Environmental Remediation of Toxic Organic Pollutants Using Visible-Light-Activated Cu/La/CeO2/GO Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
2.2. Synthesis of Cu/La/CeO2/GO Nanocomposites
2.3. Characterization of Cu/La/CeO2/GO Nanocomposites
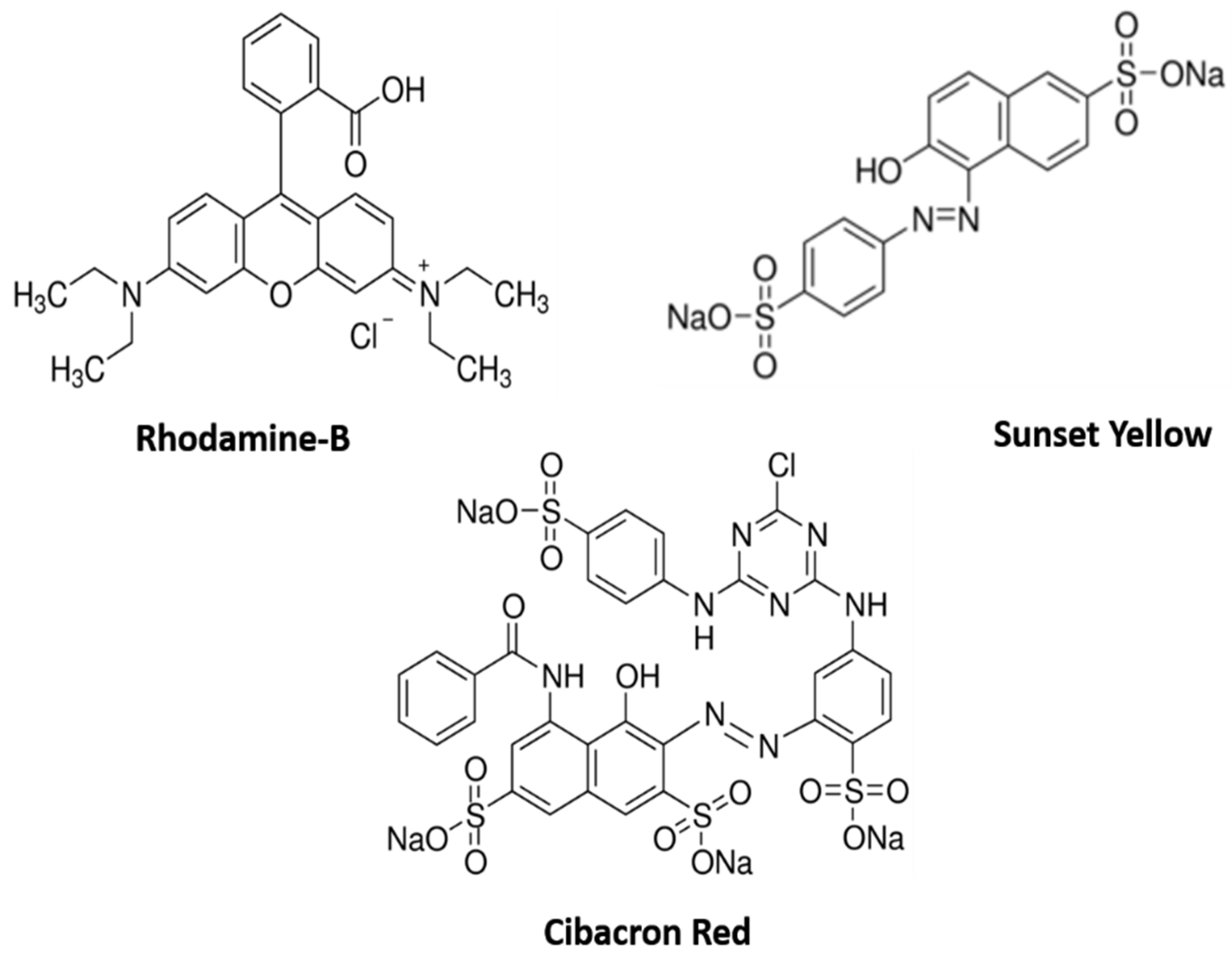
2.4. Photocatalytic Activity
3. Results and Discussion
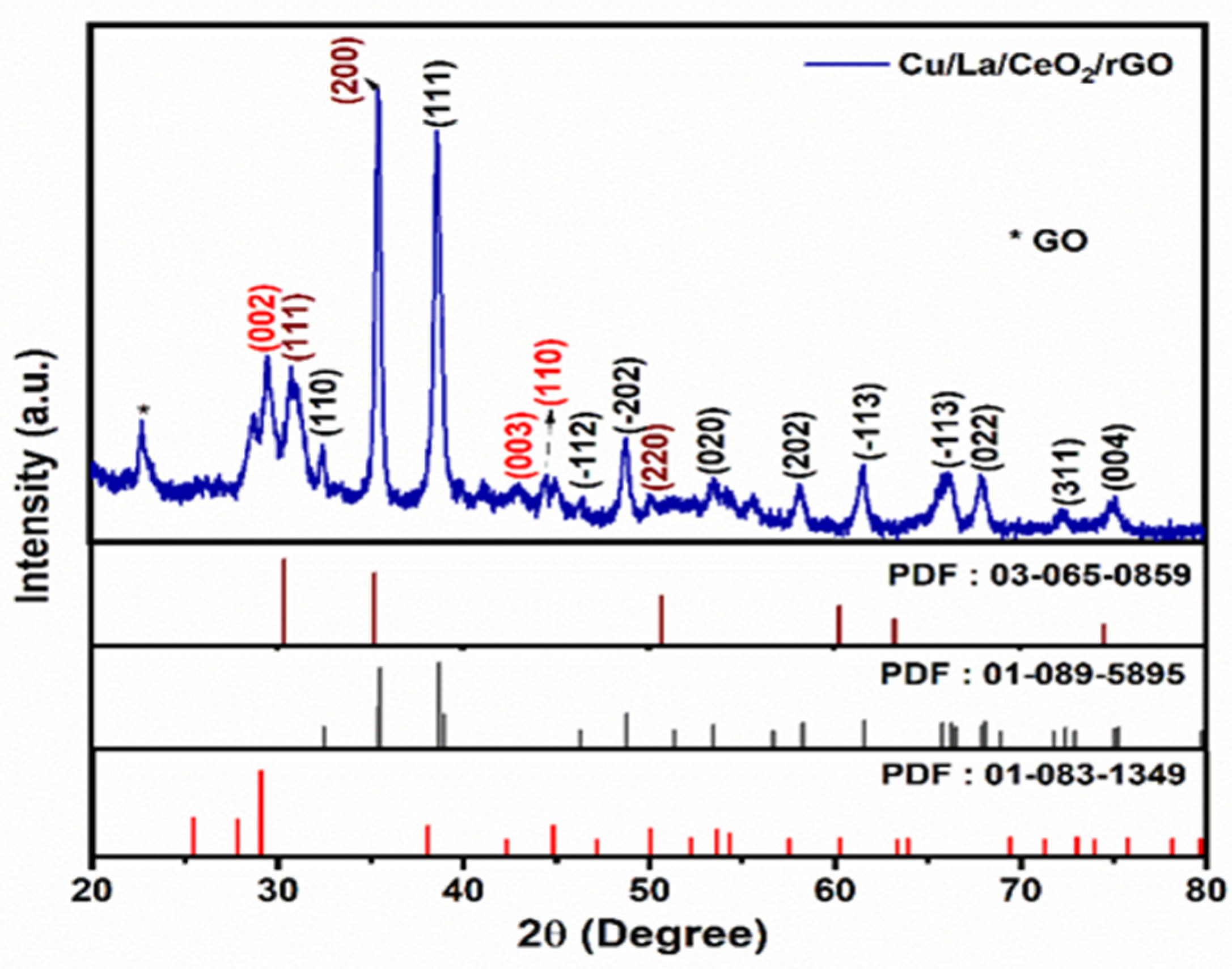
3.1. Study of the Crystalline Structure
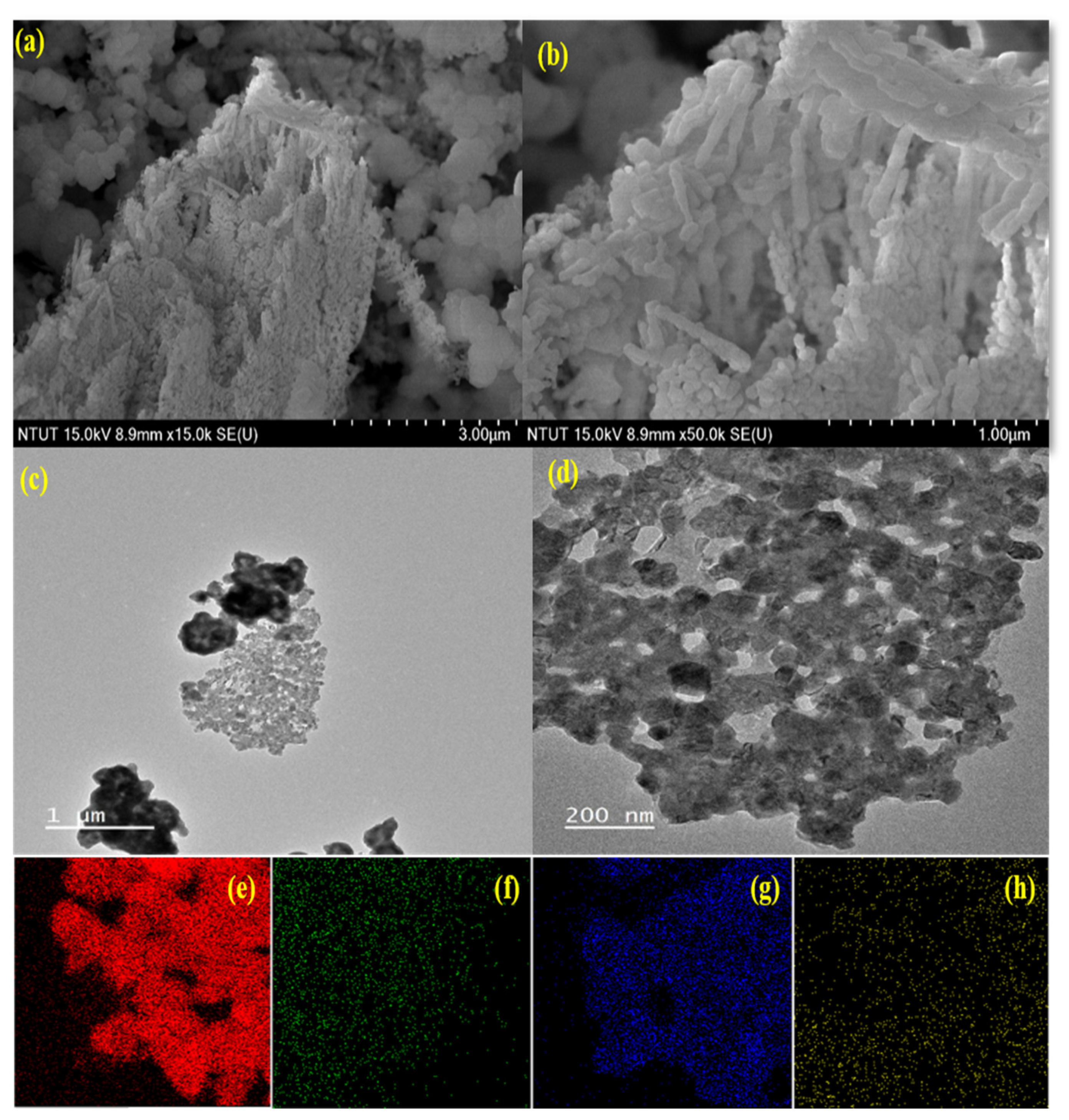
3.2. Surface Morphology and Topography Studies of Nanocomposites
3.3. Nanocomposites Surface Chemistry Studies
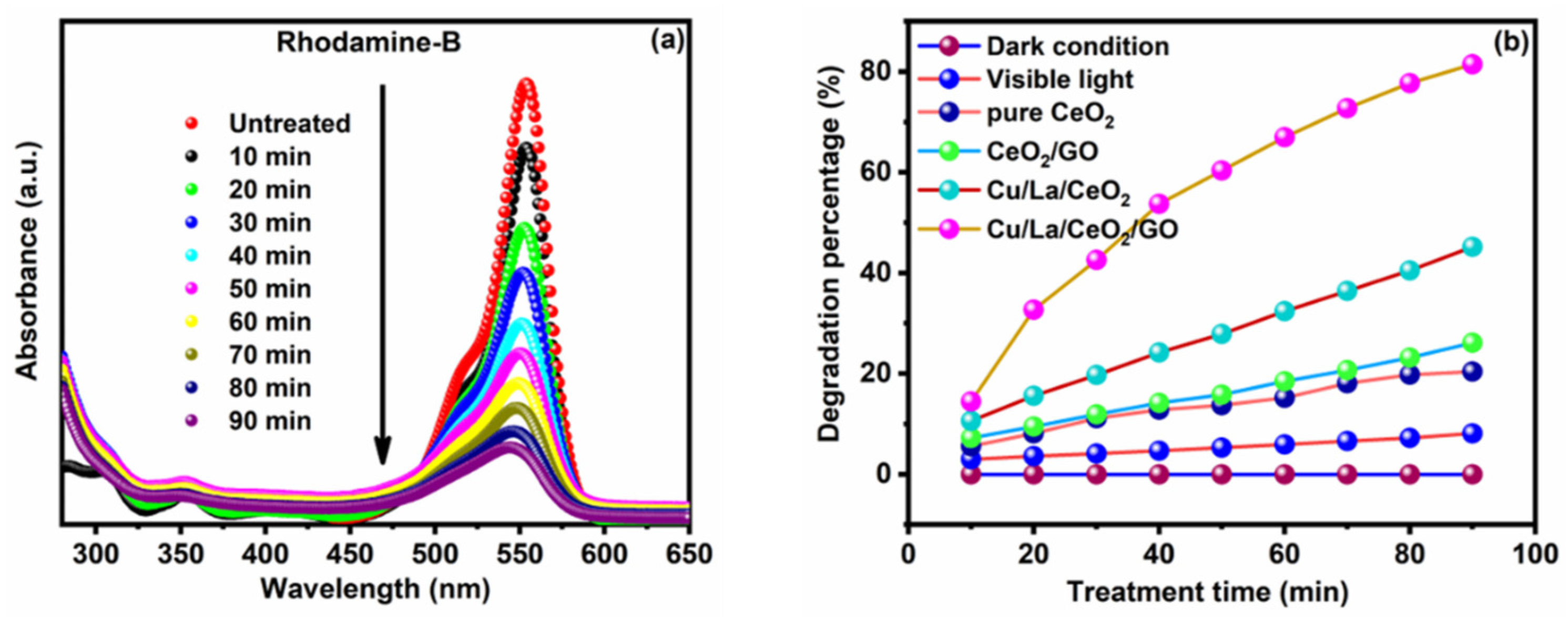
3.4. Photocatalytic Activity of Synthesized Cu/La/CeO2/GO Nanocomposites
3.4.1. Photocatalytic Degradation Mechanisms
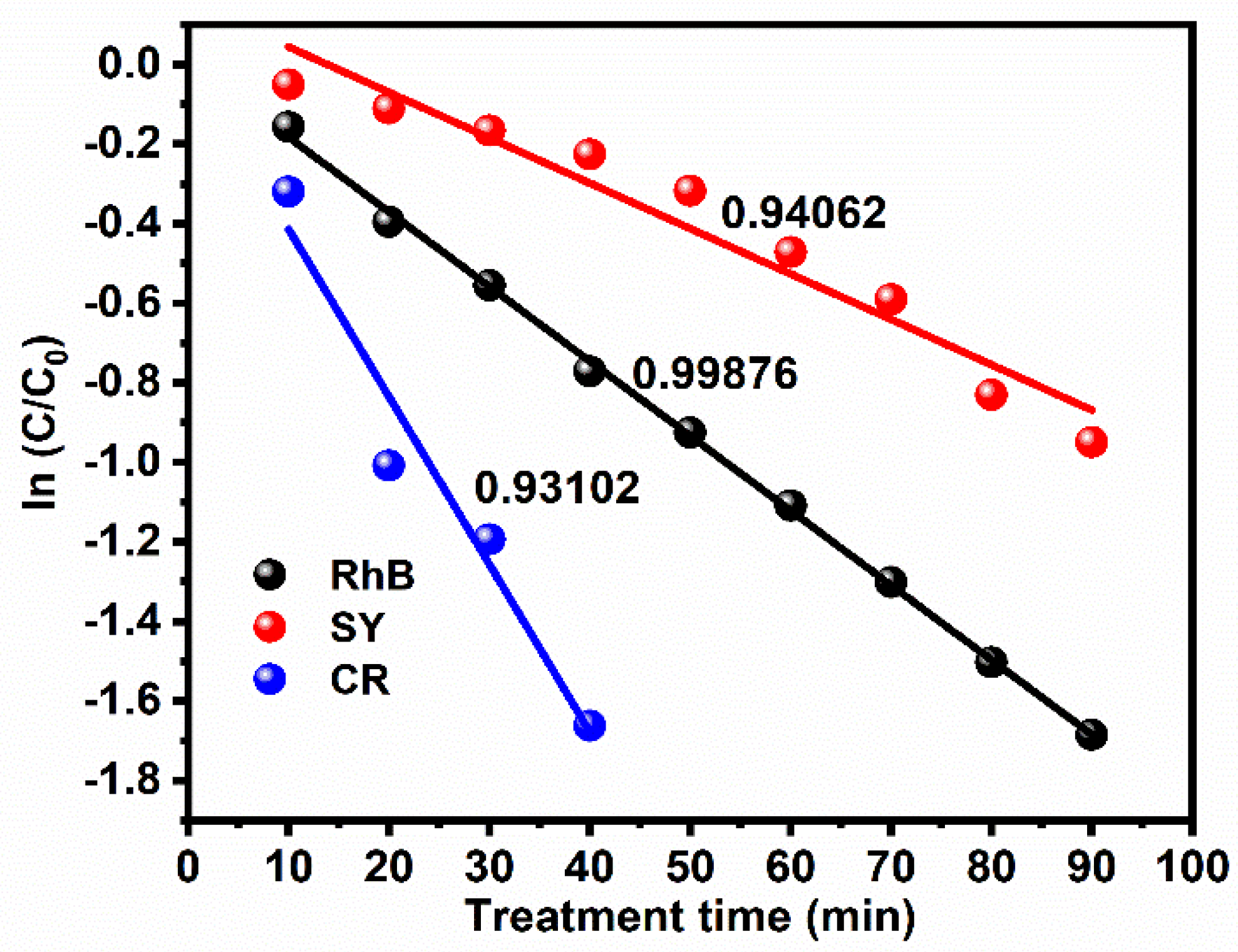
3.4.2. First-Order Kinetic Rate Constant Reaction
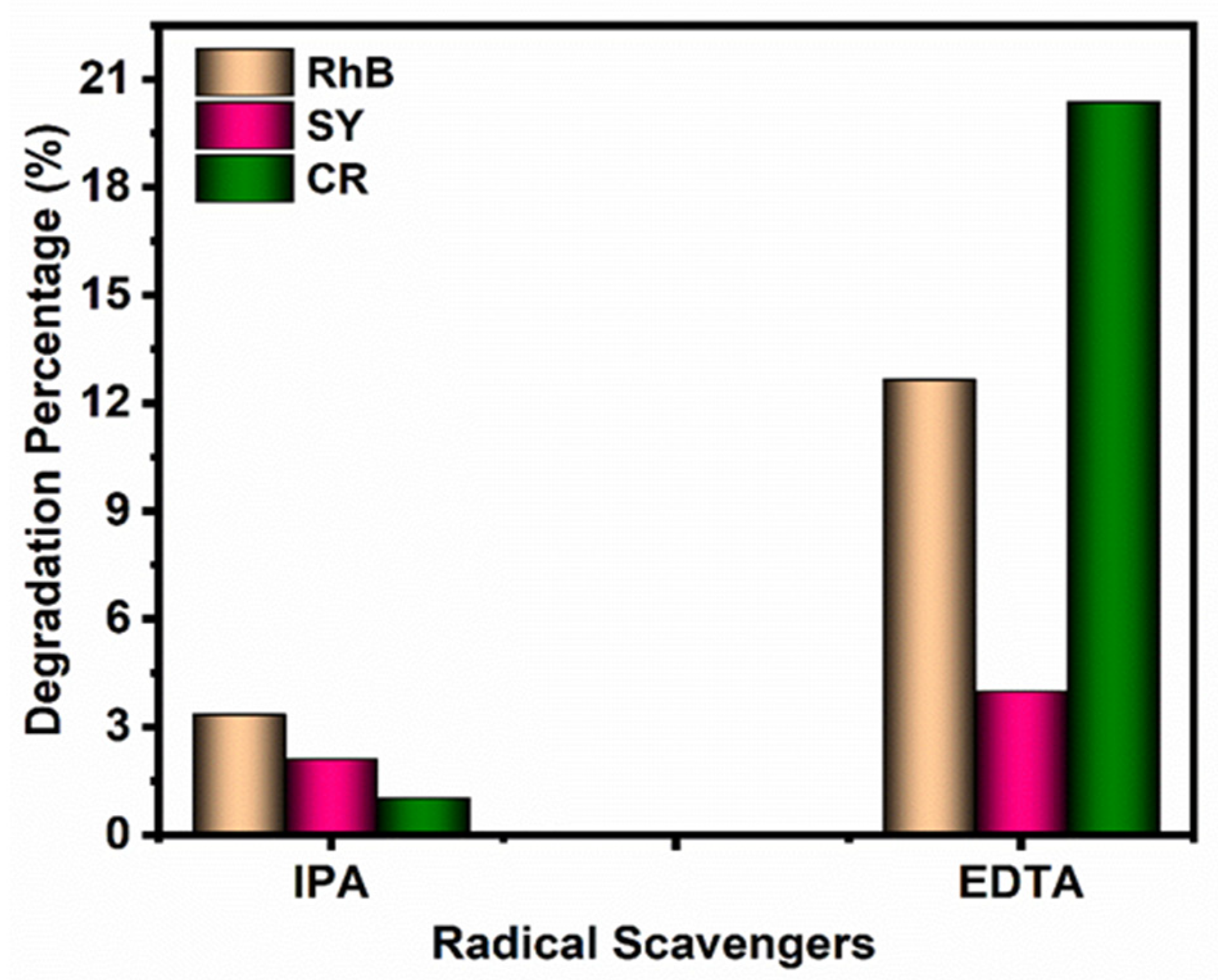
3.4.3. Radical-Trapping Experiments
3.4.4. Nanocomposite Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, T.; Sun, X.; Xian, T.; Yi, Z.; Li, R.; Wang, X.; Yang, H. Tert-butylamine/oleic acid-assisted morphology tailoring of hierarchical Bi4Ti3O12 architectures and their application for photodegradation of simulated dye wastewater. Opt. Mater. 2021, 112, 110781. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Byrappa, K.; Khanum, S.A. Sunlight-assisted synthesis of cerium (IV) oxide nanostructure with enhanced photocatalytic activity. Optik 2021, 245, 167236. [Google Scholar] [CrossRef]
- Thilagavathi, T.; Venugopal, D.; Thangaraju, D.; Marnadu, R.; Baskaran, P.; Mohd, I.; Mohd, S.; Mohd, U.; Al Faify, S. A facile co-precipitation synthesis of novel WO3/NiWO4 nanocomposite with improved photocatalytic activity. Mater. Sci. Semicond. Process. 2021, 133, 105970. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yang, Y.; Xin, B. Magnetic NiFe2O4 3D nanosphere photocatalyst: Glycerol-assisted microwave solvothermal synthesis and photocatalytic activity under microwave electrodeless discharge lamp. Ceram. Int. 2021, 47, 14594–14602. [Google Scholar] [CrossRef]
- Hassani, A.; Soltani, R.D.C.; Karaca, S.; Khataee, A. Preparation of montmorillonite–alginate nano biocomposite for adsorption of a textile dye in aqueous phase: Isotherm, kinetic and experimental design approaches. J. Ind. Eng. Chem. 2015, 21, 1197–1207. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Yang, L. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Chang, J.-S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Ya, K.; Meyyanathan, S.N.; Nageswara, R.R. Methods for the analysis of azo dyes employed in food industry. Food Chem. 2016, 192, 813–824. [Google Scholar]
- Rovina, K.; Prabakaran, P.P.; Siddiquee, S.; Shaarani, S.M. Methods for the analysis of Sunset Yellow FCF (E110) in food and beverage products-a review. TrAC Trends Anal. Chem. 2016, 85, 47–56. [Google Scholar] [CrossRef]
- Nagaraja, R.; Kottam, N.; Girija, C.R.; Nagabhushana, B.M. Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nanopowder synthesized by solution combustion route. Powder Technol. 2012, 215, 91–97. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; Zidan, N.M. Electro-Fenton oxidation of Sunset Yellow FCF azo-dye in aqueous solutions. Desalination 2011, 274, 22–30. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an azo dye Sunset Yellow under UV-A light using TiO2/CAC composite catalysts. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bernardino, T.S.; Junior, F.E.; Lanza, M.R.; Barros, W.R. Enhanced electrodegradation of the Sunset Yellow dye in acid media by heterogeneous Photoelectro-Fenton process using Fe3O4 nanoparticles as a catalyst. J. Environ. Chem. Eng. 2020, 8, 103621. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, S.; Zhang, W.; Wang, W.; Yang, C. Heterogeneous photo Fenton-like degradation of cibacron brilliant red 3B-A dye using amorphous Fe78Si9B13 and Fe73.5Si13.5B9Cu1Nb3 alloys: The influence of adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 128–136. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process. Eng. J. Water Process. Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Kumar, M.J.K.; Kalathi, J.T. Low-temperature sonochemical synthesis of high dielectric Lanthanum doped Cerium oxide nanopowder. J. Alloys Compd. 2018, 748, 348–354. [Google Scholar] [CrossRef]
- Tinwala, H.; Shah, P.; Siddhapara, K.; Shah, D.; Menghani, J. Investigation of ionic conductivity of lanthanum cerium oxide nano crystalline powder synthesized by co precipitation method. J. Cryst. Growth 2016, 452, 54–56. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Dong, C.-L.; Lu, Y.-R.; Huang, Y.-C.; Chen, C.-L.; Saravanan, P.; Asokan, K.; Kumar, R.T.R. Evolution of visible photocatalytic properties of Cu-doped CeO2 nanoparticles: Role of Cu2+-mediated oxygen vacancies and the mixed-valence states of Ce ions. ACS Sustain. Chem. Eng. 2018, 6, 8536–8546. [Google Scholar] [CrossRef]
- Rostami, M. Photodecomposition and adsorption of hazardous organic pollutants by Ce-doped ZnO@ Ce-doped TiO2-N/S-dual doped RGO ternary nano-composites photocatalyst for water remediation. J. Mol. Struct. 2019, 1185, 191–199. [Google Scholar] [CrossRef]
- Mai, H.; Zhang, D.; Shi, L.; Yan, T.; Li, H. Highly active Ce1−xCuxO2 nanocomposite catalysts for the low temperature oxidation of CO. Appl. Surf. Sci. 2011, 257, 7551–7559. [Google Scholar] [CrossRef]
- Zhang, D.; Mai, H.; Huang, L.; Shi, L. Pyridine-thermal synthesis and high catalytic activity of CeO2/CuO/CNT nanocomposites. Appl. Surf. Sci. 2010, 256, 6795–6800. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, Y.; Shi, L.; Mai, H.; Gao, R.; Zhang, J.; Yu, W.; Cao, W. Cu-doped CeO2 spheres: Synthesis, characterization, and catalytic activity. Catal. Commun. 2012, 26, 164–168. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Xing, X.; Chen, N.; Deng, D.; Wang, Y. Catalytic activity for CO oxidation of Cu–CeO2 composite nanoparticles synthesized by a hydrothermal method. Anal. Methods 2015, 7, 3238–3245. [Google Scholar] [CrossRef]
- Lim, W.F.; Cheong, K.Y. Oxygen vacancy formation and annihilation in lanthanum cerium oxide as a metal reactive oxide on 4H-silicon carbide. Phys. Chem. Chem. Phys. 2014, 16, 7015–7022. [Google Scholar] [CrossRef]
- Velliyan, S.; Rajendran, V. Study on the effect of Ce3+ doping on structural, morphological and optical properties of CuO nanoparticles synthesized via combustion technique. Phys. B Condens. Matter 2021, 613, 413015. [Google Scholar] [CrossRef]
- Deus, R.; Cortés, J.; Ramirez, M.; Ponce, M.A.; Andres, J.; Rocha, L.; Longo, E.; Simões, A. Photoluminescence properties of cerium oxide nanoparticles as a function of lanthanum content. Mater. Res. Bull. 2015, 70, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Singh, S.; Kumar, A.; Kumar, P. Oxygen-deficient lanthanum doped cerium oxide nanoparticles for potential applications in spintronics and photocatalysis. Vacuum 2020, 177, 109395. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Keyan, A.K.; Rajakumaran, R.; Chen, S.M.; Chiu, T.W.; Dong, C.; Vinothini, S. Synthesis of N-rGO-MWCNT/CuCrO2 Catalyst for the Bifunctional Application of Hydrogen Evolution Reaction and Electrochemical Detection of Bisphenol-A. Catalysts 2021, 11, 301. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Arunkumar, P.; Mahesh, A.; Dhayalan, A.; Babu, K.S. Size-and defect-controlled anti-oxidant enzyme mimetic and radical scavenging properties of cerium oxide nanoparticles. New J. Chem. 2018, 42, 18810–18823. [Google Scholar] [CrossRef]
- Janoš, P.; Henych, J.; Pfeifer, J.; Zemanová, N.; Pilařová, V.; Milde, D.; Opletal, T.; Tolasz, J.; Malý, M.; Štengl, V. Nanocrystalline cerium oxide prepared from a carbonate precursor and its ability to breakdown biologically relevant organophosphates. Environ. Sci. Nano 2017, 4, 1283–1293. [Google Scholar] [CrossRef]
- Eloirdi, R.; Cakir, P.; Huber, F.; Seibert, A.; Konings, R.; Gouder, T. X-ray photoelectron spectroscopy study of the reduction and oxidation of uranium and cerium single oxide compared to (U-Ce) mixed oxide films. Appl. Surf. Sci. 2018, 457, 566–571. [Google Scholar] [CrossRef]
- Sun, H.; Zelekew, O.A.; Chen, X.; Guo, Y.; Kuo, D.-H.; Lu, Q.; Lin, J. A noble bimetal oxysulfide CuVOS catalyst for highly efficient catalytic reduction of 4-nitrophenol and organic dyes. RSC Adv. 2019, 9, 31828–31839. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.X.; Zhu, S.L.; Mao, J.; Cui, Z.D.; Yang, X.J.; Liang, Y.Q.; Li, Z.Y. Synthesis of CuO/Co3O4 coaxial heterostructures for efficient and recycling photodegradation. Int. J. Photoenergy 2015, 2015, 183468. [Google Scholar] [CrossRef] [Green Version]
- Yadav Anuja, A.; Vaibhav, C.; Lokhande Ravindra, N.; Chandrakant, D. Amperometric CO2 gas sensor based on interconnected web-like nanoparticles of La2O3 synthesized by ultrasonic spray pyrolysis. Microchim. Acta 2017, 184, 3713–3720. [Google Scholar] [CrossRef]
- Hu, S.; Chi, B.; Pu, J.; Jian, L. Novel heterojunction photocatalysts based on lanthanum titanate nanosheets and indium oxide nanoparticles with enhanced photocatalytic hydrogen production activity. J. Mater. Chem. A 2014, 2, 19260–19267. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Chen, S.-M.; Karuppiah, C. Simultaneous voltammetric determination of acetaminophen, naproxen, and theophylline using an in-situ polymerized poly (acrylic acid) nanogel covalently grafted onto a carbon black/La2O3 composite. Microchim. Acta 2019, 186, 651. [Google Scholar] [CrossRef]
- El-Berry, M.F.; Sadeek, S.A.; Abdalla, A.M.; Nassar, M.Y. Microwave-assisted fabrication of copper nanoparticles utilizing different counter ions: An efficient photocatalyst for photocatalytic degradation of safranin dye from aqueous media. Mater. Res. Bull. 2021, 133, 111048. [Google Scholar] [CrossRef]
- Ghorai, K.; Panda, A.; Bhattacharjee, M.; Mandal, D.; Hossain, A.; Bera, P.; Seikh, M.; Gayen, A. Facile synthesis of CuCr2O4/CeO2 nanocomposite: A new Fenton like catalyst with domestic LED light assisted improved photocatalytic activity for the degradation of RhB, MB and MO dyes. Appl. Surf. Sci. 2021, 536, 147604. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasu, D.; Fu, Y.; Keyan, A.K.; Sakthinathan, S.; Chiu, T.-W. Environmental Remediation of Toxic Organic Pollutants Using Visible-Light-Activated Cu/La/CeO2/GO Nanocomposites. Materials 2021, 14, 6143. https://doi.org/10.3390/ma14206143
Vasu D, Fu Y, Keyan AK, Sakthinathan S, Chiu T-W. Environmental Remediation of Toxic Organic Pollutants Using Visible-Light-Activated Cu/La/CeO2/GO Nanocomposites. Materials. 2021; 14(20):6143. https://doi.org/10.3390/ma14206143
Chicago/Turabian StyleVasu, Dhanapal, Yongsheng Fu, Arjunan Karthi Keyan, Subramanian Sakthinathan, and Te-Wei Chiu. 2021. "Environmental Remediation of Toxic Organic Pollutants Using Visible-Light-Activated Cu/La/CeO2/GO Nanocomposites" Materials 14, no. 20: 6143. https://doi.org/10.3390/ma14206143





