Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Study of Digestate
2.1.2. Pre-Treatment of Digestate
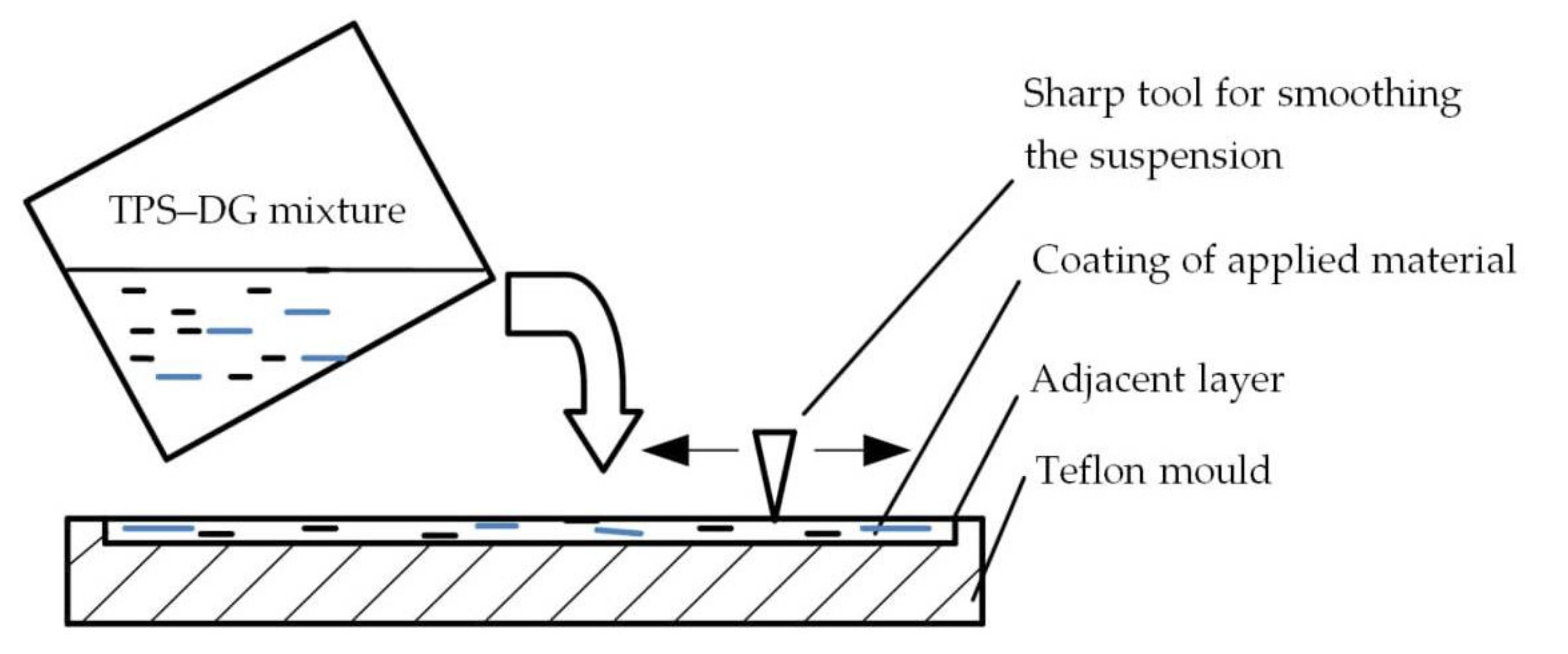
2.2. Biocomposite Production
2.3. Mechanical Properties
2.4. Water Contact Angle
2.5. Colour
2.6. Scanning Electron Microscopy (SEM)
2.7. Thermogravimetric Analysis
2.8. Differential Scanning Calorimetry (DSC)
2.9. Moisture Absorption
2.10. Statistical Analysis
3. Results
3.1. Study of Digestate
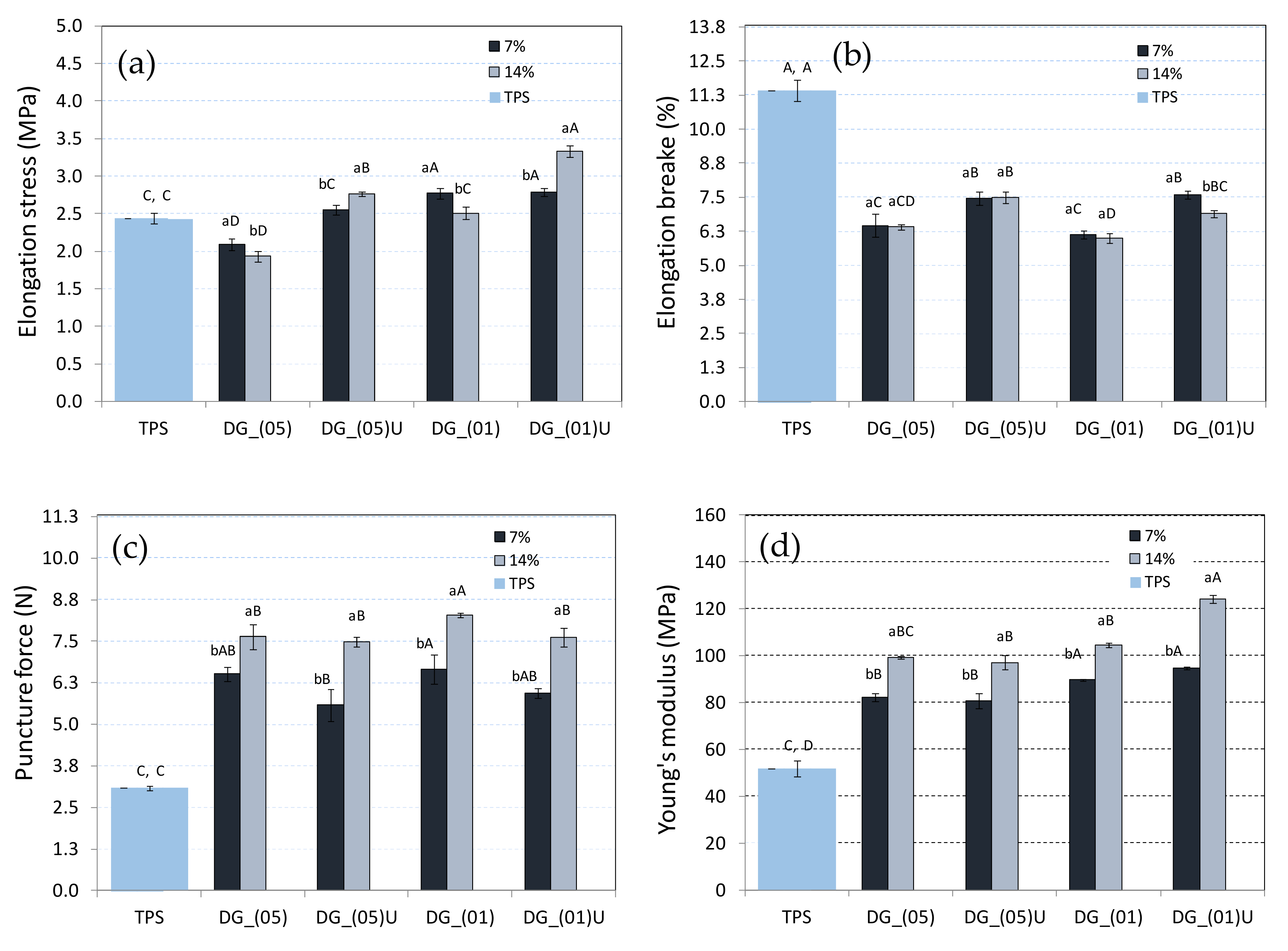
3.2. Mechanical Properties of TPS–DG
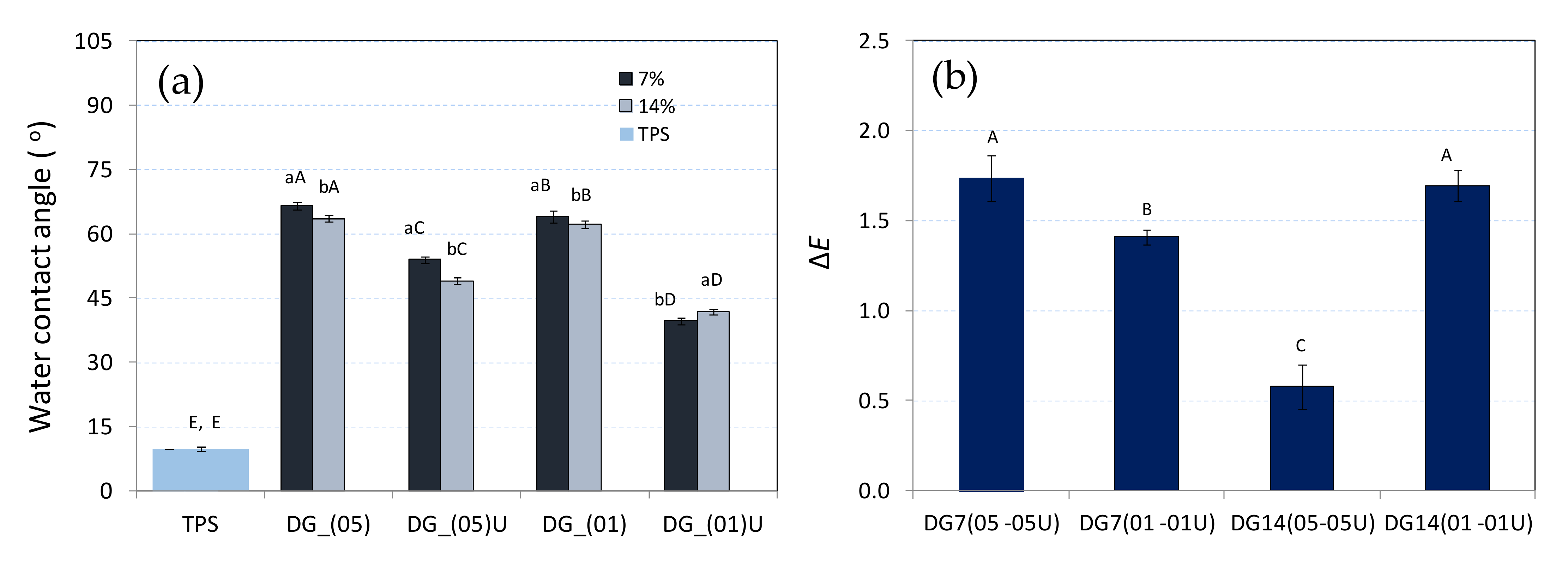
3.3. Water Contact Angle
3.4. Colour
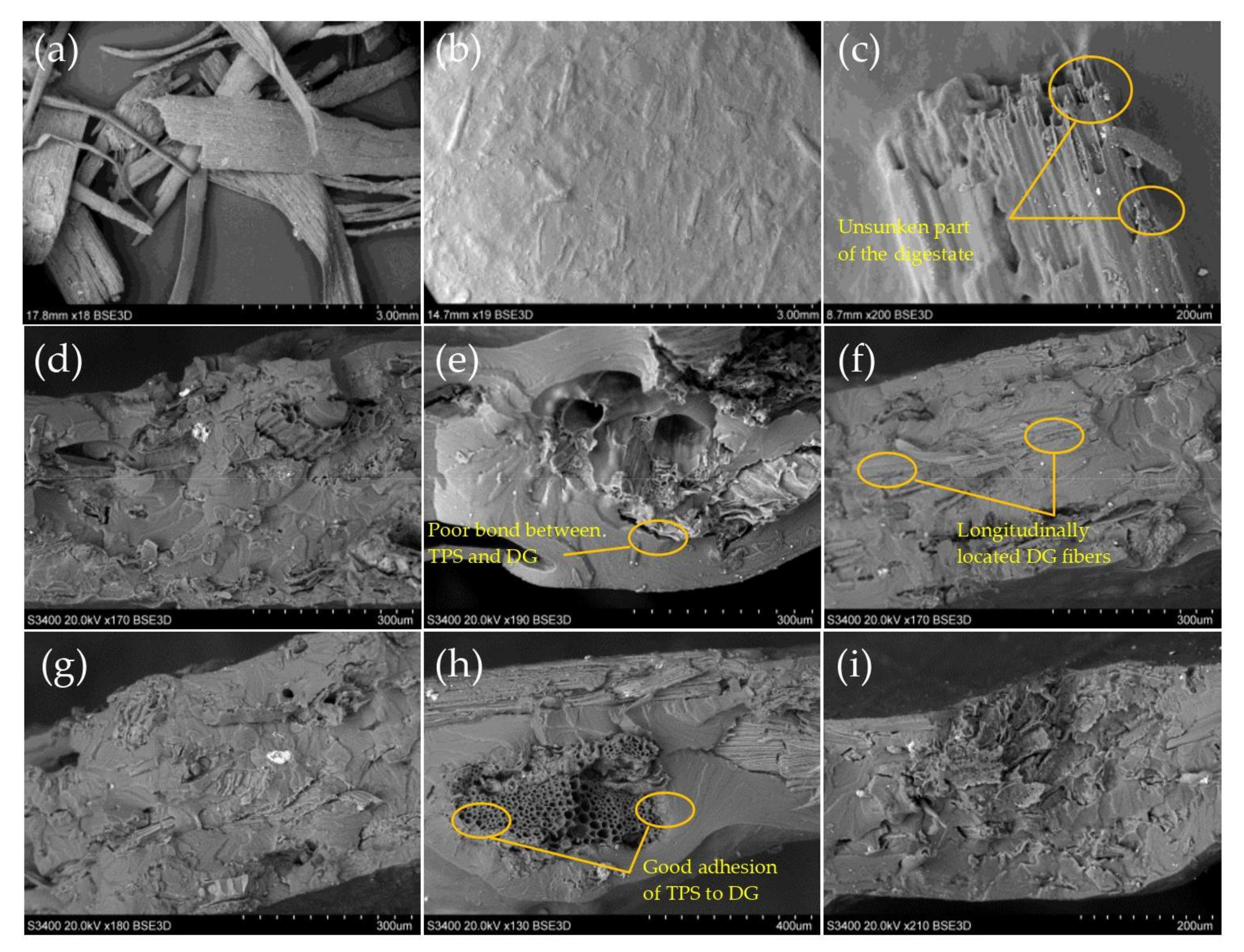
3.5. Scanning Electron Microscopy (SEM)
3.6. Thermogravimetric Analysis
3.7. Differential Scanning Calorimetry (DSC)
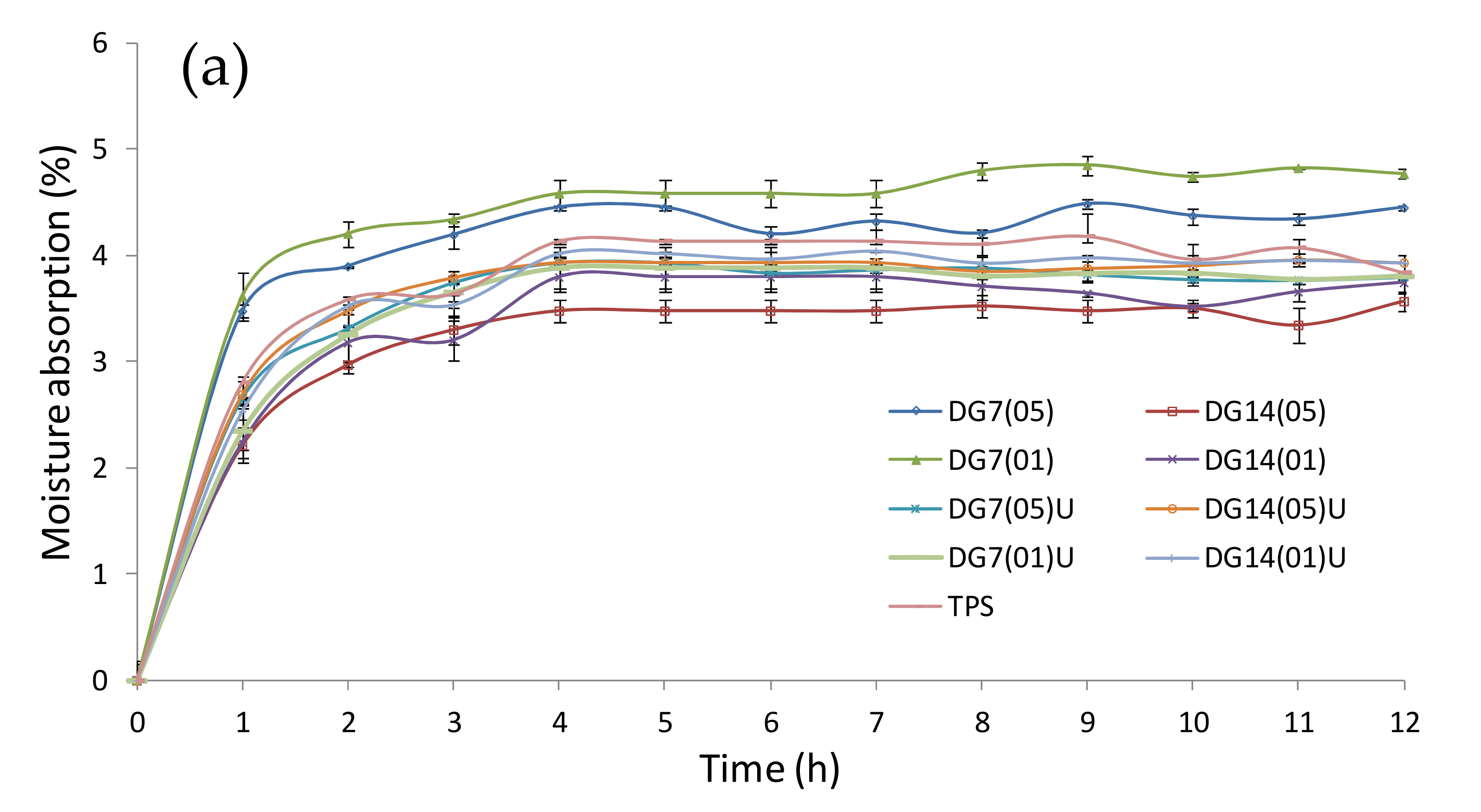
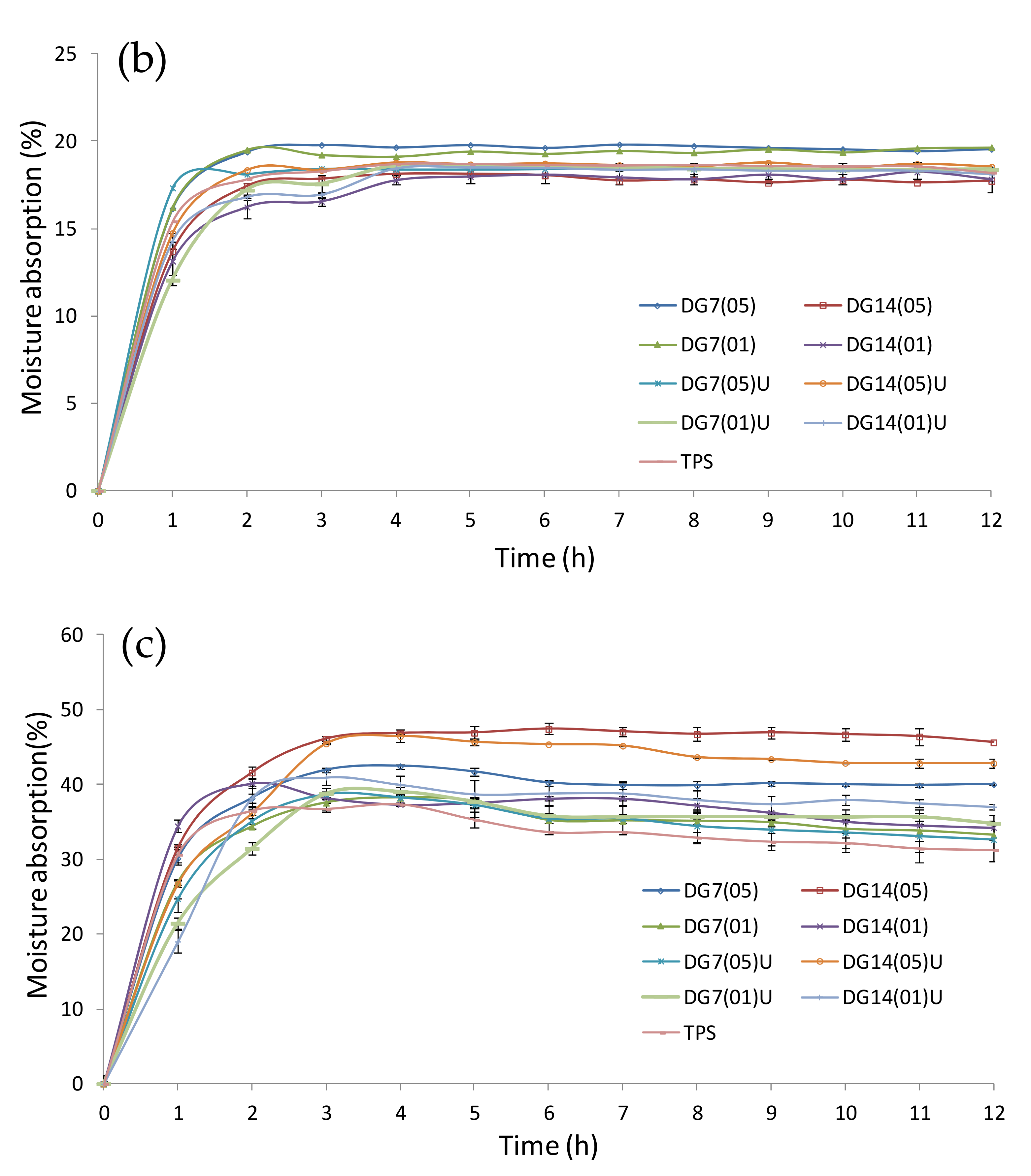
3.8. Moisture Absorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorigato, A.; Perin, D.; Pegoretti, A. Effect of the temperature and of the drawing conditions on the fracture behaviour of thermoplastic starch films for packaging applications. J. Polym. Environ. 2020, 28, 3244–3255. [Google Scholar] [CrossRef]
- Gu, F.; Kim, C.S. On the strategy of ecological design from the perspective of ecological civilization construction. E3S Web Conf. 2020, 179, 01002. [Google Scholar] [CrossRef]
- Kacorzyk, P.; Strojny, J.; Białczyk, B. The impact of biodegradable geotextiles on the effect of sodding of difficult terrain. Sustainability 2021, 13, 5828. [Google Scholar] [CrossRef]
- Kremensas, A.; Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Balčiūnas, G. Mechanical performance of biodegradable thermoplastic polymer-based biocomposite boards from hemp shivs and corn starch for the building industry. Materials 2019, 12, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angellier-Coussy, H.; Guillard, V.; Gastaldi, E.; Peyron, S.; Gontard, N. Lignocellulosic fibres-based biocomposites materials for food packaging. In Lignocellulosic Composite Materials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 389–413. [Google Scholar]
- Rodriguez, L.J.; Peças, P.; Carvalho, H.; Orrego, C.E. A literature review on life cycle tools fostering holistic sustainability assessment: An application in biocomposite materials. J. Environ. Manag. 2020, 262, 110308. [Google Scholar] [CrossRef]
- Lisowski, A.; Pajor, M.; Świętochowski, A.; Da browska, M.; Klonowski, J.; Mieszkalski, L.; Ekielski, A.; Stasiak, M.; Piatek, M. Effects of moisture content, temperature, and die thickness on the compaction process, and the density and strength of walnut shell pellets. Renew. Energy 2019, 141, 770–781. [Google Scholar] [CrossRef]
- Nanthananon, P.; Seadan, M.; Pivsa-Art, S.; Hamada, H.; Suttiruengwong, S. Reactive compatibilization of short-fiber reinforced poly (lactic acid) biocomposites. J. Renew. Mater. 2018, 6, 573–583. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus pomace biomass as a source of pectin and lignocellulose fibers: From waste to upgraded biocomposites for mulching applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef]
- Stciklen, M. Plant genetic engineering to improve biomass characteristics for biofuel. Science 2006, 17, 315–319. [Google Scholar]
- Chen, R.; Blagodatskaya, E.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Kuzyakov, Y. Decomposition of biogas residues in soil and their effects on microbial growth kinetics and enzyme activities. Biomass Bioenergy 2012, 45, 221–229. [Google Scholar] [CrossRef]
- Nielsen, K.; Roß, C.L.; Hoffmann, M.; Muskolus, A.; Ellmer, F.; Kautz, T. The chemical composition of biogas digestates determines their effect on soil microbial activity. Agriculture 2020, 10, 244. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Börjesson, J.; Bruun, M.H.; Tjerneld, F.; Jørgensen, H. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzym. Microb. Technol. 2007, 40, 888–895. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Doussot, J.; Blondeau, J.P.; Lainé, E. Characterization of ultrasonic impact on coir, flax and hemp fibers. Mater. Lett. 2014, 129, 137–141. [Google Scholar] [CrossRef]
- Syafri, E.; Yulianti, E.; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar] [CrossRef]
- Ekielski, A.; Wojcik, G.P. Carrot pomace as an additive to pressed PLA/TPS blends products: Preliminary studies. Int. Multidiscip. Sci. Geo Conf. SGEM 2017, 17, 577–584. [Google Scholar]
- Taurino, R.; Lancellotti, I.; Tatàno, F.; Carchesio, M.; Pozzi, P. Mechanical and chemical resistance of composite materials with addition of anaerobic digestate. Compos. Part B Eng. 2016, 92, 259–264. [Google Scholar] [CrossRef]
- Nair, S.S.; Chen, H.; Peng, Y.; Huang, Y.; Yan, N. Polylactic acid biocomposites reinforced with nanocellulose fibrils with high lignin content for improved mechanical, thermal, and barrier properties. ACS Sustain. Chem. Eng. 2018, 6, 10058–10068. [Google Scholar] [CrossRef]
- Żelazinski, T.; Ekielski, A.; Tulska, E.; Vladut, V.; Durczak, K. Wood dust application for improvement of selected properties of thermoplastic starch. Inmateh. Agric. Eng. 2019, 58, 37–44. [Google Scholar]
- Adeeyo, O.A.; Oresegun, O.M.; Oladimeji, T.E. Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am. J. Eng. Res. 2015, 4, 14–19. [Google Scholar]
- Margulis, M.A.; Margulis, I.M. Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrason. Sonochemistry 2003, 10, 343–345. [Google Scholar] [CrossRef]
- PN-EN ISO 527-1: 2020-01, Plastics—Determination of Mechanical Properties under Static Stretching—Part 1: General Principles; Polish Standardization Committee: Warsaw, Poland, 2020; Available online: https://sklep.pkn.pl/pn-en-iso-527-1-2020-01e.html (accessed on 26 August 2021).
- PN-EN ISO 527-2: 2012. Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; Polish Standardization Committee: Warsaw, Poland, 2013; Available online: https://www.iso.org/standard/56046.html (accessed on 26 August 2021).
- Sirviö, J.A.; Visanko, M.; Ukkola, J.; Liimatainen, H. Effect of plasticizers on the mechanical and thermomechanical properties of cellulose-based biocomposite films. Ind. Crop. Prod. 2018, 122, 513–521. [Google Scholar] [CrossRef]
- Giri, J.; Lach, R.; Grellmann, W.; Susan, M.A.; Saiter, J.M.; Henning, S.; Katiyar, V.; Adhikari, R. Compostable composites of wheat stalk micro-and nanocrystalline cellulose and poly (butylene adipate-co-terephthalate): Surface properties and degradation behavior. J. Appl. Polym. Sci. 2019, 136, 48149. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, B.Q.; Lv, Z.K.; Liang, F.Q. Improvement of hemp fiber flexibility by ultrasonic pretreatment. Dalian Gongye Daxue Xuebao 2012, 31, 292–294. [Google Scholar]
- Teixeira, E.D.M.; Curvelo, A.A.; Corrêa, A.C.; Marconcini, J.M.; Glenn, G.M.; Mattoso, L.H. Properties of thermoplastic starch from cassava bagasse and cassava starch and their blends with poly (lactic acid). Ind. Crop. Prod. 2012, 37, 61–68. [Google Scholar] [CrossRef]
- Bilck, A.P.; Grossmann, M.V.; Yamashita, F. Biodegradable mulch films for strawberry production. Polym. Test 2010, 29, 471–476. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic starch/wood biocomposites processed with deep eutectic solvents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Valencia, G.A.; Luciano, C.G.; Lourenco, R.V.; do Amaral Sobral, P.J. Microstructure and physical properties of nano-biocomposite films based on cassava starch and laponite. Int. J. Biol. Macromol. 2018, 107, 1576–1583. [Google Scholar] [CrossRef]
- Aydin, I.; Colakoglu, G. Variation in surface roughness, wettability and some plywood properties after preservative treatment with boron compounds. Build. Environ. 2007, 42, 3837–3840. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly (lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Marcilhac, C.; Sialve, B.; Pourcher, A.M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.D.; Duc Vu, C.; Viet Nguyen, H. Lignin and cellulose extraction from vietnam’s rice straw using ultrasound-assisted alkaline treatment method. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W. Ultrasonic vibration-assisted (UV-A) manufacturing processes: State of the art and future perspectives. J. Manuf Process. 2020, 51, 174–190. [Google Scholar] [CrossRef]
- Osman, A.F.; Ashafee, A.M.T.; Adnan, S.A.; Alakrach, A. Influence of hybrid cellulose/bentonite fillers on structure, ambient, and low temperature tensile properties of thermoplastic starch composites. Polym. Eng. Sci. 2020, 60, 810–822. [Google Scholar] [CrossRef]
- Angelopoulou, D.; Meunier, V.; Forny, L.; Niederreiter, G.; Palzer, S.; Salman, A.D. Particle surface design for enhanced reconstitution of fat-based food powders. Powder Technol. 2021, 393, 397–404. [Google Scholar] [CrossRef]
- Irwin, A. How to solve a problem like plastics. New Sci. 2018, 238, 25–31. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Mardones, C.; Von Baer, D.; Bautista-Baños, S.; Dublan Garcia, O. Physical-chemical evaluation of active food packaging material based on thermoplastic starch loaded with grape cane extract. Molecules 2020, 25, 1306. [Google Scholar] [CrossRef] [Green Version]
- Zain, A.M.; Kahar, A.W.M.; Noriman, N.Z. Chemical-mechanical hydrolysis technique of modified thermoplastic starch for better mechanical performance. Procedia Chem. 2016, 19, 638–645. [Google Scholar] [CrossRef]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O. Development of biodegradable composite chitosan-based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Yang, H.S.; Wolcott, M.P.; Kim, H.S.; Kim, H.J. Thermal properties of lignocellulosic filler-thermoplastic polymer bio-composites. J. Therm. Anal. Calorim. 2005, 82, 157–160. [Google Scholar] [CrossRef]
- Kim, H.S.; Yang, H.S.; Kim, H.J.; Lee, B.J.; Hwang, T.S. Thermal properties of agro-flour-filled biodegradable polymer bio-composites. J. Therm. Anal. Calorim. 2005, 81, 299–306. [Google Scholar] [CrossRef]
- Yang, S.; Tang, Y.; Wang, J.; Kong, F.; Zhang, J. Surface treatment of cellulosic paper with starch-based composites reinforced with nanocrystalline cellulose. Ind. Eng. Chem. Res. 2014, 53, 13980–13988. [Google Scholar] [CrossRef]
- Corradini, E.; Carvalho, A.J.F.D.; Curvelo, A.A.D.S.; Agnelli, J.A.M.; Mattoso, L.H.C. Preparation and characterization of thermoplastic starch/zein blends. Mater. Res. 2007, 10, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Bryś, A.; Bryś, J.; Ostrowska-Ligęza, E.; Kaleta, A.; Górnicki, K.; Głowacki, S.; Koczoń, P. Wood biomass characterization by DSC or FT-IR spectroscopy. J. Therm. Anal. Calorim. 2016, 126, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Asrofi, M.; Abral, H.; Putra, Y.K.; Sapuan, S.M.; Kim, H.J. Effect of duration of sonication during gelatinization on properties of tapioca starch water hyacinth fiber biocomposite. Int. J. Biol. Macromol. 2018, 108, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Cellulose nanofibrils reinforced PBAT/TPS blends: Mechanical and rheological properties. Int. J. Biol. Macromol. 2021, 183, 267–275. [Google Scholar] [CrossRef] [PubMed]



| Basic Components of TPS | Addition of Digestate DG to the Mixture (wt%) | Parameters of the Digestate (DG) | Acronym of the Obtained Film/Biocomposite TPS–DG | |
|---|---|---|---|---|
| Fraction (mm) | Ultrasonic Treatment | |||
| Starch 50 g + glycerol 25 g | 7 | <0.1 | Yes | DG_(01)U |
| 14 | <0.1 | Yes | DG_(01)U | |
| 7 | 0.1–0.5 | Yes | DG_(05)U | |
| 14 | 0.1–0.5 | Yes | DG_(05)U | |
| 7 | <0.1 | no | DG_(01) | |
| 14 | <0.1 | no | DG_(01) | |
| 7 | 0.1–0.5 | no | DG_(05) | |
| 14 | 0.1–0.5 | no | DG_(05) | |
| 0 | - | - | TPS | |
| Biogas Plant | Dry Matter (%) | Cellulose % DM | Lignin % DM |
|---|---|---|---|
| A * | 39 ± 1.25 | 20.3 ± 0.58 | 24.8 ± 1.37 |
| B | 28 ± 2.63 | 17.2 ± 1.58 | 32.6 ± 2.98 |
| C | 29 ± 1.84 | 17.9 ± 2.66 | 30.2 ± 3.82 |
| Sample | L* | a* | b* |
|---|---|---|---|
| DG7(01) | 37.40 (±0.1) | 3.52 (±0.03) | 11.13 (±0.06) |
| DG7(01)U | 36.26 (±0.53) | 3.90 (±0.18) | 10.41 (±0.44) |
| DG7(05) | 46.09 (±0.18) | 2.31 (±0.03) | 10.50 (±1.11) |
| DG7(05)U | 47.63 (±1.04) | 2.54 (±0.21) | 9.74 (±0.73) |
| DG14(01) | 25.18 (±0.45) | 1.11 (±0.13) | 3.19 (±0.60) |
| DG14(01)U | 24.47 (±0.75) | 1.83 (±0.29) | 4.55 (±0.31) |
| DG14(05) | 30.36 (±0.87) | 2.11 (±0.09) | 6.96 (±0.30) |
| DG14(05)U | 30.86 (±0.94) | 2.14 (±0.30) | 6.66 (±0.43) |
| TPS | 76.17 (±0.70) | −2.06 (±0.13) | 4.97 (±0.13) |
| Measurement | DG14(01) | DG14(01)U | TPS | ||
|---|---|---|---|---|---|
| Enthalpy 1 | Enthalpy 2 | Enthalpy 3 | Enthalpy 4 | Enthalpy 5 | |
| Onset (°C) | 139.23 | 153.34 | 138.68 | 167.55 | 138.22 |
| Peak Height (Wg−1) | 0.15 | 1.01 | 0.15 | 1.54 | 3.75 |
| Peak (°C) | 140.2 | 170.92 | 139.65 | 171.43 | 139.53 |
| Extrapol peak (°C) | 140.31 | 157.92 | 139.73 | 171.08 | 138.76 |
| Endset (°C) | 141.97 | 217.61 | 141.84 | 196.85 | 150.52 |
| Peak temperature range (°C) | 1.39 | 36.06 | 1.59 | 22.51 | 7.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekielski, A.; Żelaziński, T.; Mishra, P.K.; Skudlarski, J. Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics. Materials 2021, 14, 6092. https://doi.org/10.3390/ma14206092
Ekielski A, Żelaziński T, Mishra PK, Skudlarski J. Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics. Materials. 2021; 14(20):6092. https://doi.org/10.3390/ma14206092
Chicago/Turabian StyleEkielski, Adam, Tomasz Żelaziński, Pawan Kumar Mishra, and Jacek Skudlarski. 2021. "Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics" Materials 14, no. 20: 6092. https://doi.org/10.3390/ma14206092
APA StyleEkielski, A., Żelaziński, T., Mishra, P. K., & Skudlarski, J. (2021). Properties of Biocomposites Produced with Thermoplastic Starch and Digestate: Physicochemical and Mechanical Characteristics. Materials, 14(20), 6092. https://doi.org/10.3390/ma14206092








