Electrochemically Driven Phase Transition in LiCoO2 Cathode
Abstract
:1. Introduction
2. Experimental Procedure and Computational Method
2.1. Samples Preparation
2.2. Characterization
2.3. Density Functional Theory Calculations
3. Results and Discussion
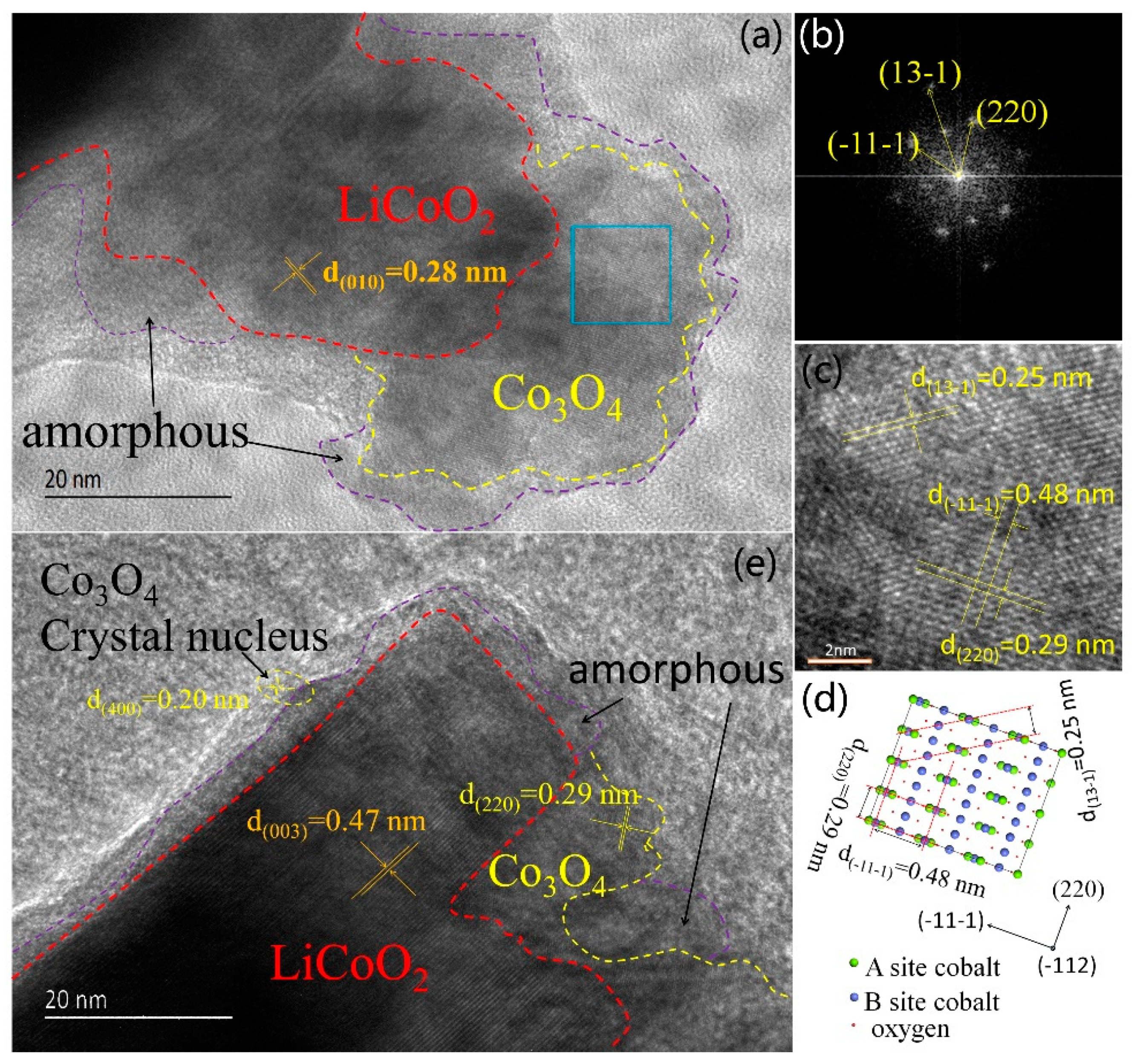
3.1. Microstructure Characterization
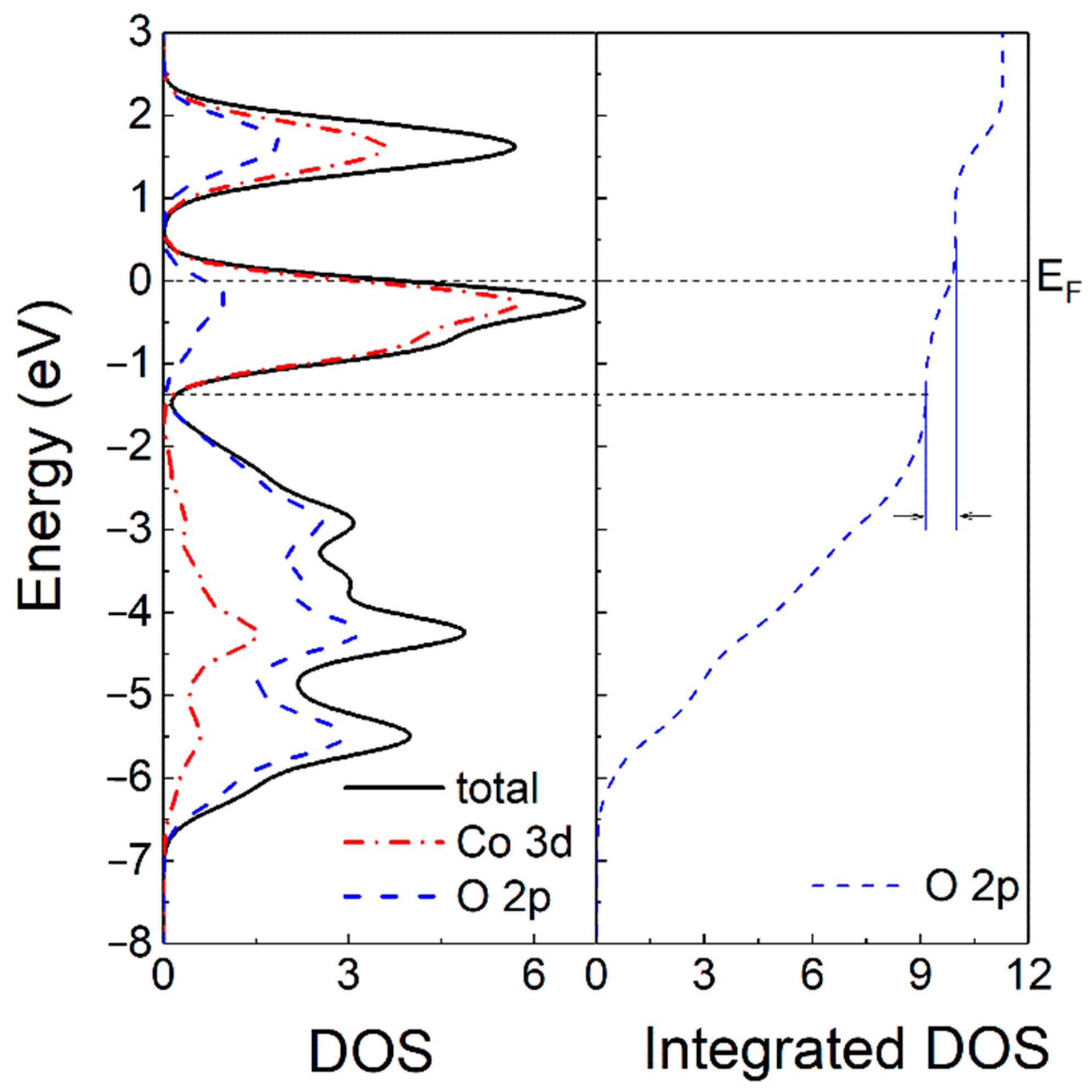
3.2. Modeling and Density Functional Theory Calculations:
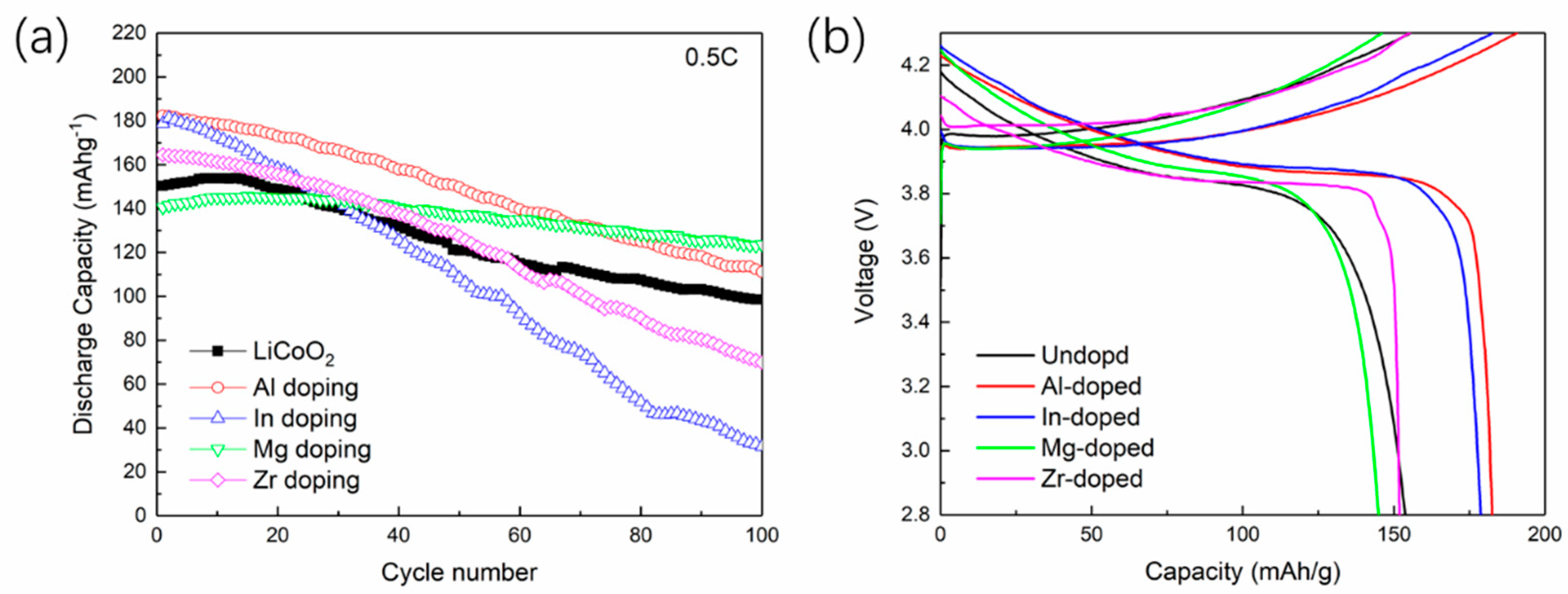
3.3. Experimental Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Neale, Z.G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L. A comprehensive review of lithium-ion batteries used in hybrid andelectric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Horiba, T.; Maeshima, T. Applications of high power density lithium ion batteries. J. Power Sour. 2005, 146, 107–110. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sour. 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Kale, B. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J. Tuning charge–discharge induced unit cell breathing in layer-structured cath-ode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 5381. [Google Scholar] [CrossRef]
- Konishi, H.; Hirano, T. Evaluation of Stability of Charged Lithium-rich Layer-structured Cath-ode Material at Elevated Temperature. Electrochim. Acta 2015, 169, 310–316. [Google Scholar] [CrossRef]
- Konishi, H.; Hirano, T. Origin of hysteresis between charge and discharge processes in lithiu-m-rich layer-structured cathode material for lithium-ion battery. J. Power Sour. 2015, 298, 144–149. [Google Scholar] [CrossRef]
- Goodenough, J.; Park, K. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, A.; Klerk, N. Toward Optimal Performance and In-Depth Understanding of Spine-l Li4Ti5O12 Electrodes through Phase Field Modeling. Adv. Funct. Mater. 2018, 28, 1705992. [Google Scholar] [CrossRef]
- Sharma, N.; Peterson, V. Structural changes in a commercial lithium-ion battery during electr-ochemical cycling: An in situ neutron diffraction study. J. Power Sour. 2010, 195, 8258–8266. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.Q. Phase Transformation of Doped LiCoPO4 during Galvanostatic Cycling. Materials 2020, 13, 3810. [Google Scholar] [CrossRef]
- Wang, A.; Hou, C. Exploration of the evolution of nanotechnology from a patent co-classification perspective. Nanotechnol. Rev. 2018, 7, 233–245. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K. Recent development of Supercapacitor Electrode Based on Carbon Materials. Nanotechnol. Rev. 2019, 8, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Li, X. Synthesis and Characterization of Mg Substituted LiCoO2. J. Electrochem. Soc. 2010, 157, A782–A790. [Google Scholar] [CrossRef]
- Xu, H.J.; Deng, S.N. Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material. J. Mater. Chem. A 2014, 2, 15015–15021. [Google Scholar] [CrossRef]
- An, J.; Shi, L.Y. Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 19738–19744. [Google Scholar] [CrossRef]
- Antonio, E.G.; Ulissi, U. A Long-Life Lithium Ion Battery with Enhanced Electrode/Electrolyt-e Interface by Using an Ionic Liquid Solution. Chem. A Eur. J. 2016, 22, 6808–6814. [Google Scholar] [CrossRef]
- Van der Ven, A.; Aydinol, K. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B. 1998, 58, 2975–2987. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lu, Z.H. Staging Phase Transitions in LixCoO2. J. Electrochem. Soc. 2002, 149, A1604–A1609. [Google Scholar] [CrossRef]
- Dahéron, L.; Dedryvère, R. Electron Transfer Mechanisms upon Lithium Deintercalation from LiCoO2 to CoO2 Investigated by XPS. Chem. Mater. 2008, 20, 583–590. [Google Scholar] [CrossRef]
- Ohnishi, M.; Matsuoka, O. Investigation of the surface degradation of LiCoO2 particles in the cathode materials of Li-ion batteries using FIB-TOF-SIMS. J. Surf. Anal. 2013, 20, 99–110. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nityananda, R.; Hohenberg, P. Inhomogeneous electron gas. Resonance 2017, 22, 809–811. [Google Scholar] [CrossRef]
- Eschrig, H.; Seifert, G. Current density functional theory of quantum electrodynamics. Solid State Commun. 1985, 56, 777–780. [Google Scholar] [CrossRef]
- Melot, B.C.; Tarascon, J.M. Design and preparation of materials for advanced electrochemical storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef]
- Xiang, Q.X.; Li, L. Inhibiting degradation of LiCoO2 cathode material by anisotropic strain during delithiation. Mater. Res. Express 2020, 7, 025501. [Google Scholar] [CrossRef]
- Amdouni, N.; Zarrouk, H. Structural and electrochemical properties of LiCoO2 and LiAlyCo1−yO2 (y=0.1 and 0.2) oxides: A comparative study of electrodes prepared by the citrate precursor route. Ionics 2003, 9, 47–55. [Google Scholar] [CrossRef]
- Xie, M.; Hu, T. Synthesis of high-voltage (4.7 V) LiCoO2 cathode materials with Al doping a-nd conformal Al2O3 coating by atomic layer deposition. RSC Adv. 2016, 6, 63250–63255. [Google Scholar] [CrossRef]
- Needham, S.A.; Wang, G.X. Synthesis and electrochemical performance of doped LiCoO2 materials. J. Power Sources 2007, 174, 828–831. [Google Scholar] [CrossRef]




| Doping Ion | Ion Radius (Å) | Effect | Theoretical Prediction | Experimental Results | Literature Results |
|---|---|---|---|---|---|
| Al3+ | 0.50 | Inhibit lattice expansion | Improved | Better | Better [37,38] |
| In3+ | 0.81 | Promote lattice expansion | Deteriorated | Worse | - |
| Mg2+ | 0.72 | Inhibit oxidation | Improved | Better | Better [20] |
| Zr4+ | 0.72 | Promote oxidation | Deteriorated | Worse | Worse [39] |
| Co3+ (Reference) | 0.745 | Reference | |||
| Doped Element | Lattice a (Å) | Δa | Lattice c | Δc (Å) |
|---|---|---|---|---|
| Al | 2.8147(4) | −0.051% | 14.0458(5) | −0.042% |
| In | 2.8169(7) | 0.028% | 14.0569(3) | 0.037% |
| Mg | 2.8160(4) | −0.005% | 14.0535(9) | 0.013% |
| Zr | 2.8159(6) | −0.005% | 14.0493(5) | −0.018% |
| undoped (Reference) | 2.8161(8) | - | 14.0517(8) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Wang, Z.; Li, G.; Hu, H.; Li, J.; Han, R.; Zhang, D. Electrochemically Driven Phase Transition in LiCoO2 Cathode. Materials 2021, 14, 242. https://doi.org/10.3390/ma14020242
Tan J, Wang Z, Li G, Hu H, Li J, Han R, Zhang D. Electrochemically Driven Phase Transition in LiCoO2 Cathode. Materials. 2021; 14(2):242. https://doi.org/10.3390/ma14020242
Chicago/Turabian StyleTan, Jinhui, Zhongzui Wang, Guangzhao Li, Huicong Hu, Jie Li, Rui Han, and Dongyan Zhang. 2021. "Electrochemically Driven Phase Transition in LiCoO2 Cathode" Materials 14, no. 2: 242. https://doi.org/10.3390/ma14020242
APA StyleTan, J., Wang, Z., Li, G., Hu, H., Li, J., Han, R., & Zhang, D. (2021). Electrochemically Driven Phase Transition in LiCoO2 Cathode. Materials, 14(2), 242. https://doi.org/10.3390/ma14020242







