Comparative Investigations of AlCrN Coatings Formed by Cathodic Arc Evaporation under Different Nitrogen Pressure or Arc Current
Abstract
1. Introduction
2. Experimental
2.1. Deposition of the Coating
- (a)
- Substrate bias voltage −100 V, arc current 80 A, and nitrogen pressure (pN2) in the range from 0.8 Pa to 5 Pa.
- (b)
- Substrate bias voltage −100 V, nitrogen pressure of 4 Pa, and arc current (Ic) in the range from 50 A to 100 A.
2.2. Coating Investigations
- (a)
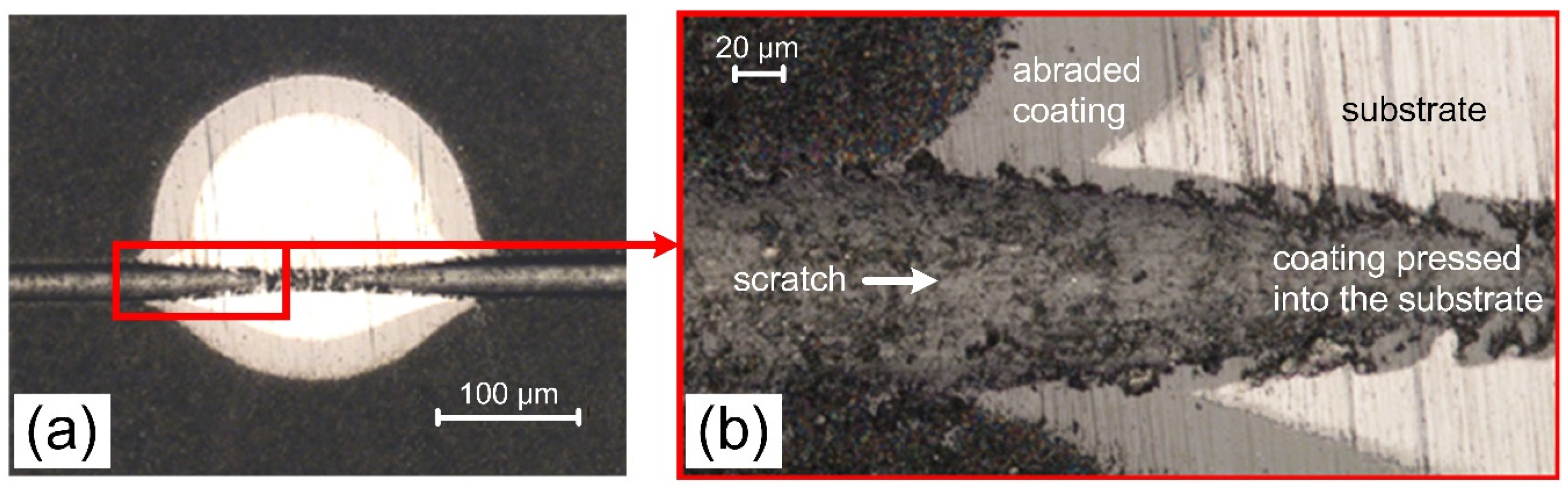
- The scratch method (Revetest® by CSEM Instruments, Peseux, Switzerland) with Rockwell C type diamond indenter with a tip radius of 200 µm. At least three scratches, each of a length of 10 mm and a distance at least 3 mm from each other, were done by moving the indenter at a speed of 10 mm/min, and simultaneously increasing the load linearly from 0 to 100 N. Two characteristic damages were observed and marked: Lc1 for first cracks of the coatings appeared and Lc2 for total delamination of the coating,
- (b)
- Daimler–Benz test. In this method, it was assumed that the assessment of coating adhesion is determined on the basis of the form and intensity of damage resulting from the indentation [34]. In this test the Rockwell indenter is pressed into the sample (coating) with the normal load of 1471 N. It is a comparative method (six-grade scale of adhesion quality) in which it was assumed that damage in the coating corresponding to the HF1-HF4 patterns (cracks and small chips) proves the proper adhesion of the coating. The occurrence of coating defects in the area surrounding the indentation, corresponding to the HF5-HF6 patterns, is evidence of insufficient adhesion of the coating.
3. Results
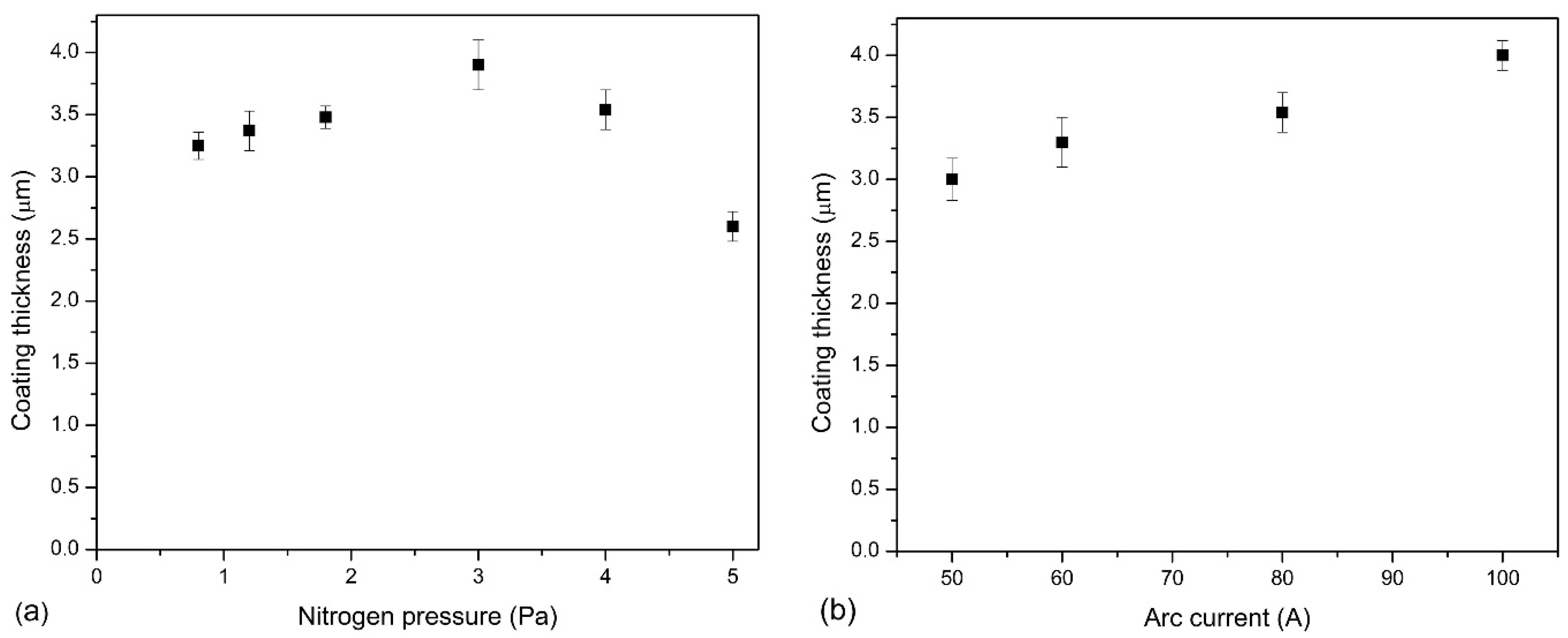
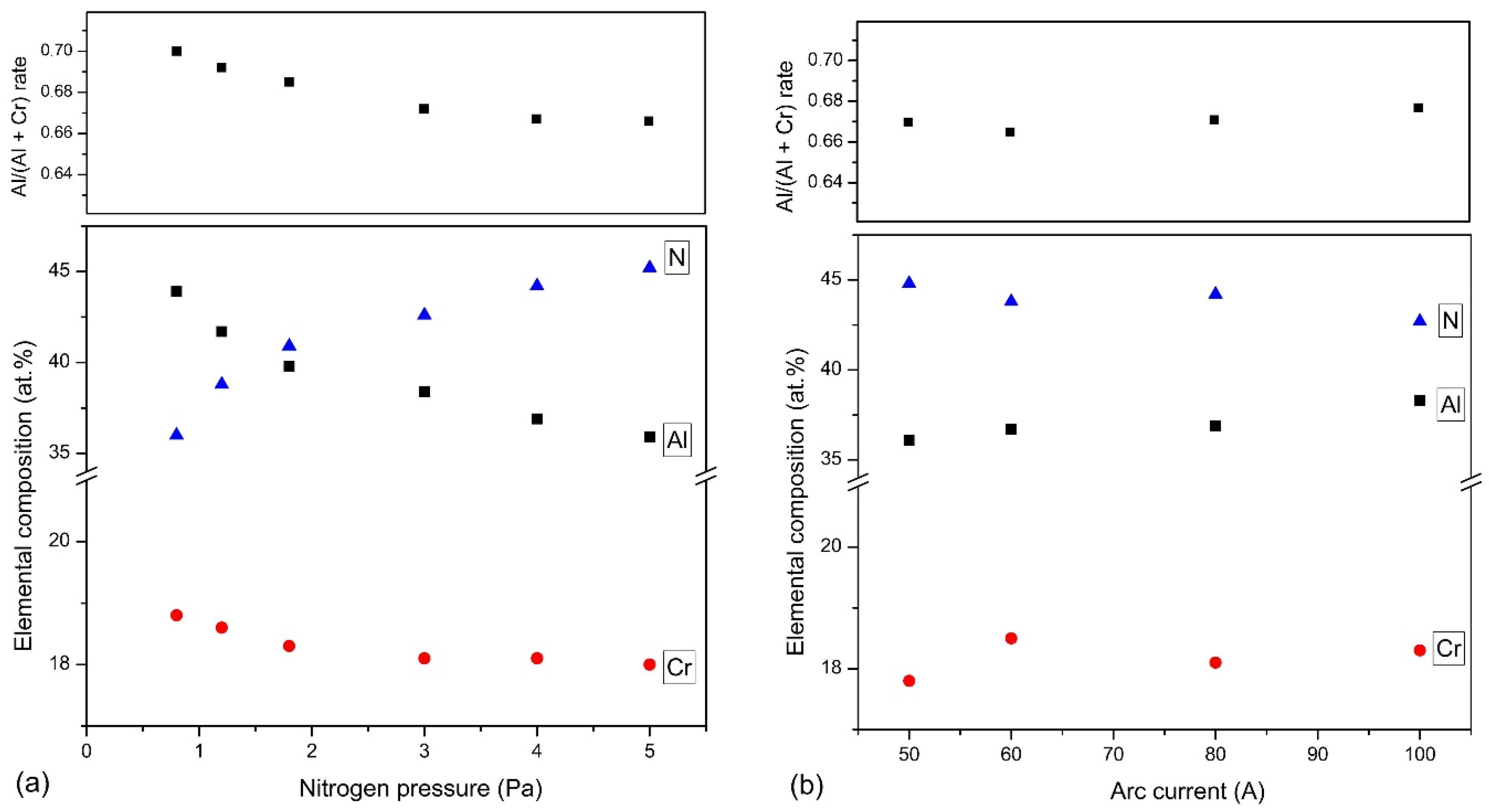
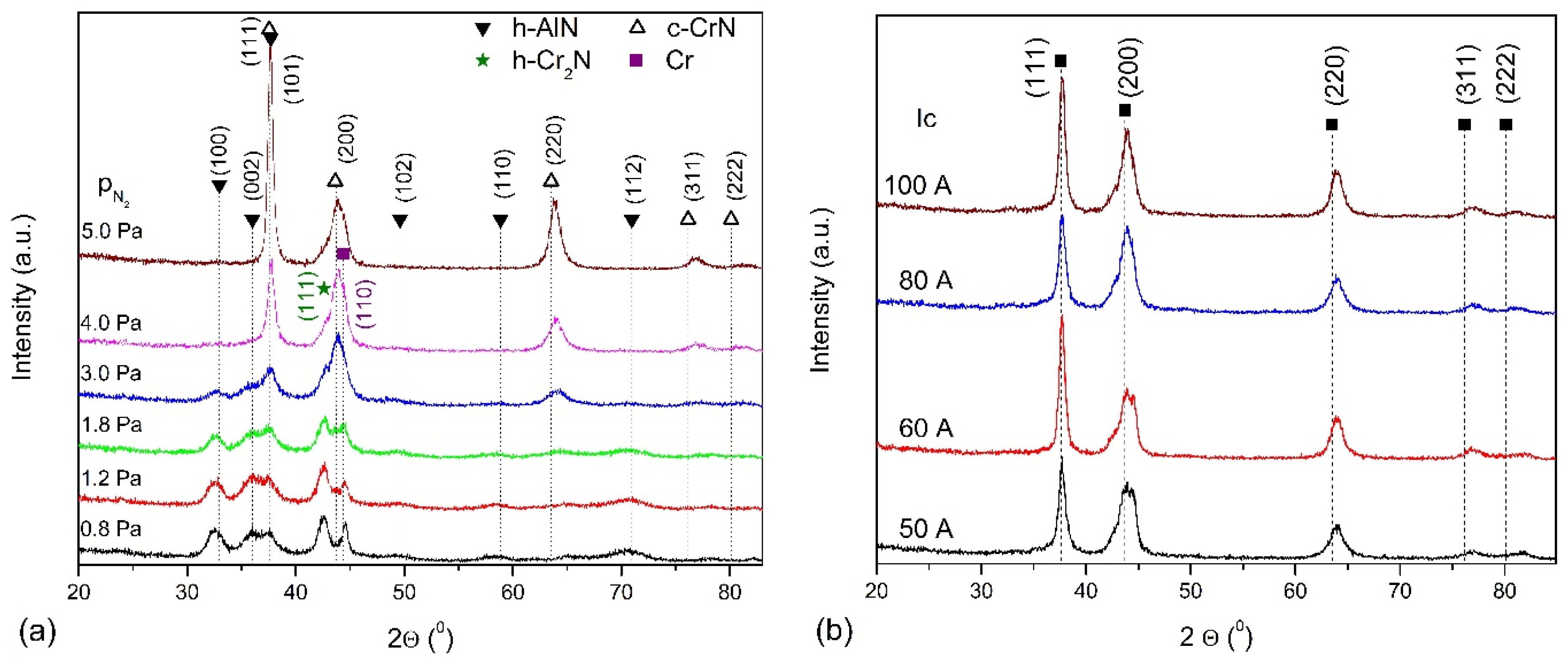
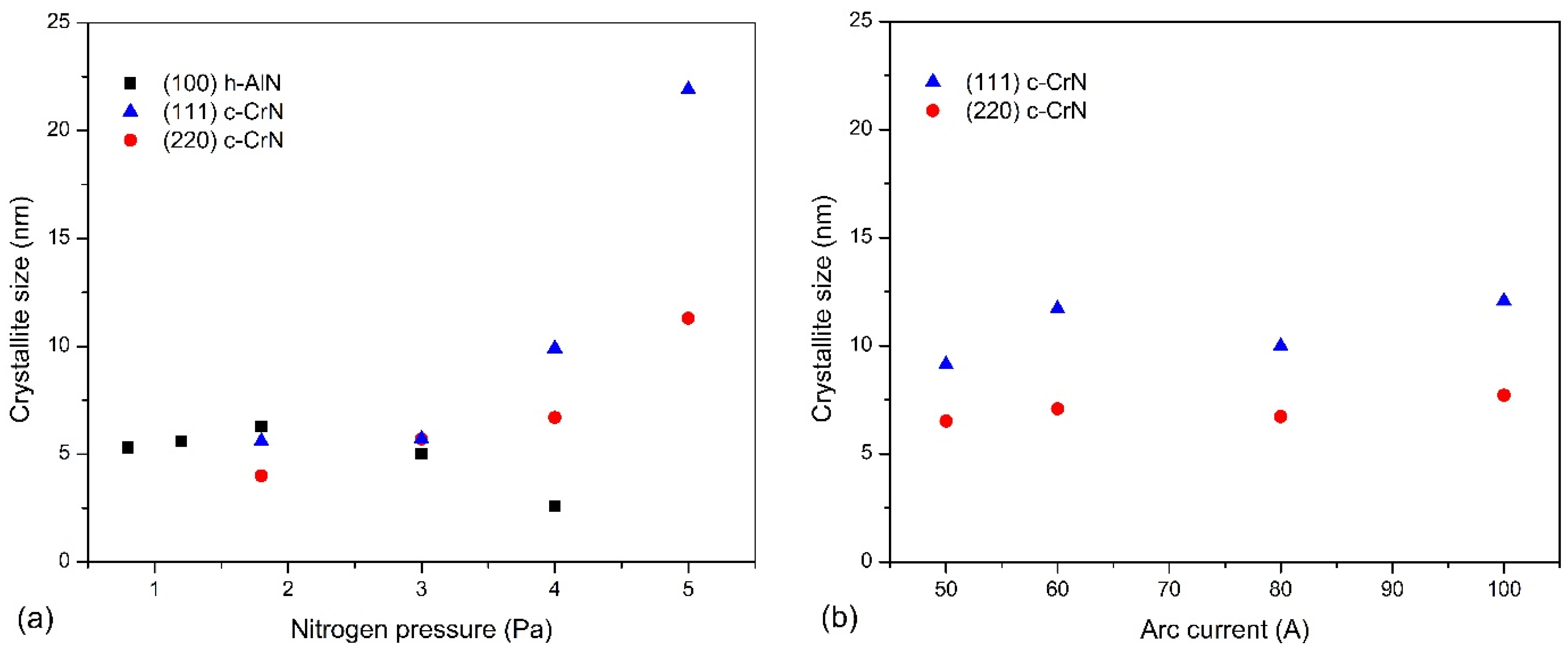
3.1. Chemical and Phase Composition of AlCrN Coatings
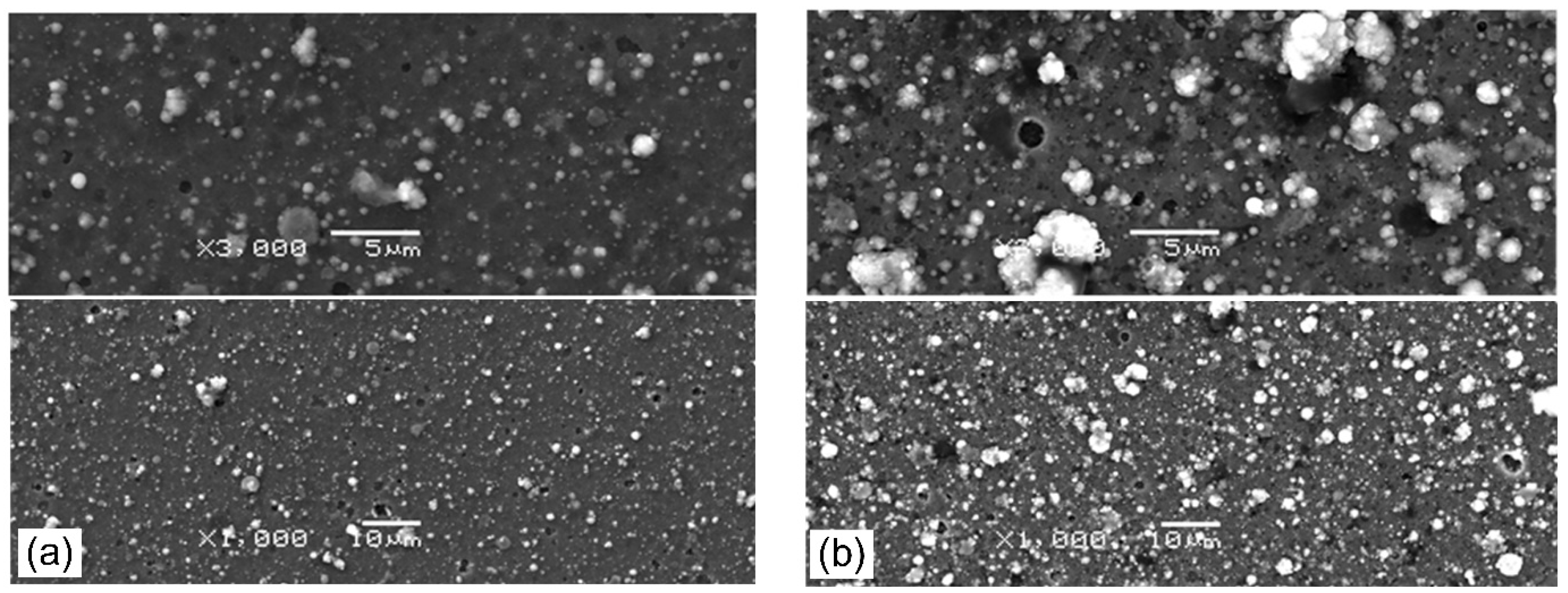
3.2. Surface Morphology
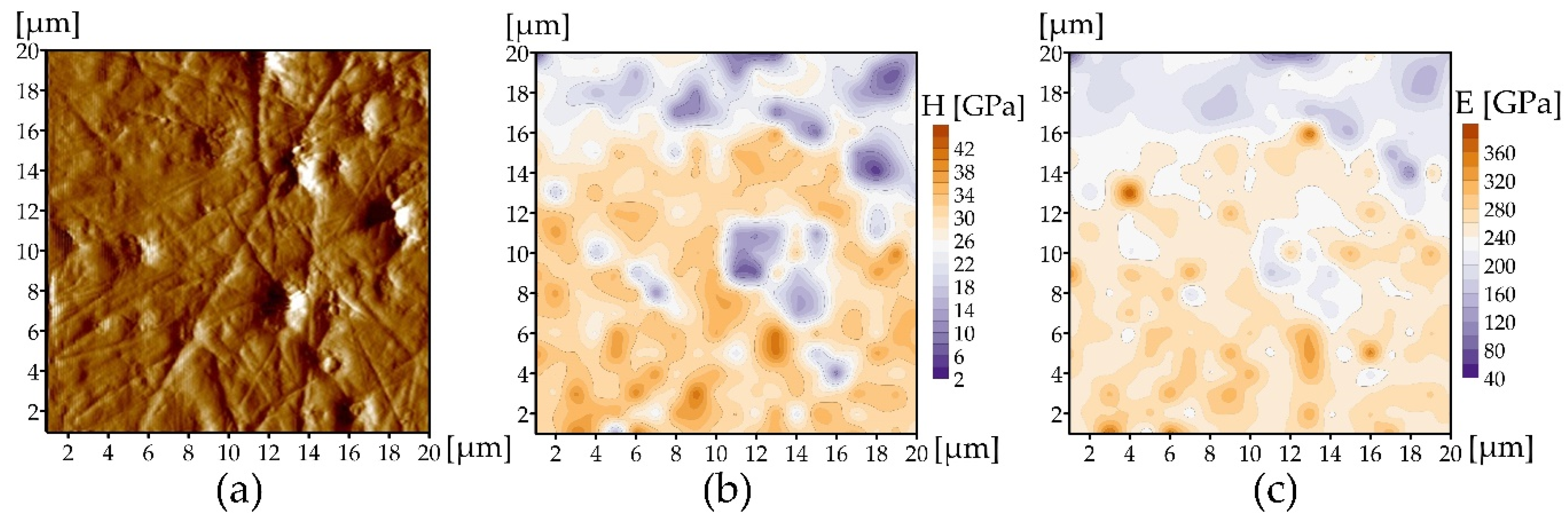
3.3. Mechanical Properties
3.4. Friction and Wear
4. Discussion
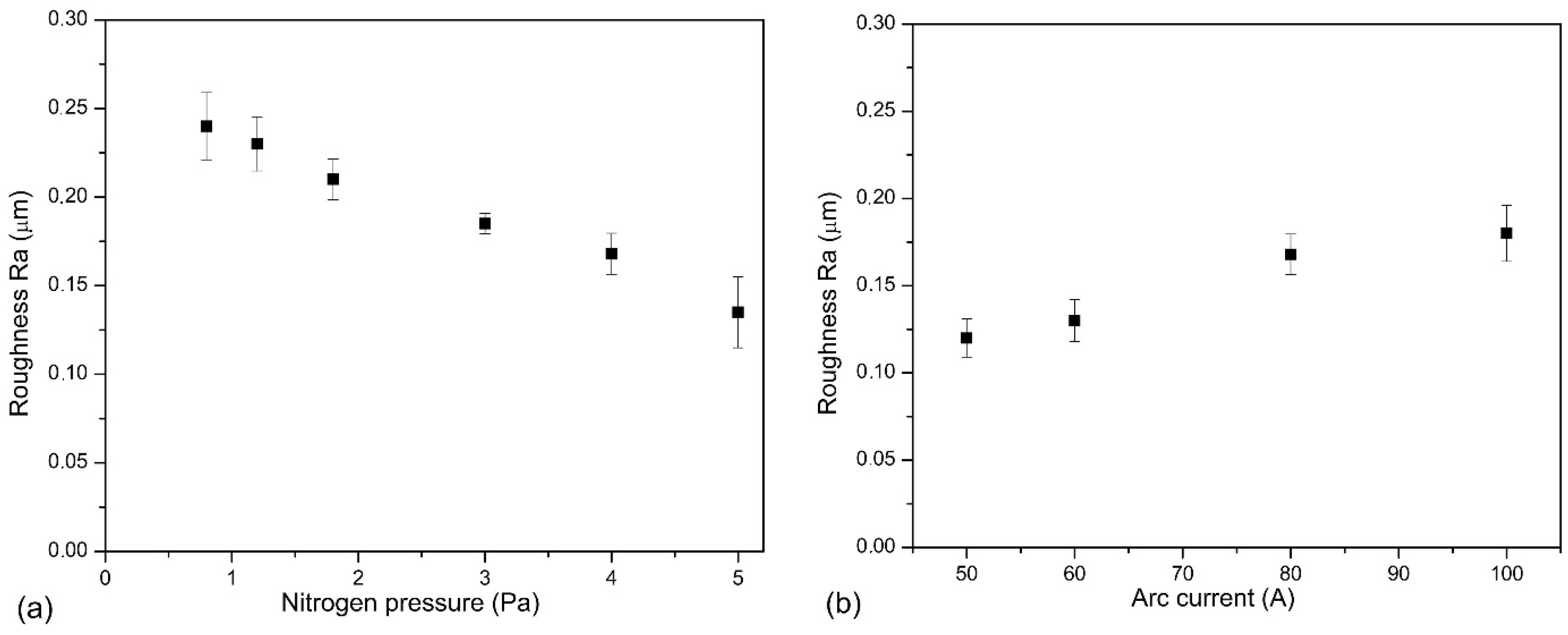
4.1. Effect of Nitrogen Pressure
4.2. Effect of Arc Current
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bobzin, K. High-performance coatings for cutting tools. CIRP J. Manuf. Sci. Technol. 2017, 18, 1–9. [Google Scholar]
- Aizikovich, S.; Krenev, L.; Sevostianov, I.; Trubchik, I.; Evich, L. Evaluation of the elastic properties of a functionally-graded coating from the indentation measurements. ZAMM J. Appl. Math. Mech. Z. Angew. Math. Mech. 2011, 91, 493–515. [Google Scholar] [CrossRef]
- Navinšek, B.; Panjan, P.; Cvelbar, A. Characterization of low temperature CrN and TiN (PVD) hard coatings. Surf. Coat. Technol. 1995, 74–75, 155–161. [Google Scholar] [CrossRef]
- Ürgen, M.; Cakır, A. The effect of heating on corrosion behavior of TiN- and CrN-coated steels. Surf. Coat. Technol. 1997, 96, 236–244. [Google Scholar]
- Fox-Rabinovich, G.S.; Kovalev, A.I.; Afanasyev, S.N. Characteristic features of wear in tools made of high-speed steels with surface engineered coatings I. Wear characteristics of surface engineered high-speed steel cutting tools. Wear 1996, 201, 38–44. [Google Scholar]
- Hurkmans, T.; Lewis, D.B.; Paritong, H.; Brooks, J.S.; Munz, W.D. Influence of ion bombardment on structure and properties ofunbalanced magnetron grown CrNx coatings. Surf. Coat. Technol. 1999, 114, 52–59. [Google Scholar]
- Mo, J.L.; Zhu, M.H. Tribological characterization of chromium nitride coating deposited by filtered cathodic vacuum arc. Appl. Surf. Sci. 2009, 25, 7627–7634. [Google Scholar] [CrossRef]
- Kudish, I.I.; Volkov, S.S.; Vasiliev, A.S.; Aizikovich, S.M. Some Criteria for Coating Effectiveness in Heavily Loaded Line Elastohydrodynamically Lubricated Contacts—Part I: Dry Contacts. J. Tribol. 2016, 138. [Google Scholar] [CrossRef]
- van Essen, P.; Hoy, R.; Kamminga, J.D.; Ehiasarian, A.P.; Janssen, G.C.A.M. Scratch resistance and wear of CrNx coatings. Surf. Coat. Technol. 2006, 200, 3496–3502. [Google Scholar]
- Kudish, I.I.; Volkov, S.S.; Vasiliev, A.S.; Aizikovich, S.M. Effectiveness of coatings with constant, linearly, and exponentially varying elastic parameters in heavily loaded line elastohydrodynamically lubricated contacts. J. Tribol. 2017, 139. [Google Scholar] [CrossRef]
- Navinsek, B.; Panjan, P.; Milosev, I. Industrial applications of CrN (PVD) coatings, deposited at high and low temperatures. Surf. Coat. Technol. 1997, 97, 182–191. [Google Scholar]
- Rech, J.; Kusiak, A.; Battaglia, J.L. Tribological and thermal functions of cutting tool coatings. Surf. Coat. Technol. 2004, 186, 364–371. [Google Scholar] [CrossRef]
- Benlatreche, Y.; Nouveau, C.; Aknouche, H.; Imhoff, L.; Martin, N.; Gavoille, J.; Rousselot, C.; Rauch, J.Y.; Pilloud, D. Physical and Mechanical Properties of CrAlN and CrSiN Ternary Systems for Wood Machining Applications. Plasma Process. Polym. 2009, 6, S113–S119. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhou, S.; Wang, Y.; Wang, C.; Wang, Y.; Sui, Y.; Lan, J.; Xue, Q. Improvement in the tribocorrosion performance of CrCN coating bymultilayered design for marine protective application. Appl. Surf. Sci. 2020, 528, 147061. [Google Scholar]
- Chen, W.; Hu, T.; Hong, Y.; Zhang, D.; Meng, X. Comparison of microstructures, mechanical and tribological properties of arc-deposited AlCrN, AlCrBN and CrBN coatings on Ti-6Al-4V alloy. Surf. Coat. Technol. 2020, 404, 126429. [Google Scholar] [CrossRef]
- Uchida, M.; Nihira, N.; Mitsuo, A.; Toyoda, K.; Kubota, K.; Aizawa, T. Friction and wear properties of CrAlN and CrVN films deposited by cathodic arc ion plating method. Surf. Coat. Technol. 2004, 177–178, 627–630. [Google Scholar] [CrossRef]
- Castaldi, L.; Kurapov, D.; Reiter, A.; Shklover, V.; Schwaller, P.; Patscheider, J. Effect of the oxygen content on the structure, morphology and oxidation resistance of Cr–O–N coatings. Surf. Coat. Technol. 2008, 203, 545–549. [Google Scholar]
- Barshilia, H.C.; Selvakumar, N.; Deepthi, B.; Rajam, K.S. A comparative study of reactive direct current magnetron sputtered CrAlN and CrN coatings. Surf. Coat. Technol. 2006, 201, 2193–2201. [Google Scholar]
- Vityaz’, P.A.; Komarov, A.I.; Komarova, V.I.; Kuznetsova, T.A. Peculiarities of triboformation of wear-resistant layers on the surface of a MAO-coating1 modified by fullerenes. J. Frict. Wear 2011, 32, 231–241. [Google Scholar]
- Reiter, A.E.; Derflinger, V.H.; Hanselmann, B.; Bachmann, T.; Sartory, B. Investigation of the properties of Al1-xCrxN coatings prepared by cathodic arc evaporation. Surf. Coat. Technol. 2005, 200, 2114–2122. [Google Scholar]
- Haršáni, M.; Ghafoor, N.; Calamba, K.; Zacková, P.; Sahul, M.; Vopát, T.; Satrapinskyy, L.; Čaplovičová, M.; Čaplovič, L. Adhesive-deformation relationships and mechanical properties of nc-AlCrN/a-SiNx hard coatings deposited at different bias voltages. Thin Solid Films 2018, 650, 11–19. [Google Scholar]
- Lin, J.; Mishra, B.; Moore, J.J.; Sproul, W.D. Microstructure, mechanical and tribological properties of Cr1−xAlxN films deposited by pulsed-closed field unbalanced magnetron sputtering (P-CFUBMS). Surf. Coat. Technol. 2006, 201, 4329–4334. [Google Scholar] [CrossRef]
- Li, T.; Li, M.; Zhou, Y. Phase segregation and its effect on the adhesion of Cr–Al–N coatings on K38G alloy prepared by magnetron sputtering method. Surf. Coat. Technol. 2007, 201, 7692–7698. [Google Scholar] [CrossRef]
- Romero, J.; Gómez, M.A.; Esteve, J.; Montalà, F.; Carreras, L.; Grifol, M.; Lousa, A. CrAlN coatings deposited by cathodic arc evaporation at different substrate bias. Thin Solid Films 2006, 515, 113–117. [Google Scholar] [CrossRef]
- Tang, J.F.; Lin, C.Y.; Yang, F.C.; Chang, C.L. Influence of Nitrogen Content and Bias Voltage on Residual Stress and the Tribological and Mechanical Properties of CrAlN Films. Coatings 2020, 10, 546. [Google Scholar]
- Antonov, M.; Afshari, H.; Baronins, J.; Adoberg, E.; Raadik, T.; Hussainova, I. The effect of temperature and sliding speed on friction and wear of Si3N4, Al2O3, and ZrO2 balls tested against AlCrN PVD coating. Tribol. Int. 2018, 118, 500–514. [Google Scholar] [CrossRef]
- Mo, J.L.; Zhu, M.H. Sliding tribological behavior of AlCrN coating. Tribol. Int. 2008, 41, 1161–1168. [Google Scholar]
- Sabitzer, C.; Paulitsch, J.; Kolozsvári, S.; Rachbauer, R.; Mayrhofer, P.H. Impact of bias potential and layer arrangement on thermal stability of arc evaporated Al-Cr-N coatings. Thin Solid Films 2016, 610, 26–34. [Google Scholar]
- Tillmann, W.; Kokalj, D.; Stangier, D.; Paulus, M.; Sternemann, C.; Tolan, M. Investigation of the influence of the vanadium content on the high temperature tribo-mechanical properties of DC magnetron sputtered AlCrVN thin films. Surf. Coat. Technol. 2017, 328, 172–181. [Google Scholar]
- Wang, L.; Zhang, S.; Chen, Z.; Li, J.; Li, M. Influence of deposition parameters on hard Cr–Al–N coatings deposited by multi-arc ion plating. Appl. Surf. Sci. 2012, 258, 3629–3636. [Google Scholar]
- Lan, R.; Wang, C.; Ma, Z.; Lu, G.; Wang, P.; Han, J. Effects of arc current and bias voltage on properties of AlCrN coatings by arc ion plating with large target. Mater. Res. Express 2019, 6, 116457. [Google Scholar]
- Gong, Z.; Chen, R.; Li, J.; Cao, P.; Geng, H. Effect of N2 Flow Rate on Structure and Corrosion Resistance of AlCrN Coatings Prepared by Multi-Arc Ion Plating. Int. J. Electrochem. Sci. 2020, 15, 1117–1127. [Google Scholar]
- Cullity, B.D.; Weymouth, J.W. Elements of X-Ray Diffraction. Am. J. Phys. 1957, 25, 394–395. [Google Scholar] [CrossRef]
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 indentation test evaluation of a reliable qualitative control for layered compounds. J. Mater. Process. Technol. 2003, 143, 481–485. [Google Scholar]
- Lapitskaya, V.A.; Kuznetsova, T.A.; Chizhik, S.A.; Sudzilouskaya, K.A.; Kotov, D.A.; Nikitiuk, S.A.; Zaparozhchanka, Y.V. Lateral force microscopy as a method of surface control after low-temperature plasma treatment. J. Phys. Conf. Ser. 2018, 443, 012019. [Google Scholar]
- Anders, A. A review comparing cathodic arcs and high power impulse magnetron sputtering (HiPIMS). Surf. Coat. Technol. 2014, 257, 308–325. [Google Scholar]
- Chang, Y.Y.; Wang, D.Y.; Hung, C.Y. Structural and mechanical properties of nanolayered TiAlN/CrN coatings synthesized by a cathodic arc deposition process. Surf. Coat. Technol. 2005, 200, 1702–1708. [Google Scholar]
- Cai, F.; Zhang, S.; Li, J.; Chen, Z.; Li, M.; Wang, L. Effect of nitrogen partial pressure on Al–Ti–N films deposited by arc ion plating. Appl. Surf. Sci. 2011, 258, 1819–1825. [Google Scholar] [CrossRef]
- Reiter, A.E.; Mitterer, C.; de Figueiredo, M.R.; Franz, R. Abrasive and adhesive wear behavior of arc-evaporated Al1-xCrxN hard coatings. Tribol. Lett. 2010, 37, 605–611. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Ratajski, J.; Kuklinski, Z.; Rochowicz, J. An analysis of macroparticle-related defects in the CrCN and CrN coatings in dependence on the substrate bias voltage. Vacuum 2012, 86, 1235–1239. [Google Scholar]
- Nikolaev, A.L.; Mitrin, B.I.; Sadyrin, E.V.; Zelentsov, V.B.; Aguiar, A.R.; Aizikovich, S.M. Mechanical Properties of Microposit S1813 Thin Layers. Adv. Struct. Mater. 2020, 137–146. [Google Scholar]
- Vasiliev, A.S.; Sadyrin, E.V.; Mitrin, B.I.; Aizikovich, S.M.; Nikolaev, A.L. Nanoindentation of ZrN Coatings on Silicon and Copper Substrates. Russ. Eng. Res. 2018, 38, 735–737. [Google Scholar] [CrossRef]
- Sadyrin, E.; Swain, M.; Mitrin, B.; Rzhepakovsky, I.; Nikolaev, A.; Irkha, V.; Aizikovich, S. Characterization of Enamel and Dentine about a White Spot Lesion: Mechanical Properties, Mineral Density, Microstructure and Molecular Composition. Nanomaterials 2020, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimized tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar]
- Musil, J.; Kunc, F.; Zeman, H.; Polakova, H. Relationships between hardness, Young’s modulus and elastic recovery in hard nanocomposite coatings. Surf. Coat. Technol. 2002, 54, 304–313. [Google Scholar] [CrossRef]
- Pintaude, G. Introduction of the Ratio of the Hardness to the Reduced Elastic Modulus for Abrasion. In Tribology—Fundamentals and Advancements, 1st ed.; Gegner, J., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Panjan, P.; Čekada, M.; Navinšek, B. A new experimental method for studying the cracking behaviour of PVD multilayer coatings. Surf. Coat. Technol. 2003, 174–175, 55–62. [Google Scholar]
- Birol, Y.; İsler, D. Response to thermal cycling of CAPVD (Al,Cr)N-coated hot work tool steel. Surf. Coat. Technol. 2010, 205, 275–280. [Google Scholar]
- Jäger, N.; Meindlhumer, M.; Spor, S.; Hruby, H.; Julin, J.; Stark, A.; Nahif, F.; Keckes, J.; Mitterer, C.; Daniel, R.; et al. Microstructural evolution and thermal stability of AlCr(Si)N hard coatings revealed by in-situ high-temperature high-energy grazing incidence transmission X-ray diffraction. Acta Mater. 2020, 186, 545–554. [Google Scholar] [CrossRef]
- Hans, M.; Music, D.; Chen, Y.-T.; Patterer, L.; Eriksson, A.O.; Kurapov, D.; Ramm, J.; Arndt, M.; Rudigier, H.; Schneider, J.M. Crystallite size-dependent metastable phase formation of TiAlN coatings. Sci. Rep. 2017, 7, 16096. [Google Scholar]
- Pogrebnjak, A.; Beresnev, V.; Bondar, O.; Postolnyi, B.; Zaleski, K.; Coy, E.; Jurga, S.; Lisovenko, M.; Konarski, P.; Rebouta, L.; et al. Superhard CrN/MoN coatings with multilayer architecture. Mater. Des. 2018, 153, 47–59. [Google Scholar]
- Rebholz, C.; Ziegele, H.; Leyland, A.; Matthews, A. Structure, mechanical and tribological properties of nitrogen-containing chromium coatings prepared by reactive magnetron sputtering. Surf. Coat. Technol. 1999, 115, 222–229. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized physical vapor deposition (IPVD): A review of technology and applications. Thin Solid Films 2006, 513, 1–24. [Google Scholar] [CrossRef]

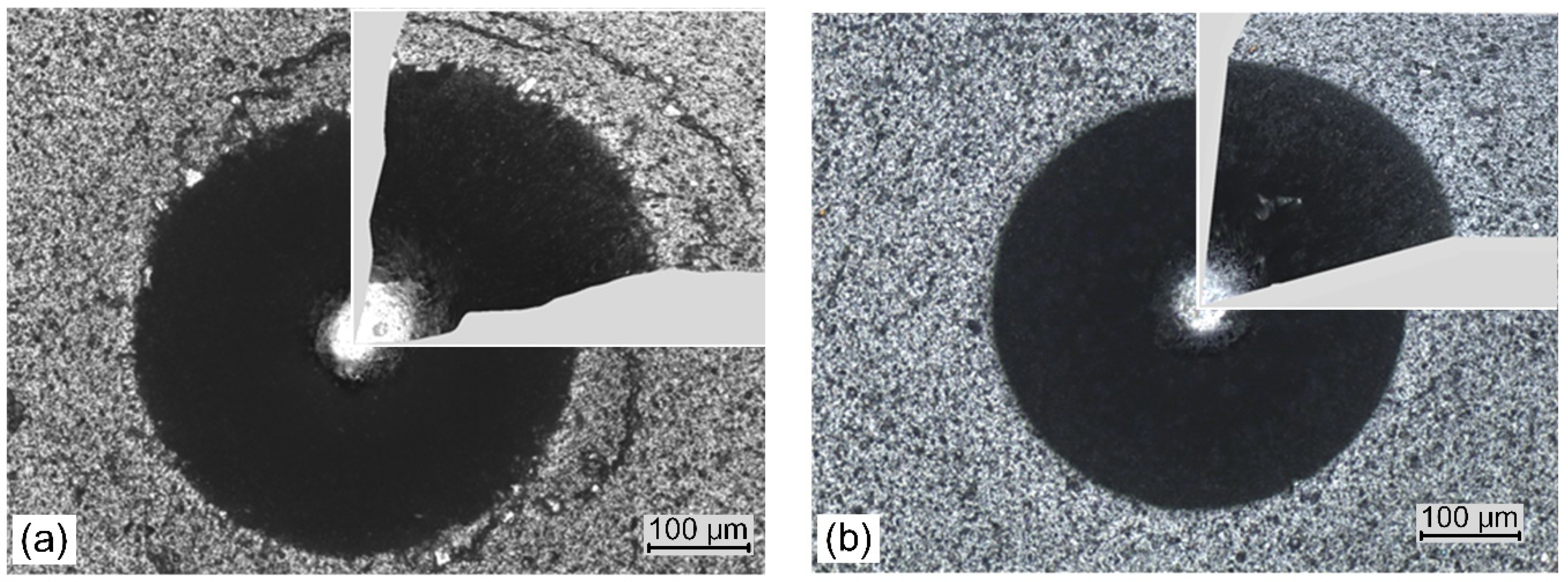
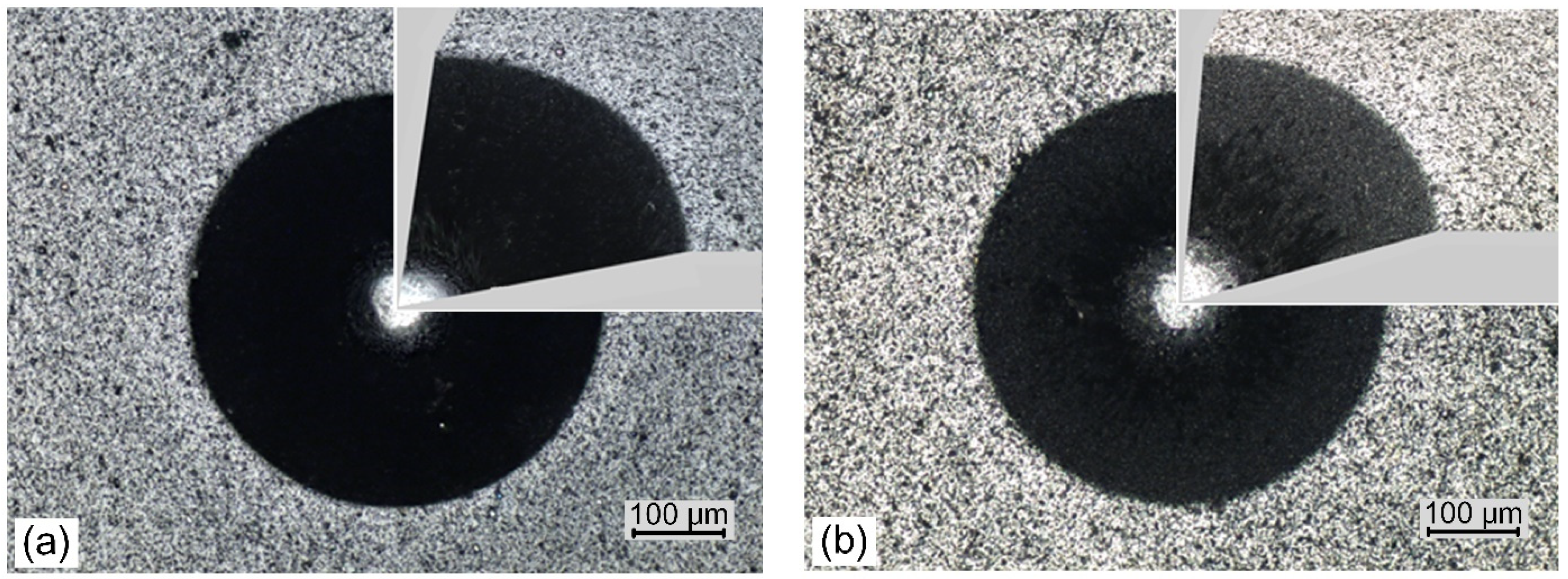

| Parameter | AlCrN(0.8) | AlCrN(1.2) | AlCrN(1.8) | AlCrN(3.0) | AlCrN(4.0) | AlCrN(5.0) |
|---|---|---|---|---|---|---|
| Hardness H (GPa) | 17.4 ± 1.3 | 18.0 ± 2.3 | 18.7 ± 2.9 | 21.8 ± 2.1 | 24.6 ± 2.2 | 27.7 ± 2.6 |
| Young’s modulus, E (GPa) | 205 ± 13 | 212 ± 15 | 218 ± 16 | 239 ± 14 | 261 ± 9 | 304 ± 16 |
| H/E | 0.085 ± 0.011 | 0.085 ± 0.017 | 0.086 ± 0.019 | 0.091 ± 0.014 | 0.094 ± 0.014 | 0.091 ± 0.013 |
| H3/E2 (GPa) | 0.12 ± 0.04 | 0.13 ± 0.07 | 0.15 ± 0.08 | 0.18 ± 0.07 | 0.22 ± 0.07 | 0.23 ± 0.09 |
| Lc2 (N) | 77.2 ± 1.8 | 85.0 ± 2.3 | 84.5 ± 3.5 | 98.0 ± 3.8 | 91.0 ± 2.1 | 79.6 ± 1.8 |
| Parameter | AlCr(50)N | AlCr(60)N | AlCr(80)N | AlCr(100)N |
|---|---|---|---|---|
| Hardness, H (GPa) | 23.3 ± 1.7 | 24.9 ± 1.9 | 24.6 ± 2.2 | 25.4 ± 1.3 |
| Young’s modulus, E (GPa) | 235 ± 9 | 252 ± 10 | 261 ± 9 | 275 ± 8 |
| H/E | 0.099 ± 0.011 | 0.099 ± 0.011 | 0.094 ± 0.014 | 0.092 ± 0.007 |
| H3/E2 (GPa) | 0.23 ± 0.07 | 0.24 ± 0.07 | 0.22 ± 0.07 | 0.22 ± 0.05 |
| Lc2 (N) | 88.1 ± 3.3 | 89.4 ± 1.0 | 91.0 ± 2.1 | 97.2 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilewicz, A.; Kuznetsova, T.; Aizikovich, S.; Lapitskaya, V.; Khabarava, A.; Nikolaev, A.; Warcholinski, B. Comparative Investigations of AlCrN Coatings Formed by Cathodic Arc Evaporation under Different Nitrogen Pressure or Arc Current. Materials 2021, 14, 304. https://doi.org/10.3390/ma14020304
Gilewicz A, Kuznetsova T, Aizikovich S, Lapitskaya V, Khabarava A, Nikolaev A, Warcholinski B. Comparative Investigations of AlCrN Coatings Formed by Cathodic Arc Evaporation under Different Nitrogen Pressure or Arc Current. Materials. 2021; 14(2):304. https://doi.org/10.3390/ma14020304
Chicago/Turabian StyleGilewicz, Adam, Tatyana Kuznetsova, Sergei Aizikovich, Vasilina Lapitskaya, Anastasiya Khabarava, Andrey Nikolaev, and Bogdan Warcholinski. 2021. "Comparative Investigations of AlCrN Coatings Formed by Cathodic Arc Evaporation under Different Nitrogen Pressure or Arc Current" Materials 14, no. 2: 304. https://doi.org/10.3390/ma14020304
APA StyleGilewicz, A., Kuznetsova, T., Aizikovich, S., Lapitskaya, V., Khabarava, A., Nikolaev, A., & Warcholinski, B. (2021). Comparative Investigations of AlCrN Coatings Formed by Cathodic Arc Evaporation under Different Nitrogen Pressure or Arc Current. Materials, 14(2), 304. https://doi.org/10.3390/ma14020304







