KCl Extracted from Chlorine Bypass Dust as Activator for Plain Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Materials
2.3. Methodology
3. Results
3.1. Mortar Flow
3.2. Paste Setting Time
3.3. Compressive Strength of Mortar
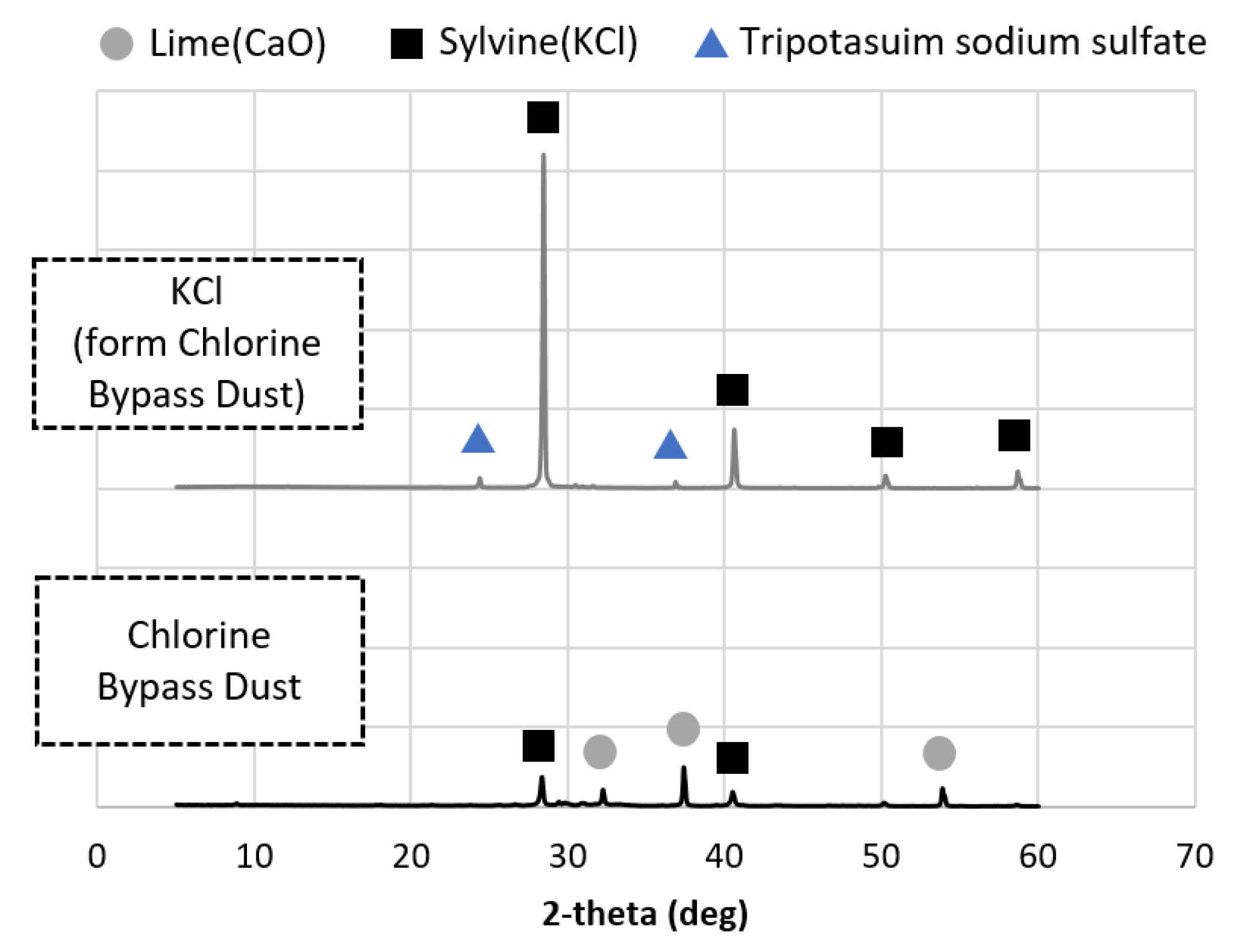
3.4. Mineral Composition
4. Conclusions
- The setting of the mortar paste due to the addition of KCl was slightly slower than pastes containing the strong alkali activators. However, setting within 24 h was achieved when the mineral admixture was used alone, and the setting of the mineral admixture and OPC mixtures was accelerated.
- The fluidity of the mortar was maintained or decreased slightly with the addition of KCl and the alkali activators due to increased mortar viscosity.
- Analysis of compressive strength demonstrated that the 100% mineral admixture samples activated with KCl were not as strong as the samples activated with NaOH and KOH. However, the activity index values of the 50% BFS and 50% OPC sample activated with KCl was 100% or more at all ages, and especially 127% at 28 d and 135% under autoclave condition.
- Water curing of the samples with KCl led to the formation of hydrocalumite, or Friedel’s salt. However, hydrocalumite decomposed during autoclave curing at 180 °C.
- The addition of KCl accelerated setting, improved the early stage hydration activity of the mineral admixtures and OPC mixtures, and increased the strength by >20% compared to the mixtures without an activator at 28 d.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.S.; Yu, G.M.; Mun, K.J. Strength Characteristics of Solidified Soil with Hardening Agents made of Industrial By-Products. J. Korean Geo-Environ. Soc. 2012, 13, 19–26. [Google Scholar]
- Flower, D.J.M.; Sanjayan, J.G. Greenhouse gas emissions due to concrete manufacture. Int. J. Life Cycle Assess. 2007, 12, 282–288. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J. Trends in Global CO2 Emission: 2013/2014/2015 Report; PBL Netherlands Environmental Assessment Agency and European Commission Joint Research Centre: Ispra, Italy; Hague, The Netherlands, 2015. [Google Scholar]
- Nazari, A.; Shadi, R. Splitting tensile strength of concrete using ground granulated blast furnace slag and SiO2 nano particles as binder. Energy Build. 2011, 43, 864–872. [Google Scholar] [CrossRef]
- Felekoglu, B.; Türkel, S.; Kalyoncu, H. Optimization of fineness to maximize the strength activity of high-calcium ground fly ash-Portland cement composites. Constr. Build. Mater. 2009, 23, 2053–2061. [Google Scholar] [CrossRef]
- Bezjak, A. On the Determination of Rat Constants for Hydration Processes in Cement pastes. Cem. Concr. Res. 1981, 10, 553–563. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A. Determination of Kinetic Equations of Alkaline Activation of Blast Furnace Slag by Means of Calorimetric Data. J. Therm. Anal. Calorim. 1998, 52, 945–955. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Provis, J.L. Engineering and durability properties of concretes based on alkali-activated granulated blast furnace slag/metakaolin blends. Constr. Build. Mater. 2012, 33, 99–108. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Microcracking and strength development of alkali activated slag concrete. Cem. Concr. Compos. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Wardhono, A.; Law, D.W.; Molyneaux, T.C.K. Long term performance of alkali activated slag concrete. J. Adv. Concr. Technol. 2015, 13, 187–192. [Google Scholar] [CrossRef] [Green Version]
- El-Feky, M.S.; Kohail, M.; El-Tair, A.M.; Serag, M.I. Effect of microwave curing as compared with conventional regimes on the performance of alkali activated slag pastes. Constr. Build. Mater. 2020, 233, 117268. [Google Scholar] [CrossRef]
- Hamed, N.; El-Feky, M.S.; Kohail, M.; Nasr, E.-S.-A.R. Effect of nano-clay deagglomeration on mechanical properties of concrete. Constr. Build Mater. 2019, 205, 245–256. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. Evaluation of Using Cement in Alkali-Activated Slag Concrete. Int. J. Sci. Technol. Res. 2020, 9, 245–248. [Google Scholar]
- Angulo-Ramírez, D.E.; de Gutiérrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Al-Kutti, W.; Nasir, M.; Johari, M.A.M.; Islam, A.B.M.S.; Manda, A.A.; Blaisi, N.I. An overview and experimental study on hybrid binders containing date palm ash, fly ash, OPC and activator composites. Constr. Build. Mater. 2018, 159, 567–577. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation process of alkali-activated slag mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Ashour, A.F.; Lee, E.T. Properties of cementless mortars activated by sodium silicate. Constr. Build Mater. 2008, 22, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhang, P.; Guo, J.; Wang, W. Bonding behavior of concrete matrix and alkali-activated mortar incorporating nano-SiO2 and polyvinyl alcohol fiber: Theoretical analysis and prediction model. Ceram. Int. 2021, 47, 31638–31649. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Hu, S.; Ling, L. Mechanical properties and prediction of fracture parameters of geopolymer/alkali-activated mortar modified with PVA fiber and nano-SiO2. Ceram. Int. 2020, 46, 20027–20037. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Wang, K. Numerical modeling of rebar-matrix bond behaviors of nano-SiO2 and PVA fiber reinforced geopolymer composites. Ceram. Int. 2021, 47, 11727–11737. [Google Scholar] [CrossRef]
- Rha, C.Y.; Seong, J.W.; Kim, C.E. The preparation and characterization of blended cement used by-pass dust. J. Korean Ceram. Soc. 1999, 36, 618–624. [Google Scholar]
- Han, M.C.; Lee, D.J. Physical and Chemical Properties of Chlorine Bypass System-Dust from Cement Manufacturing. J. Korean Recycl. Constr. Resour. Inst. 2019, 7, 310–315. [Google Scholar] [CrossRef]
- Lee, Y.H.; Han, D.H.; Lee, S.M.; Eom, H.K.; Kim, S.S. A Study on the Cation Extraction and Separation in Cement Industrial By-products for Applications to the Carbonation Process. Appl. Chem. Eng. 2019, 30, 34–38. [Google Scholar] [CrossRef]
- Jang, Y.H.; Lee, Y.H.; Kim, J.Y.; Park, I.G.; Lee, J.Y.; Park, B.H.; Lee, S.M.; Kim, S.S. A study on the optimization of Ion Exchange Resin operating condition for removal of KCl from CKD extract. J. Korean Appl. Sci. Technol. 2019, 36, 1088–1095. [Google Scholar] [CrossRef]
- Lee, E.H.; Jeong, C.I.; Park, S.K.; Lee, H.H. The Influence of KCl on the Hydration Property of OPC. J. Korean Ceram. Soc. 2002, 39, 943–947. [Google Scholar] [CrossRef]
- Standard Specification for Ground Granulated Blast-Furnace Slag and Mortar; ASTM C989:2018; ASTM International: West Conshohocken, PA, USA, 2018.
- Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus; ASTM C204:2018; ASTM International: West Conshohocken, PA, USA, 2018.
- Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM C191:2019; ASTM International: West Conshohocken, PA, USA, 2019.
- Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM C1437:2020; ASTM International: West Conshohocken, PA, USA, 2020.
- Standard Test Method for Compressive Strength of Hydraulic Cement Mortars; ASTM C109:2020; ASTM International: West Conshohocken, PA, USA, 2020.
- Carde, C.; François, R.; Torrenti, J.M. Leaching of both calcium hydroxide and C-S-H from cement paste: Modeling the mechanical behavior. Cem. Concr. Res. 1996, 26, 1257–1268. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Alahrache, S.; Winnefeld, F.; Champenois, J.B.; Hesselbarth, F.; Lothenbach, B. Chemical activation of hybrid binders based on siliceous fly ash and Portland cement. Cem. Concr. Res. 2016, 66, 10–23. [Google Scholar] [CrossRef]
- Yokoyama, S.; Nakano, T.; Tsuchida, Y.; Maki, I. Influence of Chlorine on Clinker Formation from Incinerated Ash of Urban Composite Waste. Cem. Sci. Concr. Technol. 1999, 53, 140–145. [Google Scholar] [CrossRef]
- Sutou, K.; Harada, H.; Ueno, U. Chlorine By-pass System for stable Kiln Operation and the Recycling of waste. Int. Cem. Rev. 1999, 634, 28–35. [Google Scholar] [CrossRef]
- Miyahara, S.; Nakamura, A.; Sakai, E.; Daimon, M. Hydration of cement with KCl. Cem. Sci. Concr. Technol. 1999, 53, 58–62. [Google Scholar]
- Standard Specification for Coal Fly ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM C618:2019; ASTM International: West Conshohocken, PA, USA, 2019.
- Bothe, J.V., Jr.; Brown, P.W. PhreeqC modeling of Friedel’s salt equilibria at 23 ± 1 °C. Cem. Concr. Res. 2004, 34, 1057–1063. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Weiss, J. Flexural strength reduction of cement pastes exposed to CaCl2 solutions. Cem. Concr. Compos. 2018, 86, 297–305. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, I.T.; Seo, K.Y.; Park, H.J. Strength and pore characteristics of OPC-slag Cement paste mixed with polyaluminum chloride. Constr. Build. Mater. 2019, 223, 616–628. [Google Scholar] [CrossRef]
- Galan, I.; Perron, L.; Glasser, F.P. Impact of chloride-rich environments on cement paste mineralogy. Cem. Concr. Res. 2015, 68, 174–183. [Google Scholar] [CrossRef]








| Binder Type | Mineral Admixture Replacement Ratio (wt%) | Alkali Activator Type | Alkali Activator Dosage (wt%) |
|---|---|---|---|
| OPC BFS FA | 0 50 100 | None NaOH KOH KCl (by-product) | 10% to mineral admixture |
| Mixture | W/B (%) | Air (%) | Water (kg/m3) | Binder (kg/m3) | Sand (kg/m3) | Alkaline Activator (kg/m3) | Total (kg/m3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | BFS | FA | NaOH | KOH | KCl | |||||||
| None | OPC100 | 40 | 5 | 206 | 515 | - | - | 1545 | - | - | - | 2265 |
| BFS50 | 204 | 255 | 255 | - | 1532 | - | - | - | 2247 | |||
| FA50 | 201 | 251 | - | 251 | 1505 | - | - | - | 2208 | |||
| NaOH | BFS50 | 204 | 255 | 255 | - | 1532 | 26 | - | - | 2273 | ||
| BFS100 | 203 | - | 507 | - | 1520 | 51 | - | - | 2280 | |||
| FA50 | 201 | 251 | - | 251 | 1505 | 25 | - | - | 2233 | |||
| FA100 | 196 | - | - | 489 | 1468 | 49 | - | - | 2202 | |||
| KOH | BFS50 | 204 | 255 | 255 | - | 1532 | - | 26 | - | 2273 | ||
| BFS100 | 203 | - | 507 | - | 1520 | - | 51 | - | 2280 | |||
| FA50 | 201 | 251 | - | 251 | 1505 | - | 25 | - | 2233 | |||
| FA100 | 196 | - | - | 489 | 1468 | - | 49 | - | 2202 | |||
| KCl | BFS50 | 204 | 255 | 255 | - | 1532 | - | - | 26 | 2273 | ||
| BFS100 | 203 | - | 507 | - | 1520 | - | - | 51 | 2280 | |||
| FA50 | 201 | 251 | - | 251 | 1505 | - | - | 25 | 2233 | |||
| FA100 | 196 | - | - | 489 | 1468 | - | - | 49 | 2202 | |||
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | Cl | Other | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 65.84 | 17.44 | 3.84 | 3.27 | 3.20 | 3.25 | 1.44 | 0.05 | 1.67 | 100 |
| BFS | 48.13 | 30.51 | 12.93 | 0.55 | 2.70 | 2.58 | 0.60 | 0.01 | 1.99 | 100 |
| FA | 6.91 | 49.70 | 21.66 | 11.13 | 2.45 | 0.87 | 1.53 | 0.19 | 5.56 | 100 |
| CBD | 44.4 | 7.74 | 3.23 | 1.92 | 0.55 | 13.68 | 9.87 | 15.7 | 2.91 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-B.; Kim, J.-M.; Choi, S.-M.; Kim, S.-S. KCl Extracted from Chlorine Bypass Dust as Activator for Plain Concrete. Materials 2021, 14, 6091. https://doi.org/10.3390/ma14206091
Choi H-B, Kim J-M, Choi S-M, Kim S-S. KCl Extracted from Chlorine Bypass Dust as Activator for Plain Concrete. Materials. 2021; 14(20):6091. https://doi.org/10.3390/ma14206091
Chicago/Turabian StyleChoi, Hong-Beom, Jin-Man Kim, Sun-Mi Choi, and Sung-Su Kim. 2021. "KCl Extracted from Chlorine Bypass Dust as Activator for Plain Concrete" Materials 14, no. 20: 6091. https://doi.org/10.3390/ma14206091
APA StyleChoi, H.-B., Kim, J.-M., Choi, S.-M., & Kim, S.-S. (2021). KCl Extracted from Chlorine Bypass Dust as Activator for Plain Concrete. Materials, 14(20), 6091. https://doi.org/10.3390/ma14206091






