In Vitro Evaluation of Acellular Collagen Matrices Derived from Porcine Pericardium: Influence of the Sterilization Method on Its Biological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Porcine Pericardium Matrices
2.2. PP Matrices Characterization
2.3. Cell Culture Experiments
2.4. Cytotoxic Assays
2.4.1. XTT Assay
2.4.2. Clonogenic Survival Assay
2.5. Genotoxic Assay
Comet Assay
2.6. Mutagenic Assay
Cytokinesis-Blocked Micronucleus (CBMN) Assay
2.7. Statistical Analysis
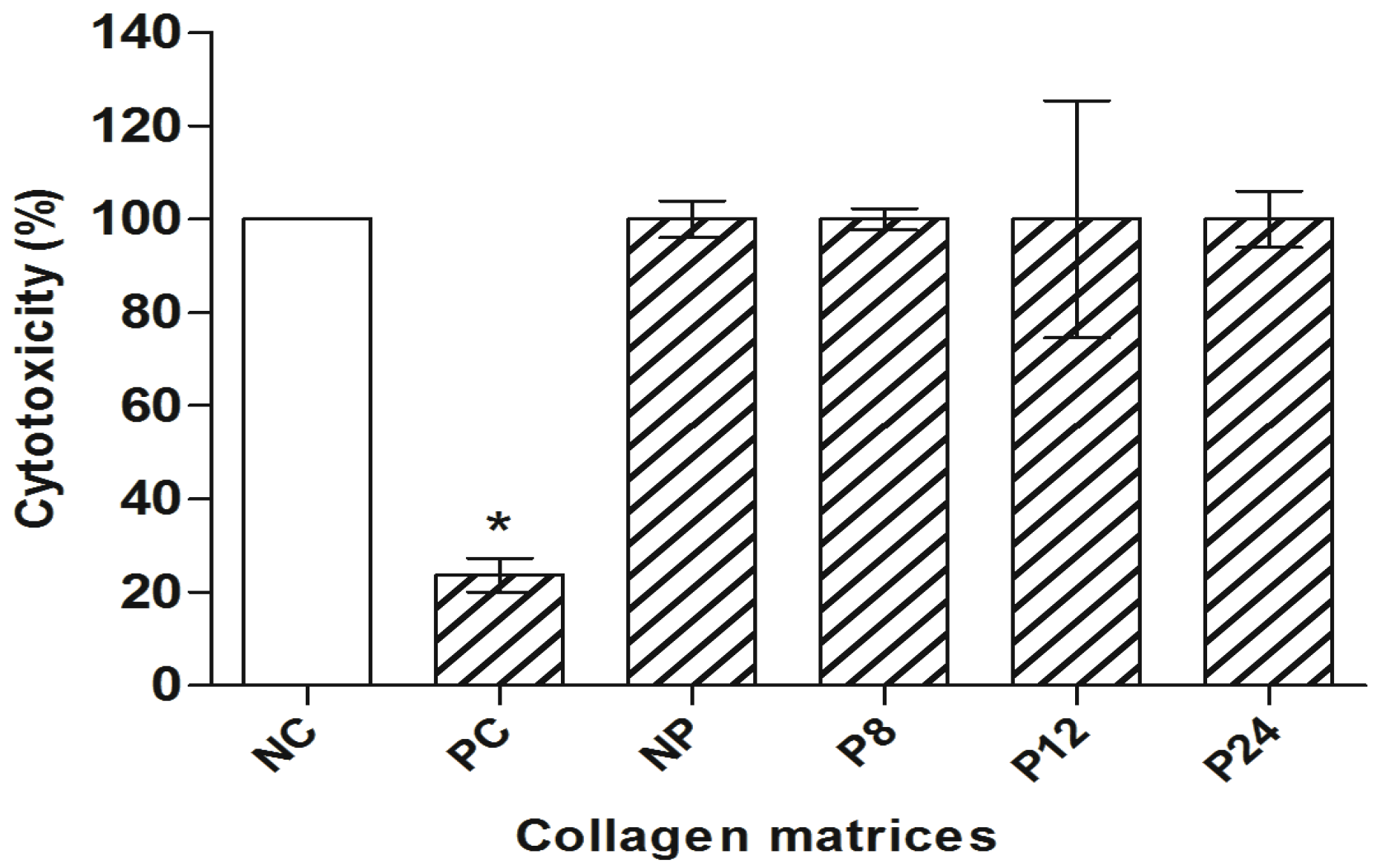
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatric Researc. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- Mahesh, L.; Kurtzman, G.M.; Shukla, S. Regeneration in Periodontics: Collagen—A Review of Its Properties and Applications in Dentistry. Compend. Contin. Educ. Dent. 2015, 36, 358–363. [Google Scholar]
- Bet, M.R.; Goissis, G.; Lacerda, C.A. Characterization of polyanionic collagen prepared by selective hydrolysis of asparagines e glutamine carboxyamide side chains. Biomacromolecules 2001, 2, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Akin, L.; Cicciu, M.; Maiorana, C.; Boyne, P.J. Use of a porcine collagen matrix as an alternative to autogenous tissue for grafting oral soft tissue defects. J. Oral Maxillofac. Surg. 2010, 68, 1463–1470. [Google Scholar] [CrossRef]
- Choe, J.A.; Jana, S.; Tefft, B.J.; Hennessy, R.S.; Go, J.; Morse, D.; Letrman, A.; Young, M.D. Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. J. Tissue Eng. Regen. Med. 2018, 12, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Stoffel, M.H.; Belyaev, Y.; Stiefel, N.G.; Vidondo, B.; Küker, S.; Mogetl, H.; Schäfer, B.; Balmer, J. Structural characterization of four different naturally occurring porcine collagen membranes suitable for medical applications. PLoS ONE 2018, 13, e0205027. [Google Scholar] [CrossRef] [PubMed]
- Dau, M.; Volprich, L.; Grambow, E.; Vollmar, B.; Frerich, B.; Al-Nawas, B.; Kämmetrer, P.W. Collagen membranes of dermal and pericardial origin—In vivo evolvement of vascularization over time. J. Biomed. Mater. Res. A 2020, 108, 2368–2378. [Google Scholar] [CrossRef]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbetck, M. In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Plepis, A.M.; Rodrigues, F.T.; Fuso, F.C.; Martins, V.C.A. Cytotoxicity and biocompatibility studies of acellular collagen matrices derived from porcine pericardium. In Proceedings of the International Conference Tissue Engineering, Leiria, Portugal, 9–11 July 2009; pp. 105–111. [Google Scholar]
- Wiegand, C.; Abel, M.; Ruth, P.; Wilhelms, T.; Schulze, D.; Norgauer, J.; Hipler, U.C. Effect of the sterilization method on the performance of collagen type I on chronic wound parameters in vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 710–719. [Google Scholar] [CrossRef]
- Vink, P.; Pleijsier, K. Aeration of ethylene oxide-sterilized polymers. Biomaterials 1986, 7, 225–230. [Google Scholar] [CrossRef]
- Lomas, R.J.; Gillan, H.L.; Matthews, J.B.; Ingham, E.; Kearney, J.N. An evaluation of the capacity of differently prepared demineralised bone matrices (DBM) and toxic residuals of ethylene oxide (EtOx) to provoke an inflammatory response in vitro. Biomaterials 2001, 22, 913–921. [Google Scholar] [CrossRef]
- Rodrígues-Benot, A.; Santamaria, R.; Martín-Malo, A.; Aljama, P. Sterilization procedures and biocompatibility. In Hemodialysis Technology: Contributions to Nephrology; Ronco, C., La Greca, G., Eds.; Karger: Basel, Switzerland, 2002; pp. 138–145. [Google Scholar]
- International Standard Organization. ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; International Standard Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rogero, S.O.; Lugão, A.B.; Ikeda, T.I.; Cruz, A.S. Teste in vito de citotoxicidade: Estudo comparativo entre duas metodologias. J. Biomed. Mater. Res. 2003, 6, 317–320. [Google Scholar]
- Eisenbrand, G.; Pool-Zobel, B.; Baker, V.; Balls, M.; Blaauboer, B.J.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.C.; Pieters, R.; et al. Methods of in vitro toxicology. Food Chem. Tox. 2002, 40, 193–236. [Google Scholar] [CrossRef]
- Saska, S.; Scarel-Caminaga, R.M.; Teixeira, L.N.; Franchi, L.P.; Dos Santos, R.A.; Gaspar, A.M.; de Oliveira, P.T.; Rosa, A.L.; Takahashi, C.S.; Messaddeq, Y.; et al. Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2253–2266. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Fenech, M. The advantages and disadvantages of the cytokinesis-block micronucleus method. Mutat. Res. 1997, 392, 11–18. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Radhika, M.; Sehgal, P.K. Studies on the desamination of bovine collagen. J. Biomed. Mater. Res. 1997, 35, 497–503. [Google Scholar] [CrossRef]
- Flandin, F.; Buffevant, C.; Herbage, D. Differential scanning calorimetry analysis of the age-related changes in the thermal stability of rate skin collagen. Biochim. Biophys. Acta 1984, 791, 205–211. [Google Scholar] [CrossRef]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomaterialia. 2018, 67, 282–294. [Google Scholar] [CrossRef]
- Horáková, J.; Mikes, P.; Saman, A.; Jencova, V.; Klapstova, A.; Svarcova, T.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. The effect of ethylene oxide sterilization on electrospun vascular grafts made from biodegradable polyesters. Mater. Sci. Eng. C 2018, 92, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Schönfelder, J.; Tugtekin, S.M.; Wetzel, C.; Hacker, M.C.; Schulz-Siegmund, M. Stabilization and sterilization of pericardial scaffolds by ultraviolet and low-energy electron irradiation. Tissue Eng. Part C Methods 2018, 24, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Pandit, A.; Zeugolis, D.I. Influence of sterilisation methods on collagen-based devices stability and properties. Expert Rev. Med. Dev. 2014, 11, 305–314. [Google Scholar] [CrossRef]
- França, R.; Mbeh, D.A.; Samani, T.D.; Le Tien, C.; Mateescu, M.A.; Yahia, L.H. The effect of ethylene oxide sterilization on the surface chemistry and in vitro cytotoxicity of several kinds of chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, J.O.; Lee, S.; Park, J.S.; Gwon, H.J.; Jeong, S.I.; Hardy, J.G.; Lim, Y.-M.; Lee, J.Y. Effective gamma-ray sterilization and characterization of conductive polypyrrole biomaterials. Sci. Rep. 2018, 8, 3721. [Google Scholar] [CrossRef] [PubMed]
- Cândido-Bacani, P.D.M.; Dos Reis, M.B.; Serpeloni, J.M.; Calvo, T.R.; Vilegas, W.; Varanda, E.A.; Colus, I.M.D.S. Mutagenicity and genotoxicity of isatin in mammalian cells in vivo. Mutat. Res. 2011, 719, 47–51. [Google Scholar] [CrossRef] [PubMed][Green Version]



| Matrix | Thickness (mm) | % Degradation * | dT (°C) |
|---|---|---|---|
| NP | 0.19 ± 0.07 | 22.1 ± 1.1 | 71.2 |
| P8 | 0.18 ± 0.07 | 32.5 ± 0.9 | 64.0 |
| P12 | 0.21 ± 0.07 | 41.4 ± 0.8 | 63.5 |
| P24 | 0.23 ± 0.11 | 69.7 ± 1.2 | 58.5 |
| Treatment | NDI (EO) | NDI (GR) | MN (EO) | MN (GR) | NPBi (EO) | NPBi (GR) |
|---|---|---|---|---|---|---|
| NC | 1.802 ± 0.2 | 1.801 ± 0.0 | 3.0 ± 1.0 | 1.0 ± 1.7 | 1.3 ± 1.5 | 0.0 ± 0.6 |
| PC | 1.882 ± 0.1 | 1.684 ± 0.0 | 353.7 ± 67.4 * | 32.0 ± 12.5 * | 12.0 ± 3.6 * | 8.0 ± 1.5 * |
| NP | 1.855 ± 0.1 | 1.849 ± 0.1 | 9.3 ± 2.9 | 2.0 ± 2.8 | 3.0 ± 1.0 | 2.0 ± 2.1 |
| P8 | 1.817 ± 0.1 | 1.831± 0.1 | 7.0 ± 1.7 | 5.0 ± 2.5 | 2.0 ± 1.0 | 1.0 ± 1.7 |
| P12 | 1.882 ± 0.1 | 1.858 ± 0.1 | 8.0 ± 2.6 | 5.0 ± 3.6 | 2.0 ± 1.0 | 1.0 ± 0.6 |
| P24 | 1.834 ± 0.1 | 1.871 ± 0.1 | 10.0 ± 2.0 | 6.0 ± 4.0 | 4.0 ± 1.7 | 2.0 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandis, R.A.D.; Miotto, L.N.; Genaro, L.E.; Migliatti Polli, L.; Plepis, A.M.d.G.; Rodrigues, F.T.; Martins, V.d.C.A.; Pereira Franchi, L.; Scarel-Caminaga, R.M.; Sidorenko de Oliveira Capote, T. In Vitro Evaluation of Acellular Collagen Matrices Derived from Porcine Pericardium: Influence of the Sterilization Method on Its Biological Properties. Materials 2021, 14, 6255. https://doi.org/10.3390/ma14216255
Grandis RAD, Miotto LN, Genaro LE, Migliatti Polli L, Plepis AMdG, Rodrigues FT, Martins VdCA, Pereira Franchi L, Scarel-Caminaga RM, Sidorenko de Oliveira Capote T. In Vitro Evaluation of Acellular Collagen Matrices Derived from Porcine Pericardium: Influence of the Sterilization Method on Its Biological Properties. Materials. 2021; 14(21):6255. https://doi.org/10.3390/ma14216255
Chicago/Turabian StyleGrandis, Rone Aparecido De, Larissa Natiele Miotto, Luis Eduardo Genaro, Larissa Migliatti Polli, Ana Maria de Guzzi Plepis, Fabiana Tessari Rodrigues, Virginia da Conceição Amaro Martins, Leonardo Pereira Franchi, Raquel Mantuaneli Scarel-Caminaga, and Ticiana Sidorenko de Oliveira Capote. 2021. "In Vitro Evaluation of Acellular Collagen Matrices Derived from Porcine Pericardium: Influence of the Sterilization Method on Its Biological Properties" Materials 14, no. 21: 6255. https://doi.org/10.3390/ma14216255
APA StyleGrandis, R. A. D., Miotto, L. N., Genaro, L. E., Migliatti Polli, L., Plepis, A. M. d. G., Rodrigues, F. T., Martins, V. d. C. A., Pereira Franchi, L., Scarel-Caminaga, R. M., & Sidorenko de Oliveira Capote, T. (2021). In Vitro Evaluation of Acellular Collagen Matrices Derived from Porcine Pericardium: Influence of the Sterilization Method on Its Biological Properties. Materials, 14(21), 6255. https://doi.org/10.3390/ma14216255






