Effect of Graphene Oxide on Properties of Alkali-Activated Slag
Abstract
:1. Introduction
- (1)
- GO influenced rheological properties
- (2)
- GO affected the mechanical properties
- (3)
- GO influenced the microstructure and its template effect
- (4)
- GO influenced the durability
- (5)
- GO endowed cement-based materials other functionality
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussions
3.1. Working Performances
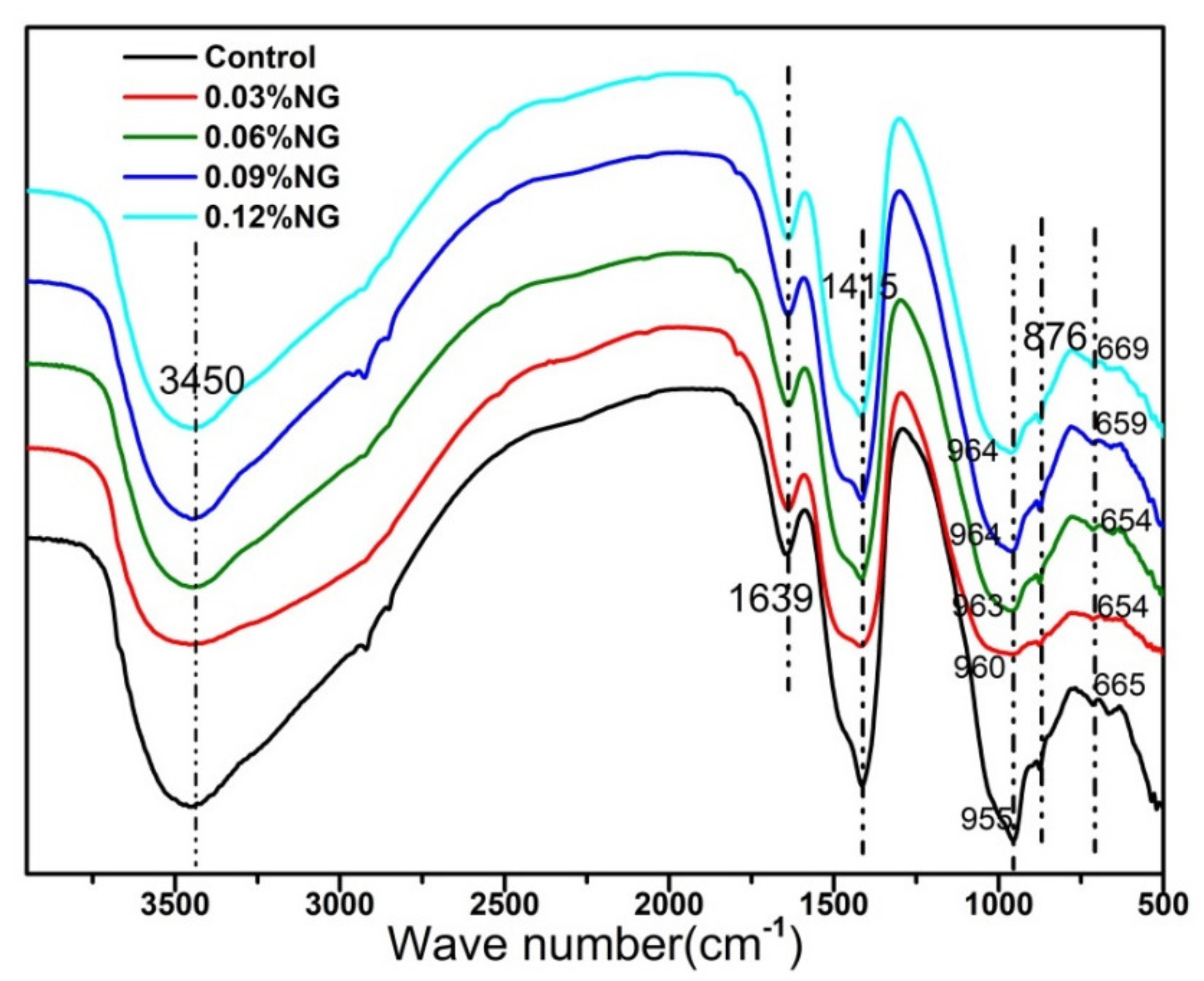
3.2. Hydration Properties
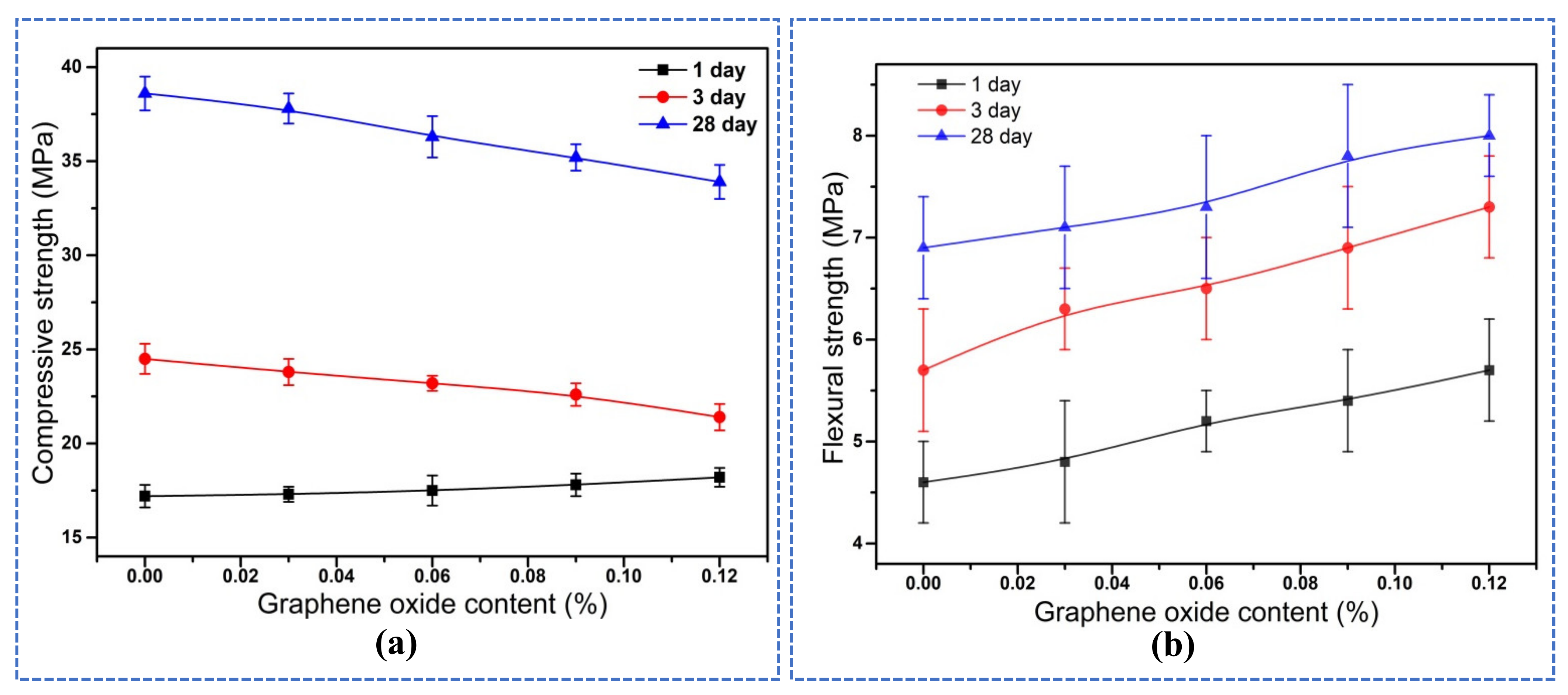
3.3. Mechanical Properties
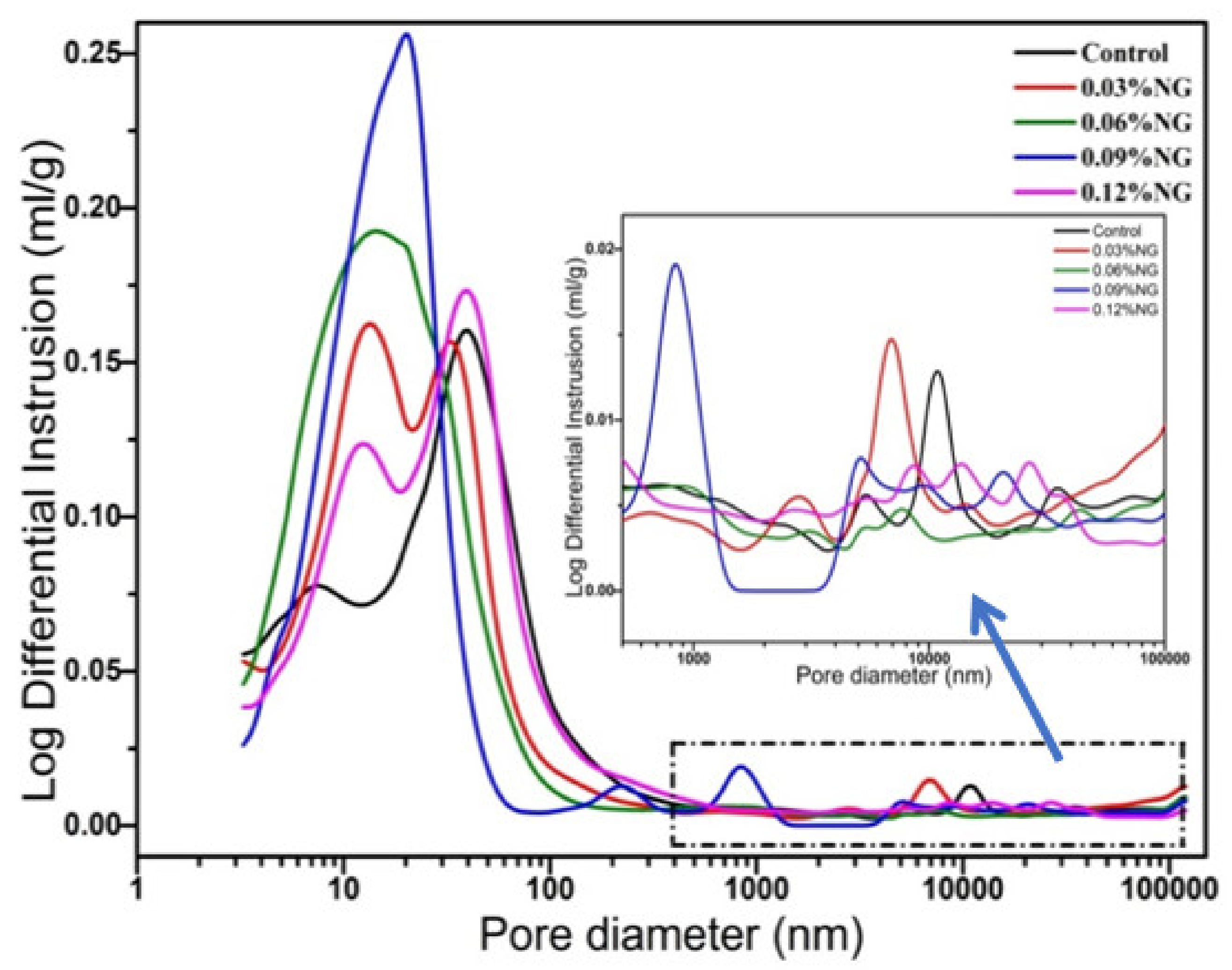
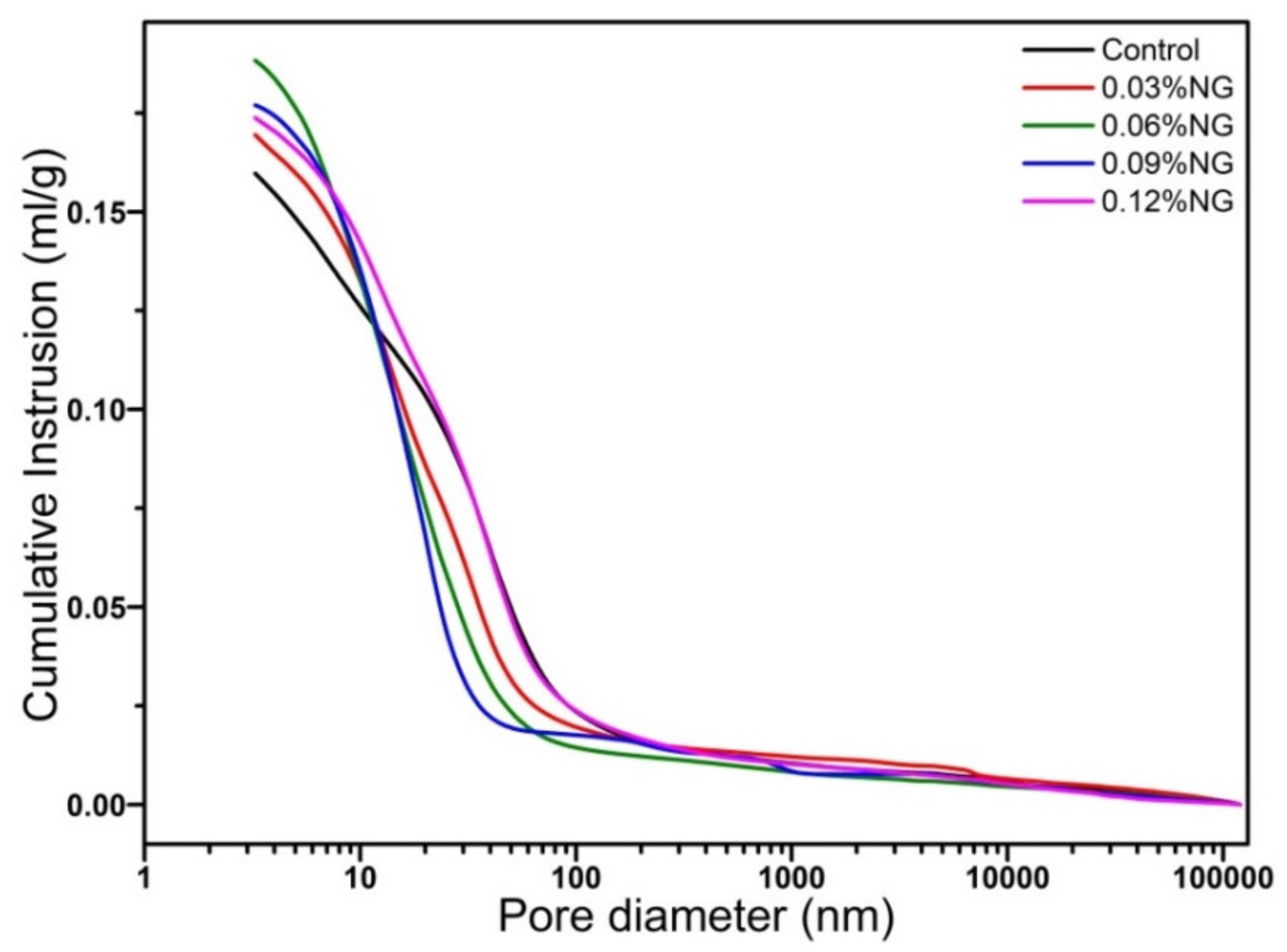
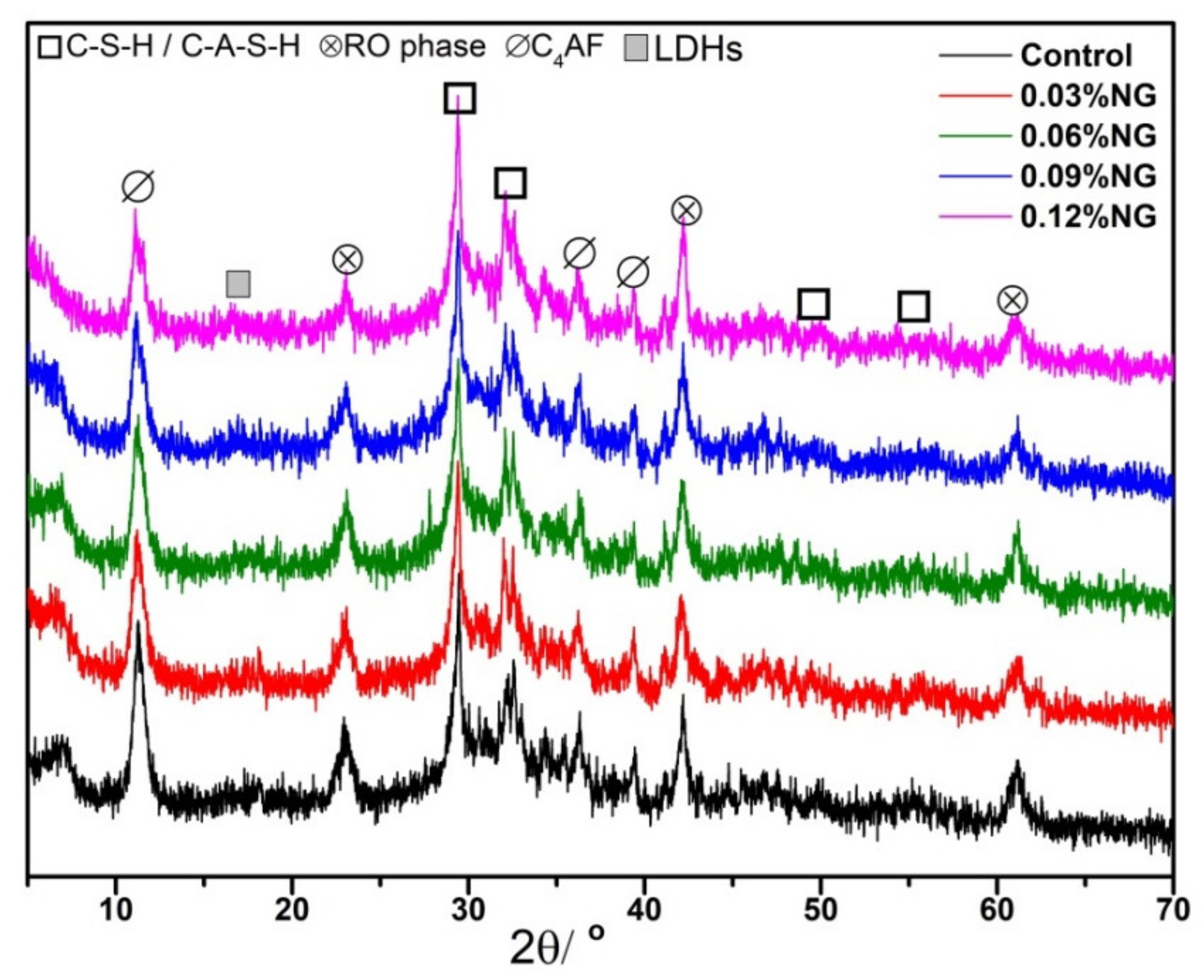
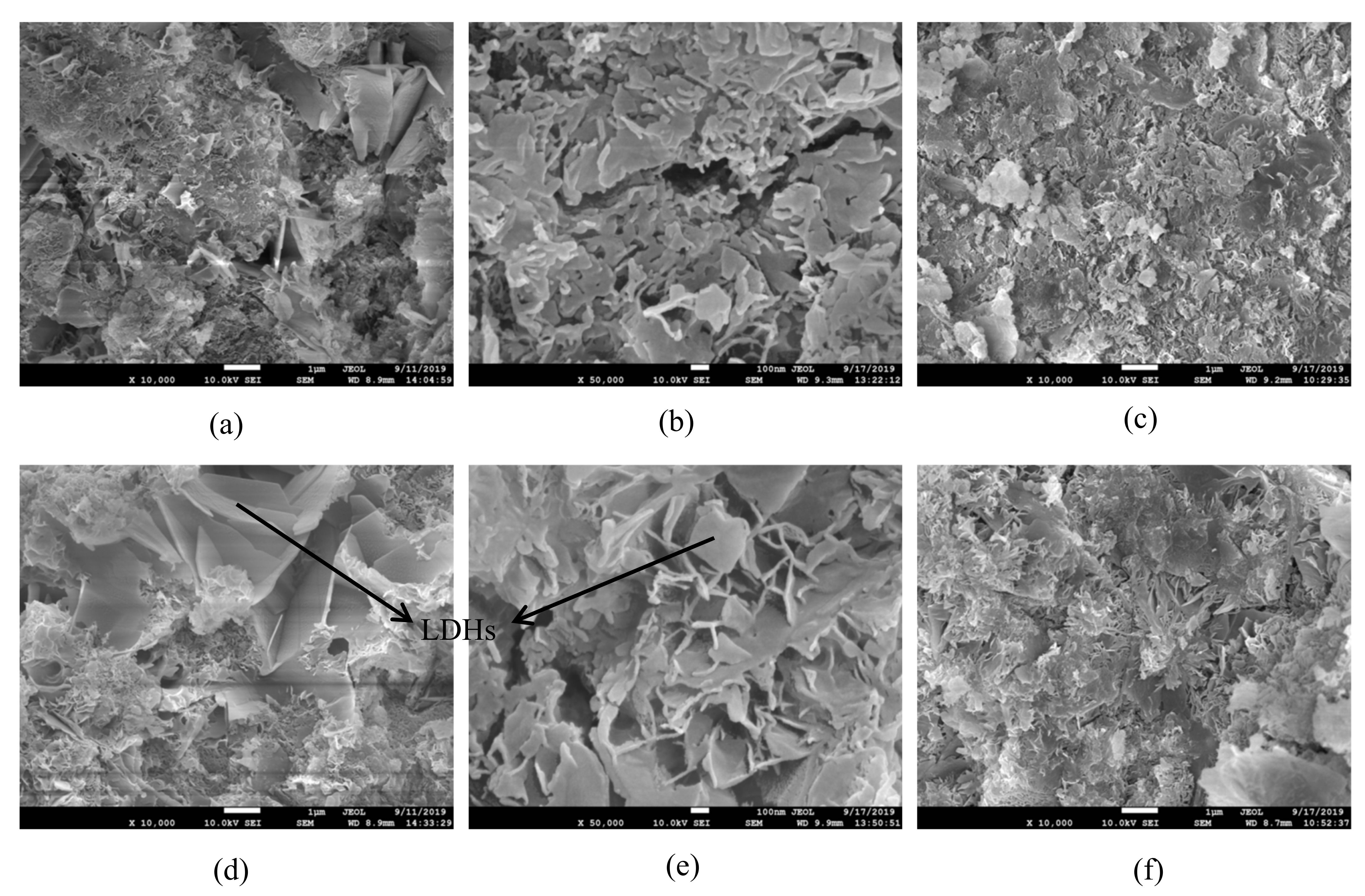
3.4. Microstructure of Hardened Paste
4. Conclusions
- (1)
- The compressive strength of mortar at 1 day was slightly increased with the addition of GO, while at 3 days and 28 days it was decreased. The flexural strength of mortars at different curing periods increased with the increase of the GO content, and the increasing rate of flexural strength reached up to 15.94% at 28 days.
- (2)
- GO adsorbed free water in paste and reduced the fluidity of mortar. GO accelerated the hydration process of alkali-activated slag, and the functional groups on surfaces provided nucleation sites to induce the generation of more hydration products.
- (3)
- The incorporation of GO enhanced the number of harmless pores and reduced the number of less harmful pores, but also introduced a large number of harmful pores, resulting in the reduction of compressive strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Use of municipal solid waste incineration bottom ashes in alkali-activated materials, ceramics and granular applications: A review. Waste Manag. 2017, 68, 207–220. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, G. Effect of reduced graphene oxide on the early-age mechanical properties of geopolymer cement. Mater. Lett. 2020, 276, 128223. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G.; Hu, J. Micro-mechanical properties of alkali-activated fly ash evaluated by nanoindentation. Constr. Build. Mater. 2017, 147, 407–416. [Google Scholar] [CrossRef]
- Zhu, W.; Bartos, P.J.M.; Porro, A. Application of nanotechnology in construction Summary of a state-of-the-art report. Mater. Struct. 2004, 37, 649–658. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Guo, S.; Lu, Y.; Wan, X.; Wu, F.; Zhao, T.; Shen, C. Preparation, characterization of highly dispersed reduced graphene oxide/epoxy resin and its application in alkali-activated slag composites. Cem. Concr. Compos. 2020, 105, 103424. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Lu, C.-x.; Liu, B.-w.; Zhang, K.; Li, C.-z. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. New Carbon Mater. 2015, 30, 349–356. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Qin, H.; Hang Hu, Y. Co-effects of graphene oxide sheets and single wall carbon nanotubes on mechanical properties of cement. J. Phys. Chem. Solids 2015, 85, 39–43. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Zhu, X.H.; Kang, X.J.; Yang, K.; Yang, C.H. Effect of graphene oxide on the mechanical properties and the formation of layered double hydroxides (LDHs) in alkali-activated slag cement. Constr. Build. Mater. 2017, 132, 290–295. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Abo-El-Enein, S.A.; Hassaan, M.Y.; Morsy, M.S.; Khalil, M.H. Mechanical performance, pore structure and micro-structural characteristics of graphene oxide nano platelets reinforced cement. Constr. Build. Mater. 2017, 138, 333–339. [Google Scholar] [CrossRef]
- Lv, S.H.; Deng, L.J.; Yang, W.Q.; Zhou, Q.F.; Cui, Y.Y. Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites. Cem. Concr. Compos. 2016, 66, 1–9. [Google Scholar] [CrossRef]
- Lu, Z.; Hanif, A.; Ning, C.; Shao, H.; Yin, R.; Li, Z. Steric stabilization of graphene oxide in alkaline cementitious solutions: Mechanical enhancement of cement composite. Mater. Des. 2017, 127, 154–161. [Google Scholar] [CrossRef]
- Horszczaruk, E.; Mijowska, E.; Kalenczuk, R.J.; Aleksandrzak, M.; Mijowska, S. Nanocomposite of cement/graphene oxide—Impact on hydration kinetics and Young’s modulus. Constr. Build. Mater. 2015, 78, 234–242. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, J.; Zhu, L.; Jia, C. Preparation of Cement Composites with Ordered Microstructures via Doping with Graphene Oxide Nanosheets and an Investigation of Their Strength and Durability. Materials 2016, 9, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, S.; Zhang, J.; Zhu, L.; Jia, C.; Luo, X. Preparation of Regular Cement Hydration Crystals and Ordered Microstructures by Doping GON and an Investigation into Its Compressive and Flexural Strengths. Crystals 2017, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Ma, Y.; Qiu, C.; Zhou, Q. Regulation of GO on cement hydration crystals and its toughening effect. Mag. Concr. Res. 2013, 65, 1246–1254. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Sun, T.; Ma, Y.; Zhou, Q. Effect of GO nanosheets on shapes of cement hydration crystals and their formation process. Constr. Build. Mater. 2014, 64, 231–239. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Tang, L.; Xing, F. Possible pitfall in sample preparation for SEM analysis—A discussion of the paper “Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites” by Lv et al. Cem. Concr. Compos. 2017, 77, 81–85. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Ho, V.D.; Gholampour, A.; Losic, D.; Ozbakkaloglu, T. Enhancing the performance and environmental impact of alkali-activated binder-based composites containing graphene oxide and industrial by-products. Constr. Build. Mater. 2021, 284, 122811. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the effects of graphene and graphene oxide nanoplatelets on the micro- and macro-properties of cementitious materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Suo, Y.; Guo, R.; Xia, H.; Yang, Y.; Yan, F.; Ma, Q. Study on modification mechanism of workability and mechanical properties for graphene oxide-reinforced cement composite. Nanomater. Nanotechnol. 2020, 10, 184798042091260. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Q.; Yu, Q.; Gao, R.; Tong, T. Experimental investigation on mechanical and piezoresistive properties of cementitious materials containing graphene and graphene oxide nanoplatelets. Constr. Build. Mater. 2016, 127, 565–576. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Narayanan, T.N.; Hashim, D.P.; Sakhavand, N.; Shahsavari, R.; Vajtai, R.; Ajayan, P.M. Hexagonal Boron Nitride and Graphite Oxide Reinforced Multifunctional Porous Cement Composites. Adv. Funct. Mater. 2013, 23, 5624–5630. [Google Scholar] [CrossRef]
- Long, W.J.; Lin, C.; Tan, X.W.; Tao, J.L.; Ye, T.H.; Luo, Q.L. Structural Applications of Thermal Insulation Alkali Activated Materials with Reduced Graphene Oxide. Materials 2020, 13, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2014, 2014, 276323. [Google Scholar] [CrossRef]
- Li, Z.; Shi, X. Graphene oxide modified, clinker-free cementitious paste with principally alkali-activated fly ash. Fuel 2020, 269, 117418. [Google Scholar] [CrossRef]
- Hou, P.; Wang, K.; Qian, J.; Kawashima, S.; Kong, D.; Shah, S.P. Effects of colloidal nanoSiO2 on fly ash hydration. Cem. Concr. Compos. 2012, 34, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Su, Y.; Du, X.; Yang, Y.; Wei, S.; Shah, S.P. Influence of nano-silica agglomeration on fresh properties of cement pastes. Constr. Build. Mater. 2013, 43, 557–562. [Google Scholar] [CrossRef]
- Test Method for Fluidity of Cement Mortar; Standardization Administration of China: Beijing, China, 2005; GB/T2419-2005.
- Cement-Test Methods-Determination of Strength; International Organization for Standardization: Bern, Switzerland, 2009; ISO679-2009.
- Li, X.; Korayem, A.H.; Li, C.; Liu, Y.; He, H.; Sanjayan, J.G.; Duan, W.H. Incorporation of graphene oxide and silica fume into cement paste: A study of dispersion and compressive strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Li, W.G.; Li, C.Y.; Sanjayan, J.G.; Duan, W.H.; Li, Z. Effects of graphene oxide agglomerates on workability, hydration, microstructure and compressive strength of cement paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Zhingwei Wu, H.L. High Performance Concrete; China Railway Press: Beijing, China, 1999. [Google Scholar]
- Pang, B.; Zhou, Z.; Xu, H. Utilization of carbonated and granulated steel slag aggregate in concrete. Constr. Build. Mater. 2015, 84, 454–467. [Google Scholar] [CrossRef]
- Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Gomez-Soberon, J.M.; Chinchillas-Chinchillas, M.J.; Corral-Higuera, R. Experimental study of XRD, FTIR and TGA techniques in geopolymeric materials. Int. J. Hous. Sci. Its Appl. 2014, 4, 221–227. [Google Scholar]
- Mollah, M.Y.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Ou, Z.H.; Ma, B.G.; Jian, S.W. Comparison of FT-IR, Thermal Analysis and XRD for Determination of Products of Cement Hydration. Adv. Mater. Res. 2010, 168–170, 518–522. [Google Scholar] [CrossRef]



| Sample | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | CaO (%) | MgO (%) | K2O (%) | Na2O (%) | Others (%) |
|---|---|---|---|---|---|---|---|---|
| Steel slag | 14.09 | 5.03 | 19.00 | 45.19 | 6.53 | 0.04 | 0.11 | 10.01 |
| Slag | 31.60 | 16.61 | 0.32 | 35.77 | 10.06 | 0.36 | 0.46 | 4.82 |
| Sequence Number | Slag and Steel Slag / (%) | Nano- GO/(%) | Liquid-Solid Ratio | Polycarboxylic Acid (%) | [NaOH]/ mol/L | Fine Aggregate/g |
|---|---|---|---|---|---|---|
| Control | 100 | 0.00 | 0.45 | 0.12 | 4.17 | 1350 |
| M1 | 99.97 | 0.03 | 0.45 | 0.12 | 4.17 | 1350 |
| M2 | 99.94 | 0.06 | 0.45 | 0.12 | 4.17 | 1350 |
| M3 | 99.91 | 0.09 | 0.45 | 0.12 | 4.17 | 1350 |
| M4 | 99.88 | 0.12 | 0.45 | 0.12 | 4.17 | 1350 |
| Sequence Number | Pores < 20 nm /(%) | Pores 20–200 nm /(%) | Pores ≥ 200 nm /(%) |
|---|---|---|---|
| Control | 43.53 | 49.02 | 7.45 |
| M1 | 51.03 | 40.48 | 8.49 |
| M2 | 60.06 | 31.61 | 8.33 |
| M3 | 64.28 | 26.49 | 9.23 |
| M4 | 47.01 | 42.94 | 10.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Wan, L.; Luan, C.; Wang, J.; Du, P. Effect of Graphene Oxide on Properties of Alkali-Activated Slag. Materials 2021, 14, 6107. https://doi.org/10.3390/ma14206107
Dong Q, Wan L, Luan C, Wang J, Du P. Effect of Graphene Oxide on Properties of Alkali-Activated Slag. Materials. 2021; 14(20):6107. https://doi.org/10.3390/ma14206107
Chicago/Turabian StyleDong, Quanwen, Lihua Wan, Congqi Luan, Jinbang Wang, and Peng Du. 2021. "Effect of Graphene Oxide on Properties of Alkali-Activated Slag" Materials 14, no. 20: 6107. https://doi.org/10.3390/ma14206107
APA StyleDong, Q., Wan, L., Luan, C., Wang, J., & Du, P. (2021). Effect of Graphene Oxide on Properties of Alkali-Activated Slag. Materials, 14(20), 6107. https://doi.org/10.3390/ma14206107





