Synthesis, Characterization, and Antibacterial Evaluation of a Cost-Effective Endodontic Sealer Based on Tricalcium Silicate-White Portland Cement
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berman, L.H.; Hargreaves, K.M. Cohen’s Pathways of the Pulp, 11th ed.; Mosby-Elsevier: Saint Louis, MO, USA, 2016; pp. 139–161. [Google Scholar]
- Torabinejad, M.; Walton, R. Endodontics: Principles and Practice, 4th ed.; Saunders-Elsevier: Philadelphia, PA, USA, 2008; pp. 61–82. [Google Scholar]
- Murray, B.E. Vancomycin-resistant enterococcal infections. N. Eng. J. Med. 2000, 342, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Q.; Zhang, C.; Cheung, G.S.; Shen, Y. Prevalence, phenotype, and genotype of Enterococcus faecalis isolated from saliva and root canals in patients with persistent apical periodontitis. J. Endod. 2010, 36, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Kutty, M.G.; Abu Kasim, N.H.; Che Ab Aziz, Z.A. Physicochemical properties of calcium phosphate-based coating on gutta-percha root canal filling. Int. J. Polym. Sci. 2015, 2015, 414521. [Google Scholar] [CrossRef]
- Singh, H.; Markan, S.; Kaur, M.; Gupta, G. Endodontic sealers: Current concepts and comparative analysis. Open Dent. J. 2015, 2, 32–37. [Google Scholar] [CrossRef]
- Orstavik, D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Cavenago, B.C.; Duarte, M.A.; Ordinola-Zapata, R.; Marciano, M.A.; Carpio-Perochena, A.E.; Bramante, C.M. Interfacial adaptation of an epoxy-resin sealer and a self-etch sealer to root canal dentin using the system B or the single cone technique. Braz. Dent. J. 2012, 23, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Darvell, B.W.; Wu, R.C.T. “MTA”—An hydraulic silicate cement: Review update and setting reaction. Dent. Mater. 2011, 27, 407–422. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Ding, S.J. Comparative physicochemical and biocompatible properties of radiopaque dicalcium silicate cement and mineral trioxide aggregate. J. Endod. 2010, 36, 1683–1687. [Google Scholar] [CrossRef]
- Liu, X.; Morra, M.; Carpi, A.; Li, B. Bioactive calcium silicate ceramics and coatings. Biomed. Pharmacother. 2008, 62, 526–529. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeek, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef]
- Marão, H.F.; Panzarini, S.R.; Aranega, A.M.; Sonoda, C.K.; Poi, W.R.; Esteves, J.C.; Silva, P.I. Periapical tissue reactions to calcium hydroxide and MTA after external root resorption as a sequela of delayed tooth replantation. Dent. Traumatol. 2012, 28, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Pelisser, F.; Steiner, L.R.; Bernardin, A.M. Recycling of porcelain tile polishing residue in portland cement: Hydration efficiency. Environ. Sci. Technol. 2012, 46, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Tomás-Catalá, C.J.; Santos, J.M.; Llena, C.; Lozano, A.; Murcia, L.; Forner, L. Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int. Endod. J. 2020, 53, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Güven, E.P.; Taşlı, P.N.; Yalvac, M.E.; Sofiev, N.; Kayahan, M.B.; Sahin, F. In vitro comparison of induction capacity and biomineralization ability of mineral trioxide aggregate and a bioceramic root canal sealer. Int. Endod. J. 2013, 46, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Bakland, L.K. Management of traumatically injured pulps in immature teeth using MTA. J. Calif. Dent Assoc. 2000, 28, 855–858. [Google Scholar] [PubMed]
- Witherspoon, D.E.; Ham, K. One visit apexification: Technique for inducing root-end barrier formation in apical closures. Pract. Proced. Aesthet. Dent. 2001, 13, 455–462. [Google Scholar] [PubMed]
- Torabinejad, M.; White, D.J. Tooth Filling Material and Method of Use. U.S. Patent No. 5, 415, 547, 1995. [Google Scholar]
- Main, C.; Mirzayan, N.; Shabahang, S.; Torabinejad, M. Repair of root perforations using mineral trioxide aggregate: A long term study. J. Endod. 2004, 30, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic portland cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar]
- Dammaschke, T.; Gerth, H.U.V.; Züchner, H.; Schäfer, E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent. Mater. 2005, 21, 731–738. [Google Scholar]
- Primathena, I.; Nurdin, D.; Adang, R.A.; Cahyanto, A. Composition and functional groups evaluation of Indonesian grey portland cement as material for dental application. Key Eng. Mater. 2018, 782, 256–261. [Google Scholar] [CrossRef]
- Nurdin, D.; Primathena, I.; Adang, R.A.; Cahyanto, A. Comparison of chemical composition between Indonesian white portland cement and MTA as dental pulp capping material. Key Eng. Mater. 2019, 829, 34–39. [Google Scholar] [CrossRef]
- Li, Q.; Coleman, N.J. The hydration chemistry of ProRoot MTA. Dent. Mater. J. 2015, 34, 458–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasheminia, S.; Norozynasab, S.; Feizianfard, M. The Effect of Three Different Calcium Hydroxide Combinations on Root Dentine Microhardness. Res. J. Biol. Sci. 2009, 4, 121–125. [Google Scholar]
- Bedoya-Hincapie, C.M.; Pinzón Cárdenas, M.J.; Alfonso, E.; Restrepo Parra, E. Physical-chemical properties of bismuth and bismuth oxides: Synthesis, characterization and application. Dyna 2012, 79, 139–148. [Google Scholar]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of Root Canal Sealers: A Systematic Review of In Vitro and In Vivo Studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing, 24th ed.; CLSI: Annapolis Junction, MD, USA, 2014. [Google Scholar]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, J.F., Jr.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Sjogren, U.; Figdor, D.; Spangberg, L.; Sundqvist, G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int. Endod. J. 1991, 24, 119–125. [Google Scholar] [CrossRef]
- Estrela, C.; Rodrigues de Araújo Estrela, C.; Bamman, L.L.; Pecora, J.D. Two methods to evaluate the antimicrobial action of calcium hydroxide paste. J. Endod. 2001, 27, 12–15. [Google Scholar] [CrossRef]
- Han, G.Y.; Park, S.H.; Yoon, T.C. Antimicrobial activity of Ca(OH)2 containing pastes with Enterococcus faecalis in vitro. J. Endod. 2001, 27, 328–332. [Google Scholar] [CrossRef]
- Estrela, C.; Pécora, J.D.; Silva, R.S. pH analysis of vehicles and calcium hydroxide pastes. Braz. Endod. J. 1998, 3, 41–47. [Google Scholar]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The emerging role of stem cells in regenerative dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar]
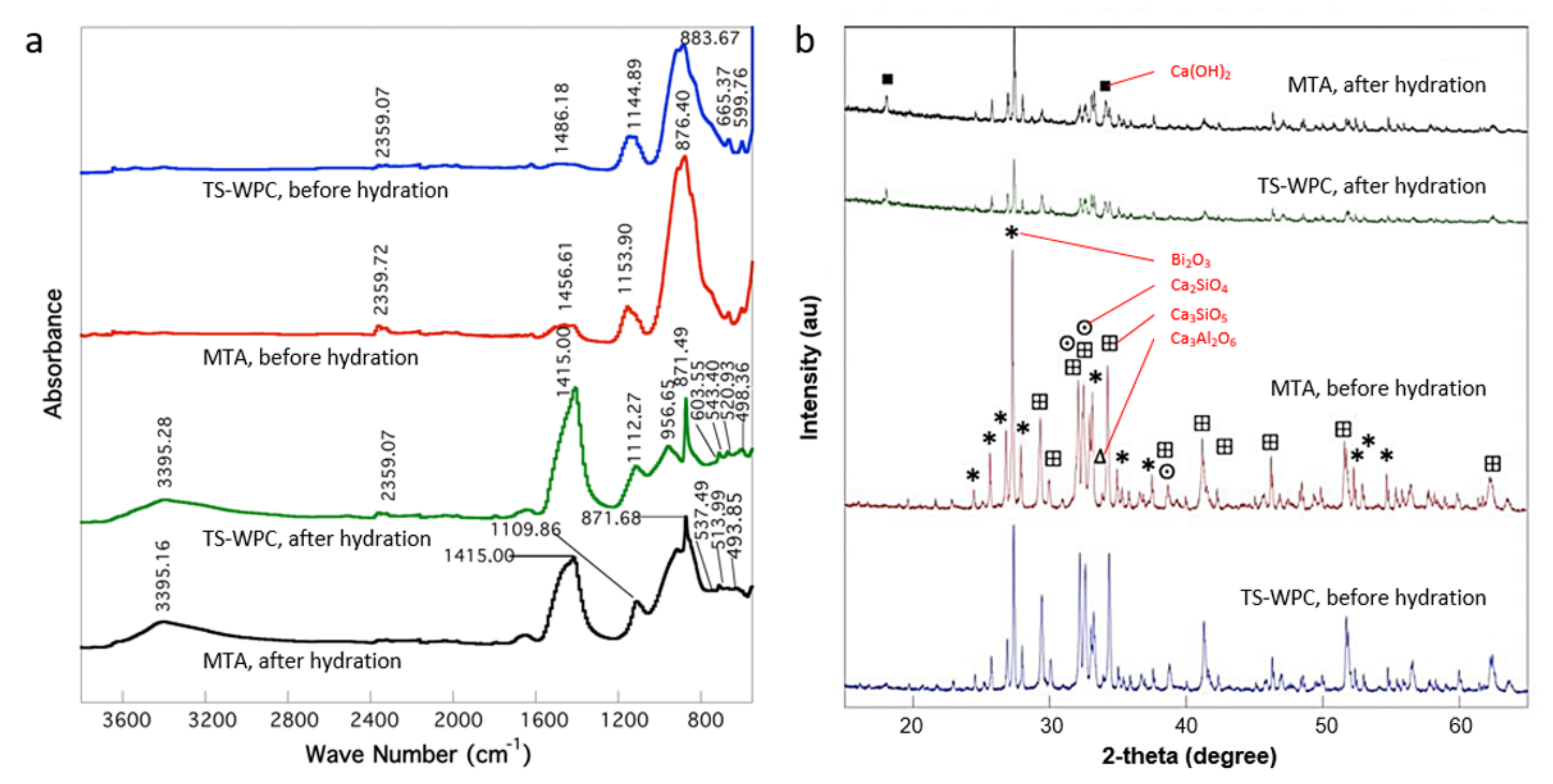
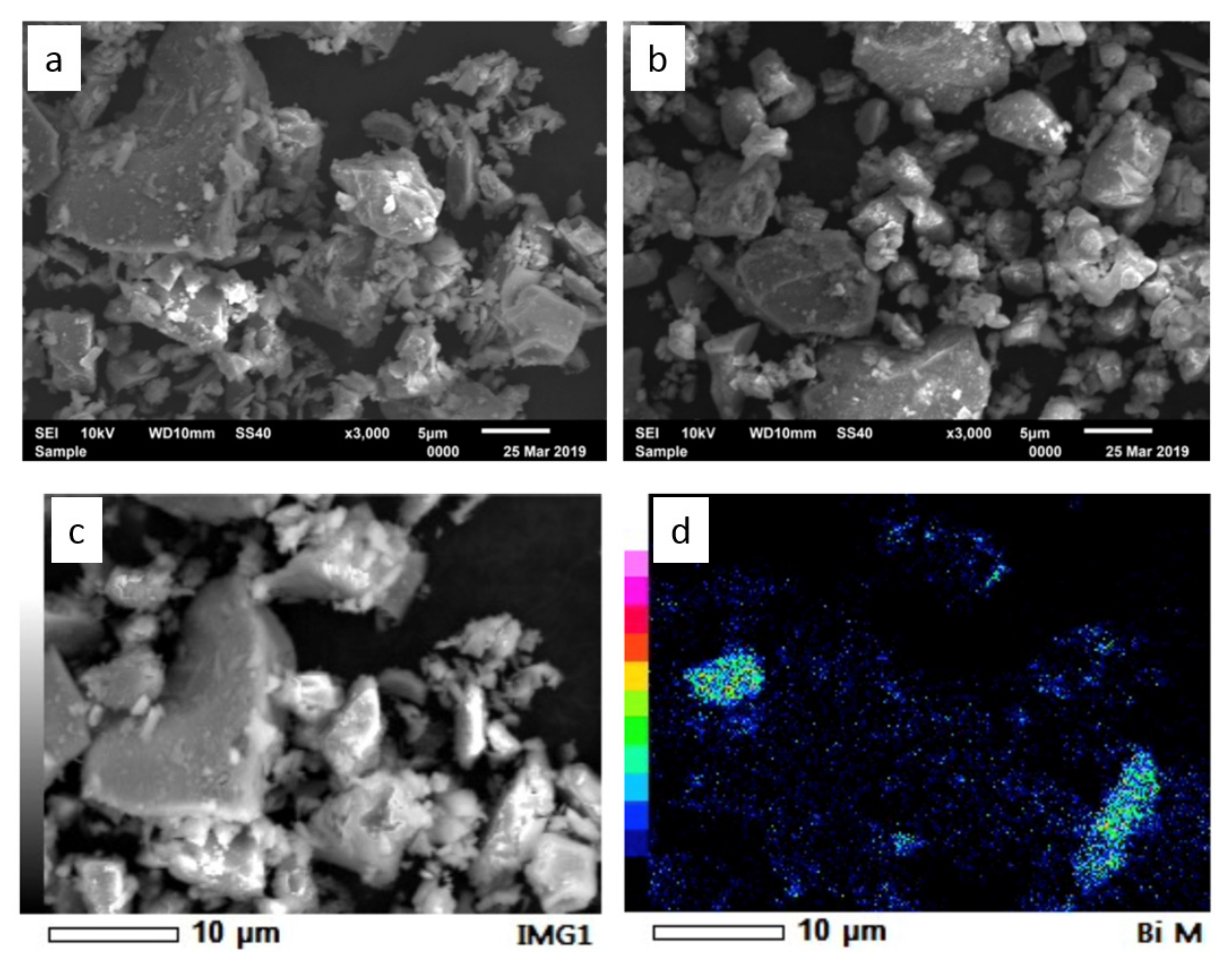
| TS-WPC | MTA | ||
|---|---|---|---|
| Wavenumber (cm−1) | Bond/Type of Functional Group | Wavenumber (cm−1) | Bond/Type of Functional Group |
| Before hydration | |||
| 599.75 | C-H (alkanes) | ||
| 665.37 | |||
| 883.67 | Si-C/C=C (alkenes) | 876.40 | Si-C/C=C (alkenes) |
| 1144.89 | Si-O | 1153.90 | Si-O |
| 1486.18 | C-H (alkanes)/C-O (alkanols) | 1456.61 | C-H (alkanes)/C-O (alkanols) |
| 2359.07 | Si-H | 2359.72 | Si-H |
| After hydration | |||
| 451.71 | Aromatic group (S-O)/sulfate ionic bonds (SO4)− | 436.77 | Aromatic group (S-O)/sulfate ionic bonds (SO4)− |
| 467.69 | 445.38 | ||
| 498.36 | 466.56 | ||
| 475.90 | |||
| 493.85 | |||
| 520.93 | Fingerprint band/Si-O (bending)/Ca=O | 513.99 | Fingerprint band/Si-O (bending)/Ca=O |
| 543.40 | 537.49 | ||
| 603.55 | Fingerprint band | ||
| 871.49 | Si-C/C=C (alkenes) | 871.68 | Si-C/C=C (alkenes) |
| 956.65 | Si-O (stretching) | ||
| 1112.27 | Si-O | 1109.86 | Si-O |
| 1408.59 | C-H (alkanes)/C-O (alkanols) | 1415.00 | C-H (alkanes)/C-O (alkanols) |
| 2359.07 | Si-H | ||
| 3395.28 | O-H | 3395.16 | O-H |
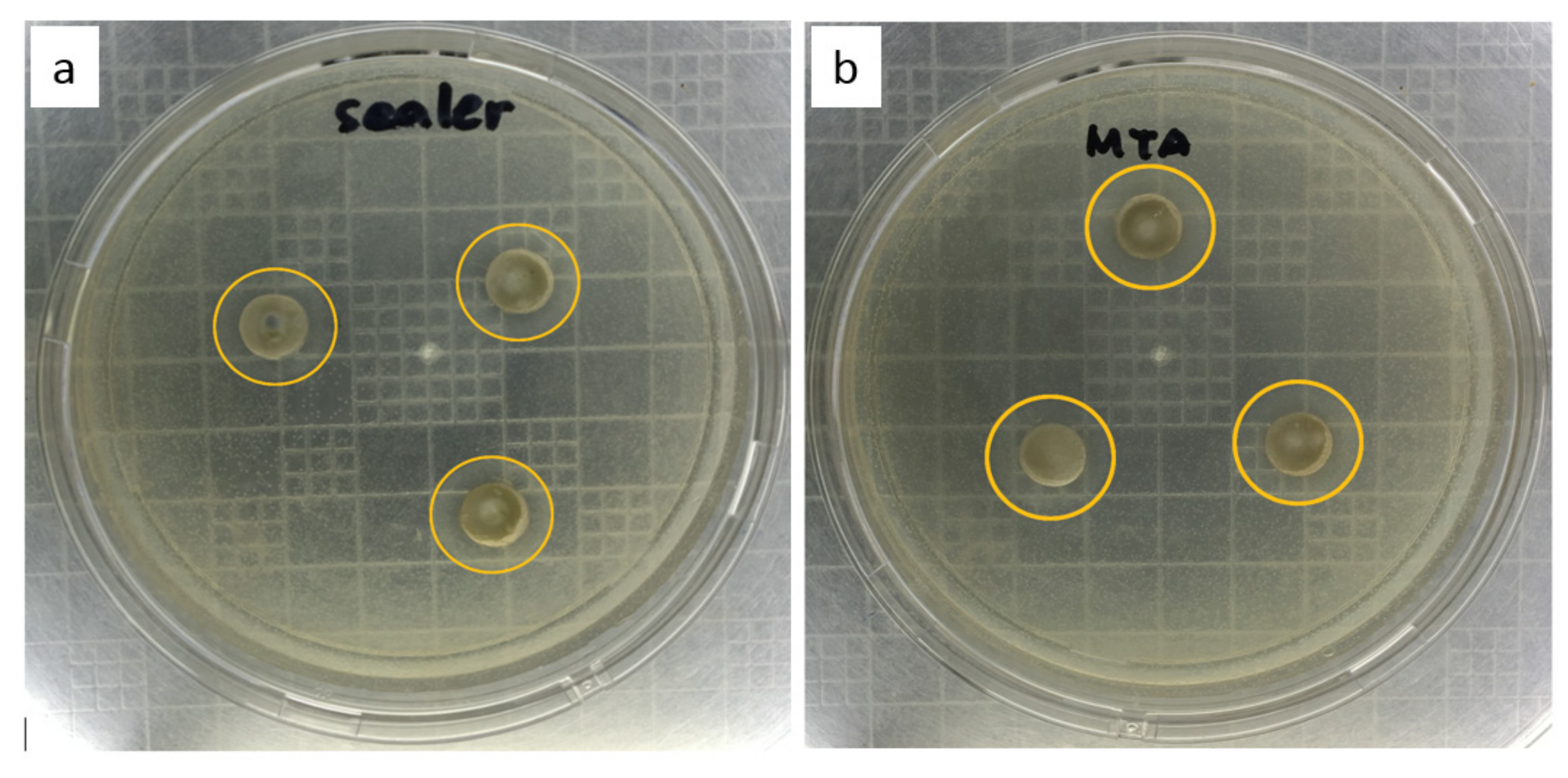
| Observation | 12,500 ppm | 25,000 ppm | 50,000 ppm | 100,000 ppm | 200,000 ppm | 400,000 ppm | Notes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | − | − | + | + | + | + | + | − | − | − | − | − | |
| B | − | − | + | + | + | + | + | − | − | − | − | − | |
| C | − | − | + | + | + | − | − | − | − | − | − | − | |
| D | − | − | − | − | − | − | − | − | − | − | − | − | * |
| E | − | − | + | + | + | + | − | − | − | − | − | − | |
| F | − | − | + | + | + | + | + | − | − | − | − | − | |
| G | − | − | + | + | + | + | + | − | − | − | − | − | |
| H | − | − | − | − | − | − | − | − | − | − | − | − | ** |
| *** | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primathena, I.; Nurdin, D.; Hermawan, H.; Cahyanto, A. Synthesis, Characterization, and Antibacterial Evaluation of a Cost-Effective Endodontic Sealer Based on Tricalcium Silicate-White Portland Cement. Materials 2021, 14, 417. https://doi.org/10.3390/ma14020417
Primathena I, Nurdin D, Hermawan H, Cahyanto A. Synthesis, Characterization, and Antibacterial Evaluation of a Cost-Effective Endodontic Sealer Based on Tricalcium Silicate-White Portland Cement. Materials. 2021; 14(2):417. https://doi.org/10.3390/ma14020417
Chicago/Turabian StylePrimathena, Indra, Denny Nurdin, Hendra Hermawan, and Arief Cahyanto. 2021. "Synthesis, Characterization, and Antibacterial Evaluation of a Cost-Effective Endodontic Sealer Based on Tricalcium Silicate-White Portland Cement" Materials 14, no. 2: 417. https://doi.org/10.3390/ma14020417
APA StylePrimathena, I., Nurdin, D., Hermawan, H., & Cahyanto, A. (2021). Synthesis, Characterization, and Antibacterial Evaluation of a Cost-Effective Endodontic Sealer Based on Tricalcium Silicate-White Portland Cement. Materials, 14(2), 417. https://doi.org/10.3390/ma14020417






