Novel Nano-Precursor Inhibiting Material for Improving Chloride Penetration Resistance of Concrete: Evaluation and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
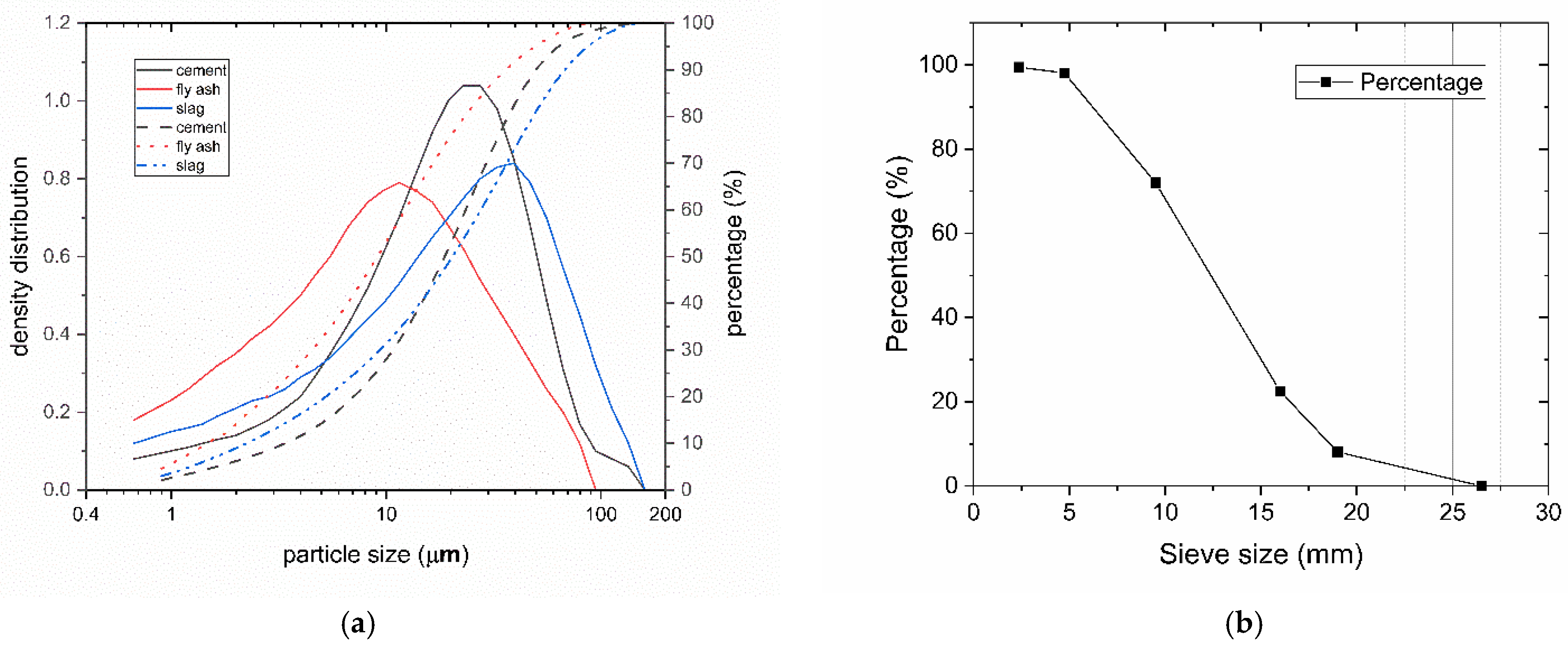
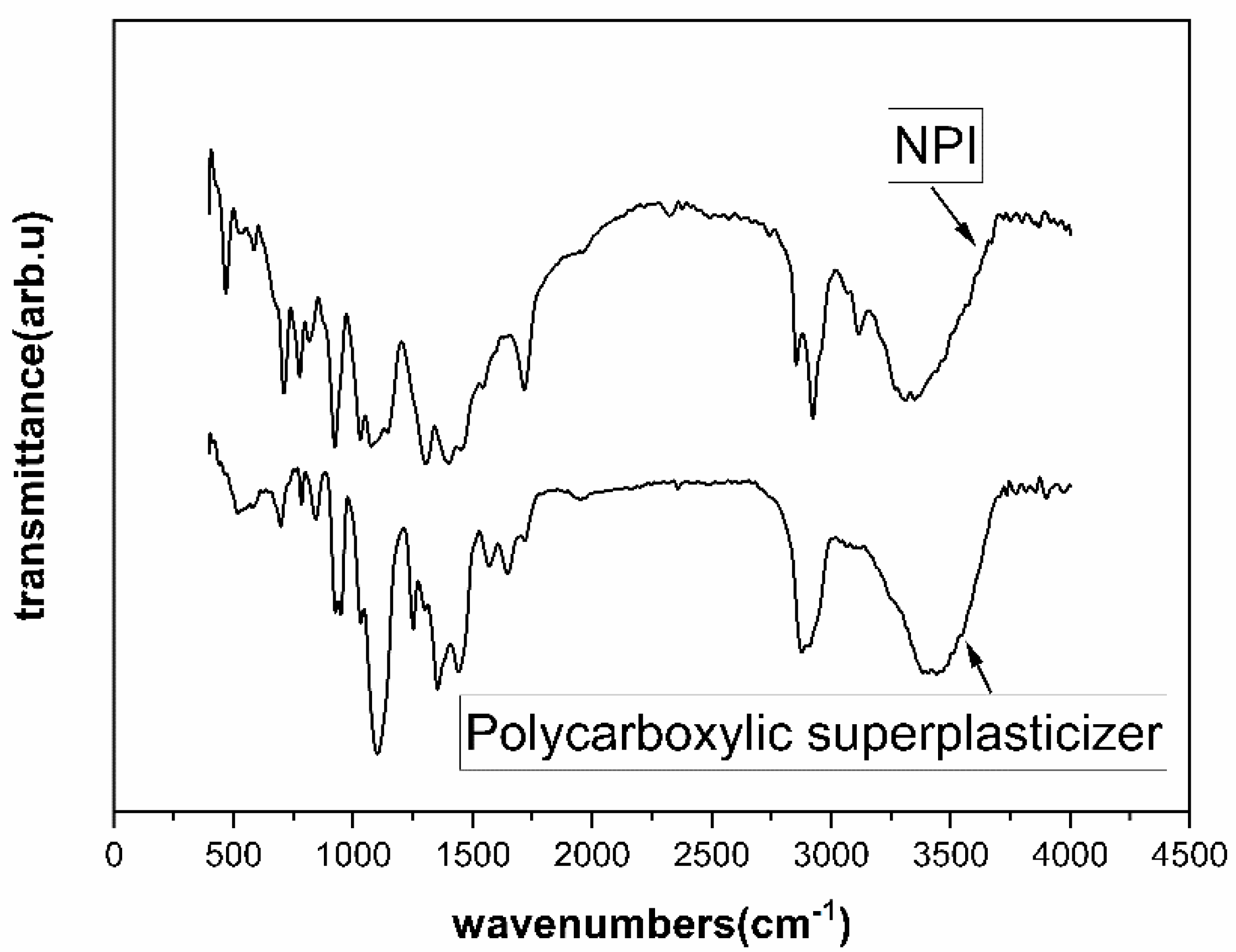
2.1. Materials
2.2. Methods
2.2.1. Specimen Preparation
2.2.2. The Compressive Strength Test
2.2.3. Isothermal Calorimetry
2.2.4. Mercury Intrusion Porosimetry (MIP)
- P is the pressure of mercury intrusion (N/m2),
- d is the test pore diameter (m),
- γ is the surface tension of mercury (N/m2), and
- θ is the contact angle between the mercury and the cement paste.
2.2.5. Scanning Electron Microscope
2.2.6. Durability Test
- Dnssm is non-steady-state migration coefficient (×10−12 m2/s);
- T is the mean value of the initial and final temperatures in the test anolyte solution (°C);
- L is the thickness of the test concrete specimen (mm);
- U is the absolute value of the applied voltage (V);
- t is the test duration time (h); and
- xd is the average value of the chloride penetration depths (mm).
2.2.7. Contact Angle Test
3. Results
3.1. Isothermal Calorimetry
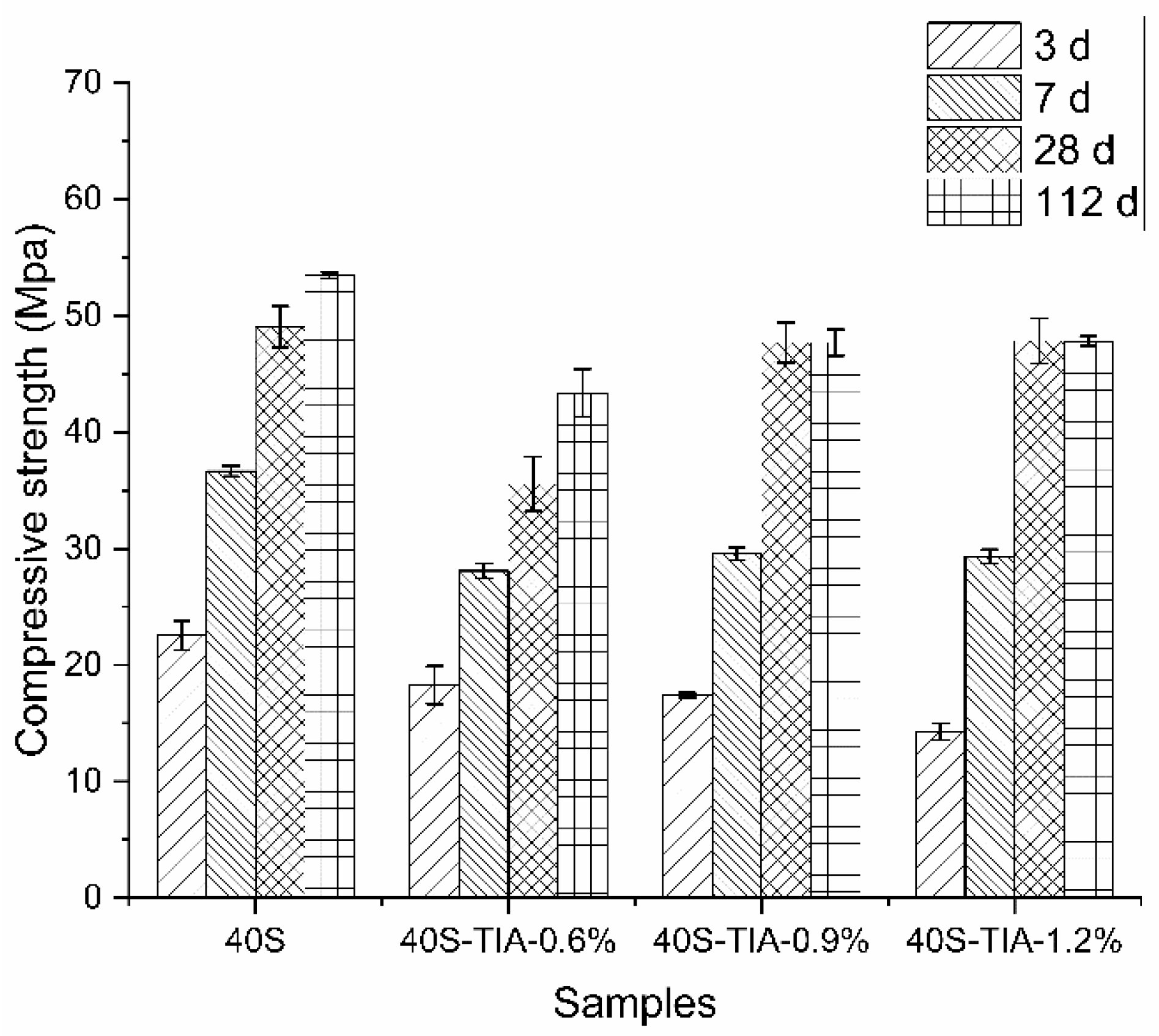
3.2. Compressive Strength
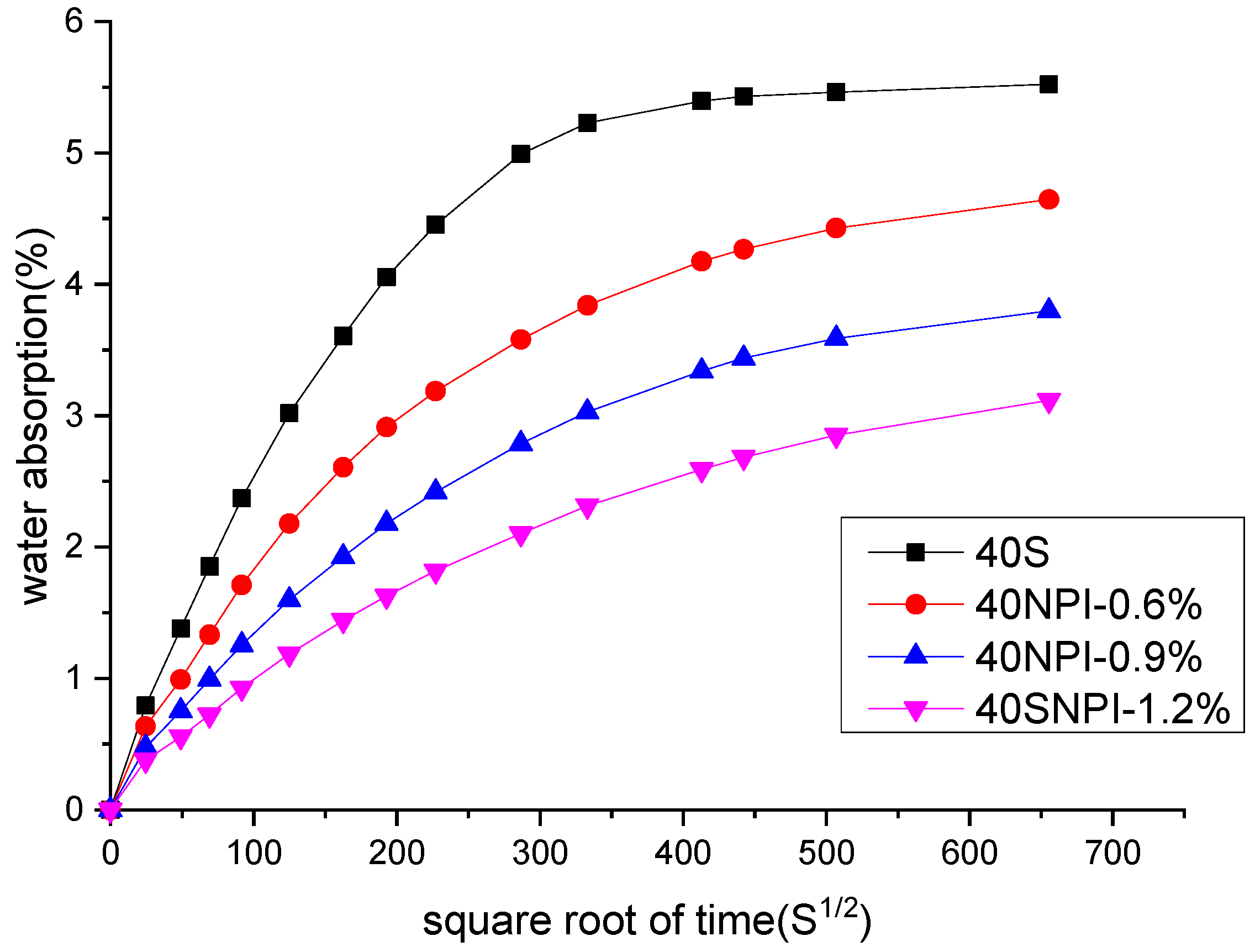
3.3. Water Absorption Ratio
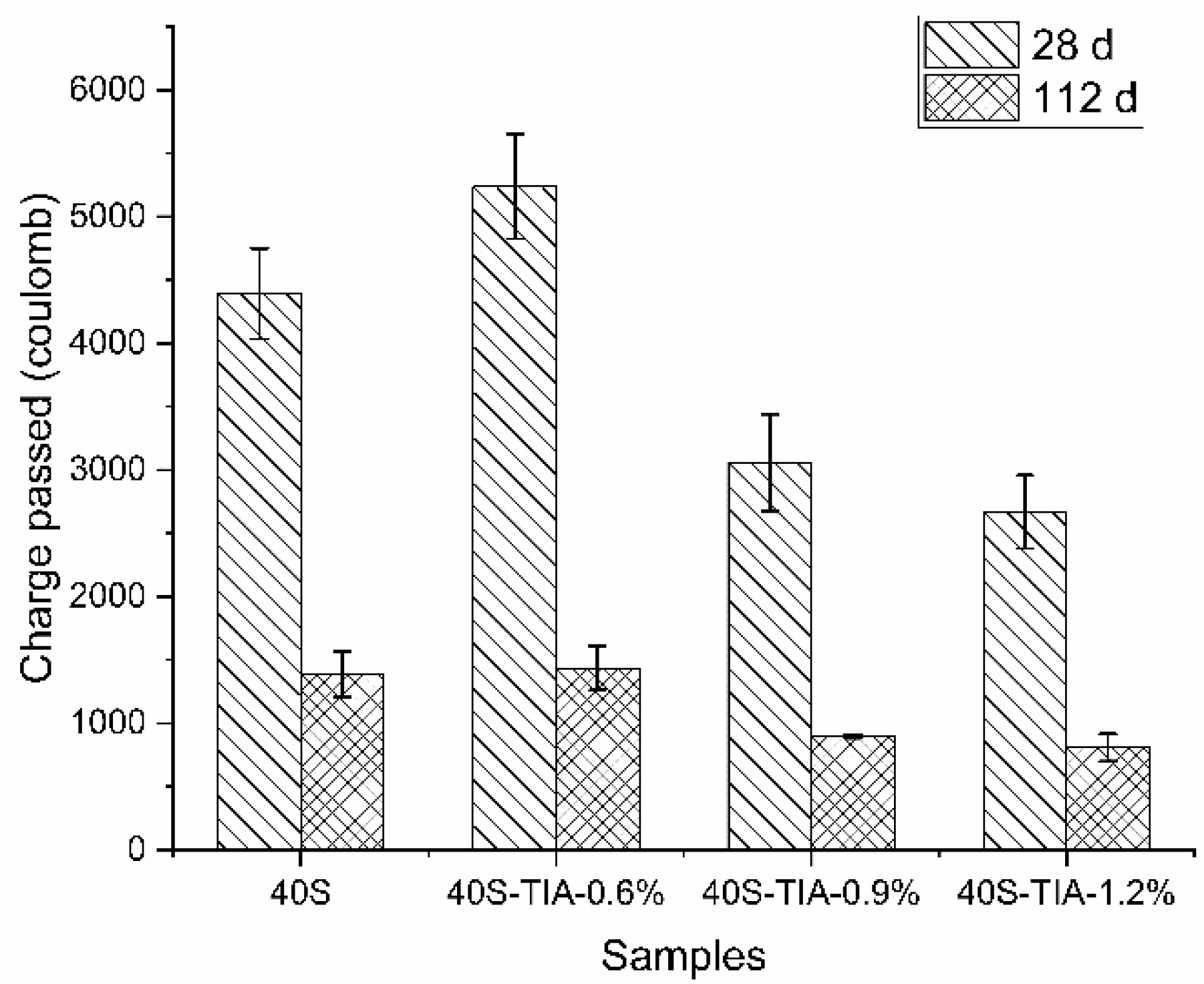
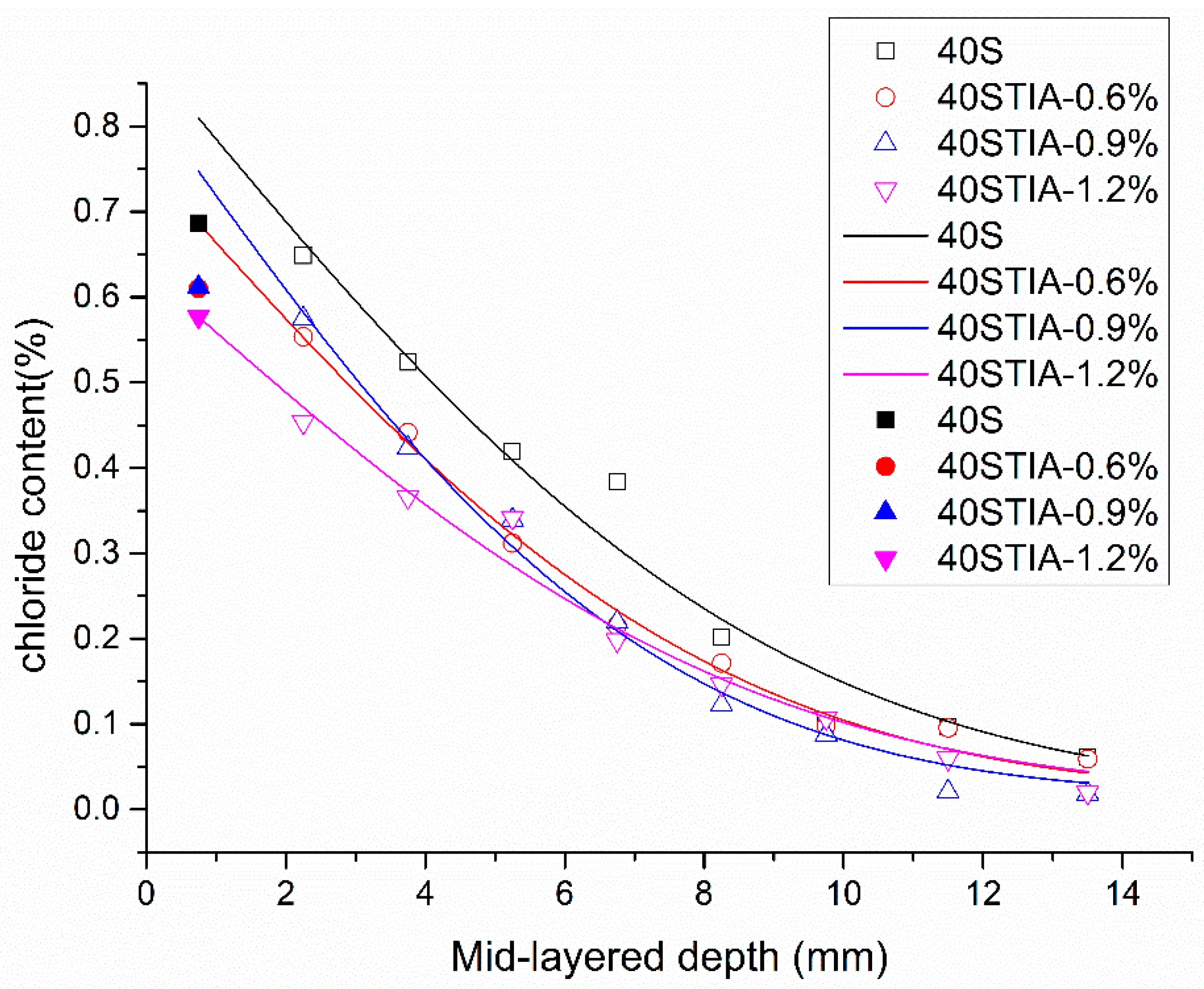
3.4. Resistance to Chloride Penetration
- Cx is the chloride content measured at a certain average depth x and exposure time t (% by mass of immersed concrete);
- Cs is the calculated chloride concentration at the exposed surface (% by mass of concrete);
- Ci is the initial chloride content of plain concrete (% by mass of concrete);
- x is the depth below the top exposed surface to the mid-point of the grounding layer (m);
- Dnss is the non-steady state chloride diffusion coefficient (m2/s); and
- t is the exposure time (s).
4. Discussion
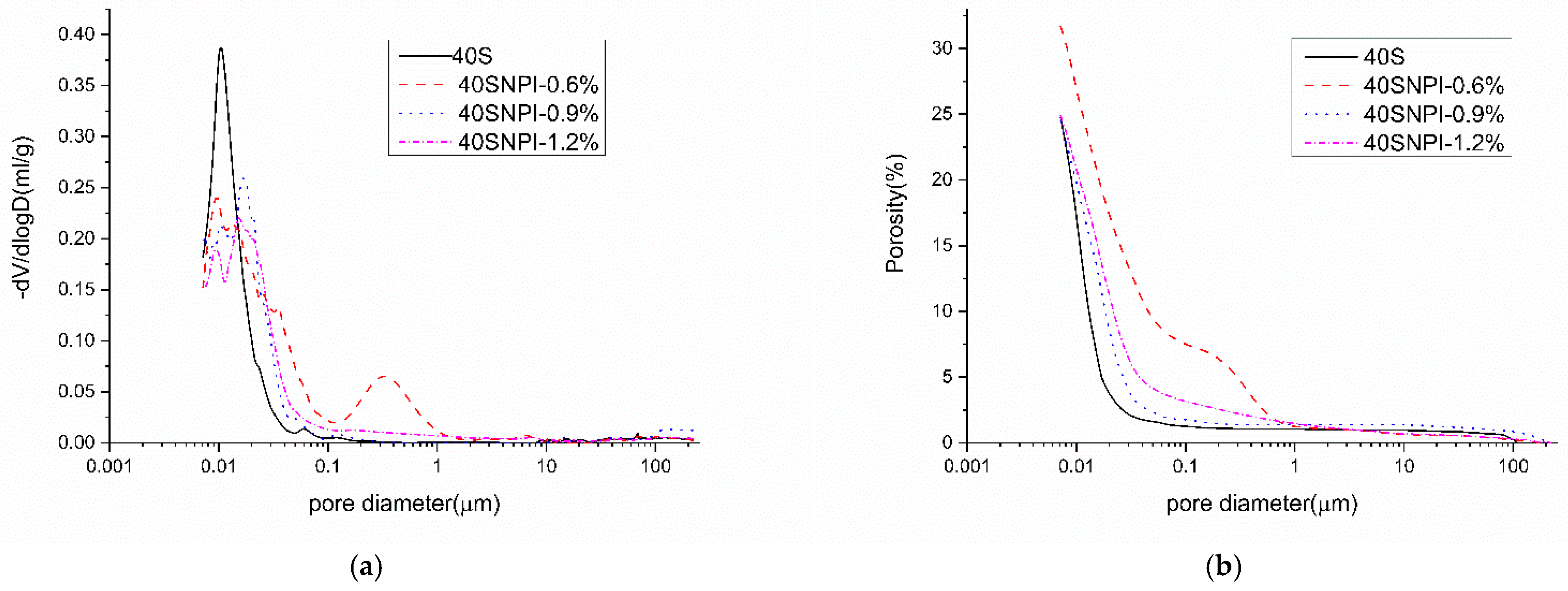
4.1. Pore Structures
4.2. Contact Angle
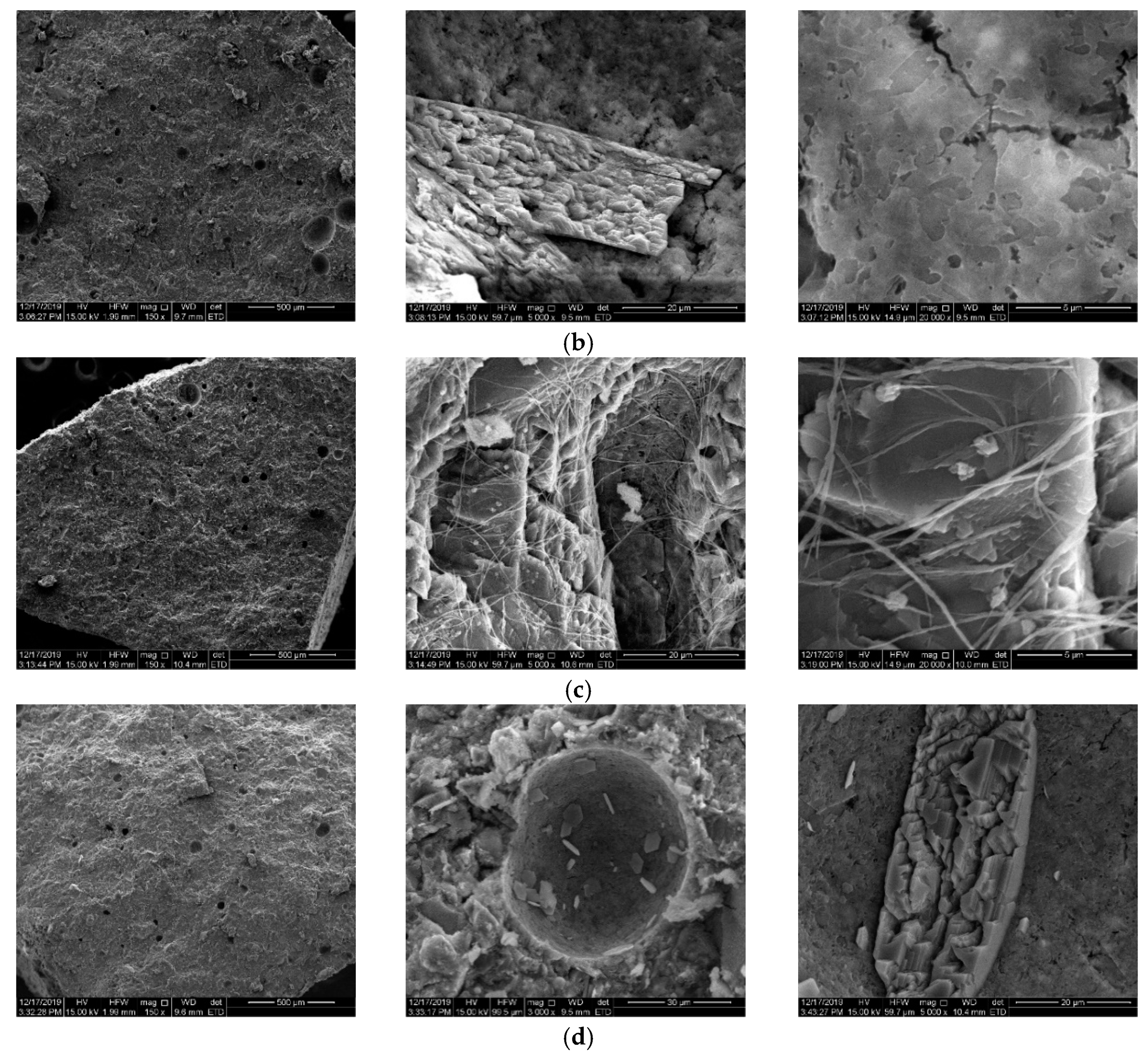
4.3. Morphology of Cement Paste
5. Conclusions
- (1)
- The NPI caused a reduction in the strength of concrete, but the strength reduction was minor at a later age. The NPI increased the total porosity and entrained big capillary pores in cement pastes, and the alkane chain resulted in a weaker ITZ in concrete. These reasons led to a decline in the compressive strength of the concrete samples.
- (2)
- The incorporation of the NPI significantly decreased water absorption and slowed down the speed of water sorptivity in concrete. The NPI also decreased the charge passed and the chloride migration coefficient of concrete. It is worth noting that the NPI effectively decreased the chloride diffusion coefficient and the chloride content at a depth lower than 5 mm and the surface chloride concentration of concrete.
- (3)
- The improvement in the transport properties of concrete was due to the incorporation with the NPI, which resulted in a gradual ascent of the contact angle from 17.8° to 85.8° when the dosage of the NPI was increased from 0% to 1.2%, and the surfaces of cement paste became less hydrophilic. Moreover, the NPI also changed the pore size distribution of hardened cement paste.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angst, U. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of surface-treatment of nano-SiO2 on the transport properties of hardened cement pastes with different water-to-cement ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Chloride induced corrosion durability of high volume fly ash concretes containing nano particles. Constr. Build. Mater. 2015, 99, 208–225. [Google Scholar] [CrossRef]
- Lincy, V.; Rao, V.V.L.K.; Lakshmy, P. A study on nanosilica- and microsilica-added concretes under different transport mechanisms. Mag. Concr. Res. 2018, 70, 1205–1216. [Google Scholar] [CrossRef]
- Mora, E.; González, G.; Romero, P.; Castellón, E. Control of water absorption in concrete materials by modification with hybrid hydrophobic silica particles. Constr. Build. Mater. 2019, 221, 210–218. [Google Scholar] [CrossRef]
- Ghassemi, P.; Toufigh, V. Durability of epoxy polymer and ordinary cement concrete in aggressive environments. Constr. Build. Mater. 2020, 234, 117887. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Li, R.; Hou, P.; Xie, N.; Ye, Z.; Cheng, X.; Shah, S.P. Design of SiO2/PMHS hybrid nanocomposite for surface treatment of cement-based materials. Cem. Concr. Compos. 2018, 87, 89–97. [Google Scholar] [CrossRef]
- Al Menhosh, A.; Wang, Y.; Wang, Y.; Augusthus-Nelson, L. Long term durability properties of concrete modified with metakaolin and polymer admixture. Constr. Build. Mater. 2018, 172, 41–51. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Fundamental interaction of hydrophobic materials in concrete with different moisture contents in saline environment. Constr. Build. Mater. 2019, 207, 122–135. [Google Scholar] [CrossRef]
- Yu, J.; Li, S.; Hou, D.; Jin, Z.; Liu, Q. Hydrophobic silane coating films for the inhibition of water ingress into the nanometer pore of calcium silicate hydrate gels. Phys. Chem. Chem. Phys. 2019, 21, 19026–19038. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, C.; Massidda, L.; Sanna, U.U.M. Capillary absorption of water in hardened cement pastes treated with silicic esters or alkylalkoxysiloxane. Mag. Concr. Res. 1993, 45, 11–15. [Google Scholar] [CrossRef]
- Aggarwal, L.K.; Thapliyal, P.C.; Karade, S.R. Properties of polymer-modified mortars using epoxy and acrylic emulsions. Constr. Build. Mater. 2007, 21, 379–383. [Google Scholar] [CrossRef]
- Franzoni, E.; Pigino, B.; Pistolesi, C. Ethyl silicate for surface protection of concrete: Performance in comparison with other inorganic surface treatments. Cem. Concr. Compos. 2013, 44, 69–76. [Google Scholar] [CrossRef]
- Holmes, N.; Obrien, R.; Basheer, P.A.M. Engineering performance of a new siloxane-based corrosion inhibitor. Mater. Struct. 2014, 47, 1531–1543. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kong, X. Influences of superplasticizer, polymer latexes and asphalt emulsions on the pore structure and impermeability of hardened cementitious materials. Constr. Build. Mater. 2014, 53, 392–402. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Chen, R.; Hou, D.; Xu, J.; Lv, K.; Zheng, Q. The design and evaluation of a smart polymer-based fluids transport inhibitor. J. Clean. Prod. 2020, 257, 120528. [Google Scholar] [CrossRef]
- Kong, X.; Liu, H.; Lu, Z.; Wang, D. The influence of silanes on hydration and strength development of cementitious systems. Cem. Concr. Res. 2015, 67, 168–178. [Google Scholar] [CrossRef]
- Feng, H.; Le, H.T.N.; Wang, S.; Zhang, M.-H. Effects of silanes and silane derivatives on cement hydration and mechanical properties of mortars. Constr. Build. Mater. 2016, 129, 48–60. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, F.; Xie, T.; Ou, J.; Xue, M.; Li, W. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Namoulniara, K.; Mahieux, P.Y.; Lux, J.; Aït-Mokhtar, A.; Turcry, P. Efficiency of water repellent surface treatment: Experiments on low performance concrete and numerical investigation with pore network model. Constr. Build. Mater. 2019, 227, 116638. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on surface treatment for concrete—Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zeng, C. Study of Cement-Based Superhydrophobic Composite Coating: New Option for Water Drainage Pipeline Rehabilitation. Materials 2020, 13, 5004. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Khan, F.M.; Dulaijan, S.U.; Al-Amoudi, O.S.B. Effectiveness of surface coatings in improving concrete durability. Cem. Concr. Compos. 2003, 25, 473–481. [Google Scholar] [CrossRef]
- Söylev, T.A.; McNally, C.; Richardson, M.G. The effect of a new generation surface-applied organic inhibitor on concrete properties. Cem. Concr. Compos. 2007, 29, 357–364. [Google Scholar] [CrossRef]
- Dai, J.-G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Wang, J.; Tittelboom, K.V.; Belie, N.D.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W. Effect of hydrophobic surface treatment on freeze-thaw durability of concrete. Cem. Concr. Compos. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- Liu, J.; Mu, S.; Cai, J.; Jiang, Q. Preparation and performance evaluation of cement hydration responsive nanomaterial. J. Build. Struct. 2019, 40, 181–187. [Google Scholar]
- China National Standardization Management Committee. Concrete Admixtures; GB 8076-2008; China Standard Press: Beijing, China, 2008. [Google Scholar]
- Ministry of Housing and Urban-Rural Development of the People’s Republic of China. Standard for Test Methods for Concrete Physical and Mechanical Properties; GB/T 50081-2002; China Building Industry Press: Beijing, China, 2002. [Google Scholar]
- European Committee for Standardization. Test Hardened Concrete—Part 3: Compressive Strength of Test Specimens; BS EN 12390-3-2009; European Committee for Standardization: London, UK, 2009. [Google Scholar]
- British Standards Institution. Testing Concrete—Part 122: Method for Determination of Water Absorption; BS 1881—122; British Standards Institution: London, UK, 2011. [Google Scholar]
- ASTM C 1202. Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- NT Build 492. Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Stead-State Migration Experiments; Nordtest: Esbo, Finland, 1999. [Google Scholar]
- NT Build 443. Concrete, Hardened: Accelerated Chloride Penetration; Nordtest: Esbo, Finland, 1995. [Google Scholar]
- Zhang, Y.; Ye, G.; Yang, Z. Pore size dependent connectivity and ionic transport in saturated cementitious materials. Constr. Build. Mater. 2020, 238, 117680. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Du, Y.; Jiang, J.; Sun, W. The effect of superhydrophobic nano-silica particles on the transport and mechanical properties of hardened cement pastes. Constr. Build. Mater. 2018, 182, 620–628. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Ma, R.; Yang, L.; Hu, Y.; Zhang, J. Water-repellent additive that increases concrete cracking resistance in dry curing environments. Constr. Build. Mater. 2020, 249, 118704. [Google Scholar] [CrossRef]
- Kong, X.M.; Lu, Z.B.; Liu, H.; Wang, D.M. Influence of triethanolamine on the hydration and the strength development of cementitious systems. Mag. Concr. Res. 2013, 65, 1101–1109. [Google Scholar] [CrossRef]
- Riding, K.A.; Silva, D.A.; Scrivener, K. Early Age Strength Enhancement of Blended Cement Systems by CaCl2 and Diethanol-isopropanolamine. Cem. Concr. Res. 2010, 40, 935–946. [Google Scholar] [CrossRef] [Green Version]





| Oxide | Cement | Fly Ash | Slag |
|---|---|---|---|
| CaO | 61.75 | 7.40 | 41.5 |
| SiO2 | 20.64 | 43.9 | 32.2 |
| Al2O3 | 4.62 | 34.8 | 14.6 |
| Fe2O3 | 2.82 | 6.13 | 0.96 |
| K2O | 0.48 | 1.09 | 0.57 |
| MgO | 2.06 | 0.65 | 6.37 |
| Na2O | 0.12 | 0.43 | 0.30 |
| SO3 | 1.20 | 2.00 | 2.12 |
| TiO2 | 0.29 | 1.51 | 0.61 |
| Sample | Cement | Slag | Fly Ash | Water | Sand | Fine Aggregate | Coarse Aggregate | NPI |
|---|---|---|---|---|---|---|---|---|
| 40S | 269.5 | 147 | 73.5 | 196 | 703 | 405 | 607 | 0 |
| 40SNPI-0.6% | 269.5 | 147 | 73.5 | 180 | 703 | 405 | 607 | 16 |
| 40SNPI-0.9% | 269.5 | 147 | 73.5 | 172 | 703 | 405 | 607 | 25 |
| 40SNPI-1.2% | 269.5 | 147 | 73.5 | 163 | 703 | 405 | 607 | 33 |
| Mark | CS | Dnss (10−12 m2/s) | R2 |
|---|---|---|---|
| 40S | 0.88 | 4.96 | 0.97 |
| 40SNPI-0.6% | 0.76 | 4.18 | 0.99 |
| 40SNPI-0.9% | 0.83 | 3.29 | 0.99 |
| 40SNPI-1.2% | 0.63 | 4.63 | 0.97 |
| Mark | Porosity (%) | Mean Pore Size (nm) |
|---|---|---|
| 40S | 24.7 | 16.1 |
| 40SNPI-0.6% | 31.7 | 20.0 |
| 40SNPI-0.9% | 24.5 | 15.2 |
| 40SNPI-1.2% | 24.9 | 17.2 |
| Sample No. | Contact Angle (°), T = 5 s | Contact Angle (°), T = 30 s |
|---|---|---|
| 40S | 17.8 ± 1.2 | 9.8 ± 0.15 |
| 40SNPI-0.6% | 19.2 ± 0.7 | 17.3 ± 0.4 |
| 40SNPI-0.9% | 39.3 ± 2.2 | 34.3 ± 4.6 |
| 40SNPI-1.2% | 85.8 ± 2.2 | 78.2 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Mu, S.; Liu, J.; Cai, J.; Xie, D.; Liu, G.; Guo, Z. Novel Nano-Precursor Inhibiting Material for Improving Chloride Penetration Resistance of Concrete: Evaluation and Mechanism. Materials 2021, 14, 5929. https://doi.org/10.3390/ma14205929
Chen R, Mu S, Liu J, Cai J, Xie D, Liu G, Guo Z. Novel Nano-Precursor Inhibiting Material for Improving Chloride Penetration Resistance of Concrete: Evaluation and Mechanism. Materials. 2021; 14(20):5929. https://doi.org/10.3390/ma14205929
Chicago/Turabian StyleChen, Ruixing, Song Mu, Jiaping Liu, Jingshun Cai, Deqing Xie, Guangyan Liu, and Zheng Guo. 2021. "Novel Nano-Precursor Inhibiting Material for Improving Chloride Penetration Resistance of Concrete: Evaluation and Mechanism" Materials 14, no. 20: 5929. https://doi.org/10.3390/ma14205929
APA StyleChen, R., Mu, S., Liu, J., Cai, J., Xie, D., Liu, G., & Guo, Z. (2021). Novel Nano-Precursor Inhibiting Material for Improving Chloride Penetration Resistance of Concrete: Evaluation and Mechanism. Materials, 14(20), 5929. https://doi.org/10.3390/ma14205929






