Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carbon Doped Black TiO2
2.3. Synthesis of Carbon and Nitrogen Co-Doped TiO2
2.4. Nanomaterials Characterization
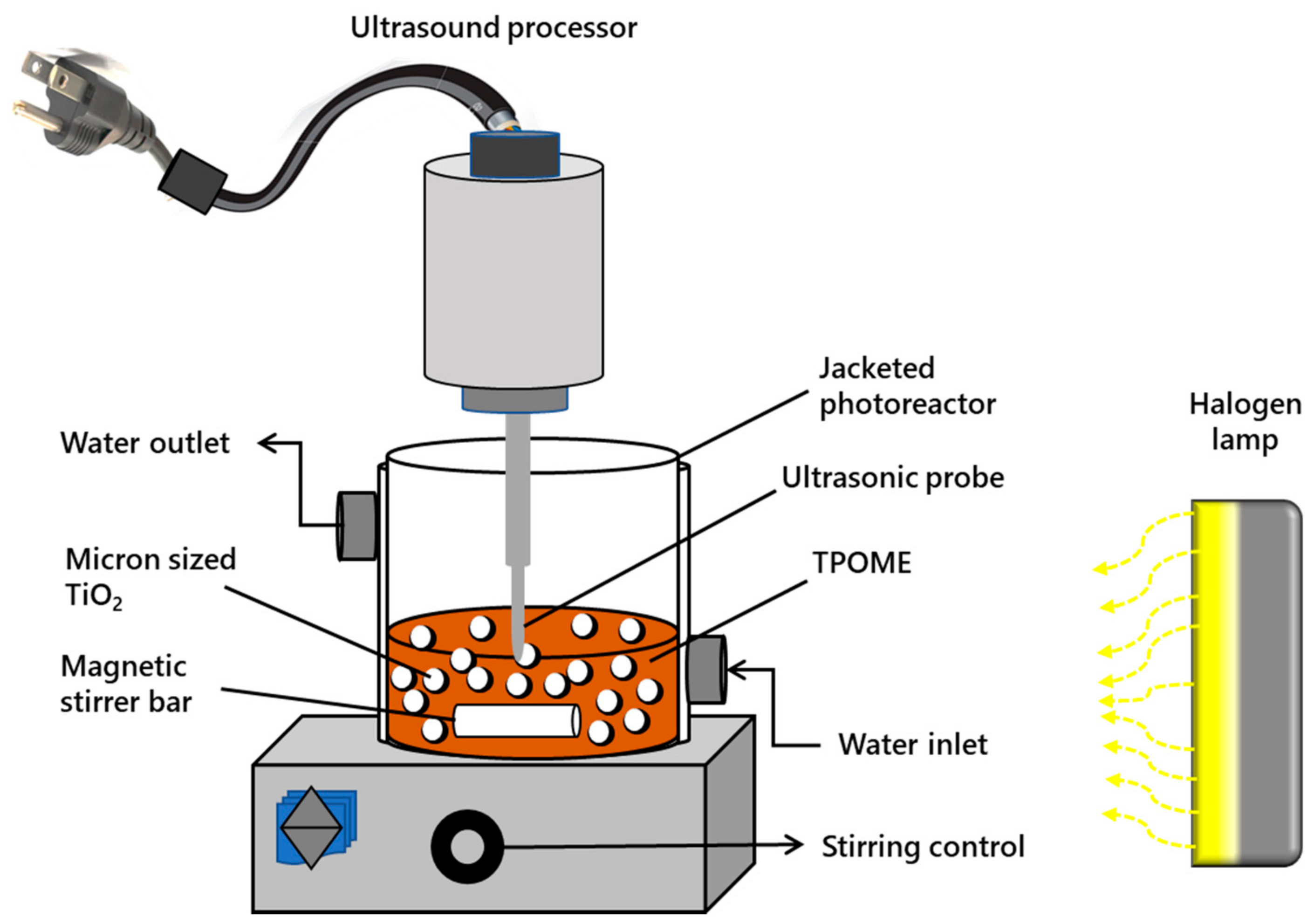
2.5. Experimental Setup
2.6. Experimental Procedures
2.7. Synergy Index
2.8. Recyclability of CNB-TiO2
3. Results and Discussion
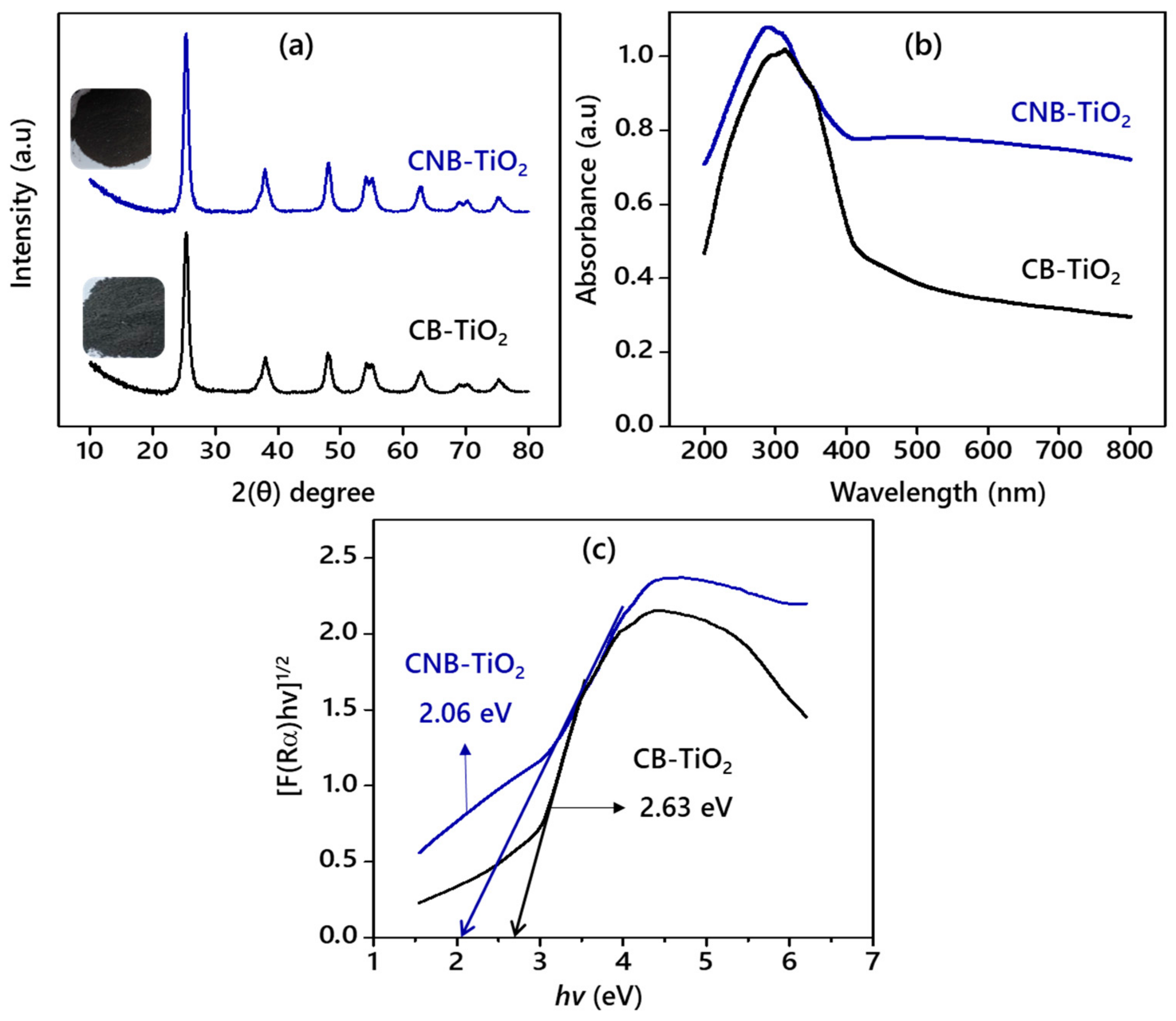
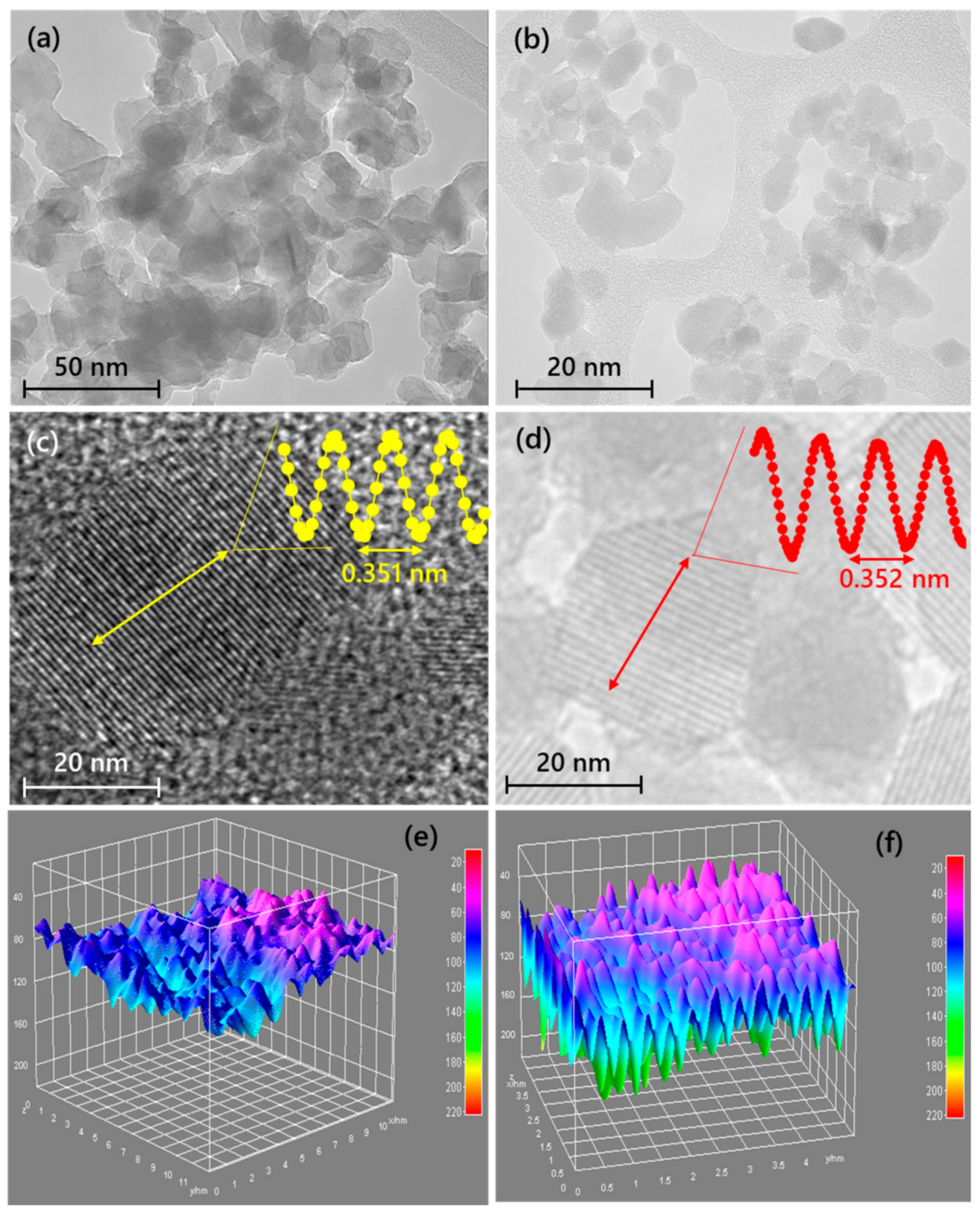
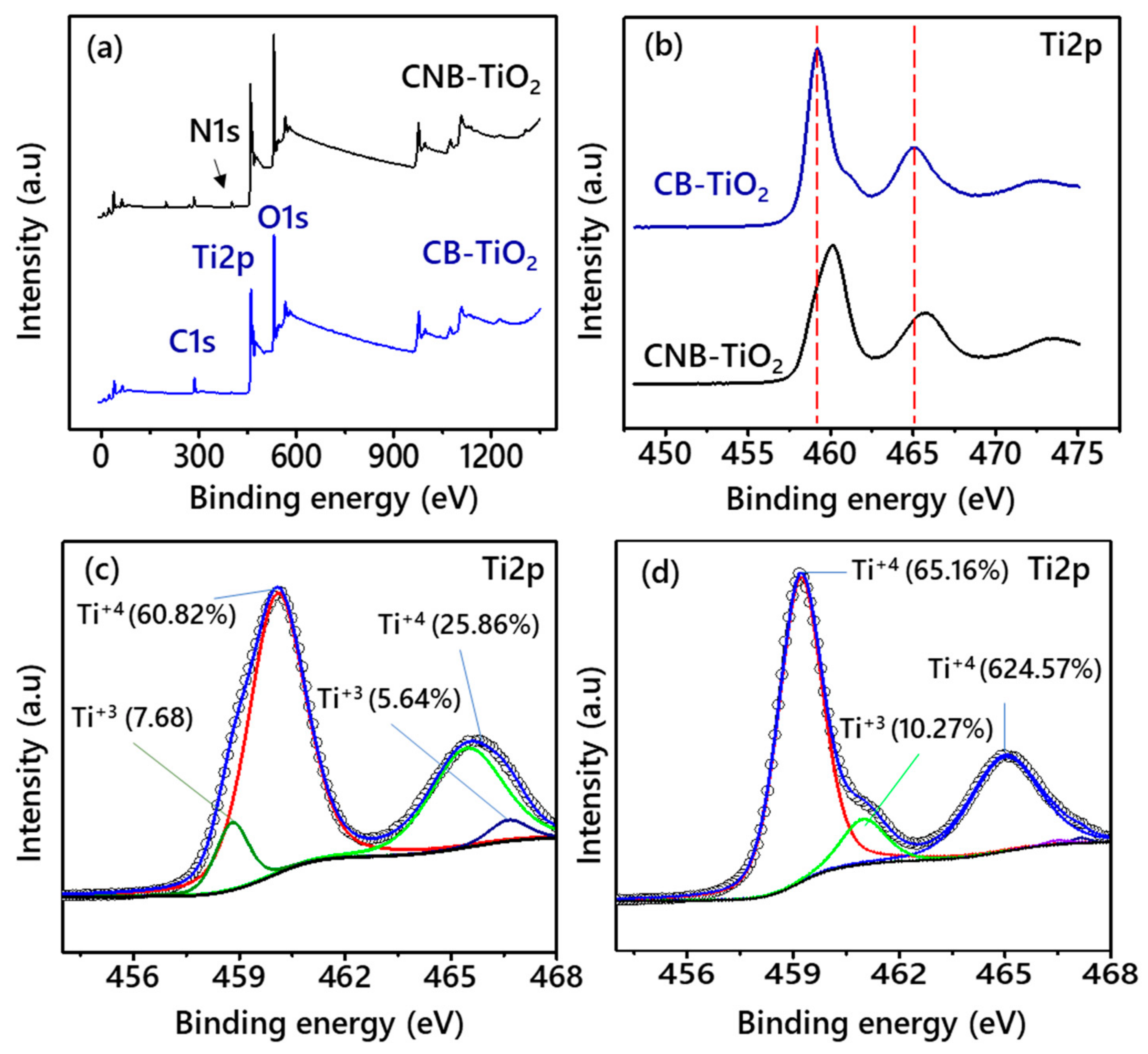
3.1. Properties of the CB-TiO2 and CNB-TiO2 Nanomaterials
3.2. Comparative Photocatalytic Performance of CB-TiO2 and CNB-TiO2 Nanomaterials
3.3. Oxidation of TPOME Using Various AOPs
3.3.1. Effect of CNB-TiO2 Loading
3.3.2. Effect of Hydrogen Peroxide (H2O2) Dosage
3.3.3. Effect of Initial COD Concentration
3.3.4. Effect of Ultrasound Power
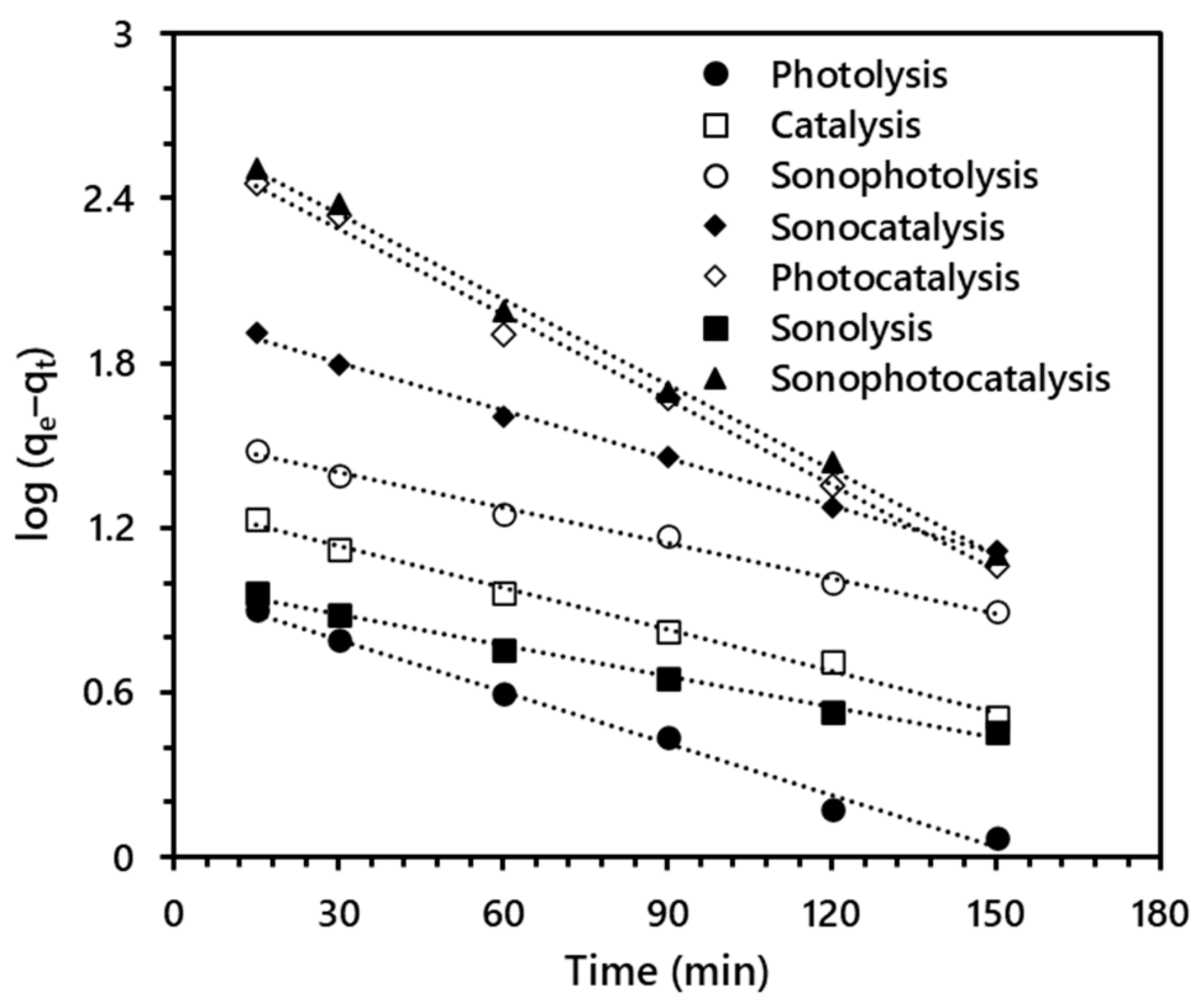
3.4. Kinetic Study Synergy Index
3.5. Energy Consumption
3.6. Recyclability of the CBN-TiO2 Nanomaterial
3.7. Sonophotocatalytic Degradation of TPOME under Optimal Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iskandar, M.J.; Baharum, A.; Anuar, F.H.; Othaman, R. Palm oil industry in South East Asia and the effluent treatment technology—A review. Environ. Technol. Innov. 2018, 9, 169–185. [Google Scholar] [CrossRef]
- Tabassum, S.; Zhang, Y.; Zhang, Z. An integrated method for palm oil mill effluent (POME) treatment for achieving zero liquid discharge—A pilot study. J. Clean. Prod. 2015, 95, 148–155. [Google Scholar] [CrossRef]
- Taha, M.R.; Ibrahim, A. COD removal from anaerobically treated palm oil mill effluent (AT-POME) via aerated heterogeneous Fenton process: Optimization study. J. Water Process. Eng. 2014, 1, 8–16. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Arifuzzaman. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sustain. Energy Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Xiong, W.; Ni, P.; Chen, Y.; Gao, Y.; Li, S.; Zhan, A. Biological consequences of environmental pollution in running water ecosystems: A case study in zooplankton. Environ. Pollut. 2019, 252, 1483–1490. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Zakaria, M.R.; Maeda, T.; Yusoff, M.Z.M.; Hassan, M.A.; Shirai, Y. Toxicity identification and evaluation of palm oil mill effluent and its effects on the planktonic crustacean Daphnia magna. Sci. Total Environ. 2020, 710, 136277. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. Pollution control technologies for the treatment of palm oil mill effluent (POME) through end-of-pipe processes. J. Environ. Manag. 2010, 91, 1467–1490. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.; Buang, A.; Shah, S.; Afzal, M. Biotechnological application of microalgae for integrated palm oil mill effluent (POME) remediation: A review. Int. J. Environ. Sci. Technol. 2019, 16, 1763–1788. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Arifin, S.N.H.; Mohd Salleh, S.N.A. Conventional and advanced treatment technologies for palm oil mill effluents: A systematic literature review. J. Dispers. Sci. Technol. 2020, 42, 1766–1784. [Google Scholar] [CrossRef]
- Khadaroo, S.N.B.A.; Poh, P.E.; Gouwanda, D.; Grassia, P. Applicability of various pretreatment techniques to enhance the anaerobic digestion of Palm oil Mill effluent (POME): A review. J. Environ. Chem. Eng. 2019, 7, 103310. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Salleh, S.F.; Baini, R.; Lim, S.F.; Rigit, A.R.H.; Said, K.A.M.; Khan, A. Photo-oxidation of pre-treated palm oil mill Effluent using cylindrical column immobilized photoreactor. Process. Saf. Environ. Prot. 2018, 117, 180–189. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Lim, S.-F.; Khan, A.; Hipolito, C.N.; Abdullah, M.O.; Bhawani, S.A.; Jamil, T. Photocatalytic treatment technology for palm oil mill effluent (POME)—A review. Process. Saf. Environ. Prot. 2016, 102, 673–686. [Google Scholar] [CrossRef]
- Aris, A.; Jusoh, M.N.H.; Wahab, N.S.A.A. Applications of Advanced Oxidation Processes in Palm Oil Mill Effluent Treatment. In Advanced Oxidation Processes (AOPs) in Water and Wastewater Treatment; IGI Global: Hershey, PA, USA, 2019; pp. 123–149. [Google Scholar]
- Ng, K.H. Adoption of TiO2-photocatalysis for Palm Oil Mill Effluent (POME) Treatment: Strengths, Weaknesses, Opportunities, Threats (SWOT) and Its Practicality Against Traditional Treatment in Malaysia. Chemosphere 2020, 270, 129378. [Google Scholar] [CrossRef]
- Charles, A.; Cheng, C.K. Photocatalytic treatment of palm oil mill effluent by visible light-active calcium ferrite: Effects of catalyst preparation technique. J. Environ. Manag. 2019, 234, 404–411. [Google Scholar] [CrossRef]
- Charles, A.; Khan, M.R.; Ng, K.H.; Wu, T.Y.; Lim, J.W.; Wongsakulphasatch, S.; Witoon, T.; Cheng, C.K. Facile synthesis of CaFe2O4 for visible light driven treatment of polluting palm oil mill effluent: Photokinetic and scavenging study. Sci. Total Environ. 2019, 661, 522–530. [Google Scholar] [CrossRef]
- Sidik, D.A.B.; Hairom, N.H.H.; Ahmad, M.K.; Madon, R.H.; Mohammad, A.W. Performance of membrane photocatalytic reactor incorporated with ZnO-Cymbopogon citratus in treating palm oil mill secondary effluent. Process. Saf. Environ. Prot. 2020, 143, 273–284. [Google Scholar] [CrossRef]
- Ng, K.H.; Gan, Y.S.; Cheng, C.K.; Liu, K.-H.; Liong, S.-T. Integration of machine learning-based prediction for enhanced Model’s generalization: Application in photocatalytic polishing of palm oil mill effluent (POME). Environ. Pollut. 2020, 267, 115500. [Google Scholar] [CrossRef]
- Ng, K.H.; Yuan, L.S.; Cheng, C.K.; Chen, K.; Fang, C. TiO2 and ZnO photocatalytic treatment of palm oil mill effluent (POME) and feasibility of renewable energy generation: A short review. J. Clean. Prod. 2019, 233, 209–225. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, W.; Liu, H.; Fu, C.; Feng, P.; Wang, Y. A study on the optimization of surfactants in the main and vice slug in weak base ASP flooding. Energies 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Bello, M.M.; Abdul Raman, A.A. Trend and current practices of palm oil mill effluent polishing: Application of advanced oxidation processes and their future perspectives. J. Environ. Manag. 2017, 198, 170–182. [Google Scholar] [CrossRef]
- Meroni, D.; Jiménez-Salcedo, M.; Falletta, E.; Bresolin, B.M.; Kait, C.F.; Boffito, D.C.; Bianchi, C.L.; Pirola, C. Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized TiO2. Ultrason. Sonochemistry 2020, 67, 105123. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Mohammed, R.R.; Fong, C.M.; Gomes, R.L.; Manickam, S. A novel hybrid approach of activated carbon and ultrasound cavitation for the intensification of palm oil mill effluent (POME) polishing. J. Clean. Prod. 2016, 112, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-Mediated Facile Synthesis of Colored Titania Nanoparticles for Visible Light Photodegradation of Phenolic Compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Ahmad, A.; Ismail, S.; Bhatia, S. Optimization of coagulation—flocculation process for palm oil mill effluent using response surface methodology. Environ. Sci. Technol. 2005, 39, 2828–2834. [Google Scholar] [CrossRef]
- Wu, T.; Englehardt, J.D. A new method for removal of hydrogen peroxide interference in the analysis of chemical oxygen demand. Environ. Sci. Technol. 2012, 46, 2291–2298. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Rahmaniati, T.; Hafidzah, A.R.; Suherman, S.; Suratman, A. Photocatalysis over N-Doped TiO2 Driven by Visible Light for Pb (II) Removal from Aqueous Media. Catalysts 2021, 11, 945. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, A.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an electronic structure of N-TiO2 on its photocatalytic activity towards decomposition of acetaldehyde under UV and fluorescent lamps irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Kovalevskiy, N.; Selishchev, D.; Svintsitskiy, D.; Selishcheva, S.; Berezin, A.; Kozlov, D. Synergistic effect of polychromatic radiation on visible light activity of N-doped TiO2 photocatalyst. Catal. Commun. 2020, 134, 105841. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Peng, T.; Liang, J.; Li, P.; Pan, D.; Fan, Q.; Wu, W. Visible light driven Ti3+ self-doped TiO2 for adsorption-photocatalysis of aqueous U(VI). Environ. Pollut. 2020, 262, 114373. [Google Scholar] [CrossRef] [PubMed]
- Shvab, R.; Hryha, E.; Nyborg, L.J.P.M. Surface chemistry of the titanium powder studied by XPS using internal standard reference. Powder Metall. 2017, 60, 42–48. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Min, B.-K.; Cho, M.H. Microbial fuel cell assisted band gap narrowed TiO2 for visible light-induced photocatalytic activities and power generation. Sci. Rep. 2018, 8, 1723. [Google Scholar] [CrossRef] [Green Version]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N-and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, M.; Zhang, J. Gel-hydrothermal synthesis of carbon and boron co-doped TiO2 and evaluating its photocatalytic activity. J. Hazard. Mater. 2011, 192, 368–373. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, S.; Shi, W.; Wu, Y.; Zhang, R.; Leng, S. Nitrogen-doped TiO2(B) nanorods as high-performance anode materials for rechargeable sodium-ion batteries. RSC Adv. 2017, 7, 10885–10890. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.; Schumacher, R. The influence of preparation on semiconducting rutile (TiO2). J. Electrochem. Soc. 1980, 127, 1186–1188. [Google Scholar] [CrossRef]
- Henderson, M.A.; Epling, W.S.; Peden, C.H.; Perkins, C.L. Insights into photoexcited electron scavenging processes on TiO2 obtained from studies of the reaction of O2 with OH groups adsorbed at electronic defects on TiO2 (110). J. Phys. Chem. B 2003, 107, 534–545. [Google Scholar] [CrossRef]
- Finazzi, E.; Di Valentin, C.; Pacchioni, G.; Selloni, A. Excess electron states in reduced bulk anatase TiO2: Comparison of standard GGA, GGA+ U, and hybrid DFT calculations. J. Chem. Phys. 2008, 129, 154113. [Google Scholar] [CrossRef]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G. Promotion of phenol photodecomposition over TiO2 using Au, Pd, and Au–Pd nanoparticles. ACS Nano 2012, 6, 6284–6292. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Shirai, T. Structural-controlled synthesis of highly efficient visible light TiO2 photocatalyst via one-step single-mode microwave assisted reaction. Sci. Rep. 2019, 9, 4900. [Google Scholar] [CrossRef] [Green Version]
- Ghafoori, S.; Mowla, A.; Jahani, R.; Mehrvar, M.; Chan, P.K. Sonophotolytic degradation of synthetic pharmaceutical wastewater: Statistical experimental design and modeling. J. Environ. Manag. 2015, 150, 128–137. [Google Scholar] [CrossRef]
- Hartmann, J.; Bartels, P.; Mau, U.; Witter, M.; Tümpling, W.V.; Hofmann, J.; Nietzschmann, E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 2008, 70, 453–461. [Google Scholar] [CrossRef]
- Madhavan, J.; Kumar, P.S.S.; Anandan, S.; Zhou, M.; Grieser, F.; Ashokkumar, M. Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere 2010, 80, 747–752. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef]
- Mohajerani, M.; Mehrvar, M.; Ein-Mozaffari, F. Recent achievements in combination of ultrasonolysis and other advanced oxidation processes for wastewater treatment. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Ahmedchekkat, F.; Medjram, M.S.; Chiha, M.; Al-Bsoul, A.M.A. Sonophotocatalytic degradation of Rhodamine B using a novel reactor geometry: Effect of operating conditions. Chem. Eng. J. 2011, 178, 244–251. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Wong, L.-P.; Isa, M.H.; Bashir, M.J.K.; Yaqub, A. Low frequency ultrasound treatment of palm oil mill effluent for solubilization of organic matter. Desalination Water Treat. 2018, 108, 164–170. [Google Scholar] [CrossRef]
- Al-Bsoul, A.; Al-Shannag, M.; Tawalbeh, M.; Al-Taani, A.A.; Lafi, W.K.; Al-Othman, A.; Alsheyab, M. Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci. Total Environ. 2020, 700, 134576. [Google Scholar] [CrossRef]
- Cheng, C.K.; Rizauddin Derahman, M.; Khan, M.R. Evaluation of the photocatalytic degradation of pre-treated palm oil mill effluent (POME) over Pt-loaded titania. J. Environ. Chem. Eng. 2015, 3, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Zhu, W.; Zhang, L.; Zhao, B.; Ji, Y.; Li, G.; An, T. Superoxide radical enhanced photocatalytic performance of styrene alters its degradation mechanism and intermediate health risk on TiO2/graphene surface. Environ. Res. 2021, 195, 110747. [Google Scholar] [CrossRef] [PubMed]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A. Study of the effect of additives on the photocatalytic degradation of a triphenylmethane dye in the presence of immobilized TiO2/NiO nanoparticles: Artificial neural network modeling. Ind. Eng. Chem. Res. 2014, 53, 6881–6895. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.S.; Rigit, A.R.H.; Bhawani, S.A. Recent developments in immobilizing titanium dioxide on supports for degradation of organic pollutants in wastewater-A review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and optimization of photocatalytic degradation of phenol via TiO2 nanoparticles: An insight into response surface methodology and artificial neural network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Ng, K.H.; Cheng, Y.W.; Khan, M.R.; Cheng, C.K. Optimization of photocatalytic degradation of palm oil mill effluent in UV/ZnO system based on response surface methodology. J. Environ. Manag. 2016, 184, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Bsoul, A.; Assirey, E.; Al-Shannag, M. Combined ultrasonic irradiation and aerobic biodegradation treatment for olive mills wastewaters. Environ. Eng. Manag. J. 2014, 13, 2109–2118. [Google Scholar]
- Asaithambi, P.; Sajjadi, B.; Abdul Aziz, A.R.; Daud, W.M.A.B.W. Ozone (O3) and sono (US) based advanced oxidation processes for the removal of color, COD and determination of electrical energy from landfill leachate. Sep. Purif. Technol. 2017, 172, 442–449. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Cheng, C.K.; Deraman, M.R.; Ng, K.H.; Khan, M.R. Preparation of titania doped argentum photocatalyst and its photoactivity towards palm oil mill effluent degradation. J. Clean. Prod. 2016, 112, 1128–1135. [Google Scholar] [CrossRef] [Green Version]

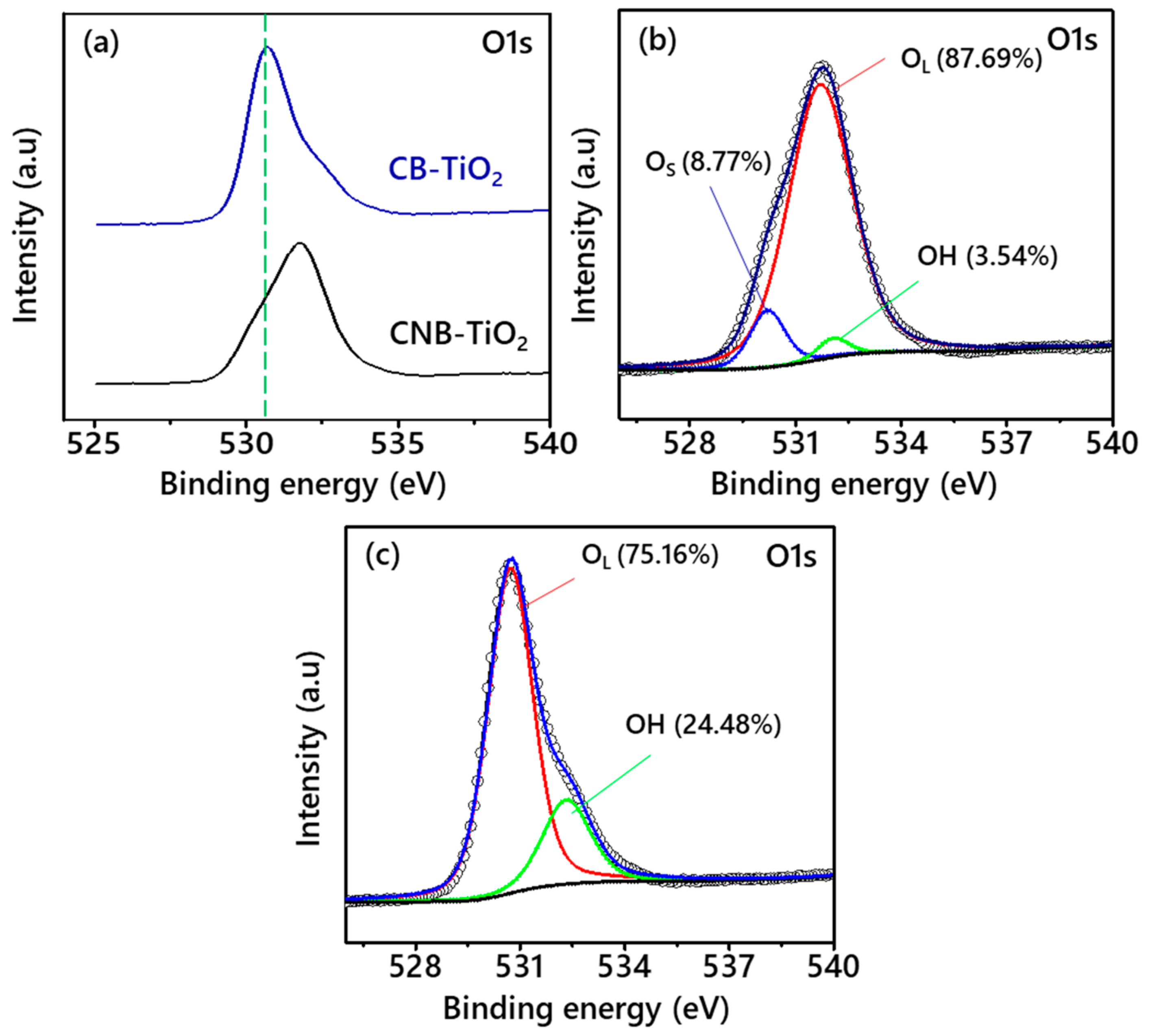
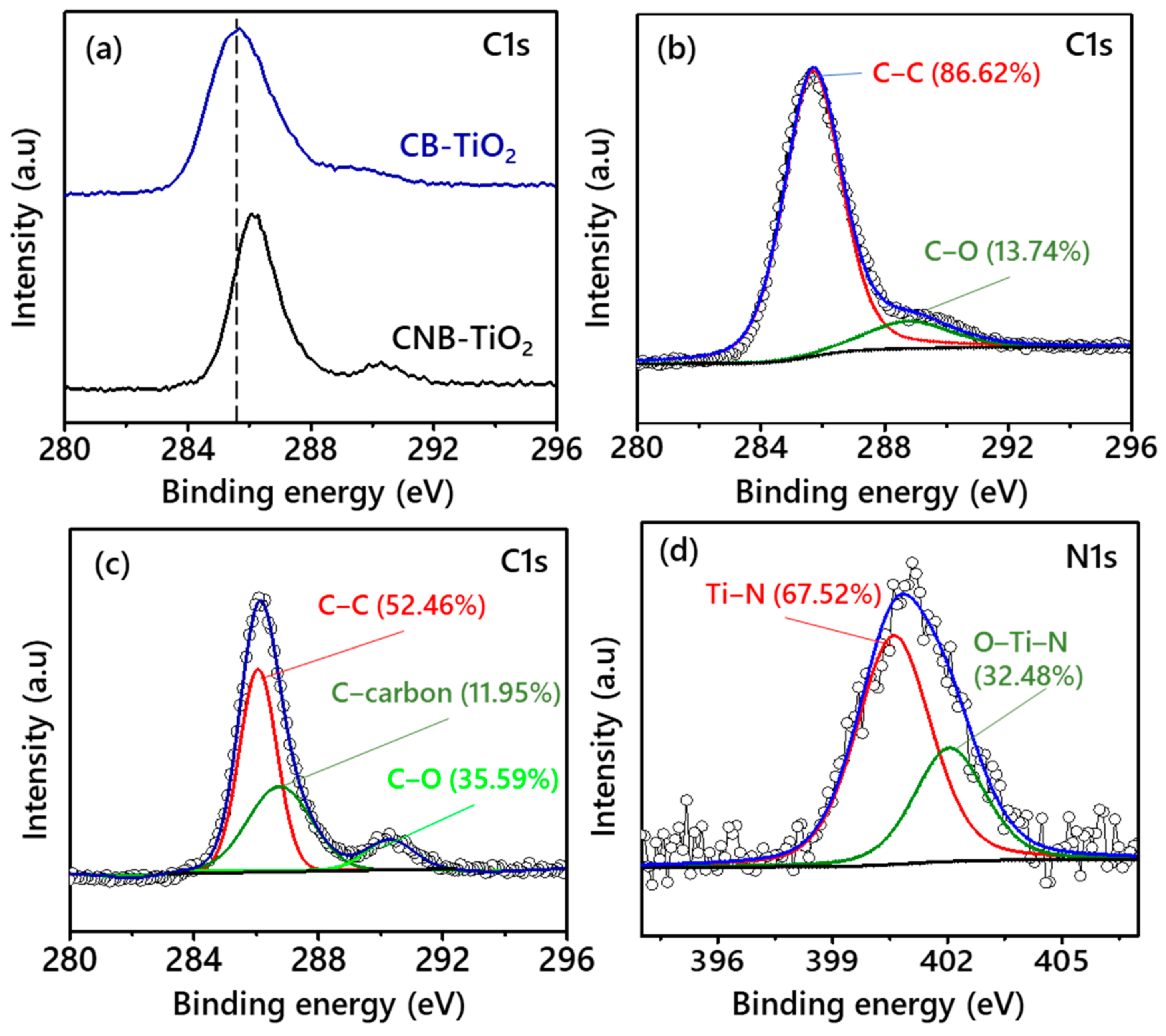
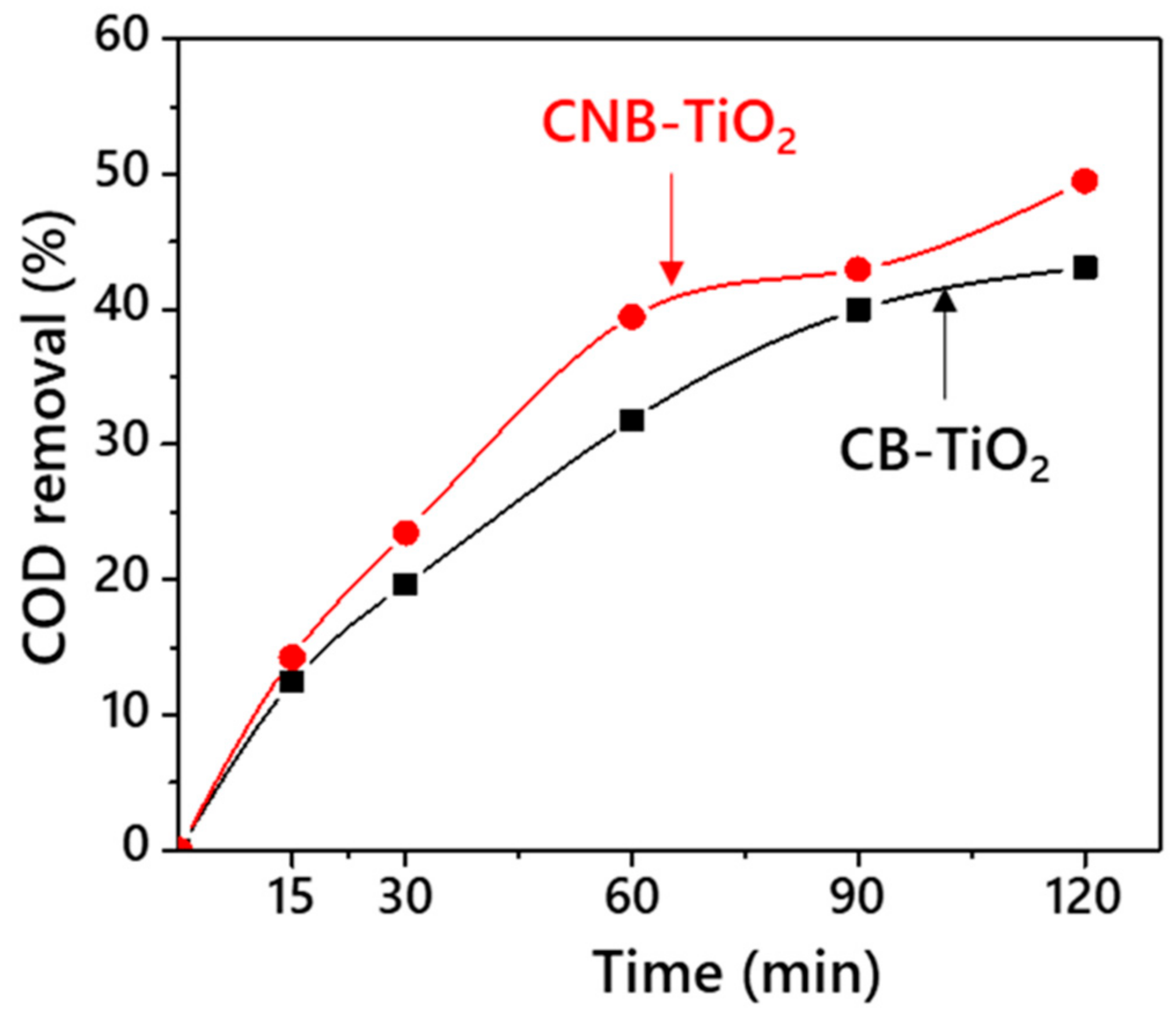
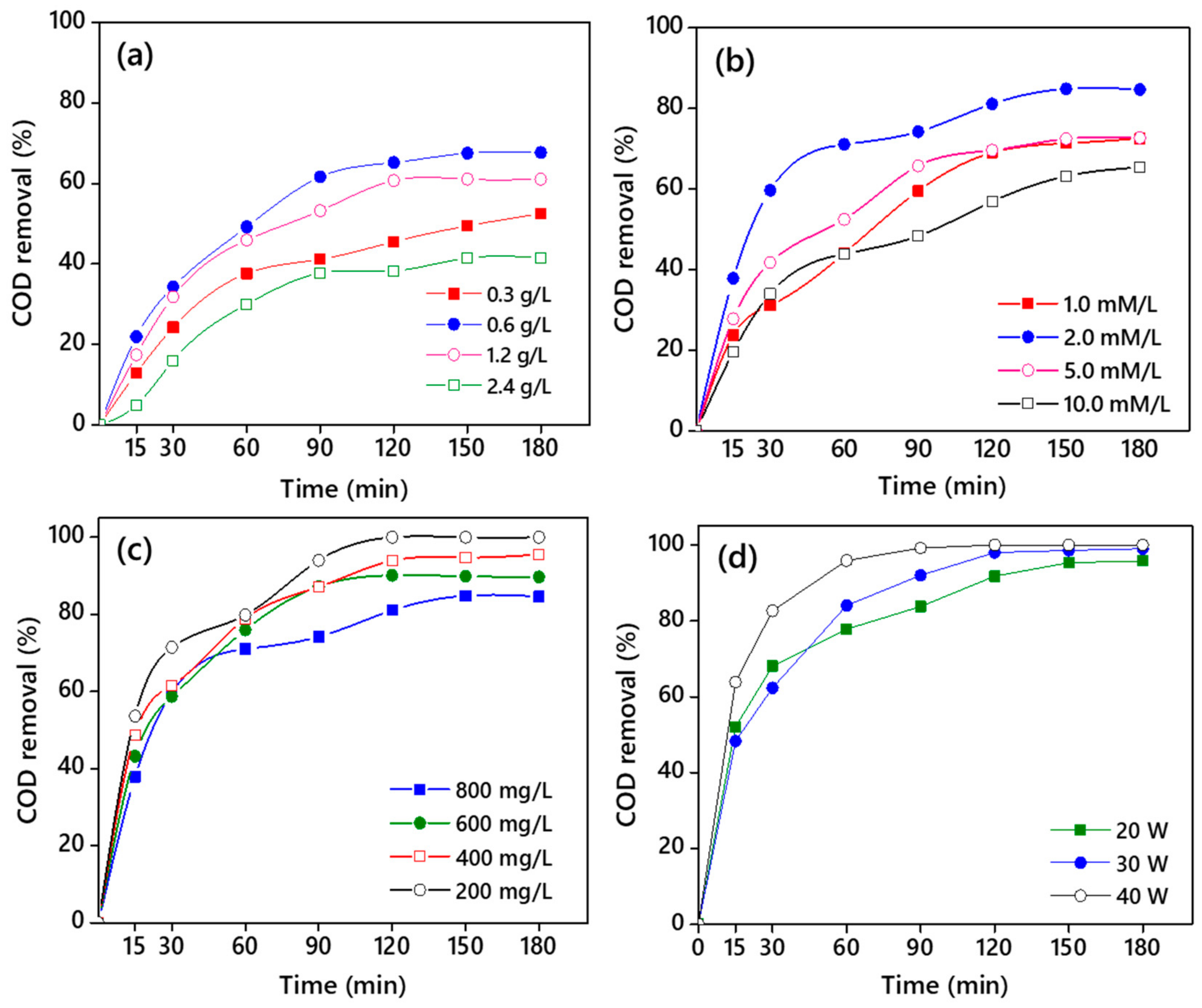

| Properties | CB-TiO2 | CNB-TiO2 |
|---|---|---|
| 2θ anatase (101) | 25.3704 | 25.2754 |
| d-spacing (nm) | 3.51073 | 3.52371 |
| FWHM | 0.3883 | 0.7675 |
| Crystallite size (nm) | 22.18 | 11.09 |
| Bandgap (eV) | 2.63 | 2.06 |
| Absorption edge (nm) | 500 | 630 |
| Surface area (m2/g) | 239.99 | 347.26 |
| Pore size (nm) | 3.7321 | 3.20380 |
| Pore volume (cm3/g) | 0.192781 | 0.254811 |
| Fitting Parameters | Binding Energy (eV) | |
|---|---|---|
| CB-TiO2 | CNB-TiO2 | |
| Ti2p3/2 (Ti4+) | 459.18 | 460.08 |
| Ti2p1/2 (Ti3+) | 464.68 | 466.63 |
| Ti2p3/2 (Ti4+) | 458.78 | - |
| Ti2p1/2 (Ti3+) | 465.48 | 460.98 |
| 1 OL | 530.18 | 530.68 |
| 2 Os | 531.68 | - |
| OH | 532.06 | 532.28 |
| C‒C | 285.68 | 285.69 |
| C‒O | 288.86 | 288.76 |
| Coke-C | - | 286.76 |
| O‒Ti‒N | - | 400.60 |
| Ti‒N | - | 402.06 |
| Advanced Oxidation Method | COD Removal Efficiency (%) | K1 (min–1) | R2 | Synergy Index |
|---|---|---|---|---|
| Photolysis (Vis) | 5.10 ± 0.48 | 1.451 × 10−2 | 0.9921 | - |
| Sonolysis (US) | 4.75 ± 0.42 | 8.751 × 10−3 | 0.9922 | - |
| Catalysis (TiO2) | 8.28 ± 0.24 | 1.175 × 10−2 | 0.9931 | - |
| Sonocatalysis | 20.34 ± 0.34 | 1.336 × 10−2 | 0.9974 | 0.6516 |
| Sonophotolysis | 17.84 ± 1.95 | 9.903 × 10−3 | 0.9913 | 0.4257 |
| Photocatalysis | 51.48 ± 0.61 | 2.394 × 10−2 | 0.9947 | 0.7326 |
| Sonophotocatalysis | 64.17 ± 0.76 | 2.395 × 10−2 | 0.9971 | 0.9118 |
| US Power (W) | COD Removal (%) | Time (min) | Electrical Energy (kWh/m3) |
|---|---|---|---|
| 20 | >90 | 120 | 1342.25 |
| 30 | >90 | 90 | 486.47 |
| 40 | >90 | 60 | 177.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.; Nawaz, R.; Khan, J.A.; Ullah, H.; Irfan, M.; Glowacz, A.; Lyp-Wronska, K.; Wzorek, L.; Asif Khan, M.K.; Jalalah, M.; et al. Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater. Materials 2021, 14, 6175. https://doi.org/10.3390/ma14206175
Rahman S, Nawaz R, Khan JA, Ullah H, Irfan M, Glowacz A, Lyp-Wronska K, Wzorek L, Asif Khan MK, Jalalah M, et al. Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater. Materials. 2021; 14(20):6175. https://doi.org/10.3390/ma14206175
Chicago/Turabian StyleRahman, Saifur, Rab Nawaz, Javed Akbar Khan, Habib Ullah, Muhammad Irfan, Adam Glowacz, Katarzyna Lyp-Wronska, Lukasz Wzorek, Mohammad Kamal Asif Khan, Mohammed Jalalah, and et al. 2021. "Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater" Materials 14, no. 20: 6175. https://doi.org/10.3390/ma14206175







