Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene
Abstract
1. Introduction
2. Fabrication of CNMs
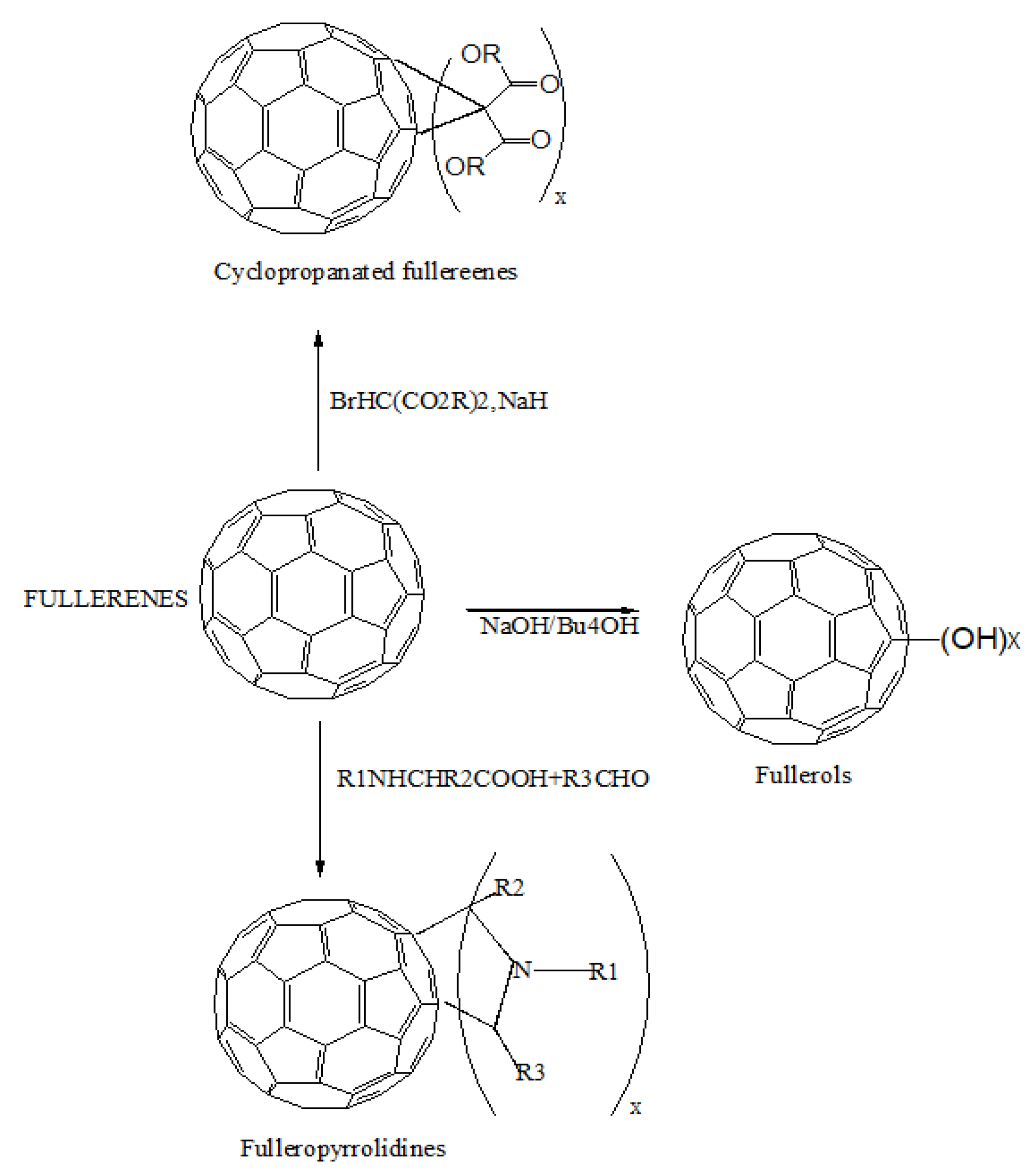
2.1. Fullerenes
2.2. Nanodiamonds
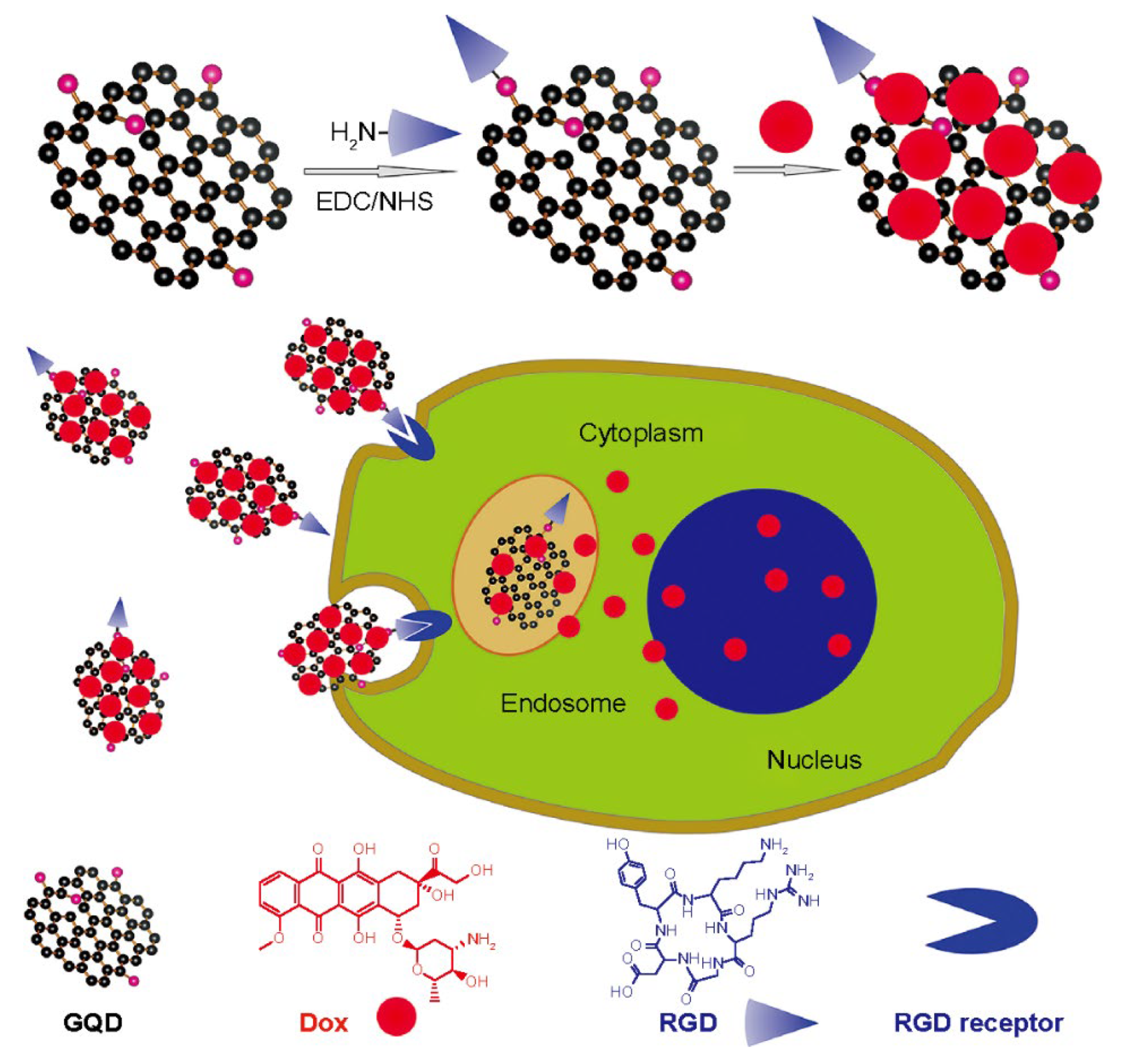
2.3. Carbon Quantum Dots
2.4. Carbon Nanotubes
2.5. Carbon Nanofibers
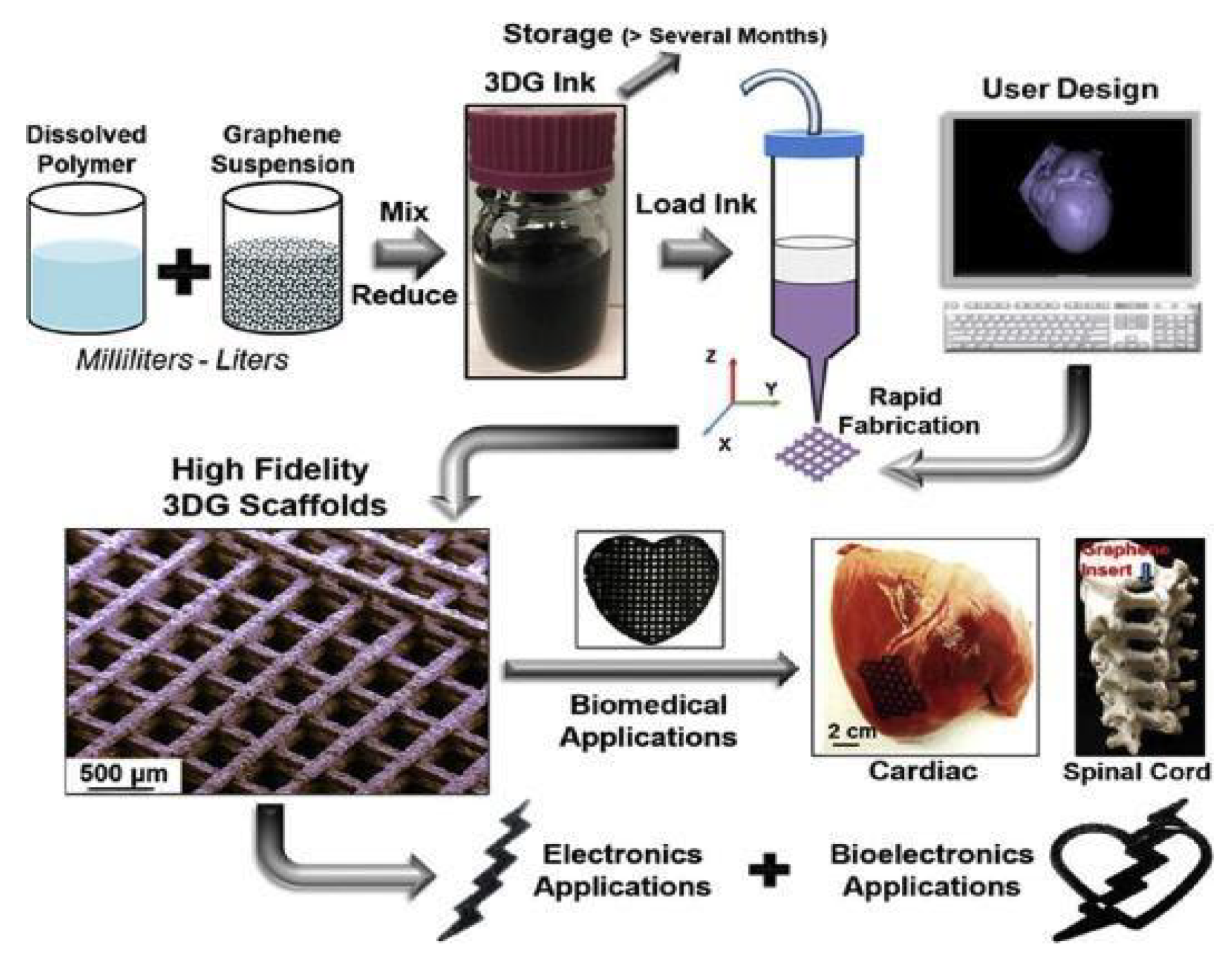
2.6. Graphene Nanosheets
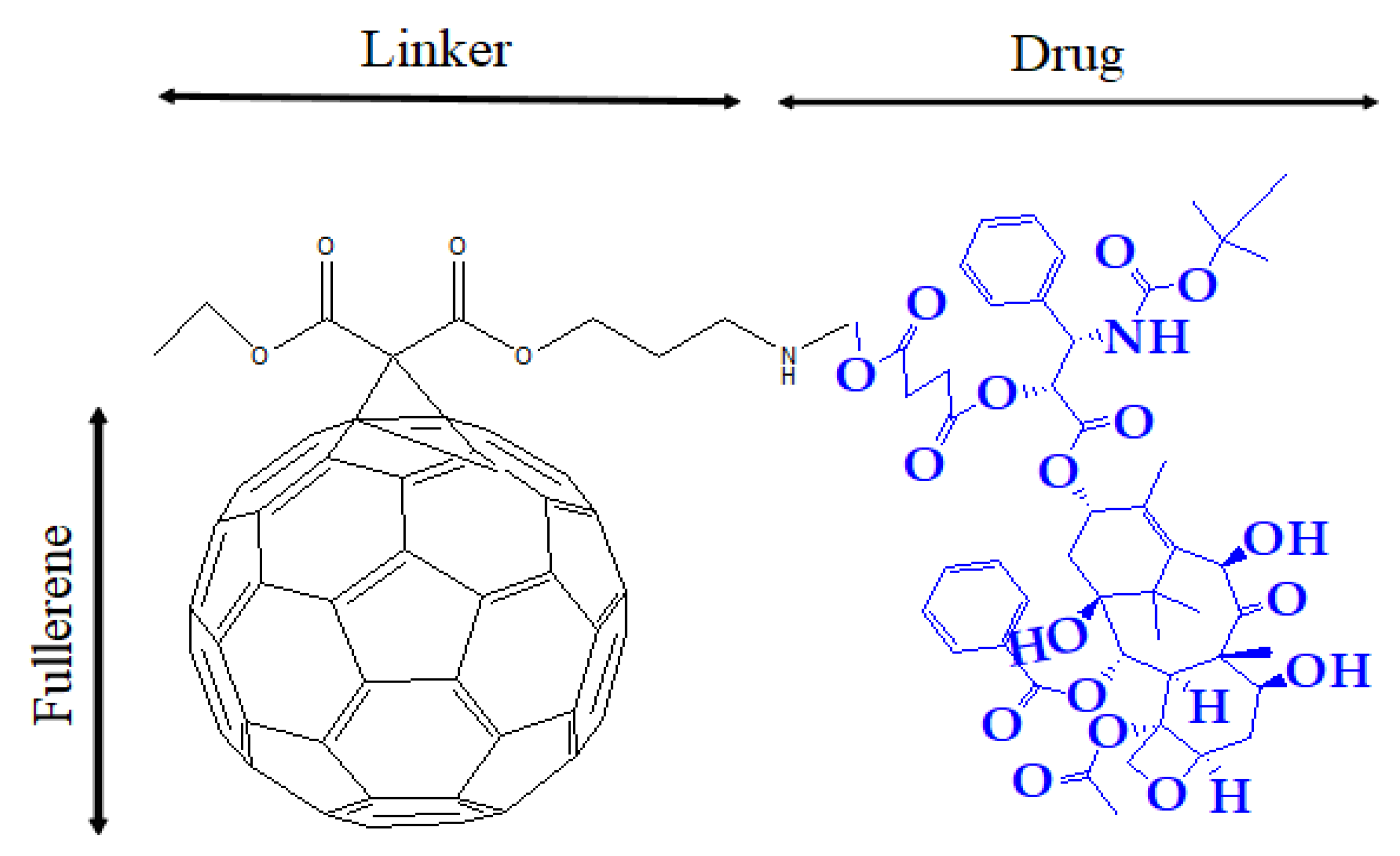
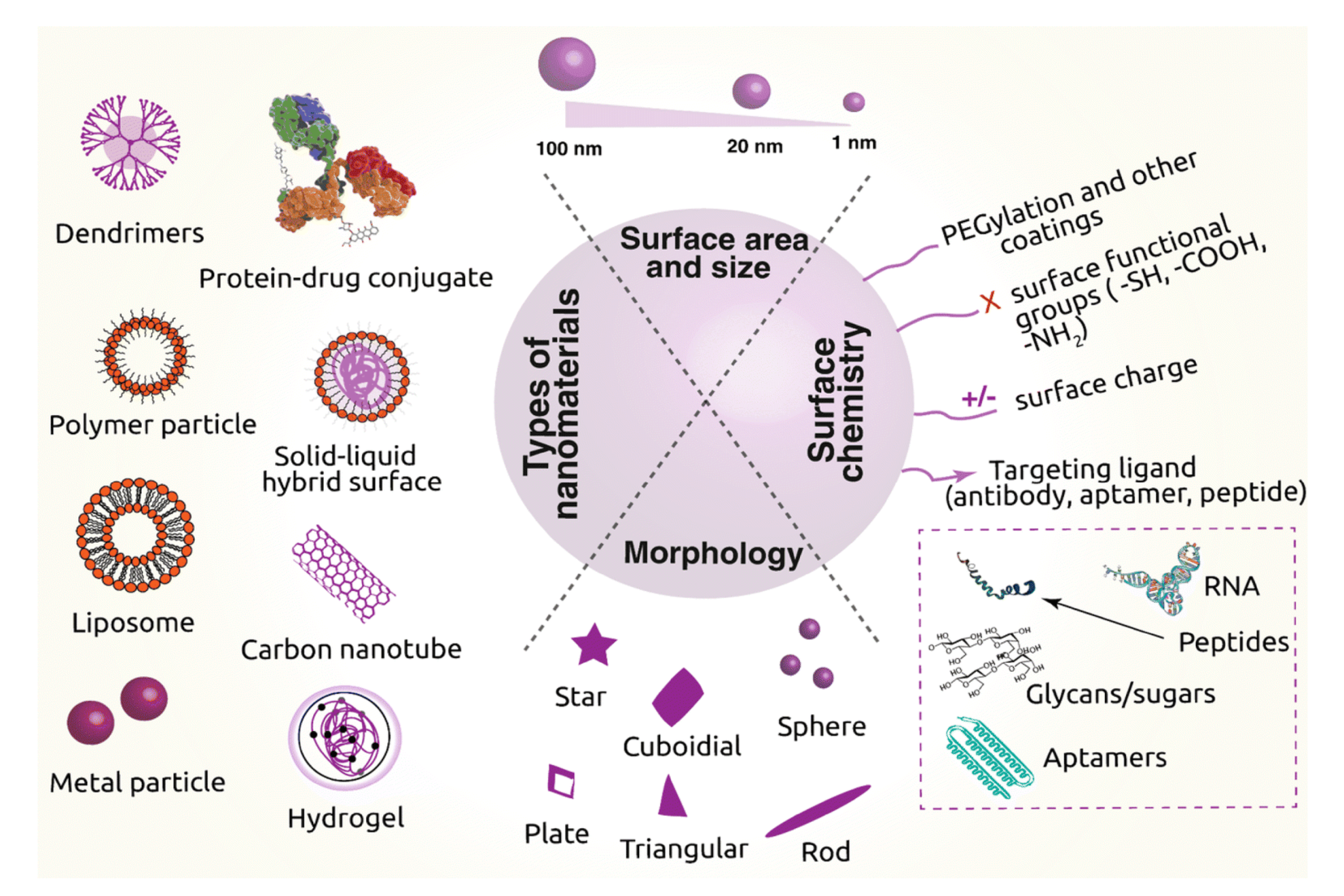
3. Drug Delivery Systems
4. Biomedical Scaffolds
5. Tissue Engineering
6. Wound Healing
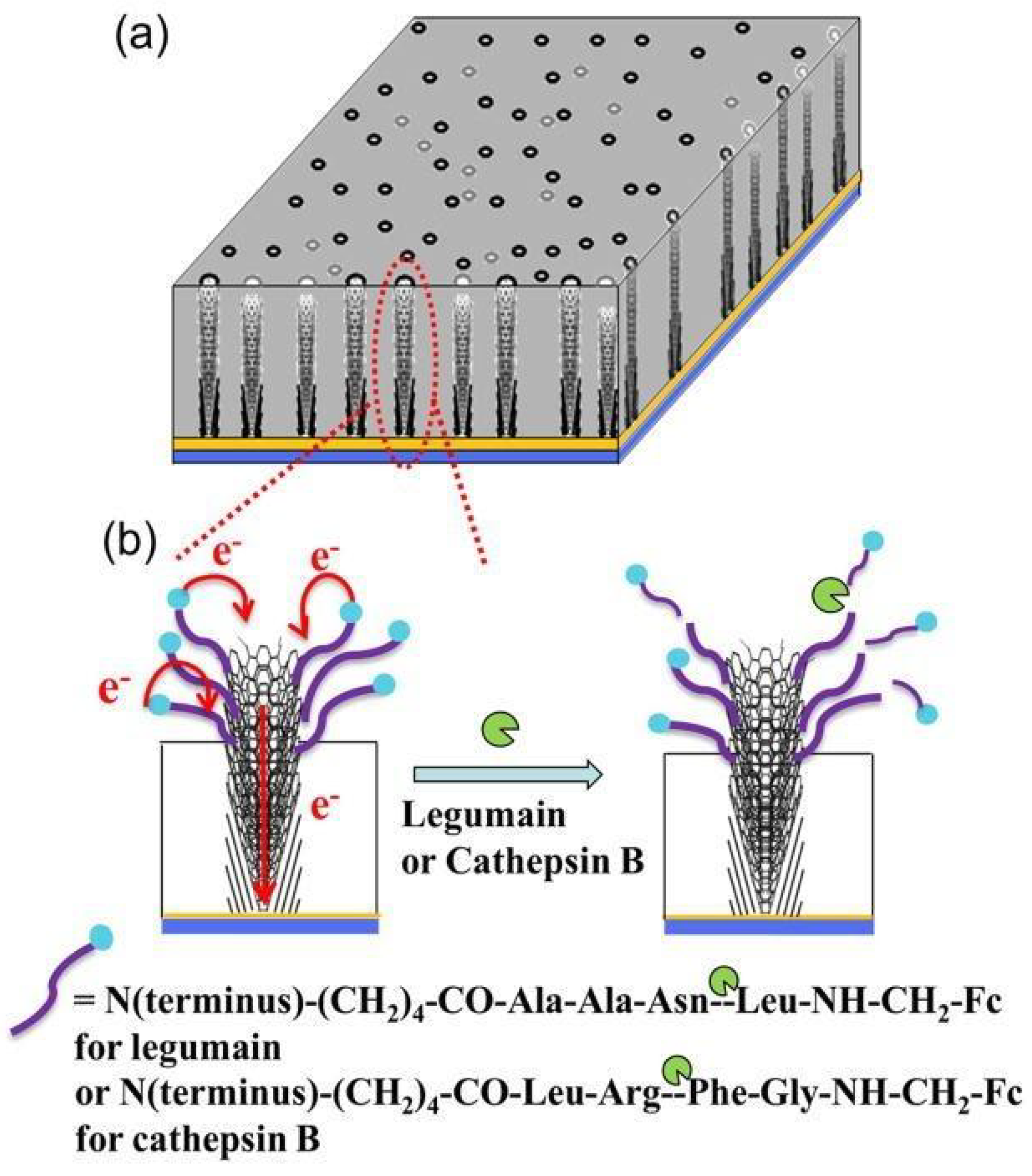
7. Biosensors
8. Bioimaging Applications
9. Vaccination
10. Photodynamic Therapy (PDT)
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Gajdosechova, Z.; Mester, Z. Recent trends in analysis of nanoparticles in biological matrices. Anal. Bioanal. Chem. 2019, 411, 4277–4292. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Ortiz, A.G.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef]
- Kumar, N.; Kumbhat, S. Carbon-Based Nanomaterials. In Essentials in Nanoscience and Nanotechnology; Wiley: Hoboken, NJ, USA, 2016; pp. 189–236. [Google Scholar]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Meireles, G.S.; Dias, A.T.; Aires, R.; Porto, M.L.; Gava, A.L.; Vasquez, E.C.; Pereira, T.M.C.; Campagnaro, B.P.; Meyrelles, S.S. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Bustos, A.R.M.; Reipa, V.; Riley, K.R.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2019, 134, 105298. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Shah, S. Progression from Graphene and Graphene Oxide to High Performance Polymer-Based Nanocomposite: A Review. Polym. Technol. Eng. 2015, 54, 173–183. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Barhoum, A.; Shalan, A.E.; El-Hout, S.I.; Ali, G.A.M.; Abdelbasir, S.M.; Abu Serea, E.S.; Ibrahim, A.H.; Pal, K. A Broad Family of Carbon Nanomaterials: Classification, Properties, Synthesis, and Emerging Applications. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–40. [Google Scholar]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef] [PubMed]
- Abdo, G.G.; Zagho, M.M.; Al Moustafa, A.; Khalil, A.; Elzatahry, A.A. A comprehensive review summarizing the recent biomedical applications of functionalized carbon nanofibers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Lakshmi, B.B.; Martin, A.C.R.; Fisher, E.R.; Ruoff, R.S. Chemical Vapor Deposition Based Synthesis of Carbon Nanotubes and Nanofibers Using a Template Method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.; Apicella, A.; Petrescu, F.I. Nano-diamond hybrid materials for structural biomedical application. Am. J. Biochem. Biotechnol. 2016, 13, 34–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Amans, D.; Chenus, A.-C.; Ledoux, G.; Dujardin, C.; Reynaud, C.; Sublemontier, O.; Masenelli-Varlot, K.; Guillois, O. Nanodiamond synthesis by pulsed laser ablation in liquids. Diam. Relat. Mater. 2009, 18, 177–180. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoumbb, A.; Gaber, S.A.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalilaf, A.S. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- El-Beshlawy, M.M.; Abdel-Haleem, F.M.; Barhoum, A. Molecularly Imprinted Potentiometric Sensor for Nanomolar Determination of Pioglitazone Hydrochloride in Pharmaceutical Formulations. Electroanalysis 2021, 33, 1244–1254. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fuller-enes. Int. J. Nanomed. 2007, 2, 639. [Google Scholar]
- Martinez, Z.S.; Castro, E.; Seong, C.-S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [PubMed]
- Ghiassi, K.B.; Olmstead, M.M.; Balch, A.L. Gadolinium-containing endohedral fullerenes: Structures and function as magnetic resonance imaging (MRI) agents. Dalton Trans. 2014, 43, 7346–7358. [Google Scholar] [CrossRef]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Mirakyan, A.L.; Wilson, L.J. Functionalization of C60 with diphosphonate groups: A route to bone-vectored fullerenes. J. Chem. Soc. Perkin Trans. 2002, 2002, 1173–1176. [Google Scholar] [CrossRef]
- Lee, D.J.; Ahn, Y.S.; Youn, Y.S.; Lee, E.S. Poly(ethylene glycol)-crosslinked fullerenes for high efficient phototherapy. Polym. Adv. Technol. 2013, 24, 220–227. [Google Scholar] [CrossRef]
- Popov, A.A. Structures and Stability of Fullerenes, Metallofullerenes, and Their Derivatives. Handb. Comput. Chem. 2016, 1, 1–66. [Google Scholar] [CrossRef]
- Bolskar, R.D. Fullerenes for Drug Delivery. Encycl. Nanotechnol. 2016, 8, 1267–1281. [Google Scholar] [CrossRef]
- Klupp, G.; Margadonna, S.; Prassides, K. Fullerenes. Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Nafisi, S. Nanotechnology in cosmetics. In Cosmetic Science and Technology; Routledge: London, UK, 2017. [Google Scholar]
- Rao, R.N. Nanomaterials in Chromatographic Sample Preparations; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Saldmann, F.; Saldmann, A.; Lemaire, M.C. Characterization and internalization of nanodiamond–trehalose conjugates into mammalian fibroblast cells of naked mole rat. Int. Nano Lett. 2020, 10, 151–157. [Google Scholar] [CrossRef]
- Mengesha, A.; Youan, B.-B. Nanodiamonds for drug delivery systems. Diam.-Based Mater. Biomed. Appl. 2013, 186–205. [Google Scholar] [CrossRef]
- Lim, D.G.; Prim, R.E.; Kim, K.H.; Kang, E.; Park, K.; Jeong, S.H. Combinatorial nanodiamond in pharmaceutical and biomedical applications. Int. J. Pharm. 2016, 514, 41–51. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.; Du, F.; Wu, Y.; Cai, R.; Xu, L.; Jin, H.; Zou, S.; Gong, A.; Du, F. Facile synthesis of cerium-doped carbon quantum dots as a highly efficient antioxidant for free radical scavenging. Nanotechnology 2019, 30, 325101. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C 2019, 5, 24. [Google Scholar] [CrossRef]
- Budimir, M.; Szunerits, S. Nanoscale Materials for the Treatment of Water Contaminated by Bacteria and Viruses; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Flores-Pacheco, A. Down-Shifting by Quantum Dots for Silicon Solar Cell Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C. 2020, 27, 111756. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.R. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int. J. Nanomed. 2015, 10, 6709–6724. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nat. Cell Biol. 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; De Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nat. Cell Biol. 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Syrgiannis, Z.; Melchionna, M.; Prato, M. Covalent Carbon Nanotube Functionalization. Encycl. Polym. Nanomater. 2014, 1–8. [Google Scholar] [CrossRef]
- Nikolaev, P. Gas-Phase Production of Single-Walled Carbon Nanotubes from Carbon Monoxide: A Review of the HiPco Process. J. Nanosci. Nanotechnol. 2004, 4, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.J.; Shanov, V.N.; Yun, Y. Nanomedicine Design of Particles, Sensors, Motors, Implants, Robots, and Devices; Artech House Publishers: Norwood, MA, USA, 2009. [Google Scholar]
- Bensghaïer, A.; Truong, S.L.; Seydou, M.; Lamouri, A.; Leroy, E.; Mičušik, M.; Forro, K.; Beji, M.; Pinson, J.; Omastová, M.; et al. Efficient Covalent Modification of Multiwalled Carbon Nanotubes with Diazotized Dyes in Water at Room Temperature. Langmuir 2017, 33, 6677–6690. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, D.; Su, Y.; Li, J.; Jiang, Z.; Jiang, Y.; Yuan, W. Covalent functionalization of multi-walled carbon nanotubes by lipase. J. Nanopart. Res. 2007, 9, 1205–1210. [Google Scholar] [CrossRef]
- Tran, P.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Harrison, B. Carbon Nanotube Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Kim, Y.A.; Hayashi, T.; Endo, M.; Dresselhaus, M.S. Carbon Nanofibers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–262. [Google Scholar] [CrossRef]
- Desmaris, V.; Saleem, A.M.; Shafiee, S.; Berg, J.; Kabir, M.S.; Johansson, A. Carbon Nanofibers (CNF) for enhanced solder-based nano-scale integration and on-chip interconnect solutions. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; pp. 1071–1076. [Google Scholar] [CrossRef]
- Han, L.; Andrady, A.L.; Ensor, D.S. Chemical sensing using electrospun polymer/carbon nanotube composite nanofibers with printed-on electrodes. Sens. Actuators B Chem. 2013, 186, 52–55. [Google Scholar] [CrossRef]
- Ozkan, T.; Naraghi, M.; Chasiotis, I. Mechanical properties of vapor grown carbon nanofibers. Carbon 2010, 48, 239–244. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Fu, S.; Liu, Y.; Shao, C. Polyacrylonitrile and Carbon Nanofibers with Controllable Nanoporous Structures by Electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.D.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Swisher, L.Z.; Syed, L.U.; Prior, A.M.; Madiyar, F.R.; Carlson, K.R.; Nguyen, T.A.; Hua, D.H.; Li, J. Electrochemical Protease Biosensor Based on Enhanced AC Voltammetry Using Carbon Nanofiber Nanoelectrode Arrays. J. Phys. Chem. C 2013, 117, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bermea, C.; Rajukumar, L.P.; Dasgupta, A.; Lei, Y.; Hashimoto, Y.; Sepulveda-Guzman, S.; Cruz-Silva, R.; Endo, M.; Terrones, M. Two-dimensional and three-dimensional hybrid assemblies based on graphene oxide and other layered structures: A carbon science perspective. Carbon 2017, 125, 437–453. [Google Scholar] [CrossRef]
- Pyun, J. Graphene Oxide as Catalyst: Application of Carbon Materials beyond Nanotechnology. Angew. Chem. Int. Ed. 2011, 50, 46–48. [Google Scholar] [CrossRef]
- Tite, T.; Chiticaru, E.A.; Burns, J.S.; Ioniţă, M. Impact of nano-morphology, lattice defects and conductivity on the performance of graphene based electrochemical biosensors. J. Nanobiotechnol. 2019, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.A.; González, D.; Quijada, R.; Basso, N.; Cerrada, M.L.; Azambuja, D.S.; Galland, G.B. Polypropylene/graphene nanosheet nanocomposites by in situ polymerization: Synthesis, characterization and fundamental properties. Compos. Sci. Technol. 2013, 84, 1–7. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Hensleigh, R.M.; Cui, H.; Oakdale, J.S.; Ye, J.C.; Campbell, P.G.; Duoss, E.B.; Spadaccini, C.M.; Zheng, X.; Worsley, M.A. Additive manufacturing of complex micro-architected graphene aerogels. Mater. Horiz. 2018, 5, 1035–1041. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Payload delivery of anticancer drug Tegafur with the assistance of graphene oxide nanosheet during biomembrane penetration: Molecular dynamics simulation survey. Appl. Surf. Sci. 2020, 517, 146186. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Zhao, X.; Kuznetsova, L.V.; Wong, S.S.; Ojima, I. Functionalized Single-Walled Carbon Nanotubes as Rationally Designed Vehicles for Tumor-Targeted Drug Delivery. J. Am. Chem. Soc. 2008, 130, 16778–16785. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Singh, R.P.; Datir, S.R.; Jain, S. Intranuclear Drug Delivery and Effective in Vivo Cancer Therapy via Estradiol–PEG-Appended Multiwalled Carbon Nanotubes. Mol. Pharm. 2013, 10, 3404–3416. [Google Scholar] [CrossRef]
- Friedman, S.H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the HIV-1 protease by fullerene derivatives: Model building studies and experimental verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene Derivatives: An Attractive Tool for Biological Applications. Eur. J. Med. Chem. 2004, 35, 913–923. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured mate-rials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H.K.F.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef]
- Thotakura, N.; Sharma, S.; Khurana, R.K.; Babu, P.V.; Chitkara, D.; Kumar, V.; Singh, B.; Raza, K. Aspartic acid tagged carbon nanotubols as a tool to deliver docetaxel to breast cancer cells: Reduced hemotoxicity with improved cytotoxicity. Toxicol. Vitr. 2019, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.; Raza, K. -desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: A synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C 2018, 89, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Lin, C.-Y.; Peng, C.-L.; Shieh, M.-J. Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater. Sci. 2016, 4, 1742–1753. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, D.; Dai, R.; Fu, D.; Li, W.; Guo, Z.; Cui, C.; Xu, J.; Shen, S.; Ma, K. PEGylated doxorubicin cloaked nano-graphene oxide for dual-responsive photochemical therapy. Int. J. Pharm. 2019, 557, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chitphet, K.; Geary, S.M.; Chan, C.H.F.; Simons, A.L.; Weiner, G.J.; Salem, A.K. Combining Doxorubicin-Loaded PEGylated Poly(Lactide-co-glycolide) Nanoparticles with Checkpoint Inhibition Safely Enhances Therapeutic Efficacy in a Melanoma Model. ACS Biomater. Sci. Eng. 2019, 6, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.; Tabakman, S.; Yang, K. Carbon Materials for Drug Delivery & Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Toudeshki, R.M.; Dadfarnia, S.; Shabani, A.M.H. Surface molecularly imprinted polymer on magnetic multi-walled carbon nanotubes for selective recognition and preconcentration of metformin in biological fluids prior to its sensitive chemiluminescence determination: Central composite design optimization. Anal. Chim. Acta 2019, 1089, 78–89. [Google Scholar] [CrossRef]
- Zakharian, T.Y.; Seryshev, A.; Sitharaman, B.; Gilbert, B.E.; Knight, V.; Wilson, L.J. A Fullerene−Paclitaxel Chemotherapeutic: Synthesis, Characterization, and Study of Biological Activity in Tissue Culture. J. Am. Chem. Soc. 2005, 127, 12508–12509. [Google Scholar] [CrossRef]
- Misra, C.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. Glycinated fullerenes for tamoxifen intracellular delivery with improved anticancer activity and pharmacokinetics. Nanomedicine 2017, 12, 1011–1023. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. A transdermal diltiazem hydrochloride delivery device using multi-walled carbon nanotube/poly(vinyl alcohol) composites. Carbon 2013, 52, 305–315. [Google Scholar] [CrossRef]
- Zhanga, X.; Meng, L.; Luab, Q.; Feic, Z.; Dyson, P. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, S.; Wang, M.-W.; Li, Y.; Shi, P.; Huang, X. Delivery of Paclitaxel Using PEGylated Graphene Oxide as a Nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 1355–1363. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.-Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3, 73ra21. [Google Scholar] [CrossRef]
- Kalita, S.J. Nanostructured Biomaterials; Springer: Stuttgart, Germany, 2008; pp. 168–219. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Zivic, F.; Affatato, S.; Trajanovic, M.; Schnabelrauch, M. Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Krul, L.; Volozhyn, A.; Belov, D.; Poloiko, N.; Artushkevich, A.; Zhdanok, S.; Solntsev, A.; Krauklis, A.; Zhukova, I. Nanocomposites based on poly-d,l-lactide and multiwall carbon nanotubes. Biomol. Eng. 2007, 24, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloys Compd. 2016, 688, 657–667. [Google Scholar] [CrossRef]
- Auciello, O.; Gurman, P.; Guglielmotti, M.B.; Olmedo, D.G.; Berra, A.; Saravia, M.J. Biocompatible ultrananocrystalline diamond coatings for implantable medical devices. MRS Bull. 2014, 39, 621–629. [Google Scholar] [CrossRef]
- Ghosh, S.; Haldar, S.; Gupta, S.; Bisht, A.; Chauhan, S.; Kumar, V.; Roy, P.; Lahiri, D. Anisotropically Conductive Biodegradable Scaffold with Coaxially Aligned Carbon Nanotubes for Directional Regeneration of Peripheral Nerves. ACS Appl. Bio Mater. 2020, 3, 5796–5812. [Google Scholar] [CrossRef]
- Goyal, A.; Goetz, S.; Stanslaski, S.; Oh, Y.; Rusheen, A.E.; Klassen, B.; Miller, K.; Blaha, C.D.; Bennet, K.E.; Lee, K. The development of an implantable deep brain stimulation device with simultaneous chronic electrophysiological recording and stimulation in humans. Biosens. Bioelectron. 2021, 176, 112888. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Buschbeck, E.; Miller, S.; Le, A.D.; Gupta, V.; Ruhunage, C.; Vilinsky, I.; Ma, Y. Carbon Nanotube Fibers for Neural Recording and Stimulation. ACS Appl. Bio Mater. 2020, 3, 6478–6487. [Google Scholar] [CrossRef]
- Kumar, A.; Suresh, B.; Ramakrishna, S. Biocompatible responsive polypyrrole/GO nanocomposite coatings for biomedical applications. RSC Adv. 2015, 5, 99866–99874. [Google Scholar] [CrossRef]
- Sirivisoot, S. Skeletal myotube formation enhanced by electrospun poly-urethane carbon nanotube scaffolds. Int. J. Nanomed. 2011, 6, 2483. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In Vitro and In Vivo Evaluation of a Three-Dimensional Porous Multi-Walled Carbon Nanotube Scaffold for Bone Regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef]
- Tashakori-Miyanroudi, M.; Rakhshan, K. Conductive Carbon Nanofibers Incorporated into Collagen Bio-Scaffold Assists Myocardial Injury Repair; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Xu, J.; Khor, K.; Sui, J. Preparation and Characterization of a Novel Hydroxyapatite/Carbon Nanotubes Composite and Its Interaction with Osteoblast-Like Cells; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in Tissue Engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in Tissue Engineering. Nat. Biotechnol. 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tis-sue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Hass, J.; Marchenkov, A.N.; Conrad, E.H.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutierrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef]
- Lu, C.-H.; Zhu, C.-L.; Li, J.; Liu, J.-J.; Chen, X.; Yang, H.-H. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Commun. 2010, 46, 3116–3118. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Kalbacova, M.H.; Brož, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R. Nanofibrous scaffolds of ϵ-polycaprolactone containing Sr/Se-hydroxyapatite/graphene oxide for tissue engineering applications. Biomed. Mater. 2021, 16, 045030. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.; Razal, J.; Wallace, G. Organic Solvent-Based Graphene Oxide Liquid Crystals: A Facile Route toward the Next Generation of Self-Assembled Layer-by-Layer Multifunctional 3D Architectures. ACS Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Khalili, R.; Zarintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-graphene oxide/chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Mohammadi, Z. Carbon nanotube doped pericardial matrix derived electro-conductivebiohybrid hydrogel for cardiac tissue engineering. Biomater. Sci. 2019, 7, 3906–3917. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.; Sakai, H.; Ariga, K. Highly Ordered 1D Fullerene Crystals for Concurrent Control of Macroscopic Cellular Orientation and Differentiation toward Large-Scale Tissue Engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.; Neitzel, I.; Knoke, I. Fluorescent PLLA-Nanodiamond Composites for Bone Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jin, L.; Ren, K.; Xu, Q.; Hong, T.; Wu, S.; Zhang, Y.; Wang, Z. Multifunctional carbon dots for live cell staining and tissue engineering applications. Polym. Compos. 2018, 39, 73–80. [Google Scholar] [CrossRef]
- Mehrabi, A.; Baheiraei, N.; Adabi, M.; Amirkhani, Z. Development of a Novel Electroactive Cardiac Patch Based on Carbon Nanofibers and Gelatin Encouraging Vascularization. Appl. Biochem. Biotechnol. 2019, 190, 931–948. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S. Engineering Stiffness in Highly Porous Biomimetic Gelatin/Tertiary Bioactive Glass Hybrid Scaffolds Using Graphene Nanosheets; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L.A. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Mathieu, D. Handbook on Hyperbaric Medicine; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-140-204-44-89. [Google Scholar]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical Potential of Ultrafine Ag/AgCl Nanoparticles Coated on Graphene with Special Reference to Antimicrobial Performances and Burn Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zou, W.; Ning, W.; Fan, J.; Li, L.; Liu, B.; Liu, X. Synthesis of DNA-guided silver nanoparticles on a graphene oxide surface: Enhancing the antibacterial effect and the wound healing activity. RSC Adv. 2018, 8, 28238–28248. [Google Scholar] [CrossRef]
- Zhou, Z.; Joslin, S.; Dellinger, A.; Ehrich, M.; Brooks, B.; Ren, Q.; Rodeck, U.; Lenk, R.; Kepley, C.L. A Novel Class of Compounds with Cutaneous Wound Healing Properties. J. Biomed. Nanotechnol. 2010, 6, 605–611. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Mansur, A.; Ciminelli, V.S.T.; Mansur, H. Nanocomposites of Poly(Vinyl Alcohol)/Functionalized-Multiwall Carbon Nanotubes Conjugated With Glucose Oxidase for Potential Application as Scaffolds in Skin Wound Healing. Int. J. Polym. Mater. 2014, 63, 185–196. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Ma, H.; Bai, H.; Liu, L.; Shu, C.; Li, H.; Wang, S.; Wang, C. Designing an Amino-Fullerene Derivative C70–(EDA)8 to Fight Superbacteria. ACS Appl. Mater. Interfaces 2019, 11, 14597–14607. [Google Scholar] [CrossRef]
- Kandra, R. Synthesis, Mechanical Properties of Fluorescent Carbon Dots Loaded Nanocomposites Chitosan Film for Wound Healing and Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fang, J.; Wang, H.; Bao, X.; Ni, Y.; Teng, Y.; Liu, J.; Carbon, X.S. Nanodiamond as Efficient Peroxidase Mimic against Periodontal Bacterial Infection; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cam, M.; Ertas, B.; Alenezi, H. Accelerated Diabetic Wound Healing by Topical Application of Combination Oral Antidiabetic Agents-Loaded Nanofibrous Scaffolds: An In Vitro and In Vivo Evaluation; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Li, Z.; Wang, H.; Yang, B.; Sun, Y. Three-Dimensional Graphene Foams Loaded with Bone Marrow Derived Mesenchymal Stem Cells Promote Skin Wound Healing with Reduced Scarring; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Jamal, M.; Shaikh, F.M.; Aslam, B.; Razeeb, K.M. Sensor and biosensor to detect vascular graft infection: Diagnosis and challenges. Anal. Methods 2012, 4, 1865–1875. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Slaughter, G. Current Advances in Biosensor Design and Fabrication. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–25. [Google Scholar]
- Dervisevic, M.; Dervisevic, E.; Şenel, M. Recent progress in nanomaterial-based electrochemical and optical sensors for hypoxanthine and xanthine. A review. Microchim. Acta 2019, 186, 749. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Shih, J.-S. Immobilized Fullerene C60-Enzyme-Based Electrochemical Glucose Sensor. J. Chin. Chem. Soc. 2011, 58, 228–235. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Gruber, A.; Dräbenstedt, A.; Tietz, C.; Fleury, L.; Wrachtrup, J.; von Borczyskowski, C. Scanning Confocal Optical Microscopy and Magnetic Resonance on Single Defect Centers. Science 1997, 276, 2012–2014. [Google Scholar] [CrossRef]
- Baptista, F.; Belhout, S.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.-S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Peairs, M. Carbon Nanotube Based Electrochemical Sensors for Biomolecules; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Zhu, L.; Deng, C.; Chen, P.; You, X.; Su, H.; Yuan, Y.; Carbon, M.Z. Glucose oxidase biosensors based on carbon nanotube non-woven fabrics. Carbon 2014, 1, 795–796. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- González-Gaitán, C.; Ruiz-Rosas, R. Effects of the surface chemistry and structure of carbon nanotubes on the coating of glucose oxidase and electrochemical biosensors performance. RSC Adv. 2017, 7, 26867–26878. [Google Scholar] [CrossRef]
- Ulissi, Z.W.; Sen, F.; Gong, X.; Sen, S.; Iverson, N.; Boghossian, A.A.; Godoy, L.C.; Wogan, G.N.; Mukhopadhyay, D.; Strano, M.S. Spatiotemporal Intracellular Nitric Oxide Signaling Captured Using Internalized, Near-Infrared Fluorescent Carbon Nanotube Nanosensors. Nano Lett. 2014, 14, 4887–4894. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.; Ebenso, E.E. Electrocatalytic oxidation of Epinephrine and Norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef]
- Kumar, A.; Sun, S.-S.; Lees, A.J. Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 2008, 252, 922–939. [Google Scholar] [CrossRef]
- Tang, L.A.L.; Wang, J.; Loh, K.P. Graphene-Based SELDI Probe with Ultrahigh Extraction and Sensitivity for DNA Oligomer. J. Am. Chem. Soc. 2010, 132, 10976–10977. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, H.; Itoh, T.; Hayashi, H.; Takagi, K. Electrochemical detection of E. coli 16S rDNA sequence using air-plasma-activated fullerene-impregnated screen printed electrodes. Bioelectrochemistry 2007, 70, 481–487. [Google Scholar] [CrossRef]
- Zhu, S.; Nanoscale, G.X. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Gong, L.; Dai, H.; Li, X.; Zhang, S.; Lu, S. Electrochemical bisphenol A sensor based on carbon nanohorns. Anal. Methods 2013, 5, 3328–3333. [Google Scholar] [CrossRef]
- Dai, H.; Gong, L.; Xu, G.; Zhang, S.; Lu, S.; Jiang, Y. An Electrochemical Sensing Platform Structured with Carbon Nanohorns for Detecting Some Food Borne Contaminants; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhu, S.; Gao, W.; Zhang, L.; Zhao, J. Simultaneous Voltammetric Determination of Dihydroxybenzene Isomers at Single-Walled Carbon Nanohorn Modified Glassy Carbon Electrode; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhu, S.; Han, S.; Zhang, L.; Parveen, S. A novel fluorescent aptasensor based on single-walled carbon nanohorns. Nanoscale 2011, 3, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Z.; Zhang, W.; Han, S.; Hu, L. Nucleic acid detection using single-walled carbon nanohorns as a fluorescent sensing platform. Chem. Commun. 2011, 47, 6099–6101. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef]
- Baldo, S.; Buccheri, S.; Ballo, A. Carbon Nanotube-Based Sensing Devices for Human Ar-Ginase-1 Detection; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Huang, Y.; Miao, Y.-E.; Ji, S.; Tjiu, W.W.; Liu, T. Electrospun Carbon Nanofibers Decorated with Ag–Pt Bimetallic Nanoparticles for Selective Detection of Dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, L.; Li, X.; Zhou, J.; Chen, Q.; Yan, S.; Li, N.; Chu, M.; Dong, Y.; Xie, Z.; et al. An Electrochemical DNA Sens-ing Platform Using Carboxyl Functionalized Graphene as the Electrode Modified Material. J. Electrochem. Soc. 2017, 164, H345–H351. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R. A novel glucose biosensor at glucose oxidase immobilized graphene and bismuth nanocomposite film modified electrode. Citeseer 2015, 10, 691–700. [Google Scholar]
- Yao, D.; He, Z.; Wen, G.; Liang, A. A facile and highly sensitive resonance Rayleigh scattering-energy transfer method for urea using a fullerene probe. RSC Adv. 2018, 8, 29008–29012. [Google Scholar] [CrossRef]
- Malik, N.; Arfin, T. Graphene Nanomaterials: Chemistry and Pharmaceutical Perspectives; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fakhri, N.; Wessel, A.D.; Willms, C.; Pasquali, M.; Klopfenstein, D.R.; MacKintosh, F.C.; Schmidt, C.F. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science 2014, 344, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Huth, K.; Glaeske, M.; Achazi, K.; Gordeev, G.; Kumar, S.; Arenal, R.; Sharma, S.K.; Adeli, M.; Setaro, A.; Reich, S.; et al. Fluorescent Polymer-Single-Walled Carbon Nanotube Complexes with Charged and Noncharged Dendronized Perylene Bisimides for Bioimaging Studies. Small 2018, 14, e1800796. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, T.K.; Reith, R.; Simonette, R.A.; Harden, M.E.; Cherukuri, P.; Tsyboulski, D.A.; Beckingham, K.M.; Weisman, R.B. Single-Walled Carbon Nanotubes in the Intact Organism: Near-IR Imaging and Biocompatibility Studies in Drosophila. Nano Lett. 2007, 7, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ohulchanskyy, T.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Yang, F.; Han, J.; Zhuo, Y.; Yang, Z.; Chai, Y.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanohorns as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-H.; Chen, Y.-W.; Hung, W.-T.; Chen, I.-W.; Chen, S.-Y. Quantum-Dot-Tagged Reduced Graphene Oxide Nanocomposites for Bright Fluorescence Bioimaging and Photothermal Therapy Monitored In Situ. Adv. Mater. 2012, 24, 1748–1754. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam. Relat. Mater. 2020, 104, 107733. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Kim, M.; Kim, B.-D.; Jo, S.; Park, S.; Son, J.; Hwang, S.; Dugasani, S.; Chang, I.; et al. Ternary and senary rep-resentations using DNA double-crossover tiles. Nanotechnology 2016, 10, 105601. [Google Scholar] [CrossRef]
- Nagai, Y.; Nakamura, K.; Yudasaka, M.; Shiraki, T.; Fujigaya, T. Radical Polymer Grafting on the Surface of Single-Walled Carbon Nanotubes Enhances Photoluminescence in the Near-Infrared Region: Implications for Bioimaging and Biosensing. ACS Appl. Nano Mater. 2020, 3, 8840–8847. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Sun, M.; Yu, J.; Chen, Y. A fluorescence–Raman dual-imaging platform based on complexes of conjugated polymers and carbon nanotubes. Nanoscale 2014, 6, 1480–1489. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Li, S.; Weng, W.; Guo, H.; Guo, T.; Zhang, X.; Chen, Y.; Huang, T.; Hong, X.; et al. Large scale synthesis of photoluminescent carbon nanodots and their application for bioimaging. Nanoscale 2013, 5, 1967–1971. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, S.; Zheng, L. Aptamer-mediated nanocomposites of semiconductor quan-tum dots and graphene oxide as well as their applications in intracellular imaging and targeted drug. J. Mater. Chem. B 2014, 2, 8558–8565. [Google Scholar] [CrossRef]
- Tan, L.; Wu, T.; Tang, Z.; Xiao, J.; Zhuo, R. Water-soluble photoluminescent fullerene capped mesoporous silica for pH-responsive drug delivery and bioimaging. Nanotechnology 2016, 27, 315104. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, T.; Ou, S.; Hu, C.; Chen, L. Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm 2018, 9, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Valentini, F.; Caminiti, R.; Petrinca, A.R.; Donia, D.; Divizia, M.; Palleschi, G. Are PEI-coated SWCNTs conjugated with hepatitis A virus? A chemical study with SEM, Z-potential, EDXD and RT-PCR. Biomed. Mater. 2010, 5, 35001. [Google Scholar] [CrossRef][Green Version]
- Yandar, N.; Pastorin, G.; Prato, M.; Bianco, A.; Patarroyo, M.E.; Lozano, J.M. Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide-carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine 2008, 26, 5864–5873. [Google Scholar] [CrossRef]
- Zeinali, M.; Jammalan, M.; Ardestani, S.K.; Mosaveri, N. Immunological and cytotoxicological characterization of tuberculin purified protein derivative (PPD) conjugated to single-walled carbon nanotubes. Immunol. Lett. 2009, 126, 48–53. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.-P.; Prato, M.; Bianco, A. Synthesis, Structural Characterization, and Immunological Properties of Carbon Nanotubes Functionalized with Peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.-P.; Muller, S.; Prato, M.; Bianco, A. Immunization with Peptide-Functionalized Carbon Nanotubes Enhances Virus-Specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; Yang, X.-D. Carbon Nanotubes Conjugated to Tumor Lysate Protein Enhance the Efficacy of an Antitumor Immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Maslak, P.G.; Dao, T.; Krug, L.M.; Chanel, S.; Korontsvit, T.; Zakhaleva, V.; Zhang, R.; Wolchok, J.D.; Yuan, J.; Pinilla-Ibarz, J.; et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood 2010, 116, 171–179. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Ma, L.; Shao, Y.; Zhao, Y.; Chen, C. Morphologically Virus-Like Fullerenol Nanoparticles Act as the Dual-Functional Nanoadjuvant for HIV-1 Vaccine. Adv. Mater. 2013, 25, 5928–5936. [Google Scholar] [CrossRef]
- Cao, W.; He, L.; Huang, X.; Jia, K.; Dai, J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]
- Li, H.; Fierens, K.; Zhang, Z.; Vanparijs, N.; Schuijs, M.J.; Van Steendam, K.; Gracia, N.F.; De Rycke, R.; De Beer, T.; De Beuckelaer, A.; et al. Spontaneous Protein Adsorption on Graphene Oxide Nanosheets Allowing Efficient Intracellular Vaccine Protein Delivery. ACS Appl. Mater. Interfaces 2016, 8, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Li, Y.; Wang, Q.; Wang, G.; Zhu, B. Carbon Nanotube-Based DNA Vaccine against Koi Herpesvirus Given by Intramuscular Injection; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- De Faria, P.C.B.; Dos Santos, L.I.; Coelho, J.P.; Ribeiro, H.B.; Pimenta, M.A.; Ladeira, L.O.; Gomes, D.A.; Furtado, C.A.; Gazzinelli, R.T. Oxidized Multiwalled Carbon Nanotubes as Antigen Delivery System to Promote Superior CD8+ T Cell Response and Protection against Cancer. Nano Lett. 2014, 14, 5458–5470. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Smyth, L.; Wang, T.-W.; da Costa, P.M.C.; Ratnasothy, K.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials 2016, 104, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, X.; Chen, Z.; Yang, X.; Shen, Z. The Adjuvant Effect of C60(OH)22 Nanoparticles Promoting Both Humoral and Cellular Immune Responses to HCV Recombinant Proteins; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, S.; Guo, Z.; Zeng, G.; Zhang, Y.; Xue, W.; Liu, Z. Polyethylenimine-Modified Fluorescent Carbon Dots As Vaccine Delivery System for Intranasal Immunization. ACS Biomater. Sci. Eng. 2018, 4, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, F.; Yan, M.; Liu, Y.; Zhu, X.; Sun, H.; Ma, G. Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 2019, 83, 390–399. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Albert, K.; Hsu, H.-Y. Carbon-Based Materials for Photo-Triggered Theranostic Applications. Molecules 2016, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- Ogbodu, R.; Limson, J.; Prinsloo, E. Photophysical Properties and Photodynamic Therapy Effect of Zinc Phthalocyanine-Spermine-Single Walled Carbon Nanotube Conjugate on MCF-7 Breast Cancer Cell; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi; Khatun, Z.; Reeck, G.R.; Lee, D.Y.; Lee, Y.-K. Photoluminescent Graphene Nanoparticles for Cancer Phototherapy and Imaging. ACS Appl. Mater. Interfaces 2014, 6, 12413–12421. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ohta, S.; Sonoda, A. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J. Control. Release 2007, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Cui, Y.; Chu, X.; Sun, B. Magnetofluorescent Fe3O4/Carbon Quantum Dots Coated Single-Walled Carbon Nanotubes as Dual-Modal Targeted Imaging and Chemo/Photodynamic/Photothermal; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Marangon, I.; Ménard-Moyon, C.; Silva, A. Synergic Mechanisms of Photothermal and Photodynamic Therapies Mediated by Photosensitizer/Carbon Nanotube Complexes; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Jiang, B.-P.; Hu, L.-F.; Shen, X.-C.; Ji, S.-C.; Shi, Z.; Liu, C.-J.; Zhang, L.; Liang, H. One-Step Preparation of a Water-Soluble Carbon Nanohorn/Phthalocyanine Hybrid for Dual-Modality Photothermal and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 18008–18017. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y. Fullerene (C60)-Based Tumor-Targeting Nanoparticles with “Off-On” State for Enhanced Treatment of Cancer; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef]
- Wang, H.; Pan, X.; Wang, X.; Wang, W.; Huang, Z.; Gu, K.; Liu, S.; Zhang, F.; Shen, H.; Yuan, Q.; et al. Degradable Carbon–Silica Nanocomposite with Immunoadjuvant Property for Dual-Modality Photothermal/Photodynamic Therapy. ACS Nano 2020, 14, 2847–2859. [Google Scholar] [CrossRef]
- Harrison, R.G.; Resasco, D.E.; Neves, L.F.F. Compositions and Methods for Cancer Treatment Using Targeted Carbon Nanotubes. U.S. Patent Application No. 9,504,745, 9 December 2013. [Google Scholar]
- Harrison, R.G.; Neves, L.F.F.; Resasco, D.E. Compositions and Methods for Cancer Treatment Using Targeted Carbon Nanotubes. U.S. Patent Application No. 8,518,870, 27 August 2013. [Google Scholar]
- Carroll, D.L.; Stewart, J.H.; Levi, N.H. Wake Forest University, Wake Forest University Health Sciences, assignee. Compositions and Methods for Treating Cancer. U.S. Patent 8,501,233, 6 August 2013. [Google Scholar]
- Wilson, L.J.; Kissell, K.R.; Hartman, K.B. Carbon Nanotube Based Imaging Agents. U.S. Patent 8,986,942, 24 March 2015. [Google Scholar]
- Hirsch, A.; Sagman, U.; Wilson, S.R. Use of Buckysome or Carbon Nanotube for Drug Delivery. U.S. Patent 7,070,810, 4 July 2006. [Google Scholar]
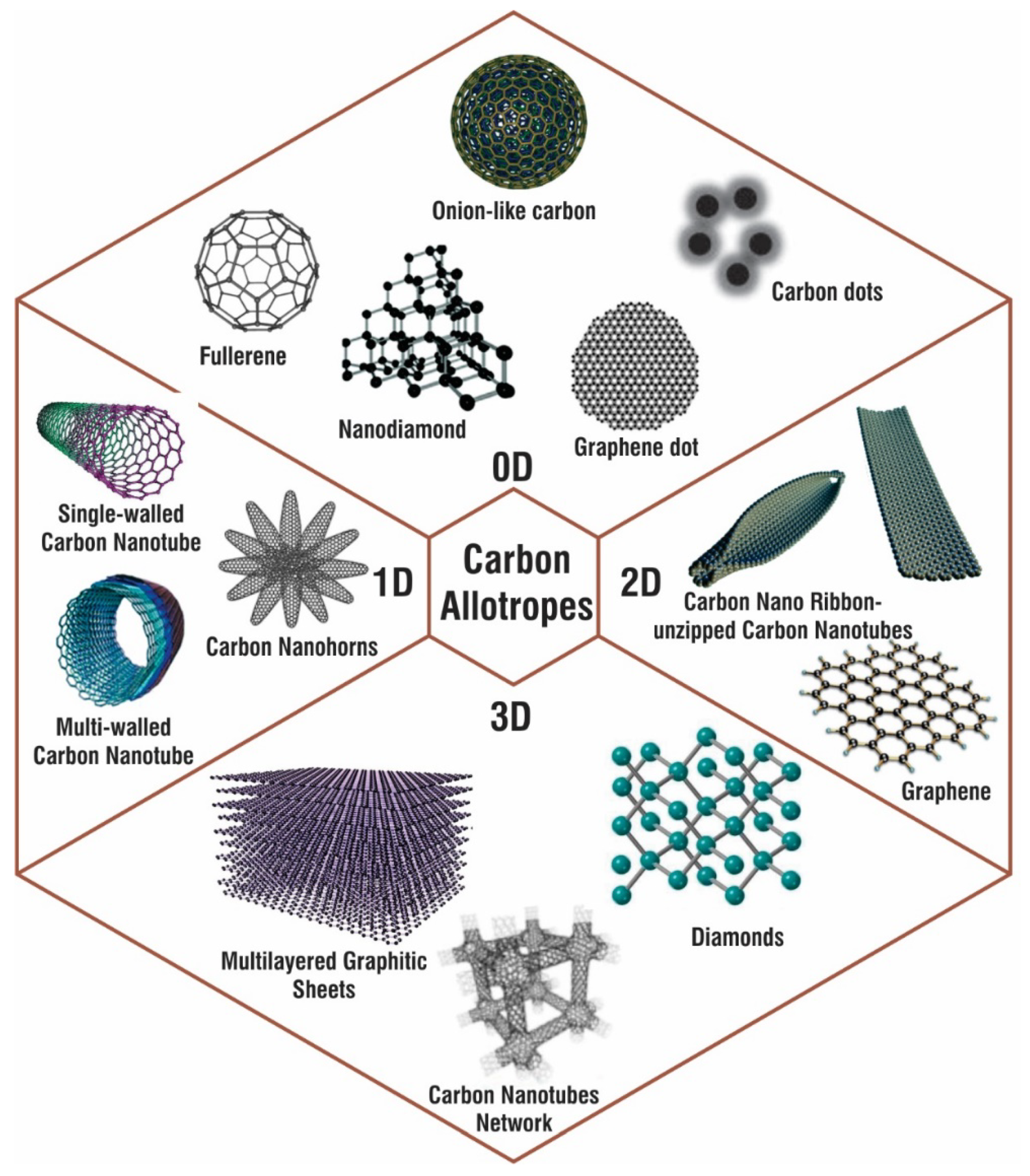

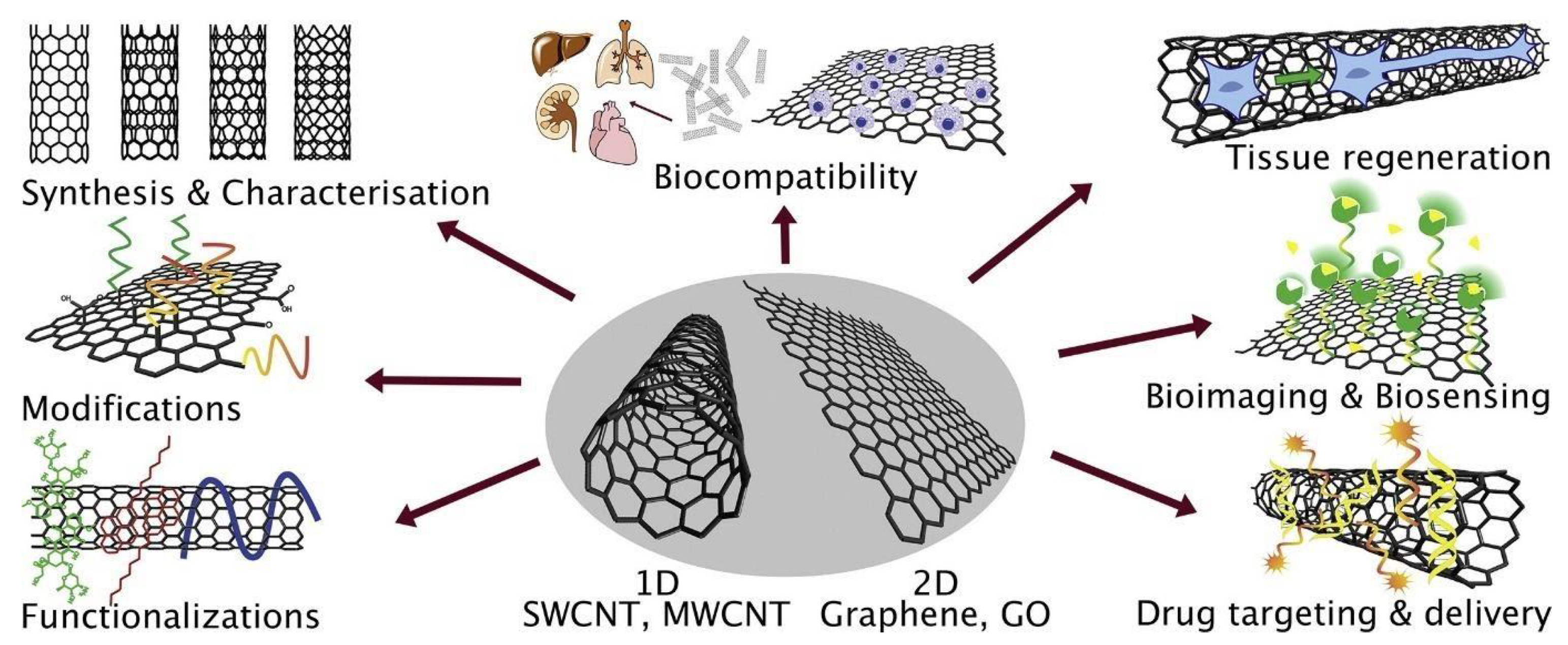
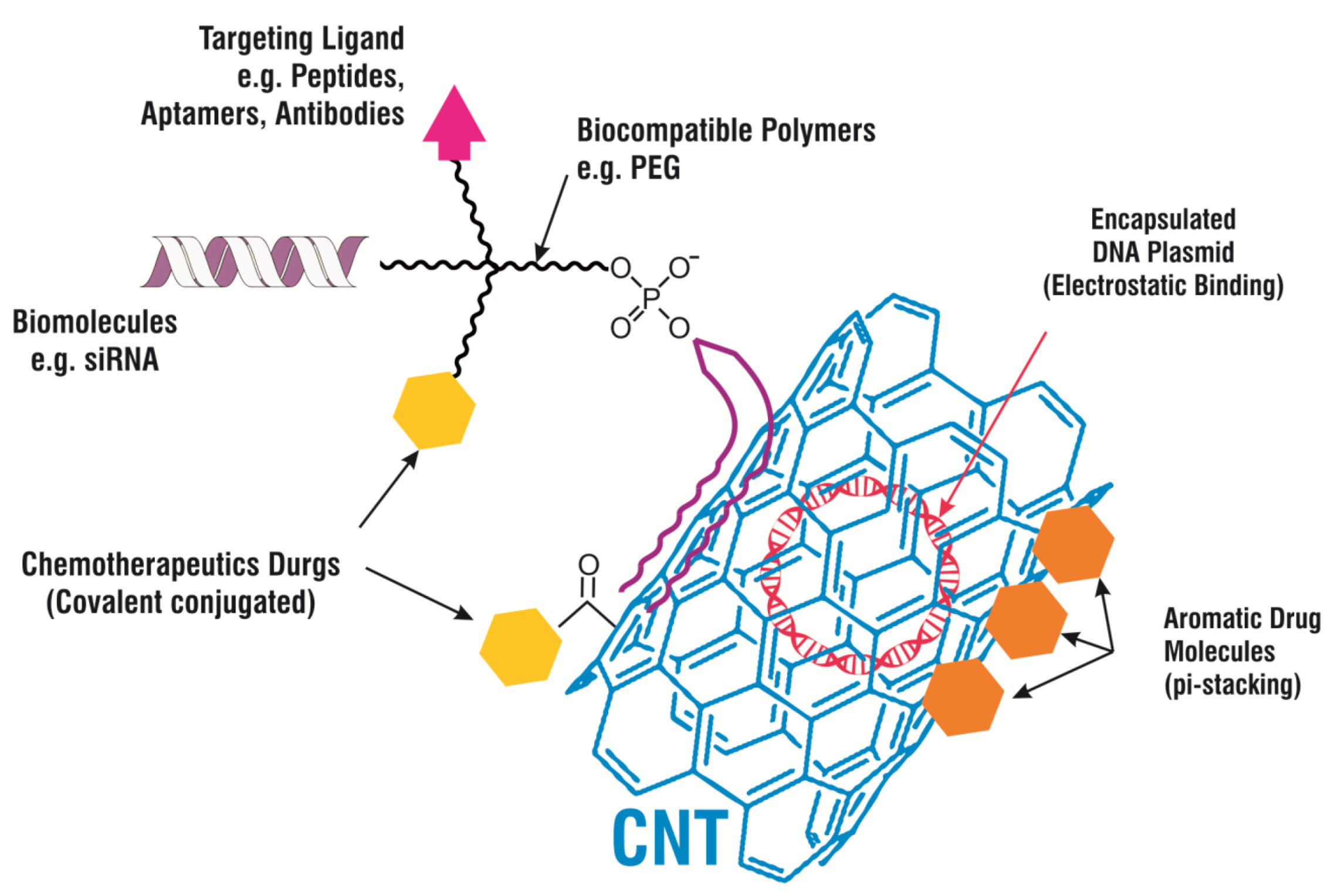
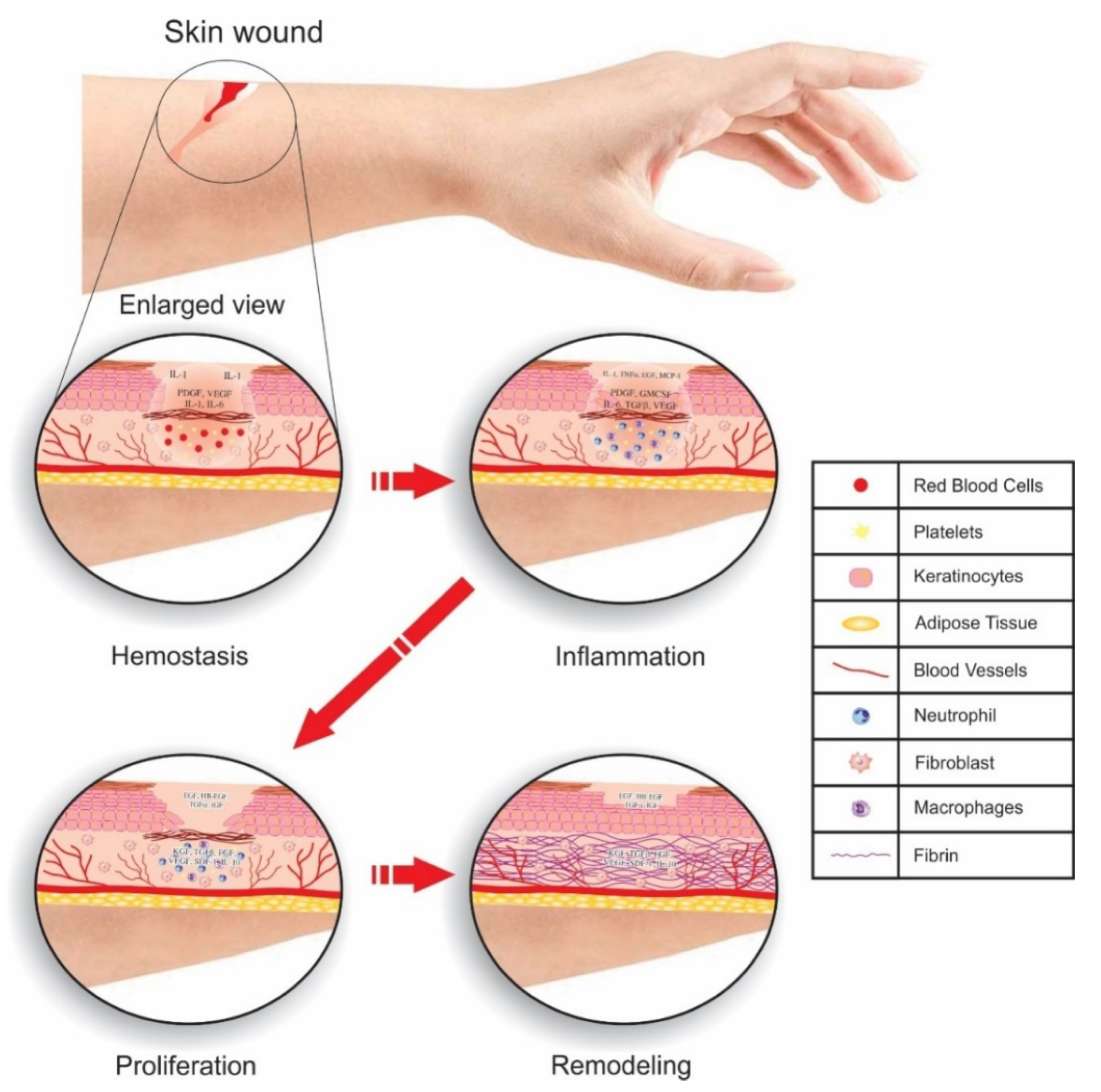


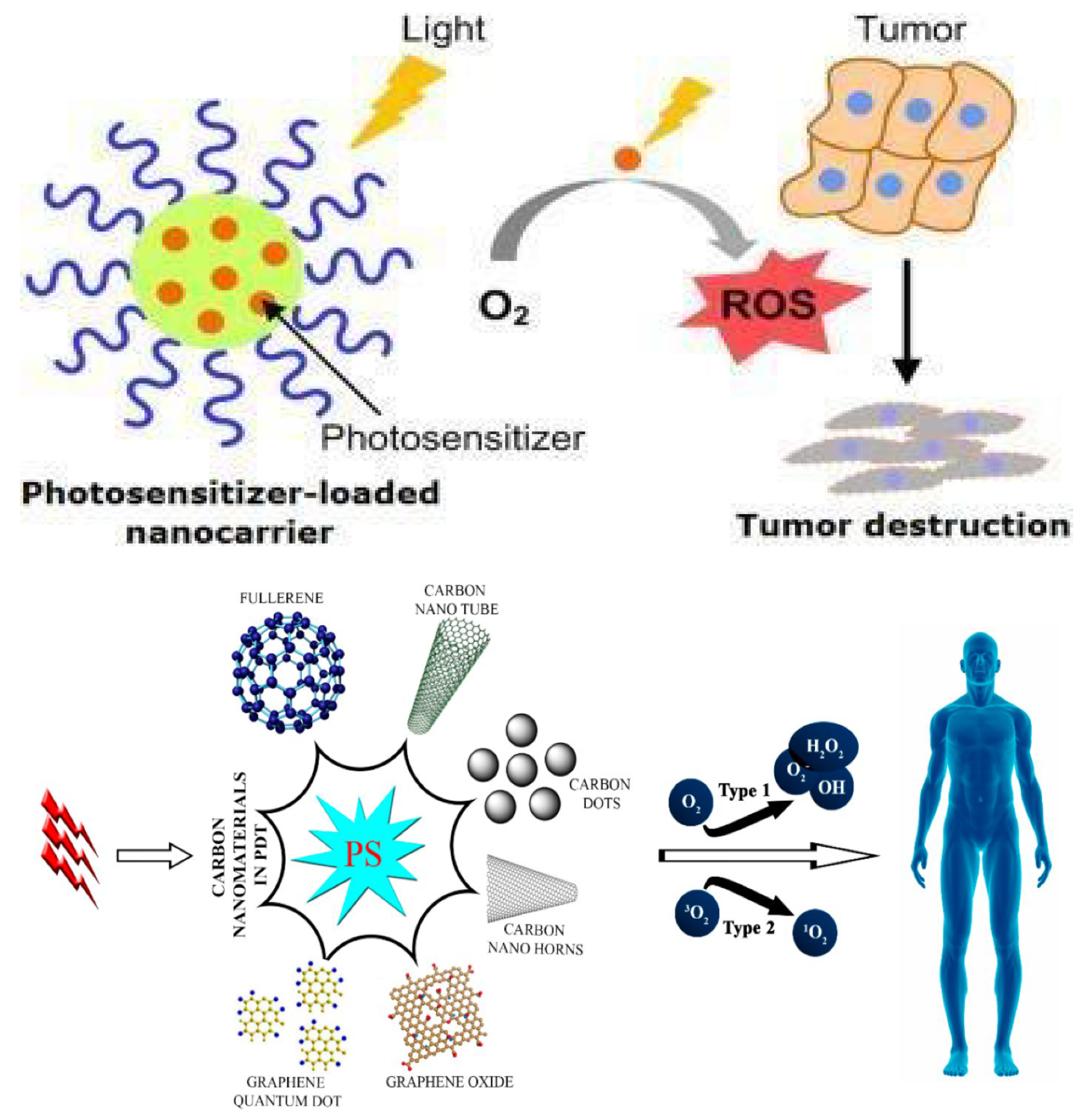
| Carbon Based Materials | Presence in Environment and Popular Synthesis Method | Properties | Applications | Ref. |
|---|---|---|---|---|
| Carbon Nanotubes | Laboratory-scale synthesis Arc discharge, Laser ablation, Chemical Vapor Deposition | High strength, Electronic properties | Biosensors, nanocomposite materials as scaffolds for tissue engineering. | [15,16] |
| Fullerenes | Manufactured at large scale in industry and laboratory. Chemical Vapor Deposition. | High strength, insoluble in water. Exhibit pi bonding between atom and are stable structure. | Pharmaceutical industry. Found to be beneficial in IT devices and diagnostic purposes. | [17] |
| Carbon Nanofibers | Laboratory production, chemical vapor deposition, phase separation electrospinning, and templatin. | The thermal conductivity of the molecules is high; they also exhibit greater strength | Cancer therapy, biosensing, tissue engineering, and wound dressing. | [18,19,20] |
| Diamond | Can be obtained naturally or by artificial means. Rapid pressurisation, pulsed laser ablation | Hard, non-volatile substance. | Used as lubricant in higher temperature. Used in jewellery design, biomedical etc. | [21,22,23] |
| Graphene | Obtained by artificial means through laboratory production Arc discharge, chemical vapor deposition, mechanical exfoliation | Most reactive form of carbon. Flammable. | Biosensing, bioimaging, bone implantation, drug delivery. | [24,25] |
| Graphite | Laboratory and industrial production, can be obtained through natural process. | Lubricity, anisotropic, electronics conductivity. | Mechanical heart valves, electrode components, lubricants. | [26] |
| Serial Number | Drug Carriers | Drug | Target Disease | Ref. |
|---|---|---|---|---|
| 1 | Carbon nanotubes | Metformin | Diabetes | [95] |
| 2 | Fullerenes | Paclitaxel Tamoxifen | Lung Cancer Breast cancer | [96,97] |
| 3 | Multiwall carbon nanotubes | Diltiazem hydrochloride | Angina Pectoris | [98] |
| 4 | Carbon nanotubes | Doxorubicin | Cervical carcinoma | [99] |
| 5 | Graphene oxide | Paclitaxel | Lung Cancer | [100] |
| 6 | Diamonds | Doxorubicin | Breast Cancer | [101] |
| Serial Number | Carbon Nanomaterial | Medical Scaffolds | Applications | Ref. |
|---|---|---|---|---|
| 1 | Carbon nanotube (CNTs) | Hydroxyapatite based CNTs composite. | Helpful in forming a strong bone–implant interface. | [112] |
| 2 | SWCNTs | Electrospun polyurethane carbon nanotube scaffolds. | Helpful in differentiation of myoblast cells. | [113] |
| 3 | MWCNTs | Polymethyl-methacrylate (PMMA) microspheres, and polyacrylonitrile-based MWCNT scaffolds. | Bone regeneration. | [114] |
| 4 | Carbon nanofibers | Collagen-carbon nanofiber scaffold. | Myocardial infarction. | [115] |
| 5 | Graphene | Electrosynthesis of polypyrrole (PPy) coating on graphene oxide (GO) nanocomposite. | Improved surface protection and biocompatibility performance in in vitro studies on MG-63 human osteoblast cells. | [116] |
| Serial Number | Carbon Nanomaterial | Formulation | Tissue Engineering Applications | Ref. |
|---|---|---|---|---|
| 1 | Carbon nanotubes | Hydrazide-functionalized carbon nanotubes–pericardial matrix derived from hydrogel. | Improved cardiac tissue engineering. | [131] |
| 2 | Fullerene whisker scaffolds | Highly aligned 1D scaffold regulates cellular differentiation to muscle cells. | Promotion of myoblast differentiation to myotube. | [132] |
| 3 | Nanodiamond | Poly(l-lactic acid) and octadecylamine-functionalized nanodiamond. | As components of bone scaffolds and surgical tools in regenerative medicine. | [133] |
| 4 | Carbon dots | CDs based composite nanofibrous mats. | Guided cell growth and enhancement of cellular activities. | [134] |
| 5 | Carbon nanofibers | Electroactive CNF/gelatin (Gel) nanofibrous cardiac patches. | Improved cellular adhesion and proliferation, as well as increased gene expressions and angiogenesis. | [135] |
| 6 | Graphene nanosheets | Biomimetic gelatin and bioactive glass scaffolds. | Excellent biocompatibility and engineered stiffness. | [136] |
| Serial Number | Carbon Nanomaterial | Wound Healing Agent | Applications | Ref. |
|---|---|---|---|---|
| 1 | MWCNTs conjugated with glucose oxidase | Glucose oxidase shows potent antimicrobial activity. | Wound cover or tissue healing matrices. | [144] |
| 2 | Fullerenes modified with amino group (C70–(EDA)8) | Amino groups interact with outer boundary of multidrug-resistant E. coli and C70 establish a potent hydrophobic interaction with bacteria, which causes cytoplast leakage. | Promising for clinical care of wound infection. | [145] |
| 3 | Fluorescent CDs loaded nanocomposites chitosan film | Chitosan, for making film and CDs as crosslinkers are taken, which are biocompatible and used in wound healing management. | Successful formulation regulates the water absorption behavior of chitosan-based film. | [146] |
| 4 | Oxygenated nanodiamonds (O-NDs) | O-NDs mimic peroxidase enzymein a rodent model. | Inhibiting and improving the course of periodontal inflammation. | [147] |
| 5 | Combination of oral antidiabetic agents-loaded nanofibrous scaffolds | Metformin, pioglitazone, and glibenclamide. | Improved diabetic wound healing on type-1 diabetic rats. | [148] |
| 6 | 3D graphene foam (GF) scaffold loaded with bone-marrow-derived mesenchymal stem cells (MSCs) | GFs loaded with MSCs clearly facilitated wound closure in animal model. | Enhanced skin wound healing. | [149] |
| Serial Number | Carbon Nanomaterial | Biosensors | Targeted Analyst | Ref. |
|---|---|---|---|---|
| 1 | SWCNTs | Conjugated aptamer-anchor polynucleotide sequence to near-infrared emissive. | Estimating protein efflux from single organisms in real-time. | [178] |
| 2 | MWCNTs deposited between electrodes | CNT resistors. | Detection of Arginase 1 (ARG-1). | [179] |
| 3 | Ag-Pt bimetallic electrospunnanoporous CNFs | Modified carbon electrode for dopamine detection. | Dopamine selectively detected in presence of uric acid and ascorbic acid. | [180] |
| 4 | Carboxyl functionalized GO (CFGR-COOH) | HRP labelled CFGR-COOH modified with Glassy carbon electrode. | DNA was successfully detected using DPV with ranges between 1 × 10−6 and 1 × 10−14. | [181] |
| 5 | Graphene-bismuth nanocomposite film modified electrode | Immobilized glucose oxidase on nanocomposite. | Successful detection of glucose with good stability and repeatability. | [182] |
| 6 | Fullerene (C60) | C60 acts as donor probe and urea (if present) reacts to DMG and formed DIK acts as receptor on RRS-ET analytical platform. | Successfully developed to detect trace amounts of urea in food. | [183] |
| Serial Number | Carbon Nanomaterial | Bioimaging Agent | Bioimaging Applications | Ref. |
|---|---|---|---|---|
| 1 | SWCNTs | Labelled recombinant thermo-stable Luciola cruciata luciferase (LcL). | Advanced powerful tool for in vivo imaging. | [195] |
| 2 | SWCNTs | SWCNT surfaces grafted with radical polymer produces brighter emission. | Bioimaging and biosensing in vivo in near-infrared region. | [196] |
| 3 | Carboxylated MWCNTs conjugated with polyelectrolytes (CPE) | MWNTs possess characteristic Raman vibration modes and CPE has optical properties; both provide fluorescence. Raman dual-imaging method. | Intracellular tracking and finding location of MWCNTs in in vitro and in vivo. | [197] |
| 4 | Carbon dots | Carbonization of sucrose with oil acid shows strong fluorescence and quantum yield. | Applicable in cell imaging. | [198] |
| 5 | Graphene oxide | GO nanosheets decorated with aptamer-labelled CdSe@ZnS QDs. | Potentially used in bio-imaging and cell-targeted drug delivery. | [199] |
| 6 | Fullerene | Fluorescent fullerene-coated mesoporous silica nanoparticles. | Fluorescent cell imaging and pH-sensitive drug release achieved. | [200] |
| Serial Number | Carbon Nanomaterial | Vaccinating Agent | Vaccine Applications | Ref. |
|---|---|---|---|---|
| 1 | SWCNTs | SWCNTs coupled with recombinant plasmid pcDNA-ORF149 (antigen). | Anti-KHV (Koi herpes virus) vaccine. | [214] |
| 2 | MWCNTs with OVA | MWCNTs (delivery system) with tumour-derived NY−ESO−1 (testis antigen). | Increased specific antibodies level in mouse model and delayed growth of tumor and prolonged survival. | [215] |
| 3 | Carboxylated MWNTs co-delivered with OVA, CpG and αCD40 | OVA (antigen) and CpG and αCD40 (adjuvants). | Elevated T cell proliferation and IFN−γ secretion and enhanced antigen-specific CTL response reduce tumor growth and prolong survival. | [216] |
| 4 | Fullerene | Multihydroxylated fullerene as adjuvant and HCV recombinant proteins as antigens. | Induce humoral and cellular immune responses. | [217] |
| 5 | Carbon Dots | Fluorescent CDs as delivery system. | Provide access to trace antigen movement from the injected site to the lymph organs. | [218] |
| 6 | Graphene Oxide | Antigen-loaded alum-based adjuvant modifies GO nanosheets and induces humoral immune response the cellular immune response. | Powerful ability to raise cellular- and humoral-type immune response and improves cancer immunotherapy efficacy. | [219] |
| Serial Number | Carbon Nanomaterial | Photodynamic Therapy Agent | Applications | Ref. |
|---|---|---|---|---|
| 1 | SWCNTs | SWCNTs coated with Fe3O4 and CQDs conjugated to a DOX-loaded sgc8c aptamer act as both NIR ROS generators and drug loading carriers. | The multifunctional delivery platform should also carry chemotherapeutic agents for multifunctional imaged-guided PDT/PTT/ chemotherapy in cancer therapy. | [229] |
| 2 | MWCNTs | mTHPC (m-tetrahydroxyphenylchlorin) as photosensitizer. | Cancer treatment with combination of PDT and PTT. | [230] |
| 3 | SWCNHs | SWCNHs nanohybrid coated with TSCuPc and MPc, in which TSCuPc acts as PDT agent. | A 650 nm laser significantly increases the anticancer efficacy of combined noninvasive PDT. | [231] |
| 4 | Fullerene | DOX conjugated to C60 attached to a hydrophilic shell provides more stability and remote control through a laser (532 nm) for PDT. | Tumor targeted with “on-off” state for strengthening the treatment of cancer through combined therapeutic effects. | [232] |
| 5 | Nano-Graphene oxide (NGO) | NGO conjugated with ICG for PDT. | Enhanced antimicrobial and anti-biofilm activity against E. faecalis. | [233] |
| 6 | Carbon−silica nanocomposite (CSN) | CSN as PDT and as immunoadjuvant. | Harbors photothermal and photodynamic properties with potent antitumoral effects. | [234] |
| Serial Number | Patent Number | Patent Description | Ref. |
|---|---|---|---|
| 1 | US20090062785 | SWCNTs were attached to proteins (including, but not limited to, annexins) or peptides and formed protein-CNT complexes. Complexes bound to specifically to tumor cells rather than to healthy cells; the cells were used to diagnose and irradiate tumors at specific wavelengths. However, an immunostimulant was also administered to intensify the immune response of the patients against antigen released by tumor cells. | [235] |
| 2 | US20080227687 | Proteins (annexins) were linked with SWCNTs to target cancerous cells, particularly tumor vasculature endothelial cells. To diagnose and destroy these tumors, a specific electromagnetic wavelength was employed. | [236] |
| 3 | US20100209479 | MWCNTs were attached to chemotherapeutic agents, such as mitomycin C. | [237] |
| 4 | US20090136987 | CNTs were loaded with contrasting agents and used as imaging agents for detection in a cell. | [238] |
| 5 | US20080193490 | CNTs employed as drug delivery vehicles for cancer drugs. CNTs were encapsulated with therapeutic agents and surface modifications were performed with different functional groups. | [239] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. https://doi.org/10.3390/ma14205978
Gaur M, Misra C, Yadav AB, Swaroop S, Maolmhuaidh FÓ, Bechelany M, Barhoum A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials. 2021; 14(20):5978. https://doi.org/10.3390/ma14205978
Chicago/Turabian StyleGaur, Manish, Charu Misra, Awadh Bihari Yadav, Shiv Swaroop, Fionn Ó. Maolmhuaidh, Mikhael Bechelany, and Ahmed Barhoum. 2021. "Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene" Materials 14, no. 20: 5978. https://doi.org/10.3390/ma14205978
APA StyleGaur, M., Misra, C., Yadav, A. B., Swaroop, S., Maolmhuaidh, F. Ó., Bechelany, M., & Barhoum, A. (2021). Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials, 14(20), 5978. https://doi.org/10.3390/ma14205978









