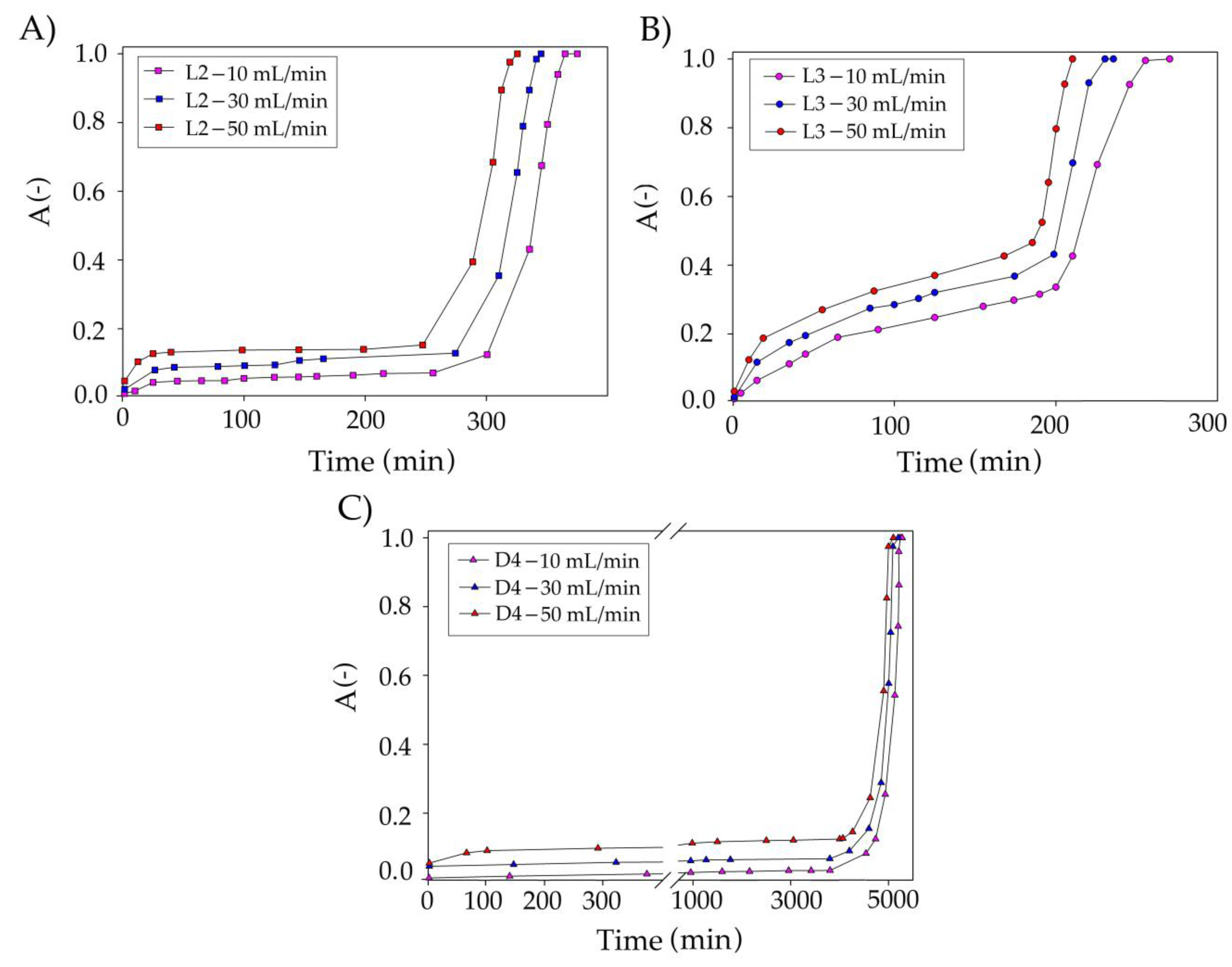
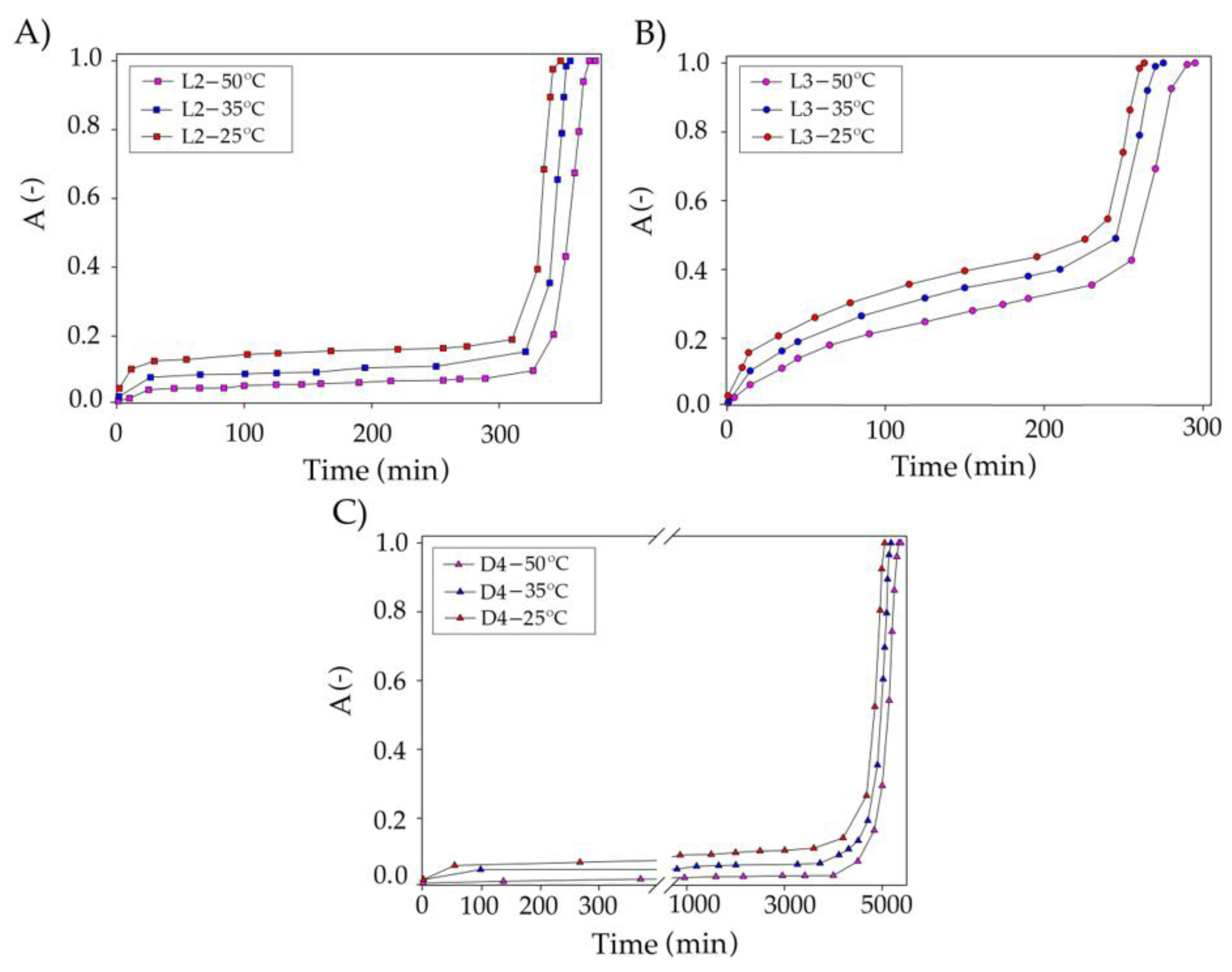
Optimization of the Absorption Process Conditions
3.4. FT-IR Studies on Absorption of Siloxane Compounds
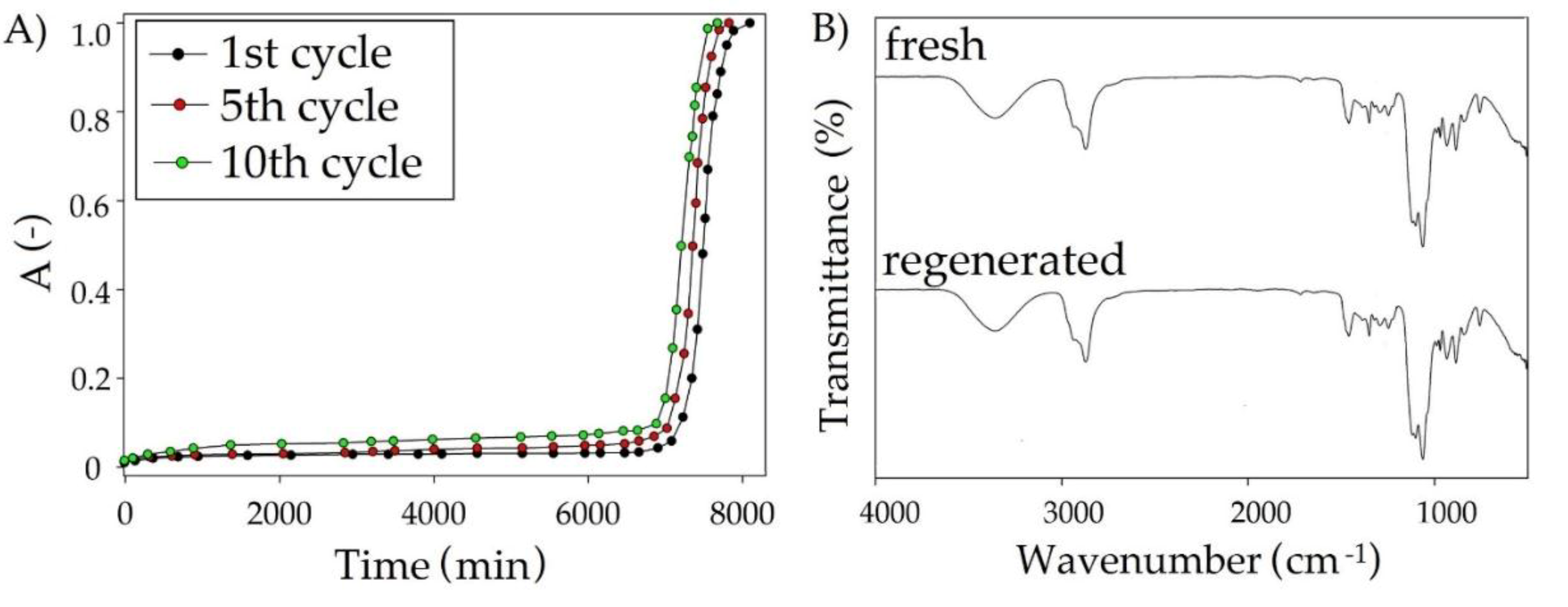
3.5. Regeneration of DES
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perea-Moreno, M.-A.; Salmeron-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Meyer, A.K.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Zhu, T.; Curtis, J.; Clancy, M. Promoting agricultural biogas and biomethane production: Lessons from cross-country studies. Renew. Sustain. Energy Rev. 2019, 114, 109332. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Barragán, E.A.; Ruiz, J.M.O.; Tigre, J.D.C.; Zalamea-León, E. Assessment of Power Generation Using Biogas from Landfills in an Equatorial Tropical Context. Sustainability 2020, 12, 2669. [Google Scholar] [CrossRef]
- Persson, M.; Jonsson, O.; Wellinger, A. Biogas Upgrading to Vehicle Fuel Standards and Grid; IEA Bioenergy Task 37; Susanne AUER: Vienna, Austria, 2007. [Google Scholar]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Wolbert, D.; Le Roux, S.; Soutrel, I.; Nunes, G. Odor concentration (OC) prediction based on odor activity values (OAVs) during composting of solid wastes and digestates. Atmos. Environ. 2019, 201, 1–12. [Google Scholar] [CrossRef]
- Rasi, S. Biogas Composition and Upgrading to Biomethane; University of Jyväskylä: Jyväskylä, Finland, 2009; ISBN 978-951-39-3618-1. [Google Scholar]
- Carrera-Chapela, F.; Donoso-Bravo, A.; Souto, J.A.; Ruiz-Filippi, G. Modeling the Odor Generation in WWTP: An Integrated Approach Review. Water Air Soil Pollut. 2014, 225, 1–15. [Google Scholar] [CrossRef]
- Sevimoğlu, O.; Tansel, B. Effect of persistent trace compounds in landfill gas on engine performance during energy recovery: A case study. Waste Manag. 2013, 33, 74–80. [Google Scholar] [CrossRef]
- Noorain, R.; Kindaichi, T.; Ozaki, N.; Aoi, Y.; Ohashi, A. Biogas purification performance of new water scrubber packed with sponge carriers. J. Clean. Prod. 2019, 214, 103–111. [Google Scholar] [CrossRef]
- Vo, T.T.; Wall, D.M.; Ring, D.; Rajendran, K.; Murphy, J.D. Techno-economic analysis of biogas upgrading via amine scrubber, carbon capture and ex-situ methanation. Appl. Energy 2018, 212, 1191–1202. [Google Scholar] [CrossRef]
- Rasi, S.; Läntelä, J.; Veijanen, A.; Rintala, J. Landfill gas upgrading with countercurrent water wash. Waste Manag. 2008, 28, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Rasi, S.; Läntelä, J.; Rintala, J. Upgrading landfill gas using a high pressure water absorption process. Fuel 2014, 115, 539–543. [Google Scholar] [CrossRef]
- Devia, C.R.; Subrenat, A. Absorption of a linear (L2) and a cyclic (D4) siloxane using different oils: Application to biogas treatment. Environ. Technol. 2013, 34, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, L.; Tatin, R.; Couvert, A. Relevance of an organic solvent for absorption of siloxanes. Environ. Technol. 2013, 35, 372–382. [Google Scholar] [CrossRef]
- Ajhar, M.; Travesset, M.; Yüce, S.; Melin, T. Siloxane removal from landfill and digester gas—A technology overview. Bioresour. Technol. 2010, 101, 2913–2923. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Hu, D.; Fan, J.; Zeng, G. A review on removal of siloxanes from biogas: With a special focus on volatile methylsiloxanes. Environ. Sci. Pollut. Res. 2018, 25, 30847–30862. [Google Scholar] [CrossRef]
- Schuur, B.; Brouwer, T.; Smink, D.; Sprakel, L.M. Green solvents for sustainable separation processes. Curr. Opin. Green Sustain. Chem. 2019, 18, 57–65. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nat. Cell Biol. 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Reddy, R.G. Novel applications of ionic liquids in materials processing. J. Phys. Conf. Ser. 2009, 165, 1–6. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Fernandes, A.; Przyjazny, A.; Boczkaj, G. Sample preparation procedure using extraction and derivatization of carboxylic acids from aqueous samples by means of deep eutectic solvents for gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2018, 1555, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Przyjazny, A.; Boczkaj, G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Słupek, E.; Gębicki, J. Extractive detoxification of feedstocks for the production of biofuels using new hydrophobic deep eutectic solvents—Experimental and theoretical studies. J. Mol. Liq. 2020, 308, 113101. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques—A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Gębicki, J. Deodorization of model biogas by means of novel non- ionic deep eutectic solvent. Arch. Environ. Prot. 2020, 46, 41–46. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P. Absorptive Desulfurization of Model Biogas Stream Using Choline Chloride-Based Deep Eutectic Solvents. Sustainability 2020, 12, 1619. [Google Scholar] [CrossRef]
- Słupek, E.; Makos, P.; Dobrzyniewski, D.; Szulczynski, B.; Gebicki, J. Process control of biogas purification using electronic nose. Chem. Eng. Trans. 2020, 82. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Unusual temperature-promoted carbon dioxide capture in deep-eutectic solvents: The synergistic interactions. Chem. Commun. 2019, 55, 3939–3942. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Intermolecular interactions upon carbon dioxide capture in deep-eutectic solvents. Phys. Chem. Chem. Phys. 2018, 20, 24591–24601. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Słupek, E.; Małachowska, A. Silica Gel Impregnated by Deep Eutectic Solvents for Adsorptive Removal of BTEX from Gas Streams. Materials 2020, 13, 1894. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Gębicki, J. Theoretical and Economic Evaluation of Low-Cost Deep Eutectic Solvents for Effective Biogas Upgrading to Bio-Methane. Energies 2020, 13, 3379. [Google Scholar] [CrossRef]
- Mu, T.; Rarey, J.; Gmehling, J. Performance of COSMO-RS with Sigma Profiles from Different Model Chemistries. Ind. Eng. Chem. Res. 2007, 46, 6612–6629. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A. Prediction of the mutual solubilities of hydrocarbons and water with COSMO-RS. Fluid Phase Equilib. 2003, 206, 223–235. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Tansel, B.; Surita, S.C. Managing siloxanes in biogas-to-energy facilities: Economic comparison of pre- vs post-combustion practices. Waste Manag. 2019, 96, 121–127. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Chu, Y.; He, X. MoDoop: An Automated Computational Approach for COSMO-RS Prediction of Biopolymer Solubilities in Ionic Liquids. ACS Omega 2019, 4, 2337–2343. [Google Scholar] [CrossRef]
- Mullins, E.; Oldland, R.; Liu, Y.A.; Wang, S.; Sandler, S.I.; Chen, C.-C.; Zwolak, A.M.; Seavey, K.C. Sigma-Profile Database for Using COSMO-Based Thermodynamic Methods. Ind. Eng. Chem. Res. 2006, 45, 4389–4415. [Google Scholar] [CrossRef]
- Jibril, B.E.-Y.; Mjalli, F.S.; Naser, J.; Gano, Z.S. New tetrapropylammonium bromide-based deep eutectic solvents: Synthesis and characterizations. J. Mol. Liq. 2014, 199, 462–469. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Santiago, R.; Moya, C.; Palomar, J. Siloxanes capture by ionic liquids: Solvent selection and process evaluation. Chem. Eng. J. 2020, 401, 126078. [Google Scholar] [CrossRef]
- Han, J.; Dai, C.; Yu, G.; Lei, Z. Parameterization of COSMO-RS model for ionic liquids. Green Energy Environ. 2018, 3, 247–265. [Google Scholar] [CrossRef]
- Aissaoui, T. Neoteric FT-IR Investigation on the Functional Groups of Phosphonium- Based Deep Eutectic Solvents. Pharm. Anal. Acta 2015, 6, 10–12. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Zhu, W.; Jiang, W.; Wang, C.; Wu, P.; Zhang, Q.; Li, H. Vibrational analysis and formation mechanism of typical deep eutectic solvents: An experimental and theoretical study. J. Mol. Graph. Model. 2016, 68, 158–175. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Lal, B.; Uemura, Y. Thermal stability and FT-IR analysis of Phosphonium-based deep eutectic solvents with different hydrogen bond donors. J. Mol. Liq. 2017, 242, 395–403. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Shahri, M.M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Satnami, M.L.; Pandey, S.; Ghosh, K.K. Self-assembly of a short-chain ionic liquid within deep eutectic solvents. RSC Adv. 2018, 8, 7969–7979. [Google Scholar] [CrossRef]
- Maheswari, A.U.; Palanivelu, K. Carbon Dioxide Capture and Utilization by Alkanolamines in Deep Eutectic Solvent Medium. Ind. Eng. Chem. Res. 2015, 54, 11383–11392. [Google Scholar] [CrossRef]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar] [CrossRef]
- Xydis, G.; Nanaki, E.A.; Koroneos, C.J. Exergy analysis of biogas production from a municipal solid waste landfill. Sustain. Energy Technol. Assess. 2013, 4, 20–28. [Google Scholar] [CrossRef]
- Yusof, R.; Abdulmalek, E.; Sirat, K.; Rahman, M.B.A. Tetrabutylammonium Bromide (TBABr)-Based Deep Eutectic Solvents (DESs) and Their Physical Properties. Molecules 2014, 19, 8011–8026. [Google Scholar] [CrossRef]
- Burrell, G.L.; Dunlop, N.F.; Separovic, F. Non-Newtonian viscous shear thinning in ionic liquids. Soft Matter 2010, 6, 2080–2086. [Google Scholar] [CrossRef]
- Basaiahgari, A.; Panda, S.; Gardas, R.L. Acoustic, volumetric, transport, optical and rheological properties of Benzyltripropylammonium based Deep Eutectic Solvents. Fluid Phase Equilib. 2017, 448, 41–49. [Google Scholar] [CrossRef]
- Verduzco, L.F.R. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sustain. Energy Rev. 2013, 19, 652–665. [Google Scholar] [CrossRef]
- Altamash, T.; Atilhan, M.; Aliyan, A.; Ullah, R.; Nasser, M.S.; Aparicio, S. Rheological, Thermodynamic, and Gas Solubility Properties of Phenylacetic Acid-Based Deep Eutectic Solvents. Chem. Eng. Technol. 2017, 40, 778–790. [Google Scholar] [CrossRef]
- Sigma Aldrich. Safety Data Sheet Tetraethylene Glycol. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=PL&language=pl&productNumber=110175&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F110175%3Flang%3Dpl (accessed on 18 November 2020).
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chu, H.; Cho, C.M. Absorption and reaction kinetics of amines and ammonia solutions with carbon dioxide in flue gas. J. Air Waste Manag. Assoc. 2003, 53, 246–252. [Google Scholar] [CrossRef]
- Yincheng, G.; Zhenqi, N.; Wenyi, L. Comparison of removal efficiencies of carbon dioxide between aqueous ammonia and Na– solution in a fine spray column. Energy Procedia 2011, 4, 512–518. [Google Scholar] [CrossRef]
- Horikawa, M.; Rossi, F.; Gimenes, M.; Costa, C.M.; Da Silva, M. Chemical absorption of H2S for biogas purification. Braz. J. Chem. Eng. 2004, 21, 415–422. [Google Scholar] [CrossRef]
- Ma, C.; Liu, C.; Lu, X.; Ji, X. Techno-economic analysis and performance comparison of aqueous deep eutectic solvent and other physical absorbents for biogas upgrading. Appl. Energy 2018, 225, 437–447. [Google Scholar] [CrossRef]
- Lemus, J.; Bedia, J.; Moya, C.; Alonso-Morales, N.; Gilarranz, M.A.; Palomar, J.; Rodriguez, J.J. Ammonia capture from the gas phase by encapsulated ionic liquids (ENILs). RSC Adv. 2016, 6, 61650–61660. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Urasaki, N.; Wong, C. Separation of low molecular siloxanes for electronic application by liquid-liquid extraction. IEEE Trans. Electron. Packag. Manuf. 1999, 22, 295–298. [Google Scholar] [CrossRef][Green Version]
- Sun, S.; Niu, Y.; Sun, Z.; Xu, Q.; Wei, X. Solubility properties and spectral characterization of sulfur dioxide in ethylene glycol derivatives. RSC Adv. 2014, 5, 8706–8712. [Google Scholar] [CrossRef]
- Sigma Aldrich. Tetraethylene Glycol. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/110175?lang=pl®ion=PL (accessed on 11 December 2020).
- Sigma Aldrich. Tetrapropylammonium Bromide. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/225568?lang=pl®ion=PL (accessed on 11 December 2020).
- Kolegaberlin Motor Oil. Available online: https://www.kolegaberlin.pl/product-pol-4300-Elf-Evolution-900-NF-5W40-5L.html (accessed on 30 November 2020).
- Hoffmann Group. Cutting Oil. Available online: https://www.hoffmann-group.com/GB/en/houk/Power-tools-and-workshop-supplies/Cooling-lubricants/High-performance-cutting-oil-chlorine-free-Alpha-93/p/084210 (accessed on 30 November 2020).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słupek, E.; Makoś-Chełstowska, P.; Gębicki, J. Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process. Materials 2021, 14, 241. https://doi.org/10.3390/ma14020241
Słupek E, Makoś-Chełstowska P, Gębicki J. Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process. Materials. 2021; 14(2):241. https://doi.org/10.3390/ma14020241
Chicago/Turabian StyleSłupek, Edyta, Patrycja Makoś-Chełstowska, and Jacek Gębicki. 2021. "Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process" Materials 14, no. 2: 241. https://doi.org/10.3390/ma14020241
APA StyleSłupek, E., Makoś-Chełstowska, P., & Gębicki, J. (2021). Removal of Siloxanes from Model Biogas by Means of Deep Eutectic Solvents in Absorption Process. Materials, 14(2), 241. https://doi.org/10.3390/ma14020241





