Softening Effects in Biological Tissues and NiTi Knitwear during Cyclic Loading
Abstract
:1. Introduction
2. Materials and Methods
- Rolling of the 20 mm diameter ingot to a 7 mm thick bar (20 cycles);
- Rotary forging of the bar from 7 mm to 3.5 mm thickness (7 cycles);
- Cold drawing of wire from 3.5 mm to 500 µm diameter (25 cycles);
- Hot drawing of wire from 500 µm to 90–40 µm diameter (50–70 cycles).
3. Results and Discussion
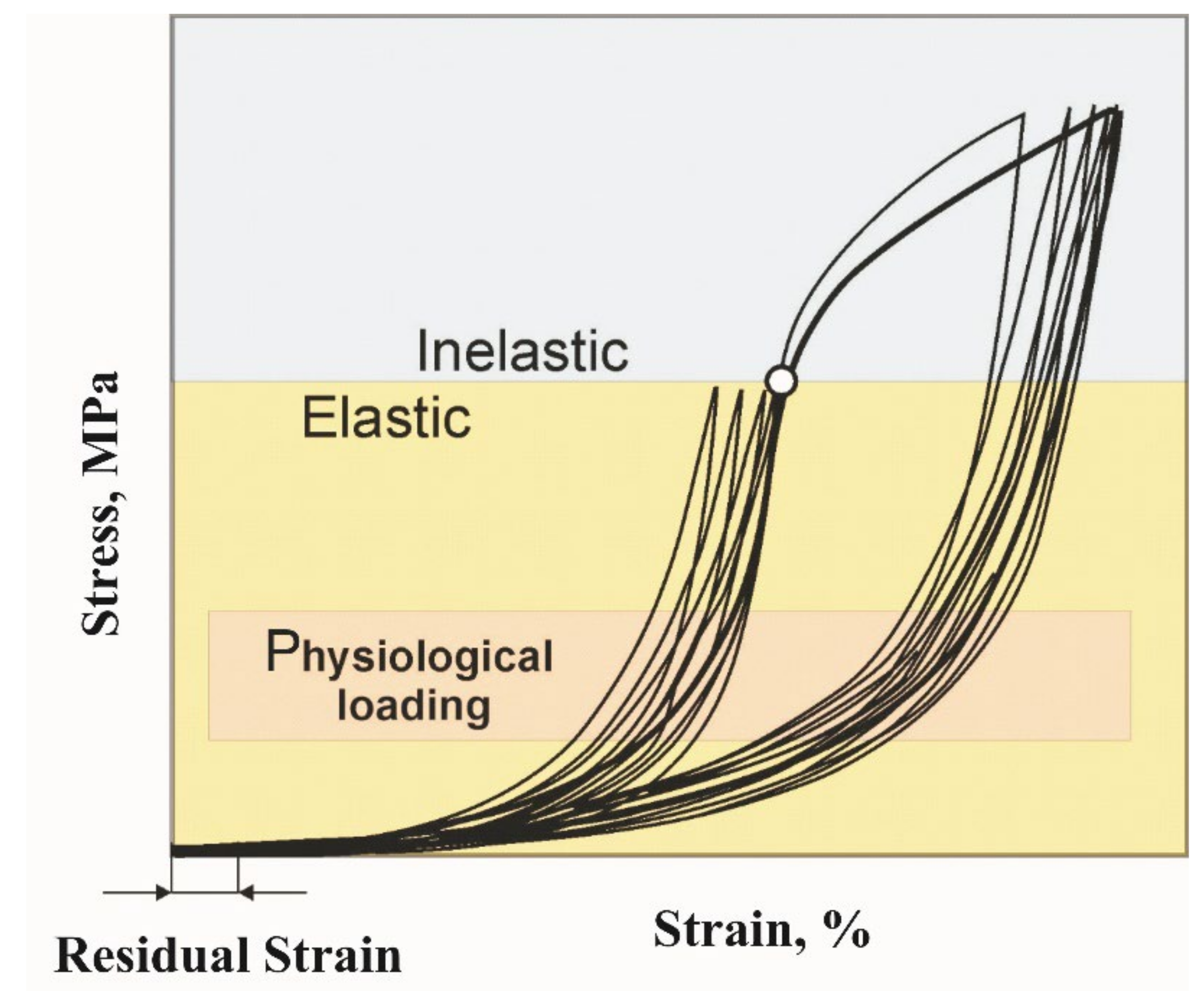
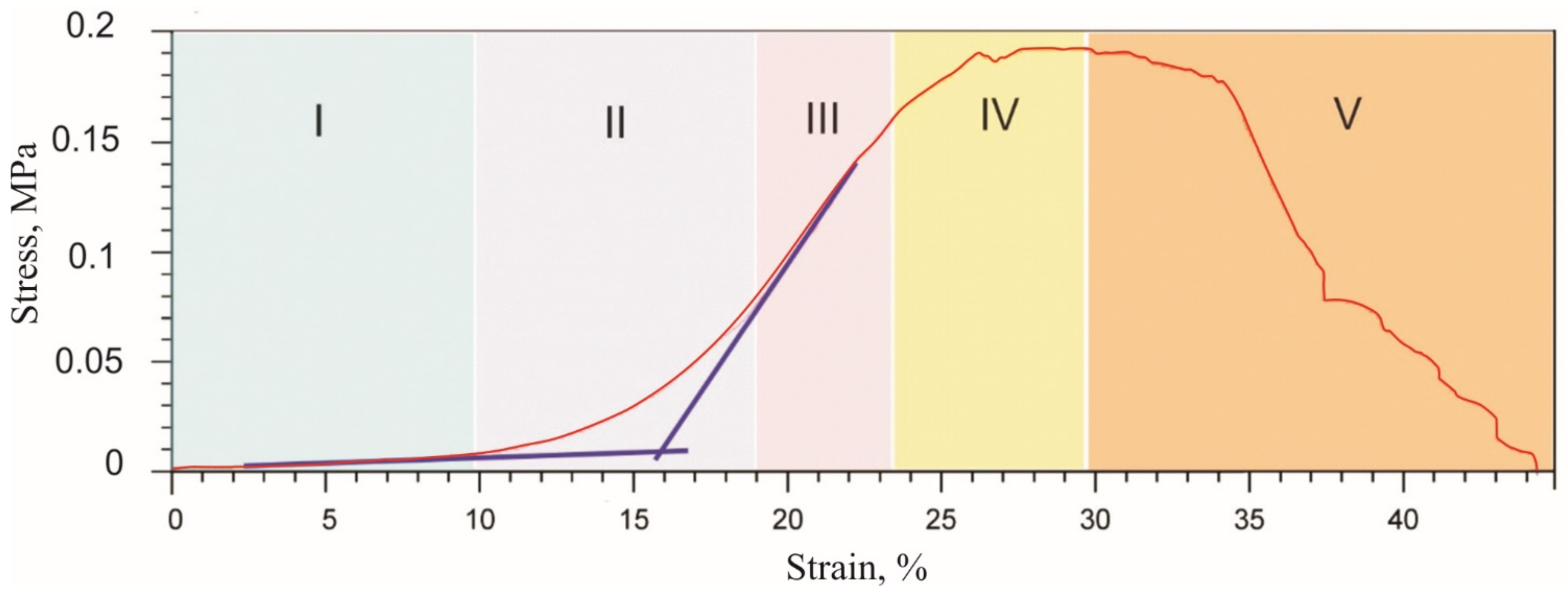
3.1. Rubber-Like Behavior of Skin, Muscles, and Tendons
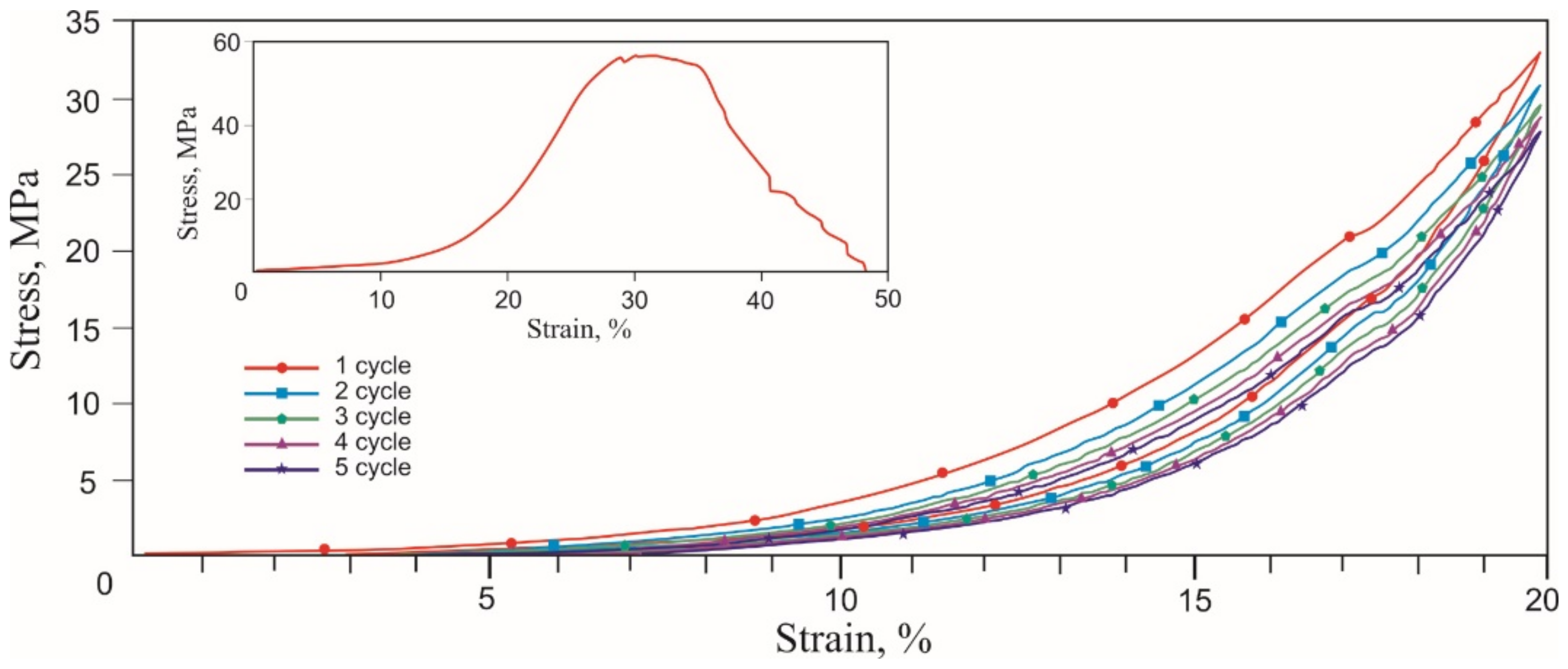
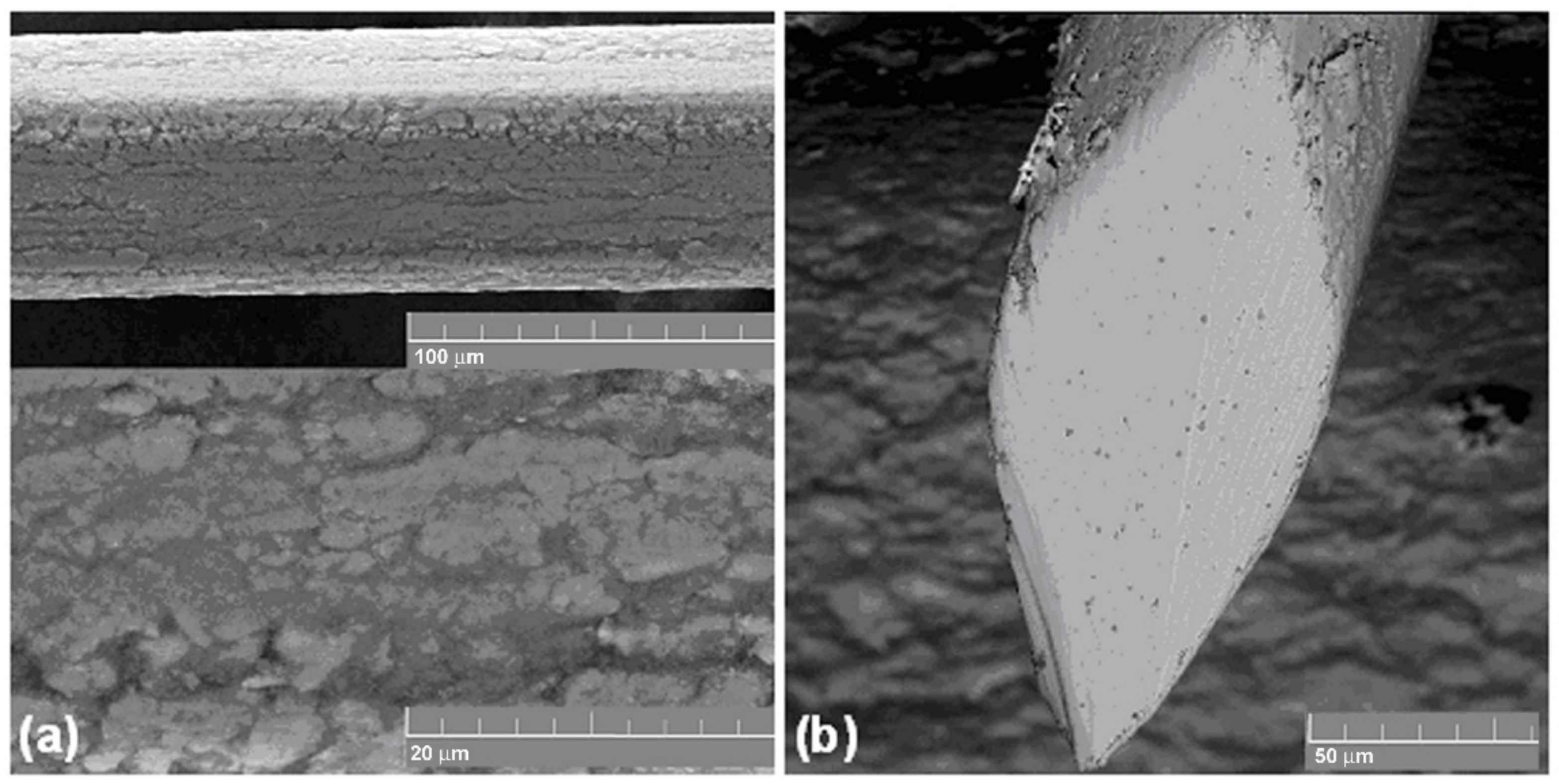
3.2. Deformation of Titanium Nickelide Knitted Mesh and Superelastic Wire
3.3. Knitted Mesh Implants Biocompatibility in Laboratory Animals
4. Conclusions
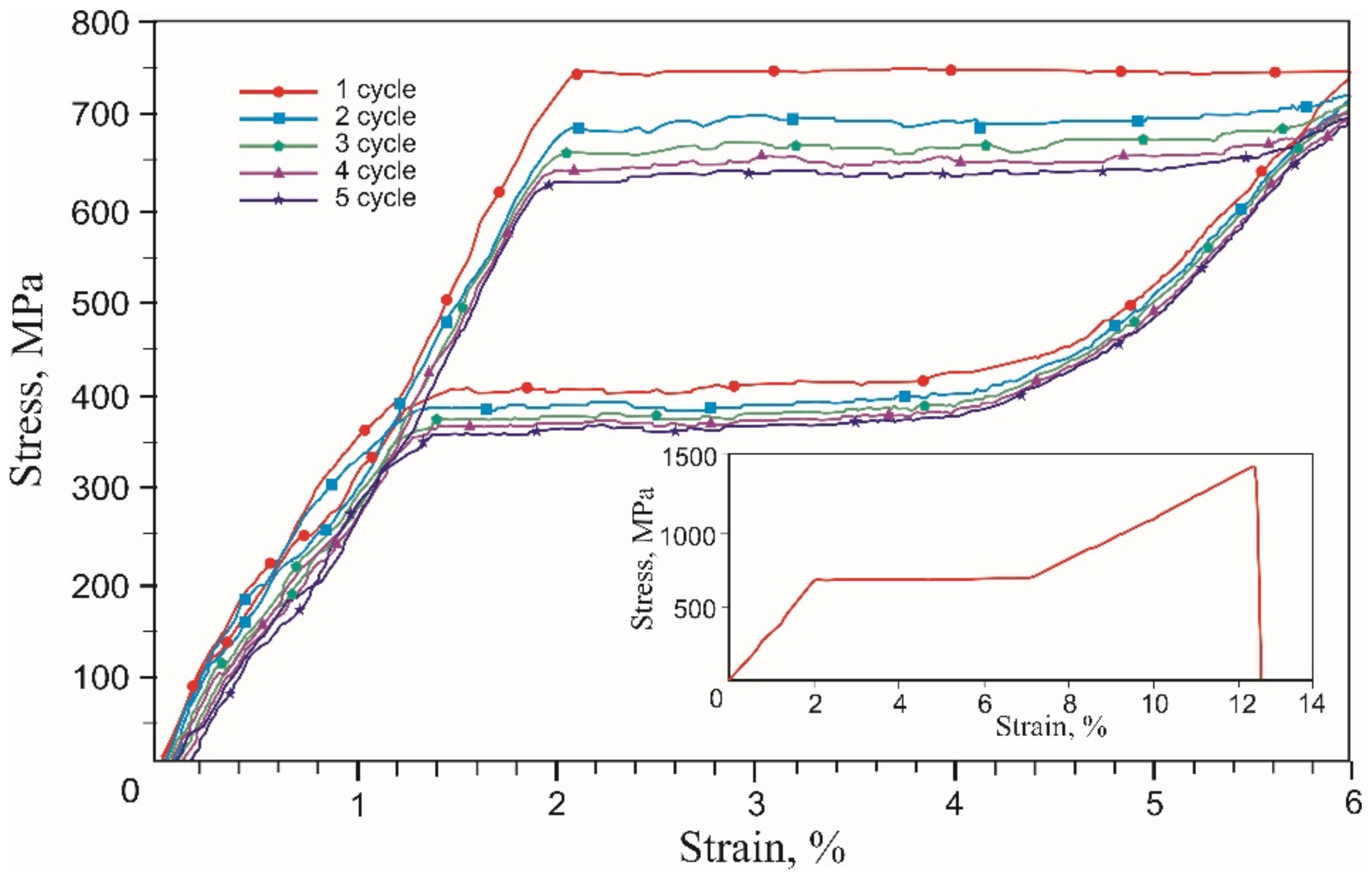
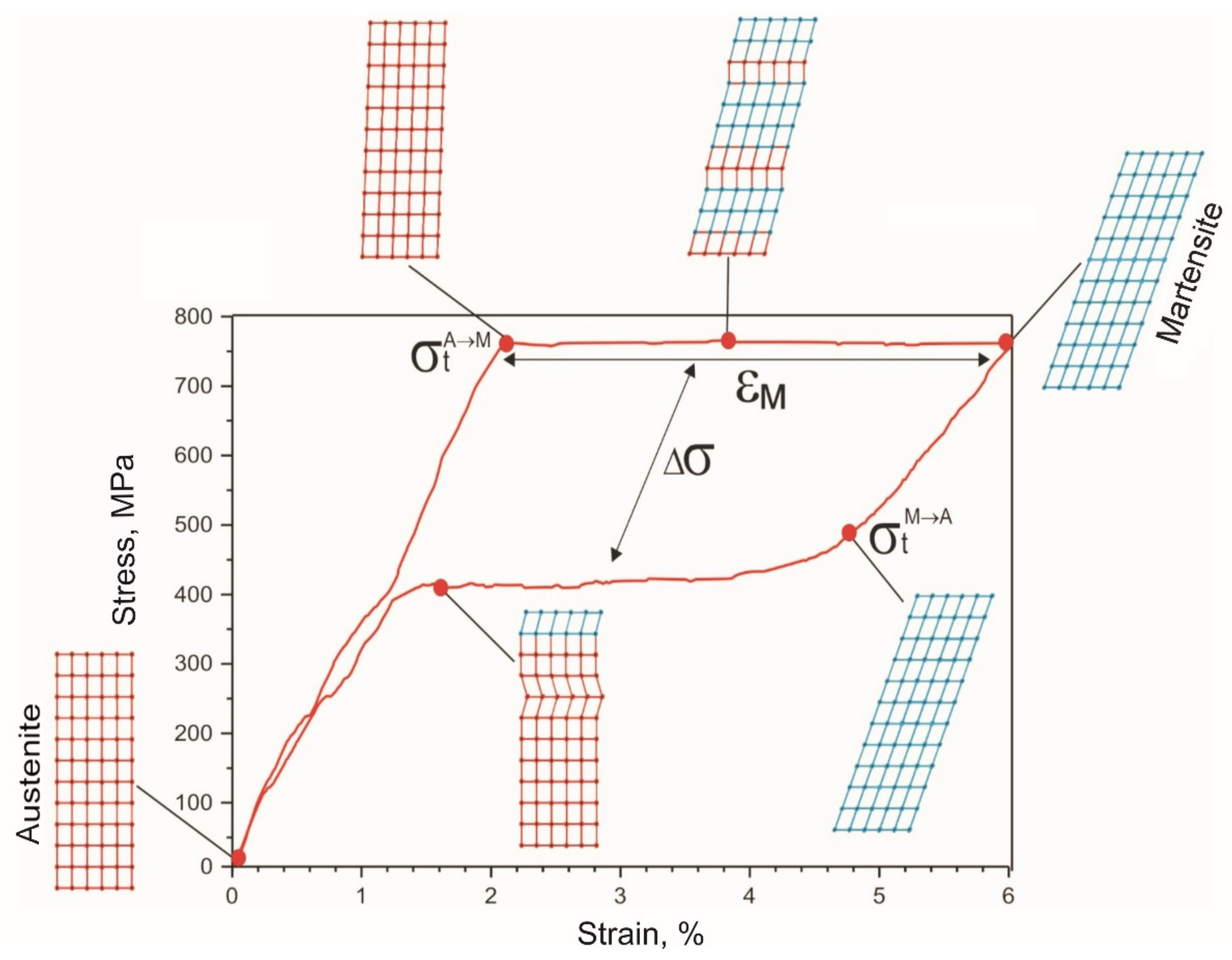
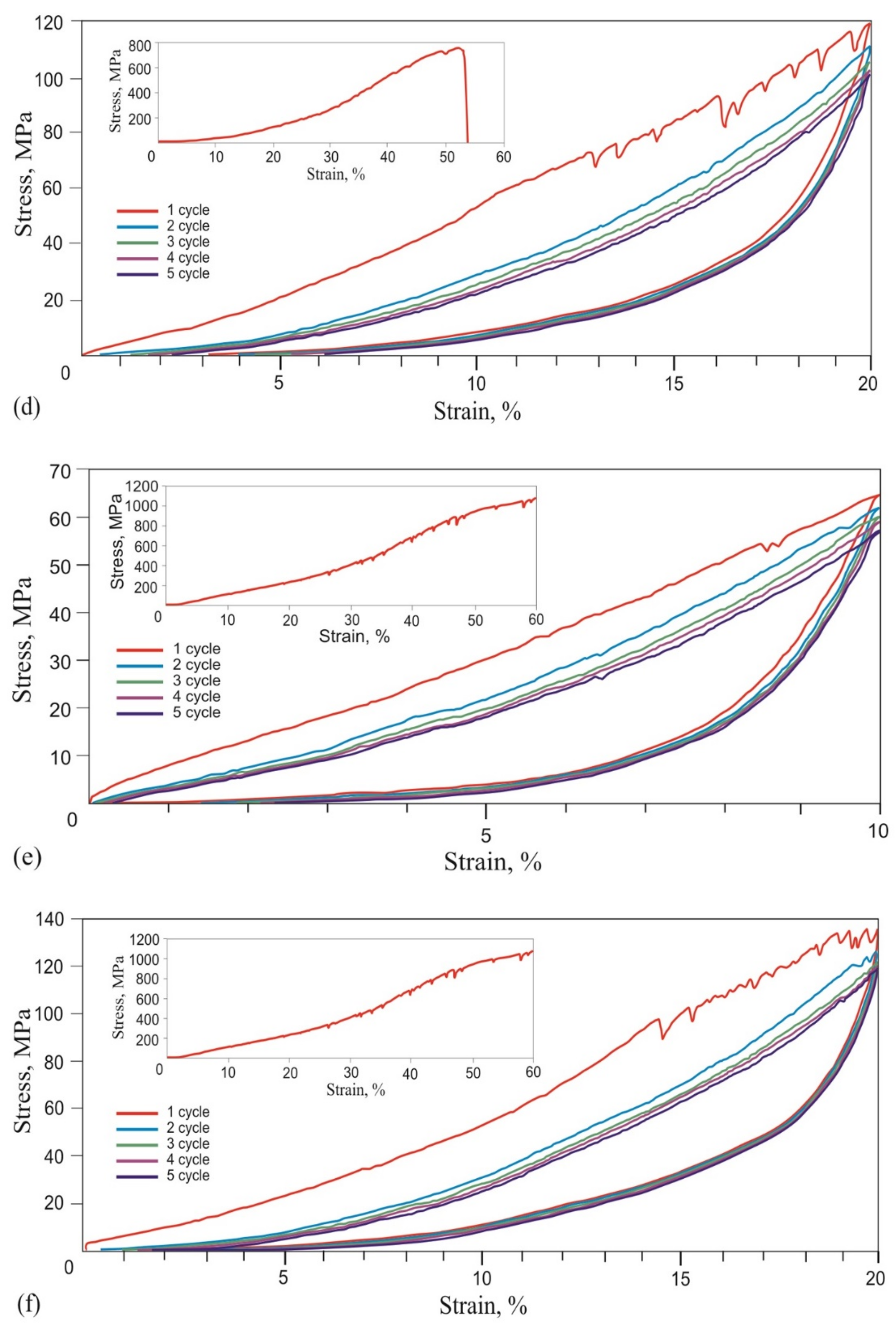
- A 60 μm diameter NiTi alloy wire reached martensitic transformation stress of 750 MPa during five loading-unloading cycles and exhibited the superelasticity effect at a 6% engineering strain. Wire rupture in the 1450–1500 MPa range had brittle fracture features.
- During the single loading of the mesh made from the NiTi wire, up to the tensile strength, and during cyclic loading up to 10% and 20% relative strain, the yield strength caused by martensitic transformation and the NiTi superelasticity effects were not found. The rupture of metal mesh in the 350–800 MPa range also exhibited a brittle fracture.
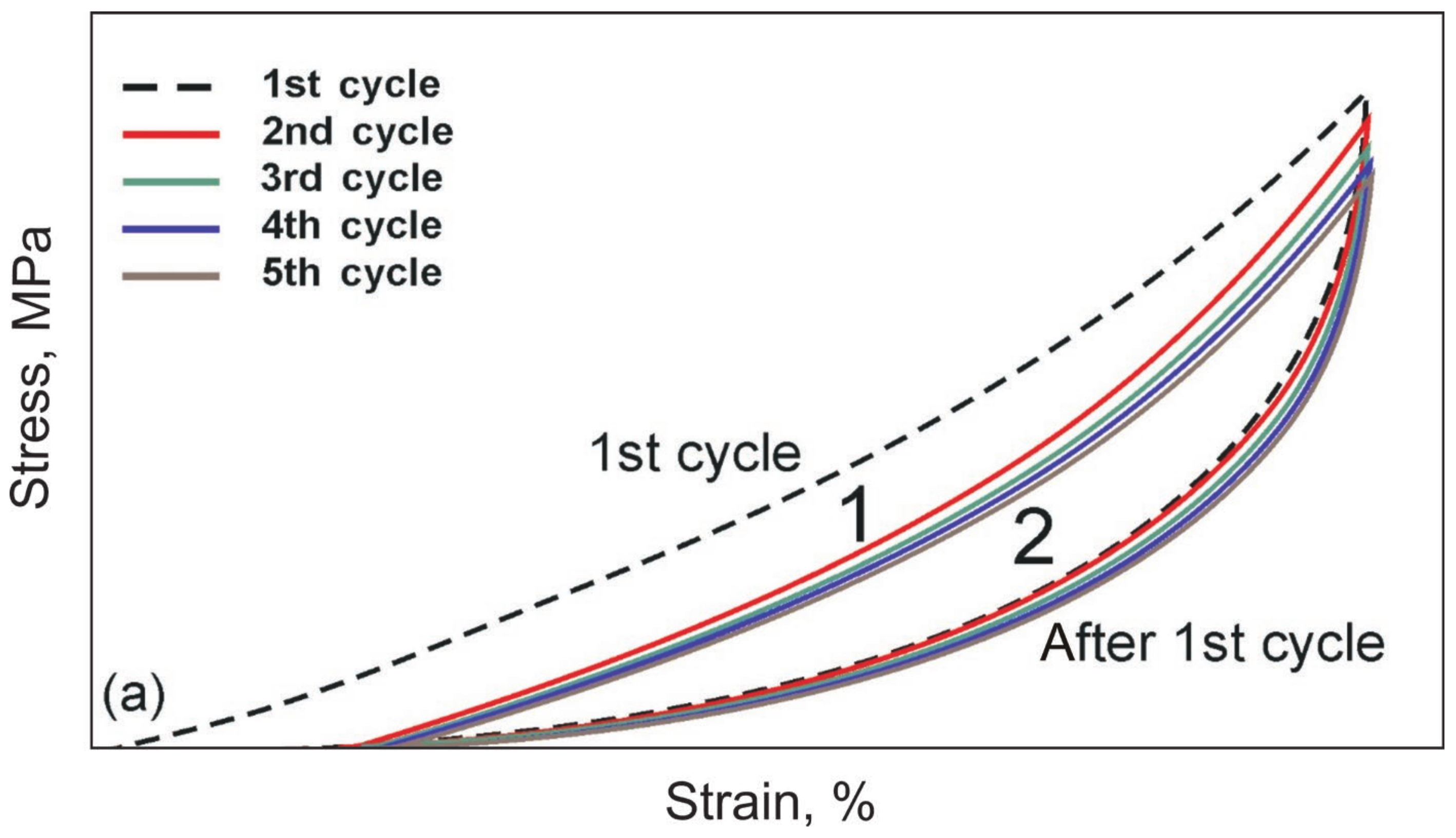
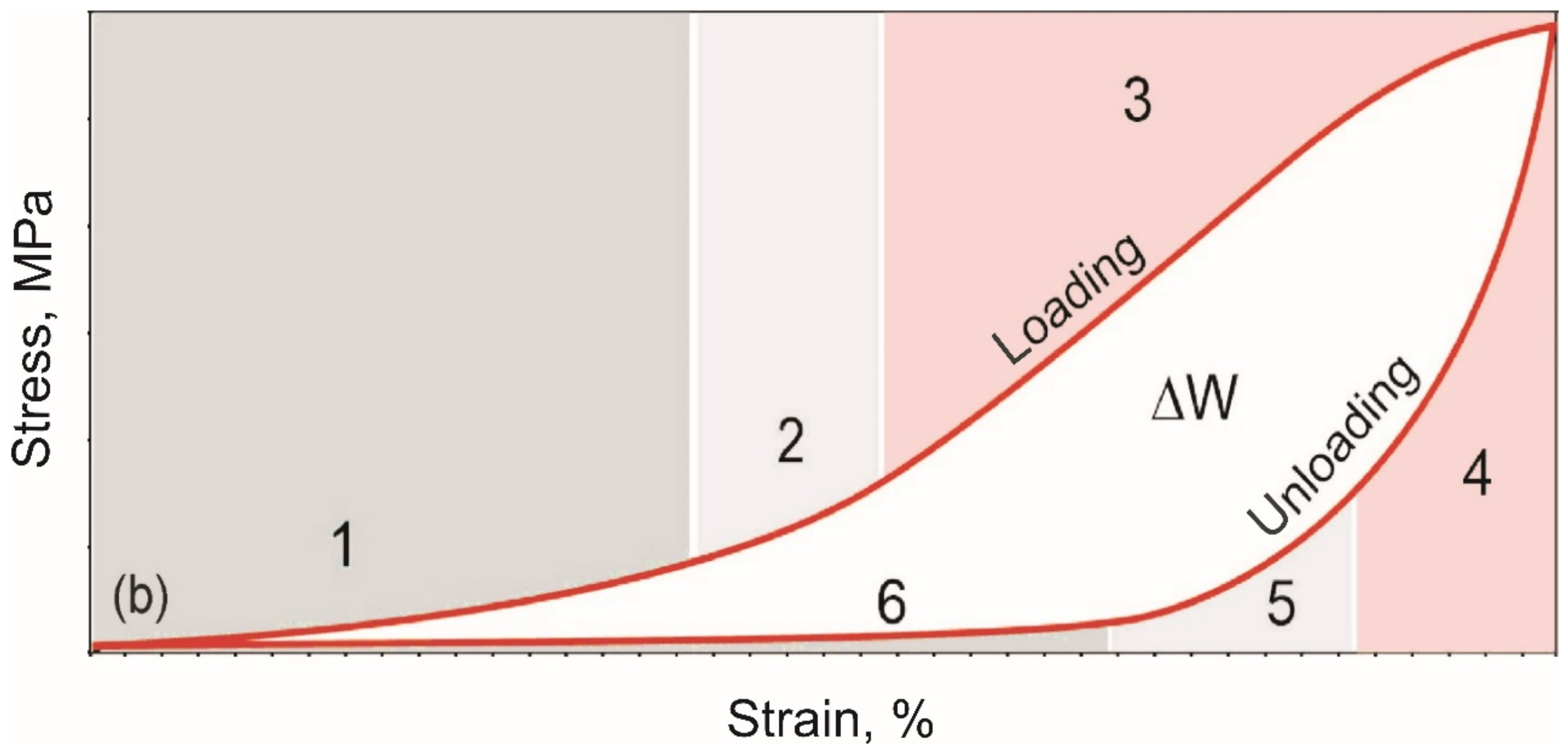
- The cyclic tension stress-strain curves of the metal mesh made from NiTi wire exhibited superelastic behavior, reversibly changing the structure under the action of external loads. A characteristic feature of superelastic behavior is the observed softening effect. The residual macro deformation of the mesh after the first two tensile cycles was due to the interaction between the contact sections of the loops: slip under load and friction, which counteracted the elastic unloading.
- The comparative analysis of cyclic loading showed that the deformation behavior of the mesh made from 40 µm, 60 µm, and 90 µm NiTi wire was similar to the superelastic behavior of skin, tendons, and muscles.
- In addition, in the knitted NiTi mesh, as well as in soft biological tissues, the effects of softening and delayed elastic unloading were observed. This effect was due to the variable modulus of elasticity of the loops, due to the inhomogeneity of the distribution of elastic stresses in the knitwear loops, the viscous slip of the loops, and viscous friction, which prevented the elastic deformation of the loops.
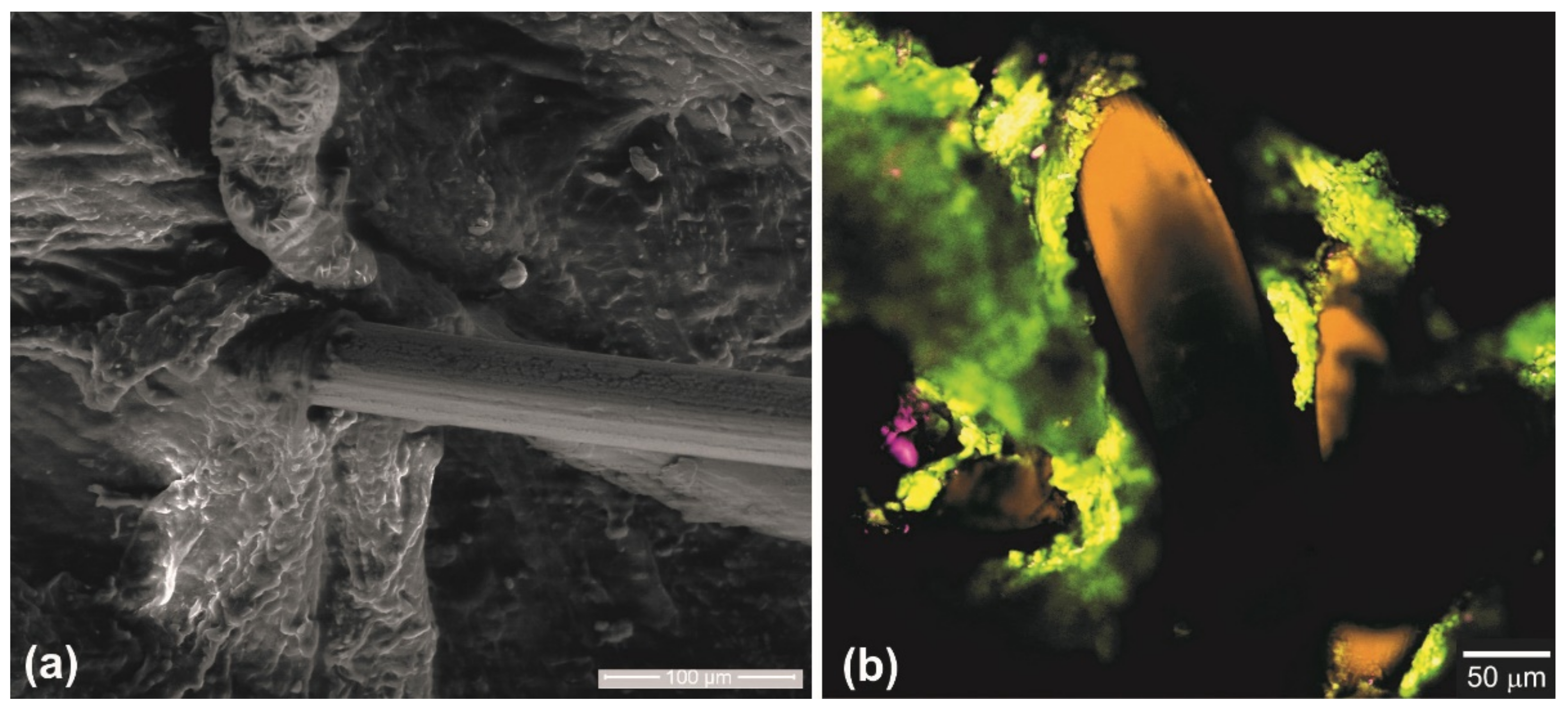
- An in vivo clinical experiment showed good integration of a superelastic knitted NiTi wire mesh into living biological tissues under normal physiological stress. Due to the similarity of the deformation behavior of the structures of the chest and abdominal walls and the mesh implant, the formed tissue-implant complex was deformed in concert, and the load was evenly distributed over the implant-biological tissue interface.
- The observed similarity of the knitted mesh stress-strain curves for all wire diameters and in the entire range of stresses and strains suggested that there was a possibility of choosing a knitted NiTi mesh with the required strength and deformation characteristics for different types of soft tissues. The main criteria for the rheological similarity of the knitted NiTi mesh and soft tissue were tensile strength, deformation range of low and high elastic moduli during loading and unloading, the value of the elastic moduli in the loading and unloading zones, and the amount of permanent deformation during the cyclic stretching of metal knitwear. Further development of the proposed methods for comparing biomechanical properties will make it possible to develop objective criteria for choosing a knitted NiTi mesh for reconstructive and organ-preserving soft tissue surgery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maccabi, A.; Shin, A.; Namiri, N.K.; Bajwa, N.; John, M.S.; Taylor, Z.D.; Grundfest, W.; Saddik, G.N. Quantitative characterization of viscoelastic behavior in tissue-mimicking phantoms and ex vivo animal tissues. PLoS ONE 2018, 13, e0191919. [Google Scholar]
- Lattore, M.; Romero, X.; Montans, F. The relevance of transverse deformation effects in modeling soft biological tissues. Int. J. Solids Struct. 2016, 99, 57–70. [Google Scholar] [CrossRef]
- Ozkaya, N.; Goldsheyder, D.; Nordin, M. Mechanical properties of biological tissues. In Fundamentals of Biomechanics, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2017; p. 454. [Google Scholar]
- Garrett, W.E.; Nikolaou, P.K.; Ribbeck, B.M.; Glisson, R.R.; Seaber, A.V. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive extension. Am. J. Sports Med. 1988, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Collinsworth, A.M.; Reichert, W.M. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech. 2001, 34, 1545–1553. [Google Scholar] [CrossRef]
- Elliott, G.F.; Worthington, C.R. Muscle contraction: Viscous-like frictional forces and the impulsive model. Int. J. Biol. Macromol. 2001, 29, 213–218. [Google Scholar] [CrossRef]
- Victor, S.; Muthu, J. Polymer ceramic composite materials for orthopedic applications—Relevance and need for mechanical match and bone regeneration. J. Mechatron. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Yadav, S.; Ganswar, S. An overview on recent progresses and future perspective of biomaterials. Mat. Sci. Eng. 2018, 404, 012013. [Google Scholar] [CrossRef]
- Borhani, S.; Hassanajili, S.; Tafti, S.-H.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef] [Green Version]
- Flynn, C.; Taberner, A.; Nielsen, P. Mechanical characterisation of in vivo human skin using a 3D-force-sensitive micro-robot and finite element analysis. Biomech. Model. Mechanobiol. 2011, 10, 27–38. [Google Scholar] [CrossRef]
- Sun, Q.-P.; Li, Z.-Q. Phase transformation in superelastic NiTi polycrystalline micro-tubes under tension and torsion-from localization to homogeneous deformation. Int. J. Solids Struct. 2002, 39, 3797–3809. [Google Scholar] [CrossRef]
- Davis, J.R. Overview of biomaterials and their use in medical devices. In Handbook of Materials for Medical Devices; ASM International: Novelty, OH, USA, 2003; pp. 1–17. [Google Scholar]
- Gunther, V.E. The directions of creation of unique technologies in medicine on the basis of new generation of biocompatible materials and implants with shape memory. KnE Mater. Sci. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- André, M.; Wriggers, P. Thermo-mechanical behaviour of rubber materials during vulcanization. Int. J. Solids Struct. 2005, 42, 4758–4778. [Google Scholar] [CrossRef]
- Ghahfarokhi, Z.M.; Zand, M.M.; Tehrani, M.S.; Wendland, B.R.; Dargazany, R. A visco-hyperelastic constitutive model of short- and long-term viscous effects on isotropic soft tissues. J. Mech. Eng. Sci. 2019, 234, 3–17. [Google Scholar]
- Khajehsaeid, H.; Arghavani, J.; Naghdabadi, R.; Sohrabpour, S. A visco-hyperelastic constitutive model for rubber-like materials: A rate-dependent relaxation time scheme. Int. J. Eng. Sci. 2014, 79, 44–58. [Google Scholar] [CrossRef]
- Chernyshova, A.; Kolomiets, L.; Chekalkin, T.; Chernov, V.; Sinilkin, I.; Gunther, V.; Marchenko, E.; Baigonakova, G.; Kang, J.H. Fertility-sparing surgery using knitted TiNi mesh implants and sentinel lymph nodes: A 10-year experience. J. Investig. Surg. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Gunther, V.; Radkevich, A.; Kang, S.B.; Chekalkin, T.; Marchenko, E.; Gunther, S.; Pulikov, A.; Sinuk, I.; Kaunietis, S.; Podgorniy, V.; et al. Study of the knitted TiNi mesh graft in a rabbit cranioplasty model. Biomed. Phys. Eng. Express 2019, 5, 027005. [Google Scholar] [CrossRef]
- Shtin, V.; Novikov, V.; Chekalkin, T.; Gunther, V.; Marchenko, E.; Choynzonov, E.; Kang, J.H.; Obrosov, A. Repair of orbital post-traumatic wall defects by custom-made TiNi mesh endografts. J. Funct. Biomater. 2019, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Gunther, V.; Marchenko, E.; Chekalkin, T.; Baigonakova, G.; Kang, J.-H.; Kim, J.-S.; Klopotov, A. Study of structural phase transitions in quinary TiNi(MoFeAg)-based alloys. Mater. Res. Exp. 2017, 4, 105702. [Google Scholar] [CrossRef]
- Yahia, L.; Ryhänen, J. Bioperformance of Shape Memory Alloys; Springer: Berlin/Heidelberg, Germany, 2000; p. 349. [Google Scholar]
- Kapoor, D. Nitinol for Medical Applications: A brief introduction to the properties and processing of nickel titanium shape memory alloys and their use in stents. Johns. Matthey Technol. Rev. 2017, 61, 66–76. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, H.; Dai, F.; Qiang, H.; Rong, L.; Chen, P.; Huang, F. Compressive behavior of 64% porosity NiTi alloy: An experimental study. Mater. Sci. Eng. A 2009, 515, 117–130. [Google Scholar] [CrossRef]
- Fercec, J.; Kos, M.; Bruncko, M.; Anzel, I.; Glisic, B.; Markovic, E.; Rudolf, R. Comparison of NiTi orthodontic archwires and a determination of the characteristic properties. Mater. Tehnol. 2014, 48, 99–104. [Google Scholar]
- Wollants, P.; Roos, J.R.; Delaey, L. Thermally- and stress-induced thermoelastic martensitic transformations in the reference frame of equilibrium thermodynamics. Prog. Mater. Sci. 1993, 37, 227–288. [Google Scholar] [CrossRef]
- Gadot, B.; Martinez, O.R.; du Roscoat, S.R.; Bouvard, D.; Rodney, D.; Orgéas, L. Entangled single-wire NiTi material: A porous metal with tunable superelastic and shape memory properties. Acta Mater. 2015, 96, 311–323. [Google Scholar] [CrossRef]
- Li, S.; Mao, C.; Li, H.; Yagebai, Z. Mechanical properties and theoretical modeling of self-centering shape memory alloy pseudo-rubber. Smart Mater. Struct. 2011, 20, 115008. [Google Scholar] [CrossRef]
- Ramo, N.L.; Puttlitz, C.M.; Troyer, K.L. The development and validation of a numerical integration method for non-linear viscoelastic modeling. PLoS ONE 2018, 13, e0190137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W. Damage models for soft tissues: A survey. J. Med. Biol. Eng. 2016, 36, 285–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, E.W.V. Notes on continuum mechanics. In Lecture Notes on Numerical Methods in Engineering and Sciences, 1st ed.; Oñate, E., Ed.; Springer: Amsterdam, The Netherlands, 2013; p. 673. [Google Scholar]
- Rivlin, R.S. Large elastic deformations of isotropic materials. IV. Further developments of the general theory. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1948, 241, 379–397. [Google Scholar]
- Diani, J.; Fayolle, B.; Gilormini, P. A review on the Mullins effect. Eur. Polym. J. 2009, 45, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Cantournet, S.; Desmorat, R.; Besson, J. Mullins effect and cyclic stress softening of filled elastomers by internal sliding and friction thermodynamics model. Int. J. Solids Struct. 2009, 46, 2255–2264. [Google Scholar] [CrossRef]
- Beatty, M.F. The Mullins effect in a pure shear. J. Elast. 2000, 59, 369–392. [Google Scholar] [CrossRef]
- Sarangi, S. On the Mullins Effect of Soft-Biological Tissues: A Comparison between Material Models with Experimental Results. In Proceedings of the 6th World Congress of Biomechanics (WCB 2010), Singapore, 1–6 August 2010; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1032–1035. [Google Scholar]
- Mohammed, M.A.P. Visco-hyperelastic model for soft rubber-like materials. Sains Malays. 2014, 43, 451–457. [Google Scholar]
- Dubruel, P.; Vlierberghe, S.V. Biomaterials for Bone Regeneration: Novel Techniques and Applications; Woodhead Publishing: Sawston, UK, 2014; p. 502. [Google Scholar]
- Miserez, A.; Guerettea, P.A. Phase transition-induced elasticity of a-helical bioelastomeric fibres and networks. Chem. Soc. Rev. 2013, 42, 1973–1995. [Google Scholar] [CrossRef]
- Baigonakova, G.; Marchenko, E.; Chekalkin, T.; Kang, J.; Weiss, S.; Obrosov, A. Influence of silver addition on structure, martensite transformations and mechanical properties of TiNi-Ag alloy wires for biomedical application. Materials 2020, 13, 4721. [Google Scholar] [CrossRef]
- Gunter, V.E.; Marchenko, E.S.; Gunter, S.V.; Baigonakova, G.A. The Influence of the surface layer on the combination of properties of thin TiNi alloy wires. Tech. Phys. Lett. 2018, 44, 811–813. [Google Scholar] [CrossRef]
- Elibol, C.; Wagner, M.F.-X. Strain rate effects on the localization of the stress-induced martensitic transformation in pseudoelastic NiTi under uniaxial tension, compression and compression—Shear. Mater. Sci. Eng. A 2015, 643, 194–202. [Google Scholar] [CrossRef]
- Chen, I.W.; Chiao, Y.H. Statistics of martensitic nucleation. Acta Met. 1985, 33, 1827–1859. [Google Scholar] [CrossRef]
- Shi, X.B.; Hu, Z.C.; Hu, X.W.; Zhang, J.S.; Cui, L.S. Effect of plastic deformation on stress-induced martensitic transformation of nanocrystalline NiTi alloy. Mater. Charact. 2017, 128, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Waitz, T.; Kazykhanov, V.; Karnthaler, H.P. Martensitic phase transformations in nanocrystalline NiTi studied by TEM. Acta Mater. 2004, 52, 137–147. [Google Scholar] [CrossRef]
- Waitz, T. The self-accommodated morphology of martensite in nanocrystalline NiTi shape memory alloys. Acta Mater. 2005, 53, 2273–2283. [Google Scholar] [CrossRef]
- Yu, C.; Kang, G.; Xie, X.; Rao, W. A micromechanical model for the grain size dependent super-elasticity degeneration of NiTi shape memory alloys. Mech. Mater. 2018, 125, 35–51. [Google Scholar] [CrossRef]
- Upadhyay, K.; Subhash, G.; Spearot, D. Visco-hyperelastic constitutive modeling of strain rate sensitive soft materials. J. Mech. Phys. Solids 2020, 135, 103777. [Google Scholar] [CrossRef]
- De Tommasi, D.; Puglisi, G.; Saccomandi, G. A micromechanics-based model for the Mullins effect. J. Rheol. 2006, 50, 495. [Google Scholar] [CrossRef]
- D’Ambrosio, P.; De Tommasi, D.; Ferri, D.; Puglisi, G. A phenomenological model for healing and hysteresis in rubber-like materials. Int. J. Eng. Sci. 2008, 46, 293–305. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Mao, C. Comparative study of two types of self-recovery shape-memory alloy pseudo-rubber isolator devices under compression—Shear interactions. J. Intell. Mater. Syst. Struct. 2019, 30, 2241–2256. [Google Scholar] [CrossRef]






| Type of Tissue | Skin | Tendon | Muscle |
|---|---|---|---|
| Tensile strength, MPa | 1.4 | 0.4 | 0.2 |
| Fracture region, % | 27–33 | 17–92 | 27–45 |
| Middle transitional region of nonlinear deformation, % | 9–12/7–8/12–13 | 6–9/6–9/15–17 | 13–17 |
| Maximum stress during cyclic loading, MPa | 0.95 | 0.27 | 0.08 |
| Minimum stress during cyclic unloading, MPa | 0.01 | 0.01 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasenchuk, Y.F.; Marchenko, E.S.; Gunter, S.V.; Baigonakova, G.A.; Kokorev, O.V.; Volinsky, A.A.; Topolnitsky, E.B. Softening Effects in Biological Tissues and NiTi Knitwear during Cyclic Loading. Materials 2021, 14, 6256. https://doi.org/10.3390/ma14216256
Yasenchuk YF, Marchenko ES, Gunter SV, Baigonakova GA, Kokorev OV, Volinsky AA, Topolnitsky EB. Softening Effects in Biological Tissues and NiTi Knitwear during Cyclic Loading. Materials. 2021; 14(21):6256. https://doi.org/10.3390/ma14216256
Chicago/Turabian StyleYasenchuk, Yuri F., Ekaterina S. Marchenko, Sergey V. Gunter, Gulsharat A. Baigonakova, Oleg V. Kokorev, Alex A. Volinsky, and Evgeny B. Topolnitsky. 2021. "Softening Effects in Biological Tissues and NiTi Knitwear during Cyclic Loading" Materials 14, no. 21: 6256. https://doi.org/10.3390/ma14216256
APA StyleYasenchuk, Y. F., Marchenko, E. S., Gunter, S. V., Baigonakova, G. A., Kokorev, O. V., Volinsky, A. A., & Topolnitsky, E. B. (2021). Softening Effects in Biological Tissues and NiTi Knitwear during Cyclic Loading. Materials, 14(21), 6256. https://doi.org/10.3390/ma14216256








