Instability of the Homogeneous Distribution of Chemical Waves in the Belousov–Zhabotinsky Reaction
Abstract
:1. Introduction
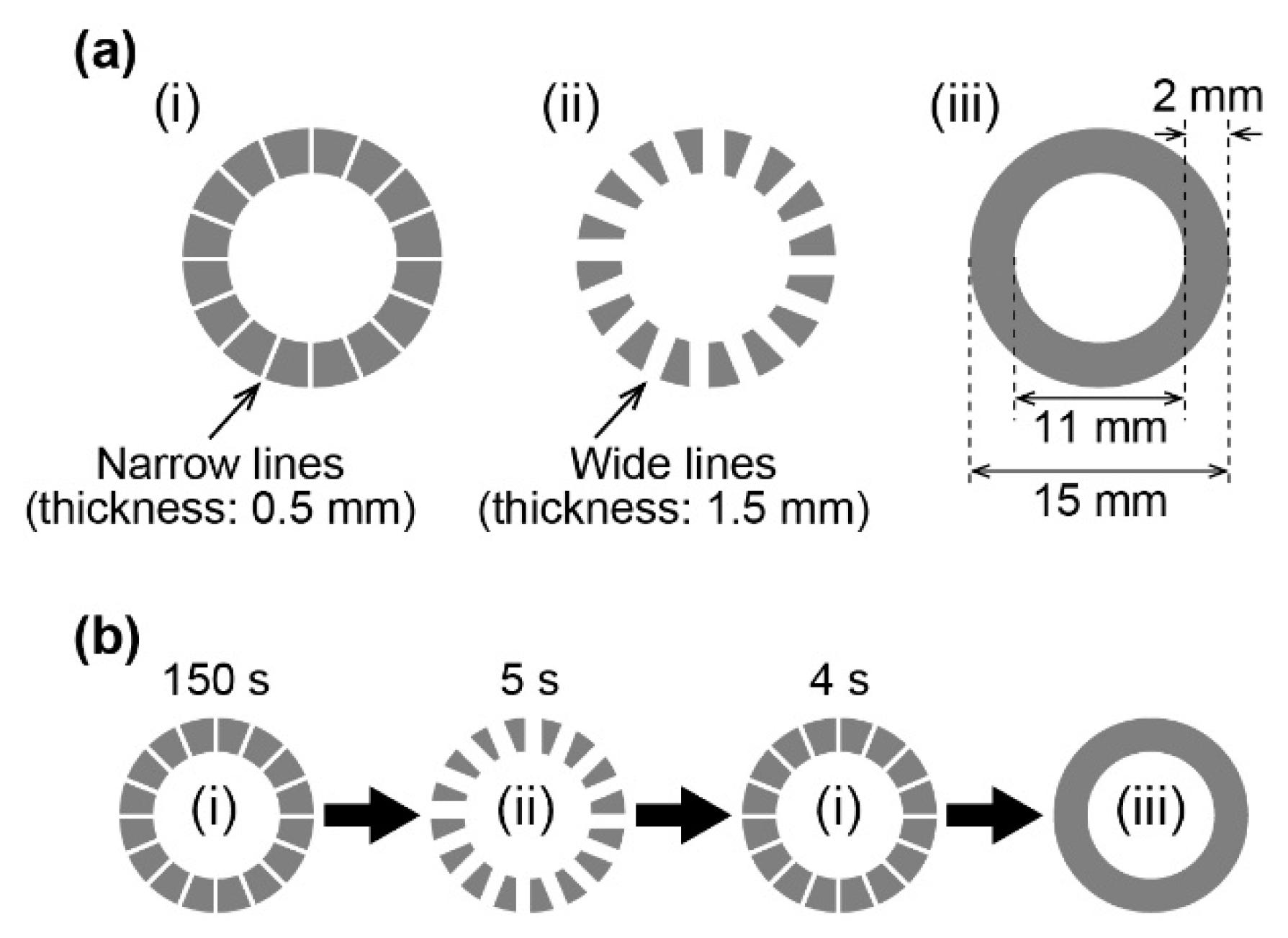
2. Materials and Methods
3. Results
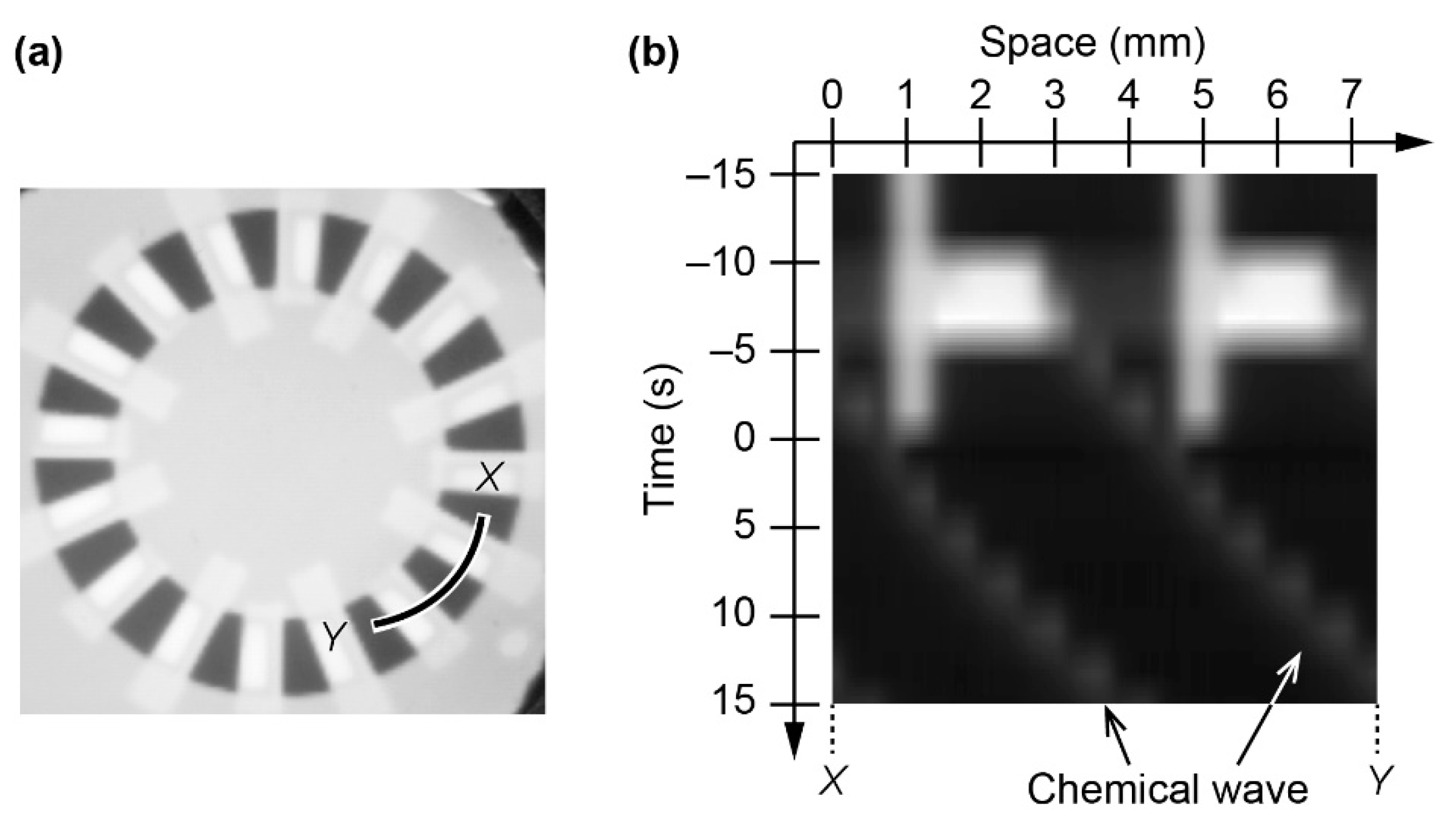
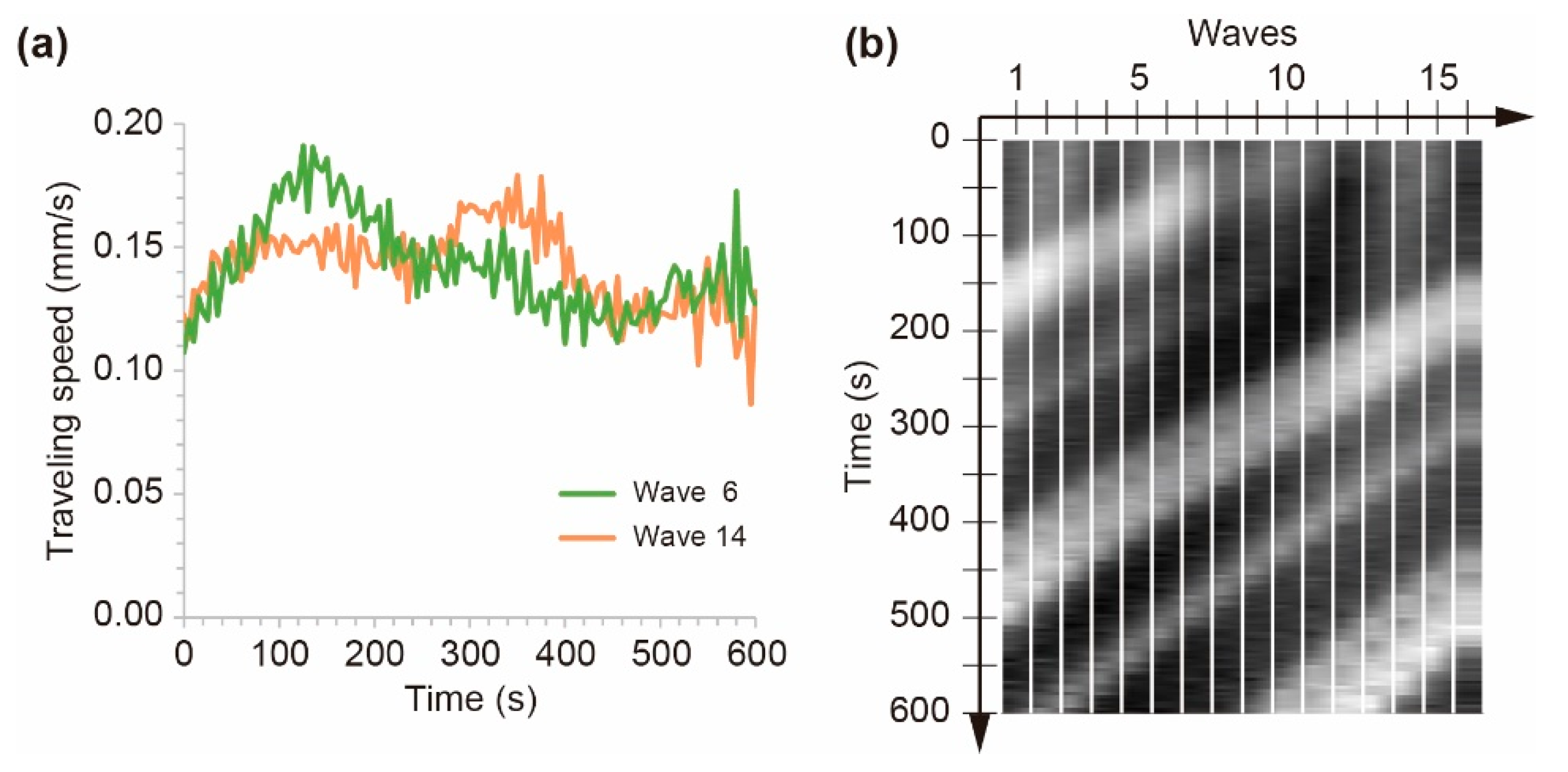
3.1. Generation of Unidirectional Chemical Waves
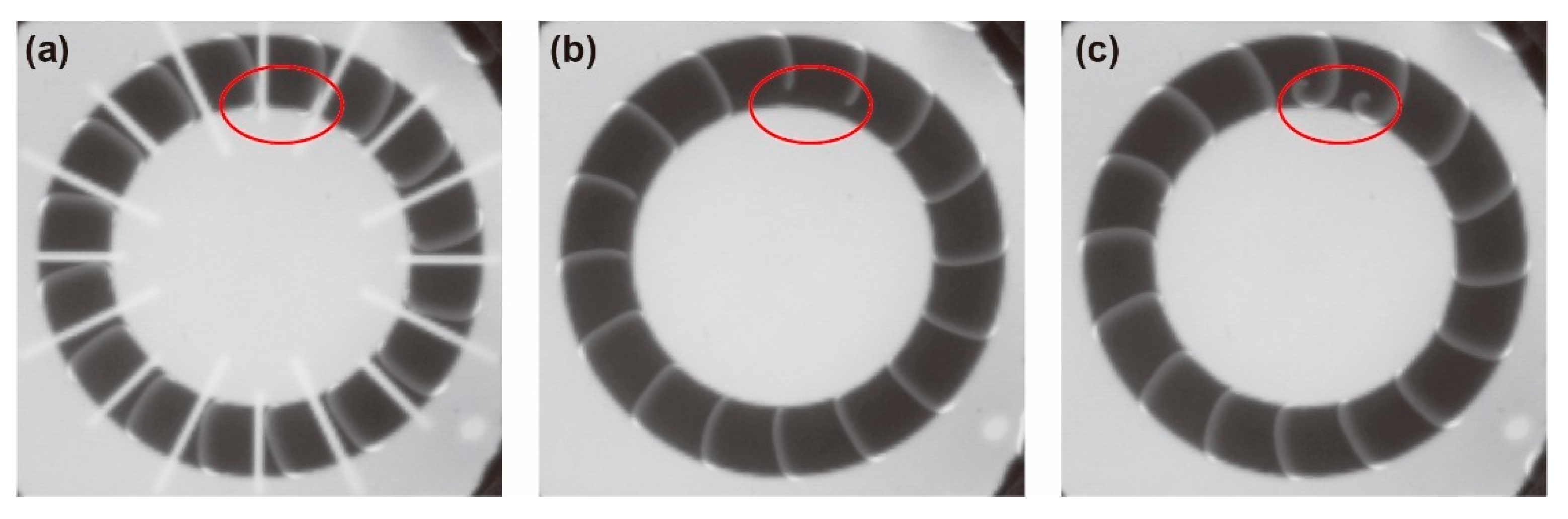
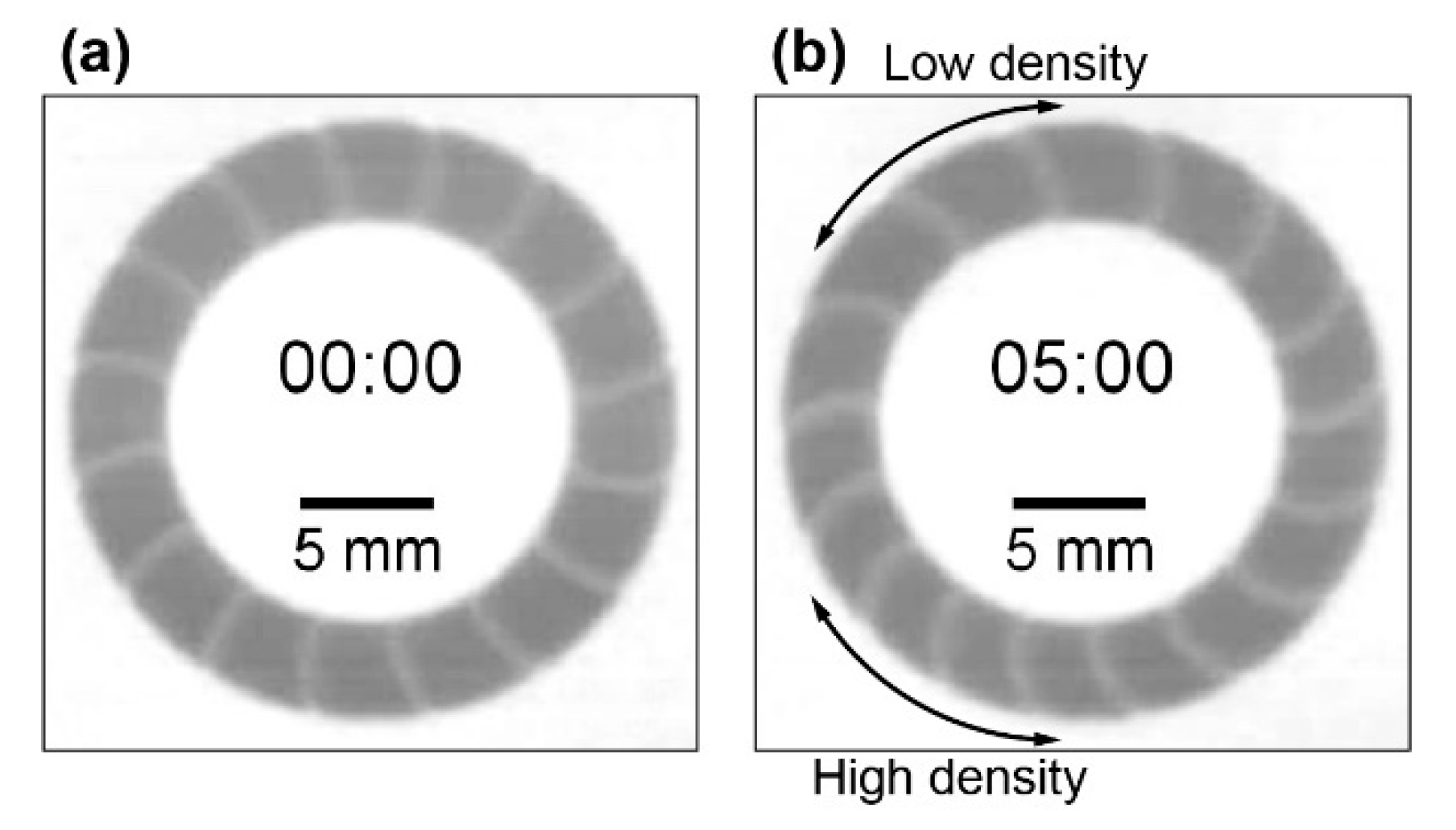
3.2. Stability of Homogeneous Distribution of Chemical Waves
4. Discussion
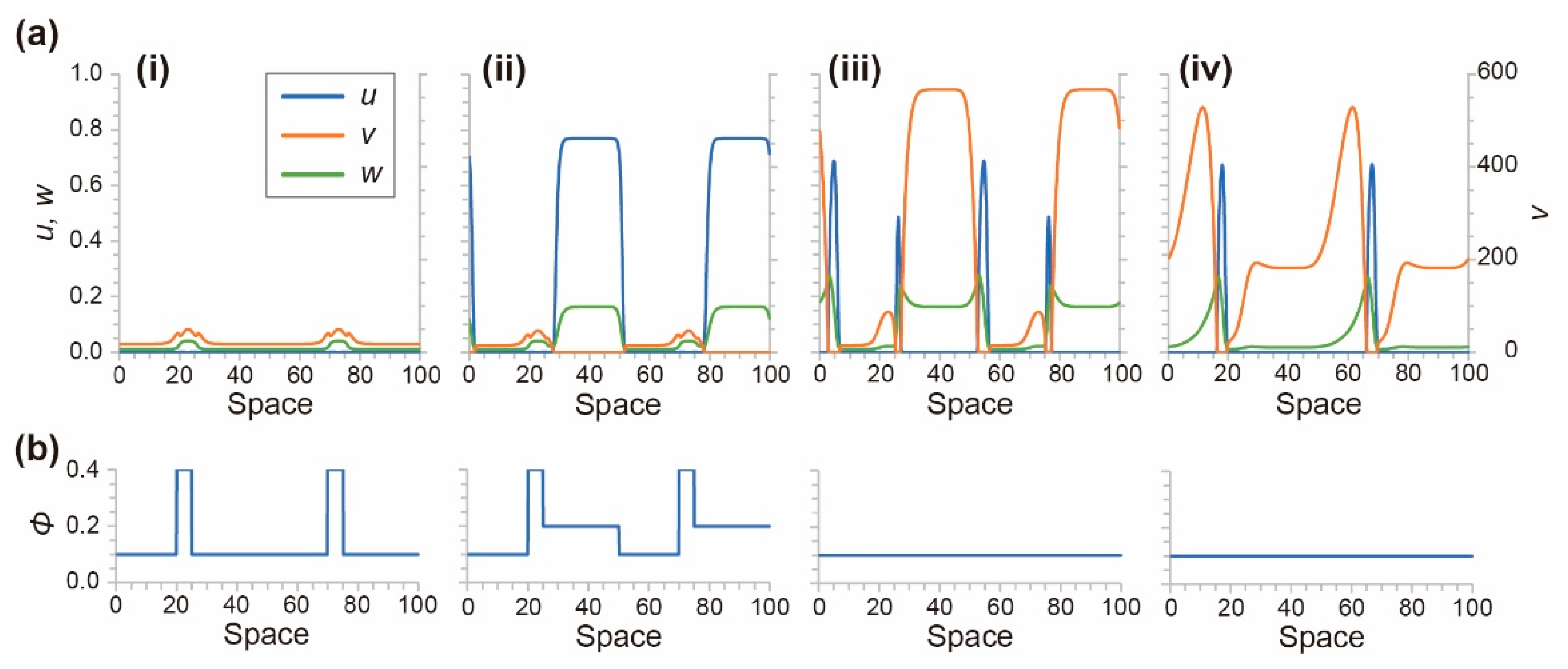
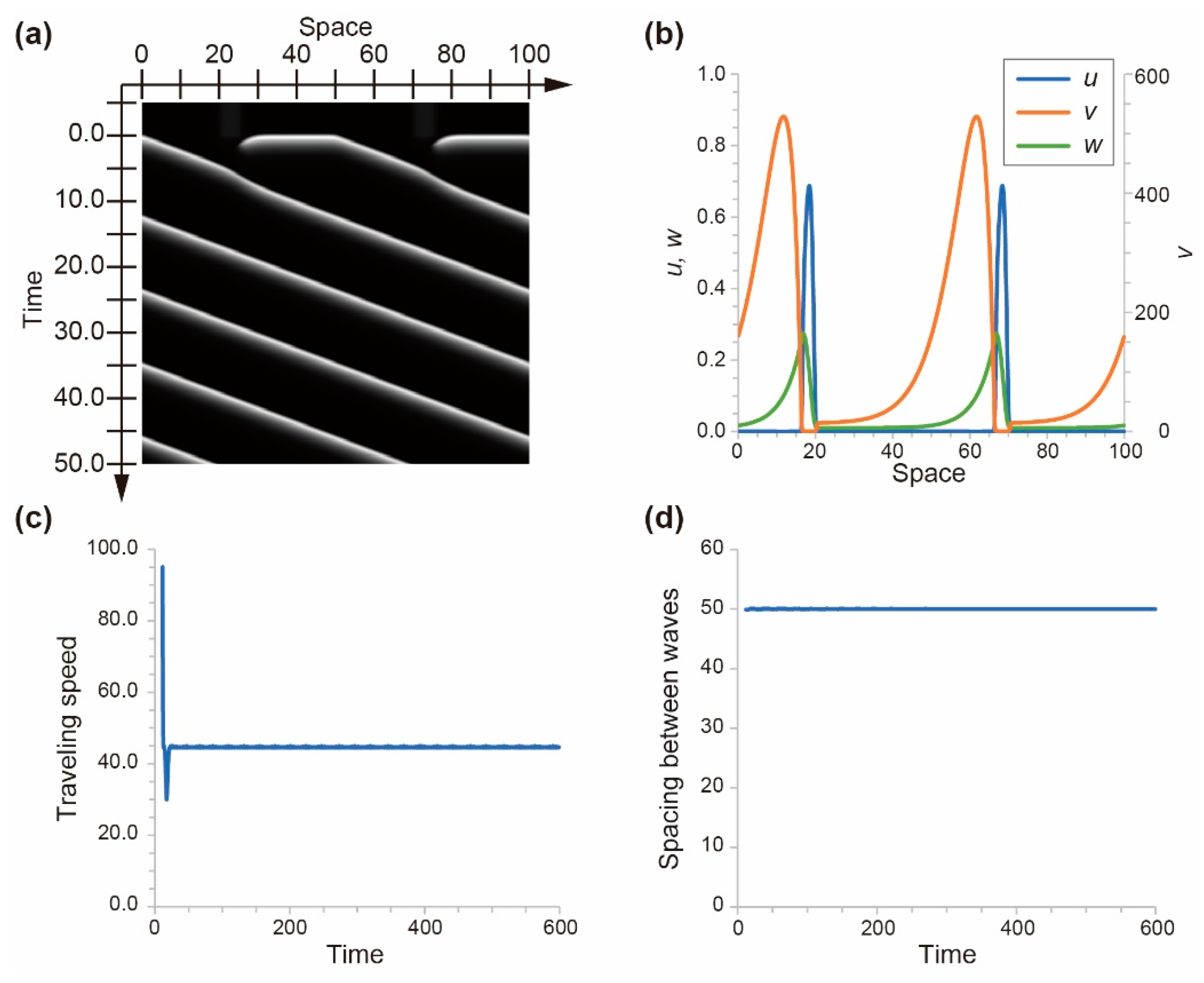
4.1. Numerical Simulation Using a Mathematical Model for Photosensitive BZ Reaction
4.2. Stability of the Homogeneous Distribution of Chemical Waves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alonso, S.; Bär, M.; Echebarria, B. Nonlinear physics of electrical wave propagation in the heart: A review. Rep. Prog. Phys. 2016, 79, 096601. [Google Scholar] [CrossRef]
- He, J.-Q.; Ma, Y.; Lee, Y.; Thomson, J.A.; Kamp, T.J. Human embryonic stem cells develop into multiple types of Cardiac Myocytes: Action Potential Characterization. Circ. Res. 2003, 93, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleber, A.G. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, R.J.; Koros, E.; Noyes, R.M. Oscillations in chemical systems. II. Thorough analysis of temporal oscillation in the bromate-cerium-malonic acid system. J. Am. Chem. Soc. 1972, 94, 8649–8664. [Google Scholar] [CrossRef]
- Taylor, A.F. Mechanism and phenomenology of an oscillating chemical reaction. Prog. React. Kinet. Mech. 2002, 27, 247–326. [Google Scholar] [CrossRef]
- Tyson, J.J. Scaling and reducing the Field-Koros-Noyes mechanism of the Belousov-Zhabotinskii reaction. J. Phys. Chem. 1982, 86, 3006–3012. [Google Scholar] [CrossRef]
- Field, R.J.; Noyes, R.M. Oscillations in chemical systems. IV. Limit cycle behavior in a model of a real chemical reaction. J. Chem. Phys. 1974, 60, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- Steinbock, O.; Kettunen, P.; Showalter, K. Anisotropy and spiral organizing centers in patterned excitable media. Science 1995, 269, 1857–1860. [Google Scholar] [CrossRef] [Green Version]
- Agladze, K.; Keener, J.P.; Müller, S.C.; Panfilov, A. Rotating spiral waves created by geometry. Science 1994, 264, 1746–1748. [Google Scholar] [CrossRef]
- Jahnke, W.; Skaggs, W.E.; Winfree, A.T. Chemical vortex dynamics in the Belousov-Zhabotlnskii reaction and in the two-variable Oregonator model. J. Phys. Chem. 1989, 93, 740–749. [Google Scholar] [CrossRef]
- Agladze, K.; Aliev, R.R.; Yamaguchi, T.; Yoshikawa, K. Chemical Diode. J. Phys. Chem. 1996, 100, 13895–13897. [Google Scholar] [CrossRef]
- Sendiña-Nadal, I.; DeCastro, M.; Gómez-Gesteira, M. Kinematic description of wave propagation through a chemical diode. Chaos 2006, 16, 033110. [Google Scholar] [CrossRef]
- Gorecka, J.N.; Gorecki, J.; Igarashi, Y. One dimensional chemical signal diode constructed with two nonexcitable barriers. J. Phys. Chem. A 2007, 111, 885–889. [Google Scholar] [CrossRef]
- Ichino, T.; Fujio, K.; Matsushita, M.; Nakata, S. Wave propagation in the photosensitive Belousov-Zhabotinsky reaction across an asymmetric gap. J. Phys. Chem. A 2009, 113, 2304–2308. [Google Scholar] [CrossRef]
- Aihara, R.; Yoshikawa, K. Size-dependent switching of the spatiotemporal structure between a traveling wave and global rhythm. J. Phys. Chem. A 2001, 105, 8445–8448. [Google Scholar] [CrossRef]
- Kuze, M.; Horisaka, M.; Suematsu, N.J.; Amemiya, T.; Steinbock, O.; Nakata, S. Chemical wave propagation in the Belousov–Zhabotinsky reaction controlled by electrical potential. J. Phys. Chem. A 2019, 123, 4853–4857. [Google Scholar] [CrossRef] [PubMed]
- Kuze, M.; Horisaka, M.; Suematsu, N.J.; Amemiya, T.; Steinbock, O.; Nakata, S. Switching between two oscillatory states depending on the electrical potential. J. Phys. Chem. B 2021, 125, 3638–3643. [Google Scholar] [CrossRef]
- Perez-Muñuzuri, V.; Aliev, R.; Vasiev, B.; Perez-Villar, V.; Krinsky, V.I. Super-spiral structures in an excitable medium. Nature 1991, 353, 740–742. [Google Scholar] [CrossRef]
- Sandstede, B.; Scheel, A. Superspiral structures of meandering and drifting spiral waves. Phys. Rev. Lett. 2001, 86, 171–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, G.S.; Swinney, H.L. Periodic to quasiperiodic transition of chemical spiral rotation. Phys. D 1991, 48, 1–16. [Google Scholar] [CrossRef]
- Flesselles, J.-M.; Belmonte, A.; Gáspár, V. Dispersion relation for waves in the Belousov–Zhabotinsky reaction. J. Chem. Soc. Faraday Trans. 1998, 94, 851–855. [Google Scholar] [CrossRef]
- Manz, N.; Ginn, B.T.; Steinbock, O. Propagation failure dynamics of wave trains in excitable systems. Phys. Rev. E 2006, 73, 066218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponboonjaroenchai, B.; Luengviriya, J.; Sutthiopad, M.; Wungmool, P.; Kumchaiseemak, N.; Müller, S.C.; Luengviriya, C. Self-organization of multiarmed spiral waves in excitable media. Phys. Rev. E 2019, 100, 042203. [Google Scholar] [CrossRef]
- Lázár, A.; Noszticzius, Z.; Försterling, H.D.; Nagy-Ungvárai, Z. Chemical waves in modified membranes I. Developing the technique. Phys. D 1995, 84, 112–119. [Google Scholar] [CrossRef]
- Suematsu, N.J.; Sato, T.; Motoike, I.N.; Kashima, K.; Nakata, S. Density wave propagation of a wave train in a closed ex-citable medium. Phys. Rev. E 2011, 84, 046203. [Google Scholar] [CrossRef]
- Nakata, S.; Matsushita, M.; Sato, T.; Suematsu, N.J.; Kitahata, H.; Amemiya, T.; Mori, Y. Photoexcited chemical wave in the ruthenium-catalyzed Belousov–Zhabotinsky reaction. J. Phys. Chem. A 2011, 115, 7406–7412. [Google Scholar] [CrossRef]
- Gani, M.O.; Ogawa, T. Instability of periodic traveling wave solutions in a modified FitzHugh–Nagumo model for ex-citable media. Appl. Math. Comput. 2015, 256, 968–984. [Google Scholar]
- Gani, M.O.; Ogawa, T. Stability of periodic traveling waves in the Aliev–Panfilov reaction–diffusion system. Commun Nonlinear Sci. Numer. Simul. 2016, 33, 30–42. [Google Scholar] [CrossRef]
- Rovinsky, A.B.; Zhabotinsky, A.M. Mechanism and mathematical model of the oscillating bromate-ferroin-bromomalonic acid reaction. J. Phys. Chem. 1984, 88, 6081–6084. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suematsu, N.J.; Nakata, S. Instability of the Homogeneous Distribution of Chemical Waves in the Belousov–Zhabotinsky Reaction. Materials 2021, 14, 6177. https://doi.org/10.3390/ma14206177
Suematsu NJ, Nakata S. Instability of the Homogeneous Distribution of Chemical Waves in the Belousov–Zhabotinsky Reaction. Materials. 2021; 14(20):6177. https://doi.org/10.3390/ma14206177
Chicago/Turabian StyleSuematsu, Nobuhiko J., and Satoshi Nakata. 2021. "Instability of the Homogeneous Distribution of Chemical Waves in the Belousov–Zhabotinsky Reaction" Materials 14, no. 20: 6177. https://doi.org/10.3390/ma14206177
APA StyleSuematsu, N. J., & Nakata, S. (2021). Instability of the Homogeneous Distribution of Chemical Waves in the Belousov–Zhabotinsky Reaction. Materials, 14(20), 6177. https://doi.org/10.3390/ma14206177






