Local Interactions of Atmospheric Oxygen with MoS2 Crystals
Abstract
:1. Introduction
2. Phenomenological Observations of Thermal MoS2 Oxidation in Air and in Water
2.1. Interactions of the MoS2 Samples with Oxygen till 300 °C
2.2. Microscopic Oxidative Etching between 300 and 400 °C
2.3. Microscopic Oxidation above 400 °C
3. Mechanistic Details of the MoS2 Oxidative Processes
3.1. Stoichiometric Considerations
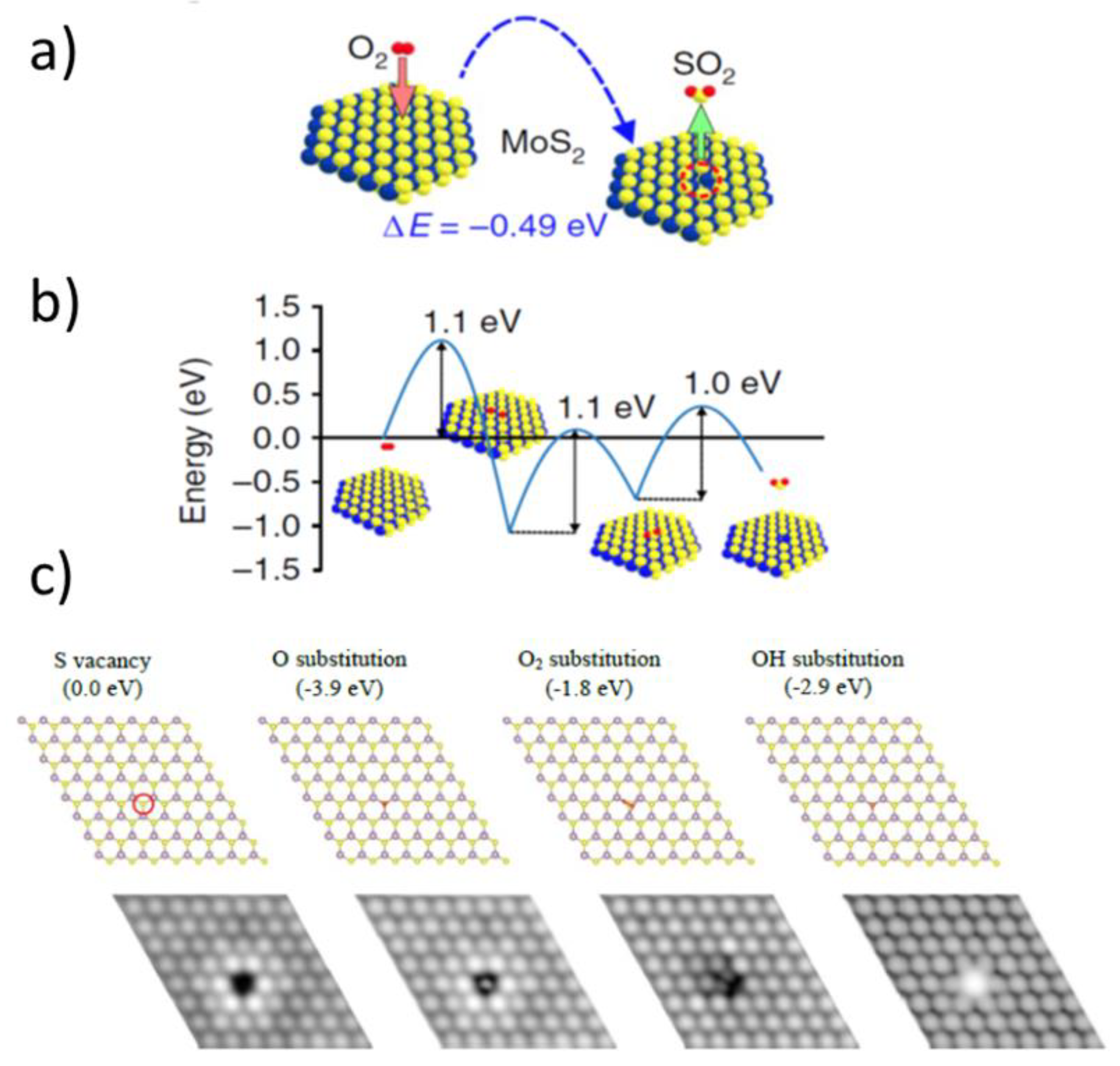
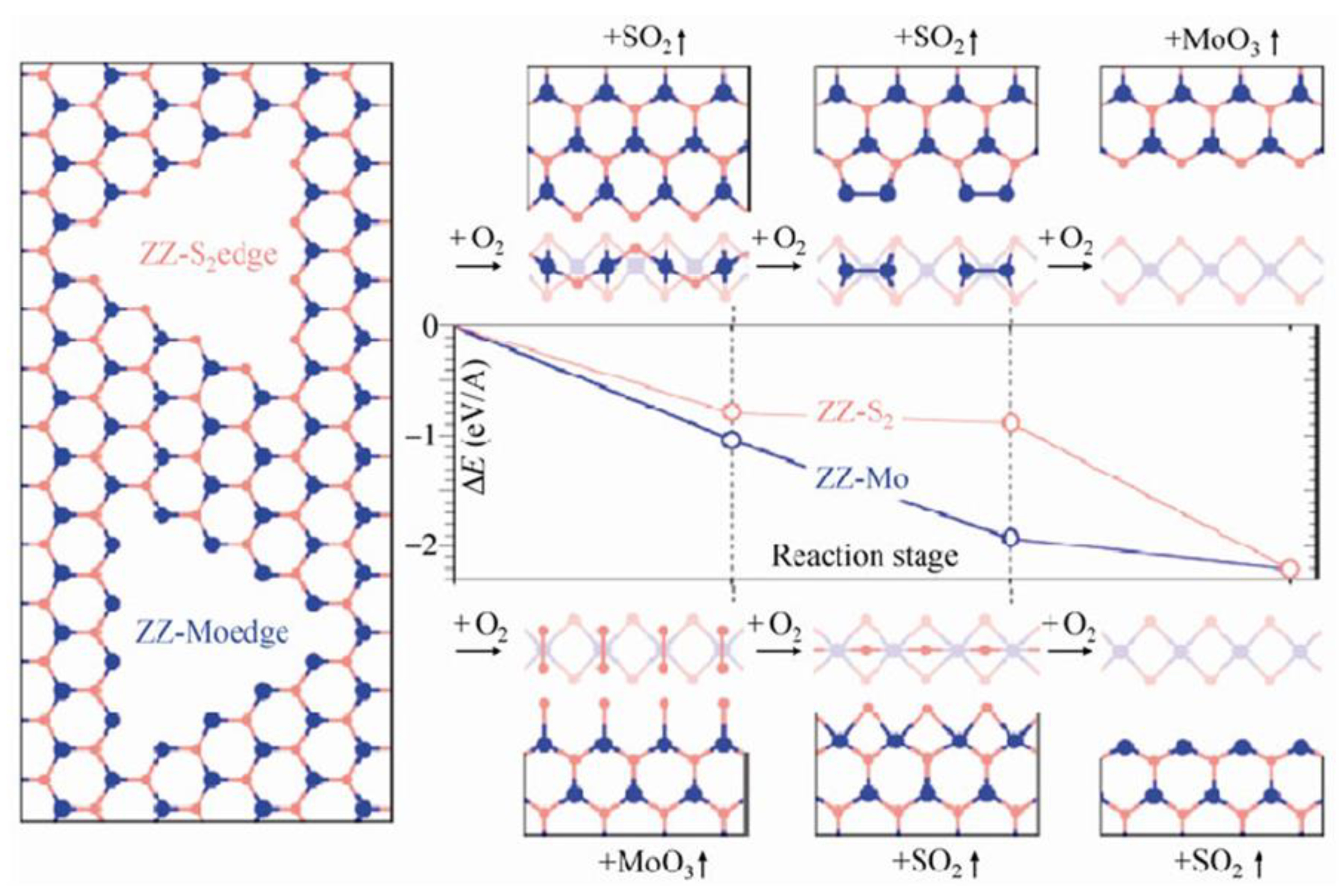
3.2. Computer Simulations
4. Mo Oxides and Their Derivatives in MoS2 Oxidation
5. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Tagawa, M.; Muromoto, M.; Hachiue, S.; Yokota, K.; Ohmae, N.; Matsumoto, K.; Suzuki, M. Hyperthermal atomic oxygen interaction with MoS2 lubricants and relevance to space environmental effects in low earth orbit—Effects on friction coefficient and wear-life. Tribol. Lett. 2005, 18, 437–443. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Fleischauer, P.D.; Lince, J. A comparison of oxidation and oxygen substitution in MoS2 solid film lubricants. Tribol. Int. 1999, 32, 627–636. [Google Scholar] [CrossRef]
- Lince, J.; Frantz, P.P. Anisotropic oxidation of MoS2 crystallites studied by angle-resolved X-ray photoelectron spectroscopy. Tribol. Lett. 2001, 9, 211–218. [Google Scholar] [CrossRef]
- Imanishi, N.; Kanamura, K.; Takehara, Z. Synthesis of MoS2 thin film by chemical vapor deposition method and discharge characteristics as a cathode of the lithium secondary battery. J. Electrochem. Soc. 1992, 139, 2082–2087. [Google Scholar] [CrossRef]
- Jäger-Waldau, A.; Lux-Steiner, M.; Bucher, E. MoS2, MoSe2, WS2 and WSe2 thin films for photovoltaics. Solid State Phenom. 1994, 37–38, 479–484. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nilsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, F.; Sun, Y.; Zhang, X.; Tang, L. Oxygen-incorporated MoS2 ultrathin nanosheets grown on graphene for efficient electrochemical hydrogen evolution. J. Power Sources 2015, 291, 195–200. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disordered engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Whitwick, M.B.; Kis, A. Integrated circuits and logic operations based on single-layer MoS2. ACS Nano 2011, 5, 9934–9938. [Google Scholar] [CrossRef] [PubMed]
- Wachter, S.; Polyushkin, D.; Bethge, O.; Mueller, T. A microprocessor based on a two-dimensional semiconductor. Nat. Commun. 2017, 8, 14948. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Pak, S.; Giraud, P.; Lee, Y.-W.; Cho, Y.; Hong, J.; Jang, A.R.; Chung, H.-S.; Hong, W.-K.; Jeong, H.Y.; et al. Thermodynamically stable synthesis of large-scale and highly crystalline transition metal dichalcogenide monolayers and their unipolar n-n heterojunction devices. Adv. Mater. 2017, 29, 1702206. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, Z.; Huang, W.; Zhang, H. Non-volatile resistive memory devices based on solution-processed ultrathin two-dimensional nanomaterials. Chem. Soc. Rev. 2015, 44, 2615–2628. [Google Scholar] [CrossRef] [Green Version]
- Marega, G.M.; Zhao, Y.; Avsar, A.; Wang, Z.; Tripathi, M.; Radenovic, A.; Kis, A. Logic-in-memory based on an atomically thin semiconductor. Nature 2020, 587, 72–77. [Google Scholar] [CrossRef]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.Y.; Mak, K.F.; Kim, C.J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Ho, P.H.; Tseng, M.F.; Lin, F.Y.; Hou, C.H.; Lin, I.K.; Wang, H.; Huang, P.P.; Chiang, C.H.; Yang, Y.C.; et al. Fast growth of large-grain and continuous MoS2 films through a self-capping vapor-liquid-solid method. Nat. Commun. 2020, 11, 3682. [Google Scholar] [CrossRef]
- Shim, J.; Bae, S.-H.; Kong, W.; Lee, D.; Qiao, K.; Nezich, D.; Park, Y.J.; Zhao, R.; Sundaram, S.; Li, X.; et al. Controlled crack propagation for atomic precision handling of wafer-scale two-dimensional materials. Science 2018, 362, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Lee, K.H.; Han, Y.; Gao, H.; Xie, S.; Muller, D.A.; Park, J. Layer-by-layer assembly of two-dimensional materials into wafer-scale heterostructures. Nature 2017, 550, 229–233. [Google Scholar] [CrossRef]
- Amani, M.; Lien, D.H.; Kiriya, D.; Xiao, J.; Azcatl, A.; Noh, J.; Madhvapathy, S.R.; Addou, R.; Kc, S.; Dubey, M.; et al. Near-unity photoluminescence quantum yield in MoS2. Science 2015, 350, 1065–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez Marin, J.F.; Unuchek, D.; Watanabe, K.; Taniguchi, T.; Kis, A. MoS2 photodetectors integrated with photonic circuits. NPJ 2D Mater. Appl. 2019, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Zhang, Y.; Chen, W.; Chu, J.; Lei, T.; Pu, J.; Dai, L.; Wu, C.; Cheng, Y.; Zhai, T.; et al. Electronic and optoelectronic applications based on 2D novel anisotropic transition metal dichalcogenides. Adv. Sci. 2017, 4, 1700231. [Google Scholar] [CrossRef] [PubMed]
- Unuchek, D.; Ciarrocchi, A.; Avsar, A.; Watanabe, K.; Taniguchi, T.; Kis, A. Room-temperature electrical control of exciton flux in a van der Waals heterostructure. Nature 2018, 560, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; He, K.; Shan, J.; Heinz, T.F. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nanotechnol. 2012, 7, 494–498. [Google Scholar] [CrossRef]
- Yalon, E.; McClellan, C.J.; Smithe, K.K.H.; Muñoz Rojo, M.; Xu, R.L.; Suryavanshi, S.V.; Gabourie, A.; Neumann, C.M.; Xiong, F.; Farimani, A.B.; et al. Energy dissipation in monolayer MoS2 electronics. Nano Lett. 2017, 17, 3429–3433. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Pan, L.; Yao, Z.; Li, J.; Shi, Y.; Wang, X.; Park, W.; Park, J.; Jang, J.; Lee, H.; et al. Electrical characterization of back-gated bi-layer MoS2 field-effect transistors and the effect of ambient on their performances. Appl. Phys. Lett. 2012, 100, 123104. [Google Scholar] [CrossRef]
- Park, W.; Park, J.; Jang, J.; Lee, H.; Jeong, H.; Cho, K.; Hong, S.; Lee, T. Oxygen environmental and passivation effects on molybdenum disulfide field effect transistors. Nanotechnology 2013, 24, 095202. [Google Scholar] [CrossRef]
- Li, T.-D.; Gao, J.; Szoszkiewicz, R.; Landman, U.; Riedo, E. Structured and viscous water in subnanometer gaps. Phys. Rev. B 2007, 75, 115415. [Google Scholar] [CrossRef] [Green Version]
- Szoszkiewicz, R.; Riedo, E. Nucleation time of nanoscale water bridges. Phys. Rev. Lett. 2005, 95, 135502. [Google Scholar] [CrossRef] [Green Version]
- Ross, S. Surface oxidation of molybdenum disulfide. J. Phys. Chem. 1955, 59, 889–892. [Google Scholar] [CrossRef]
- Ebrahimi Kahrizsangi, R.; Abbasi, M.H.; Saidi, A. Model-Fitting Approach to Kinetic Analysis of Non-Isothermal Oxidation of Molybdenite. Iran. J. Chem. Chem. Eng. 2007, 26, 119–123. [Google Scholar]
- Zhou, H.; Yu, F.; Liu, Y.; Zou, X.; Cong, C.; Qiu, C.; Yu, T.; Yan, Z.; Shen, X.; Sun, L.; et al. Thickness-dependent patterning of MoS2 sheets with well-oriented triangular pits by heating in air. Nano Res. 2013, 6, 703–711. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Yin, Z.; Li, H.; Liu, J.; Cao, X.; Zhang, Q.; Zhang, H. Layer thinning and etching of mechanically exfoliated MoS2 nanosheets by thermal annealing in air. Small 2013, 9, 3314–3319. [Google Scholar] [CrossRef]
- Yamamoto, M.; Einstein, T.L.; Fuhrer, M.S.; Cullen, W.G. Anisotropic etching of atomically thin MoS2. J. Phys. Chem. C 2013, 117, 25643–25649. [Google Scholar] [CrossRef]
- Walter, T.N.; Kwok, F.; Simchi, H.; Aldosari, H.M.; Mohney, S.E. Oxidation and oxidative vapor-phase etching of few-layer MoS2. J. Vac. Sci. Technol. B 2017, 35, 21203. [Google Scholar] [CrossRef]
- Rao, R.; E Islam, A.; Campbell, P.M.; Vogel, E.M.; Maruyama, B. In situ thermal oxidation kinetics in few layer MoS2. 2D Mater. 2017, 4, 025058. [Google Scholar] [CrossRef]
- Tang, J.; Wei, Z.; Wang, Q.; Wang, Y.; Han, B.; Li, X.; Huang, B.; Liao, M.; Liu, J.; Li, N.; et al. In situ oxygen doping of monolayer MoS2 for novel electronics. Small 2020, 16, 2–9. [Google Scholar] [CrossRef]
- Wang, G.; Pandey, R.; Karna, S.P. Physics and chemistry of oxidation of two-dimensional nanomaterials by molecular oxygen. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1280. [Google Scholar] [CrossRef]
- Ukegbu, U.; Szoszkiewicz, R. Microscopic kinetics of heat-induced oxidative etching of thick MoS2 crystals. J. Phys. Chem. C 2019, 123, 22123–22129. [Google Scholar] [CrossRef]
- Spychalski, W.L.; Pisarek, M.; Szoszkiewicz, R. Microscale Insight into Oxidation of Single MoS2 Crystals in Air. J. Phys. Chem. C 2017, 121, 26027–26033. [Google Scholar] [CrossRef]
- Szoszkiewicz, R.; Rogala, M.; Dabrowski, P. Surface-bound and volatile mo oxides produced during oxidation of single MoS2 crystals in air and high relative humidity. Materials 2020, 13, 3067. [Google Scholar] [CrossRef] [PubMed]
- KC, S.; Longo, R.C.; Wallace, R.M.; Cho, K. Surface oxidation energetics and kinetics on MoS2 monolayer. J. Appl. Phys. 2015, 117, 135301. [Google Scholar] [CrossRef]
- Krawczyk, M.; Pisarek, M.; Szoszkiewicz, R.; Jablonski, A. Surface characterization of MoS2 atomic layers mechanically exfoliated on a Si substrate. Materials 2020, 13, 3595. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Liu, C.; Yang, B.; Song, S. Microscale control of edge defect and oxidation on molybdenum disulfide through thermal treatment in air and nitrogen atmospheres. Appl. Surf. Sci. 2018, 462, 471–479. [Google Scholar] [CrossRef]
- Martincova, J.; Otyepka, M.; Lazar, P. Oxidation of metallic two-dimensional transition metal dichalcogenides: 1T-MoS2 and 1T-TaS2. 2D Mater. 2020, 7, 045005. [Google Scholar] [CrossRef]
- Park, S.; Garcia-Esparza, A.T.; Abroshan, H.; Abraham, B.; Vinson, J.; Gallo, A.; Nordlund, D.; Park, J.; Kim, T.R.; Vallez, L.; et al. Operando study of thermal oxidation of monolayer MoS2. Adv. Sci. 2021, 8, 2002768. [Google Scholar] [CrossRef]
- Rogala, M.; Sokołowski, S.; Ukegbu, U.; Mierzwa, A.; Szoszkiewicz, R. Direct identification of surface bound MoO3 on single MoS2 flakes heated in dry and humid air. Adv. Mater. Interfaces 2021, 2100328. [Google Scholar] [CrossRef]
- Ballou, E.V.; Ross, S. The adsorption of benzene and water vapors by molybdenum disulfide. J. Phys. Chem. 1953, 57, 653–657. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalan-tar-Zadeh, K. Molybdenum oxides—From fundamentals to functionality. Adv. Mater. 2017, 29, 1–31. [Google Scholar] [CrossRef]
- Åsbrink, S.; Kihlborg, L.; Malinowski, M. High-pressure single-crystal X-ray diffraction studies of MoO3. I. Lattice parameters up to 7·4 GPa. J. Appl. Crystallogr. 1988, 21, 960–962. [Google Scholar] [CrossRef]
- Bihn, J.H.; Park, J.; Kang, Y.C. Synthesis and characterization of mo films deposited by RF sputtering at various oxygen ratios. J. Korean Phys. Soc. 2011, 58, 509–514. [Google Scholar] [CrossRef]
- Smolik, G.R.; Petti, D.A.; Schuetz, S.T. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283-287, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Mirabelli, G.; McGeough, C.; Schmidt, M.; McCarthy, E.K.; Monaghan, S.; Povey, I.M.; McCarthy, M.M.; Gity, F.; Nagle, R.E.; Hughes, G.; et al. Air sensitivity of MoS2, MoSe2, MoTe2, HfS2, and HfSe2. J. Appl. Phys. 2016, 120, 125102. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.S.; Singh, A.; Yang, L.; Tiwari, S.C.; Hong, S.; Krishnamoorthy, A.; Sales, M.G.; Oliver, S.M.; Fox, J.; Cavalero, R.L.; et al. Growth kinetics and atomistic mechanisms of native oxidation of ZrSxSe2–x and MoS2 crystals. Nano Lett. 2020, 20, 8592–8599. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, B.; Tan, J.; Chow, P.; Lu, T.-M.; Koratkar, N. Aging of transition metal dichalcogenide monolayers. ACS Nano 2016, 10, 2628–2635. [Google Scholar] [CrossRef]
- Longo, R.C.; Addou, R.; Santosh, K.C.; Noh, J.Y.; Smyth, C.M.; Barrera, D.; Zhang, C.; Hsu, J.W.P.; Wallace, R.M.; Cho, K. Intrinsic air stability mechanisms of two-dimensional transition metal dichalcogenide surfaces: Basal versus edge oxidation. 2D Mater. 2017, 4, 025050. [Google Scholar] [CrossRef]
- Martincová, J.; Otyepka, M.; Lazar, P. Is single layer MoS2 stable in the air? Chem. A Eur. J. 2017, 23, 13233–13239. [Google Scholar] [CrossRef]
- Sen, H.S.; Sahin, H.; Peeters, F.M.; Durgun, E. Monolayers of MoS2 as an oxidation protective nanocoating material. J. Appl. Phys. 2014, 116, 083508. [Google Scholar] [CrossRef] [Green Version]
- Pető, J.; Ollár, T.; Vancsó, P.; Popov, Z.I.; Magda, G.Z.; Dobrik, G.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Spontaneous doping of the basal plane of MoS2 single layers through oxygen substitution under ambient conditions. Nat. Chem. 2018, 10, 1246–1251. [Google Scholar] [CrossRef]
- Wang, Z.; Bussche, A.V.D.; Qiu, Y.; Valentin, T.M.; Gion, K.; Kane, A.B.; Hurt, R.H. Chemical dissolution pathways of MoS2 nanosheets in biological and environmental media. Environ. Sci. Technol. 2016, 50, 7208–7217. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jia, F.; Yang, B.; Song, S. Oxidation of molybdenum disulfide sheet in water under in situ atomic force microscopy observation. J. Phys. Chem. C 2017, 121, 9938–9943. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Cruywagen, J.J.; Heyns, J.B.B. Solubility of yellow molybdenum(VI) oxide dihydrate (Mo03.2H20) in 3,0M-sodium perchlorate at 25 °C. S. Afr. J. Chem. 1981, 34, 118–120. [Google Scholar]
- Oyerinde, O.F.; Weeks, C.L.; Anbar, A.D.; Spiro, T.G. Solution structure of molybdic acid from Raman spectroscopy and DFT analysis. Inorganica Chim. Acta 2008, 361, 1000–1007. [Google Scholar] [CrossRef]
- Piquemal, J.-Y.; Manoli, J.-M.; Beaunier, P.; Ensuque, A.; Tougne, P.; Legrand, A.-P.; Brégeault, J.-M. Using inorganic silicate precursor/molybdenum peroxo complexes/onium salt interfaces in aqueous acidic media to design mesoporous silica with high molybdenum content and high dispersion. Microporous Mesoporous Mater. 1999, 29, 291–304. [Google Scholar] [CrossRef]
- Espinosa, F.M.; Ryu, Y.K.; Marinov, K.; Dumcenco, D.; Kis, A.; Garcia, R. Direct fabrication of thin layer MoS2 field-effect nanoscale transistors by oxidation scanning probe lithography. Appl. Phys. Lett. 2015, 106, 103503. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Shu, Y.; Wang, A.A.; Zhang, T. Green synthesis and characterization of anisotropic uniform single-crystal α-MoO3 nanostructures. J. Phys. Chem. C 2007, 111, 2401–2408. [Google Scholar] [CrossRef]
- Addou, R.; McDonnell, S.; Barrera, D.; Guo, Z.; Azcatl, A.; Wang, J.; Zhu, H.; Hinkle, C.L.; Quevedo-Lopez, M.; Alshareef, H.N.; et al. Impurities and electronic property variations of natural MoS2 crystal surfaces. ACS Nano 2015, 9, 9124–9133. [Google Scholar] [CrossRef]
- Lv, D.; Wang, H.; Zhu, D.; Lin, J.; Yin, G.; Lin, F.; Zhang, Z.; Jin, C. Atomic process of oxidative etching in monolayer molybdenum disulfide. Sci. Bull. 2017, 62, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, B.; Bera, A.; Muthu, D.V.S.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys. Rev. B 2012, 85, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Farigliano, L.M.; Paredes-Olivera, P.A.; Patrito, E.M. Initial steps of oxidative etching of MoS2 basal plane induced by O2. J. Phys. Chem. C 2020, 124, 13177–13186. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, J.; Xu, X.; Ho, Y.; Ni, G.; Zou, Q.; Koon, G.K.W.; Zhao, W.; Castro Neto, A.H.; Eda, G.; et al. An innovative way of etching MoS2: Characterization and mechanistic investigation. Nano Res. 2013, 6, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Maguire, P.; Jadwiszczak, J.; O’Brien, M.; Keane, D.; Duesberg, G.S.; McEvoy, N.; Zhang, H. Defect-moderated oxidative etching of MoS2. J. Appl. Phys. 2019, 126, 164301. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Barkelid, M.; Goossens, A.M.; Calado, V.E.; van der Zant, H.S.J.; Steele, G.A. Laser-thinning of MoS2: On demand generation of a single-layer semiconductor. Nano Lett. 2012, 12, 3187–3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunamura, K.; Page, T.R.; Yoshida, K.; Yano, T.A.; Hayamizu, Y. Laser-induced electrochemical thinning of MoS2. J. Mater. Chem. C 2016, 4, 3268–3273. [Google Scholar] [CrossRef]
- Ukegbu, U.K. Microscale Etching/Oxidation of Thick MoS2 Flakes. Master’s Thesis, University of Warsaw, Warsaw, Poland, 2018. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong photoluminescence enhancement of MoS2 through defect engineering and oxygen bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grønborg, S.S.; Thorarinsdottir, K.; Kyhl, L.; Rodríguez-Fernández, J.; E Sanders, C.; Bianchi, M.; Hofmann, P.; Miwa, J.; Ulstrup, S.; Lauritsen, J.V. Basal plane oxygen exchange of epitaxial MoS2 without edge oxidation. 2D Mater. 2019, 6, 045013. [Google Scholar] [CrossRef]
- Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Gu, L.; Mao, N.; Feng, Q.; Xie, L.; et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef]
- Ko, T.Y.; Jeong, A.; Kim, W.; Lee, J.; Kim, Y.; Lee, J.E.; Ryu, G.H.; Park, K.; Kim, D.; Lee, Z.; et al. On-stack two-dimensional conversion of MoS2 into MoO3. 2D Mater. 2016, 4, 014003. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J. Dehydration of the molybdenum trioxide hydrates MoO3·nH2O: In situ x-ray absorption spectroscopy study at the Mo K edge. J. Phys. Condens. Matter 2000, 12, 1959–1970. [Google Scholar] [CrossRef] [Green Version]
- Rice, R.H.; Mokarian-Tabari, P.; King, W.P.; Szoszkiewicz, R. Local thermomechanical analysis of a microphase-separated thin lamellar PS-b-PEO film. Langmuir 2012, 28, 13503–13511. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qin, X.; Cheng, L.; Azcatl, A.; Kim, J.; Wallace, R.M. Remote plasma oxidation and atomic layer etching of MoS2. ACS Appl. Mater. Interfaces 2016, 8, 19119–19126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, X.; Wu, J.; Zeng, Z.; Wei, J.; Zhang, H. Investigation of MoS2 and graphene nanosheets by magnetic force microscopy. ACS Nano 2013, 7, 2842–2849. [Google Scholar] [CrossRef] [Green Version]
- Lavini, F.; Calò, A.; Gao, Y.; Albisetti, E.; Li, T.D.; Cao, T.; Li, G.; Cao, L.; Aruta, C.; Riedo, E. Friction and work function oscillatory behavior for an even and odd number of layers in polycrystalline MoS2. Nanoscale 2018, 10, 8304–8312. [Google Scholar] [CrossRef]
- Kim, C.; Moon, I.; Lee, D.; Choi, M.S.; Ahmed, F.; Nam, S.; Cho, Y.; Shin, H.-J.; Park, S.; Yoo, W.J. Fermi level pinning at electrical metal contacts of monolayer molybdenum dichalcogenides. ACS Nano 2017, 11, 1588–1596. [Google Scholar] [CrossRef]
- Choi, M.S.; Qu, D.; Lee, D.; Liu, X.; Watanabe, K.; Taniguchi, T.; Yoo, W.J. Lateral MoS2 p–n junction formed by chemical doping for use in high-performance optoelectronics. ACS Nano 2014, 8, 9332–9340. [Google Scholar] [CrossRef]
- Inzani, K.; Nematollahi, M.; Vullum-Bruer, F.; Grande, T.; Reenaas, T.W.; Selbach, S.M. Electronic properties of reduced molybdenum oxides. Phys. Chem. Chem. Phys. 2017, 19, 9232–9245. [Google Scholar] [CrossRef]
- Chuang, S.; Battaglia, C.; Azcatl, A.; McDonnell, S.; Kang, J.S.; Yin, X.; Tosun, M.; Kapadia, R.; Fang, H.; Wallace, R.M.; et al. MoS2 P-type transistors and diodes enabled by high work function MoOx contacts. Nano Lett. 2014, 14, 1337–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, K.; Xiang, Y.; Jiang, M.; Creedon, D.L.; Akhgar, G.; Yianni, S.A.; Xiao, H.; Ley, L.; Stacey, A.; McCallum, J.C.; et al. MoO3 induces p-type surface conductivity by surface transfer doping in diamond. Appl. Surf. Sci. 2020, 509, 144890. [Google Scholar] [CrossRef] [Green Version]
- Kessler, M.A.; Ohrdes, T.; Wolpensinger, B.; Harder, N.-P. Charge carrier lifetime degradation in Cz silicon through the formation of a boron-rich layer during BBr3diffusion processes. Semicond. Sci. Technol. 2010, 25, 055001. [Google Scholar] [CrossRef]
- Szoszkiewicz, R.; Okada, T.; Jones, S.C.; Li, T.D.; King, W.P.; Marder, S.R.; Riedo, E. high-speed, sub-15 nm Feature size thermochemical nanolithography. Nano Lett. 2007, 7, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.V.; Martínez, J.; Garcia, R. Silicon nanowire circuits fabricated by AFM oxidation nanolithography. Nanotechnology 2010, 21, 245301. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; Knoll, A.; Riedo, E. Advanced scanning probe lithography. Nat. Nanotechnol. 2014, 9, 577–587. [Google Scholar] [CrossRef] [Green Version]

















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szoszkiewicz, R. Local Interactions of Atmospheric Oxygen with MoS2 Crystals. Materials 2021, 14, 5979. https://doi.org/10.3390/ma14205979
Szoszkiewicz R. Local Interactions of Atmospheric Oxygen with MoS2 Crystals. Materials. 2021; 14(20):5979. https://doi.org/10.3390/ma14205979
Chicago/Turabian StyleSzoszkiewicz, Robert. 2021. "Local Interactions of Atmospheric Oxygen with MoS2 Crystals" Materials 14, no. 20: 5979. https://doi.org/10.3390/ma14205979
APA StyleSzoszkiewicz, R. (2021). Local Interactions of Atmospheric Oxygen with MoS2 Crystals. Materials, 14(20), 5979. https://doi.org/10.3390/ma14205979





