Influence of Fuel Level on Properties, Productivity, and Mineralogy of Russian Vanadiferous Titanomagnetite Sinter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
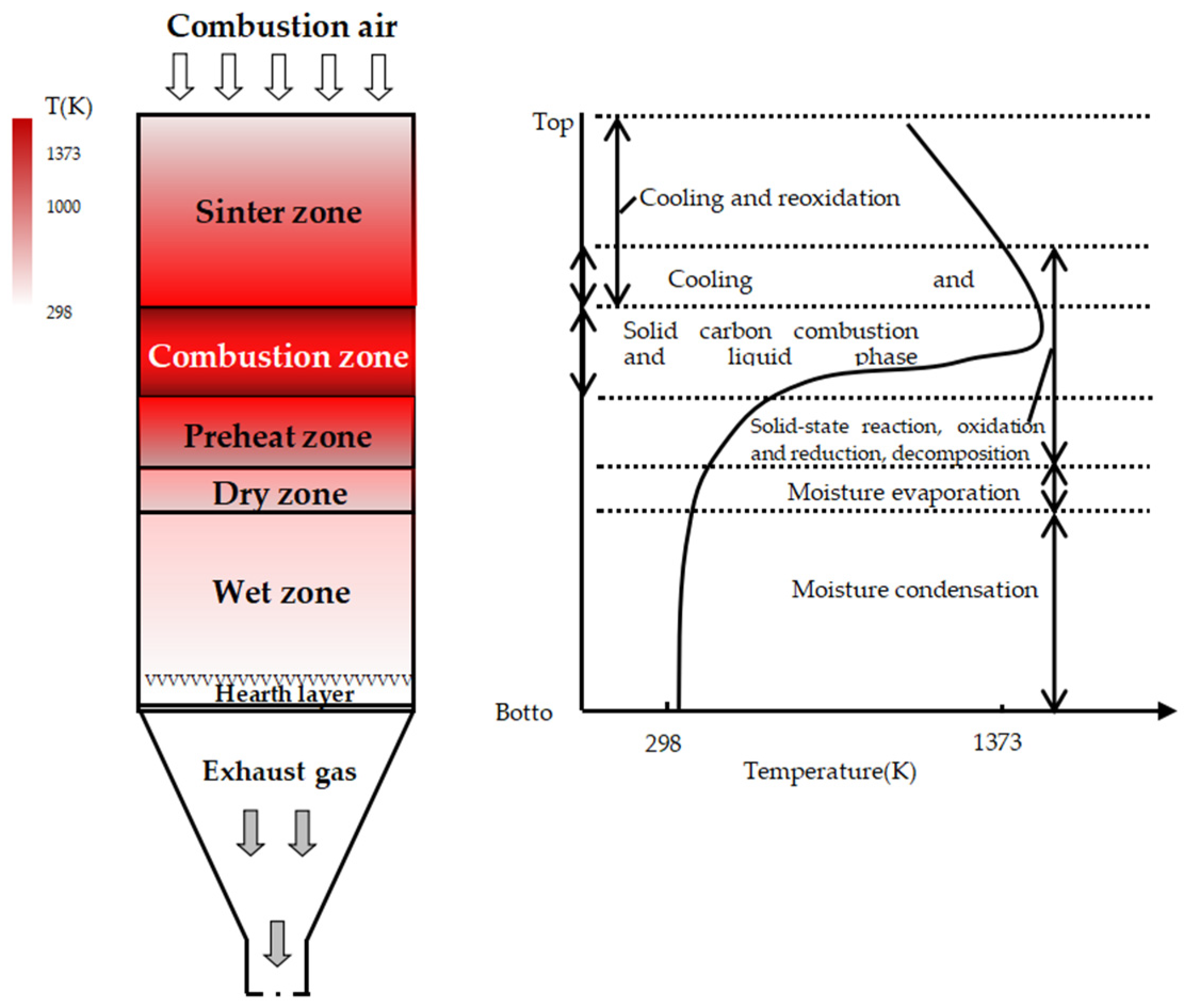
2.2. Experimental Procedure
2.2.1. Schemes of Sintering Pot Tests at Different Fuel Levels
2.2.2. Sintering Pot Tests
2.2.3. Mineralogical Investigations
3. Results
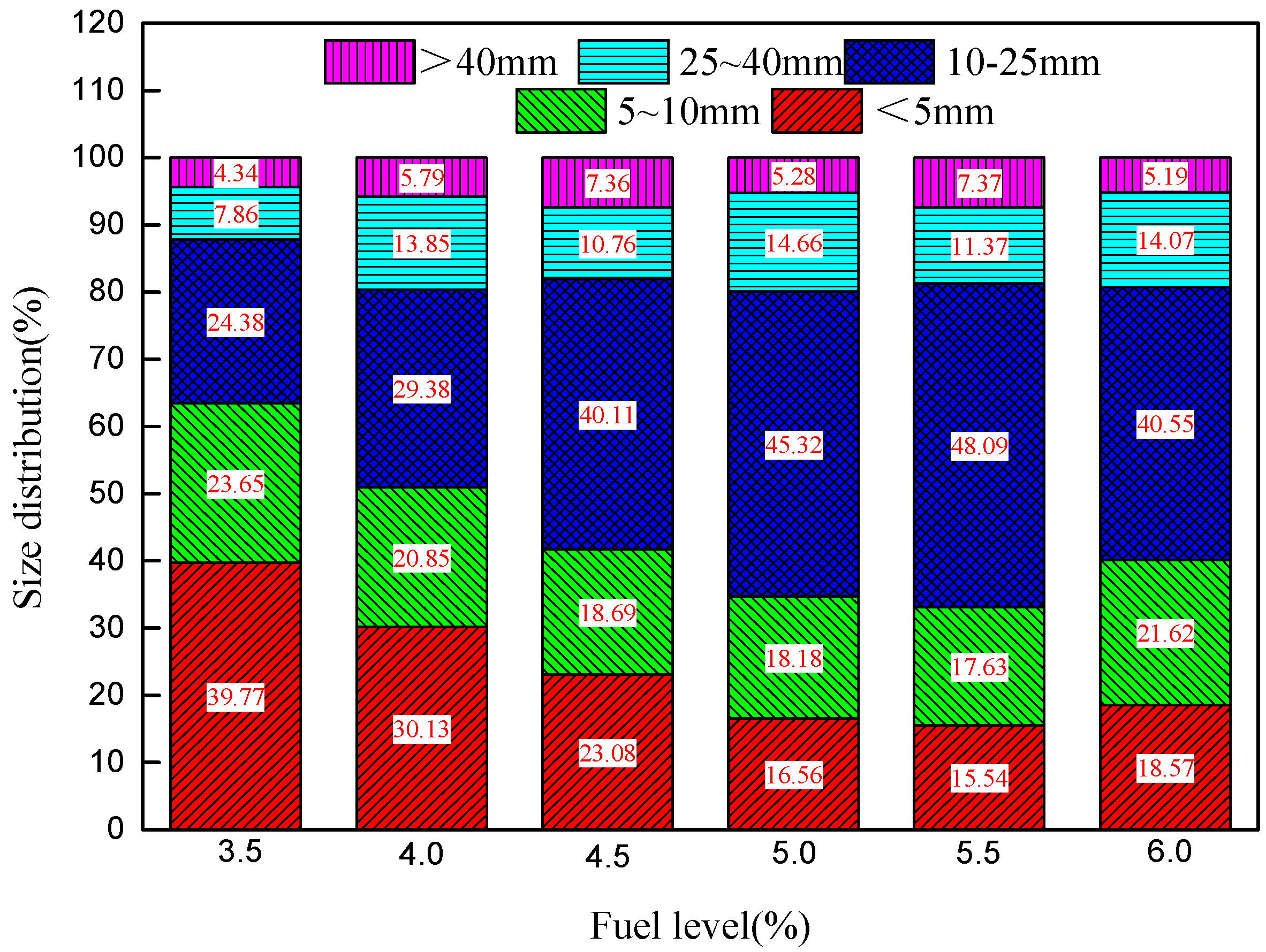
3.1. Influence of Fuel Level on Particle Size Distribution
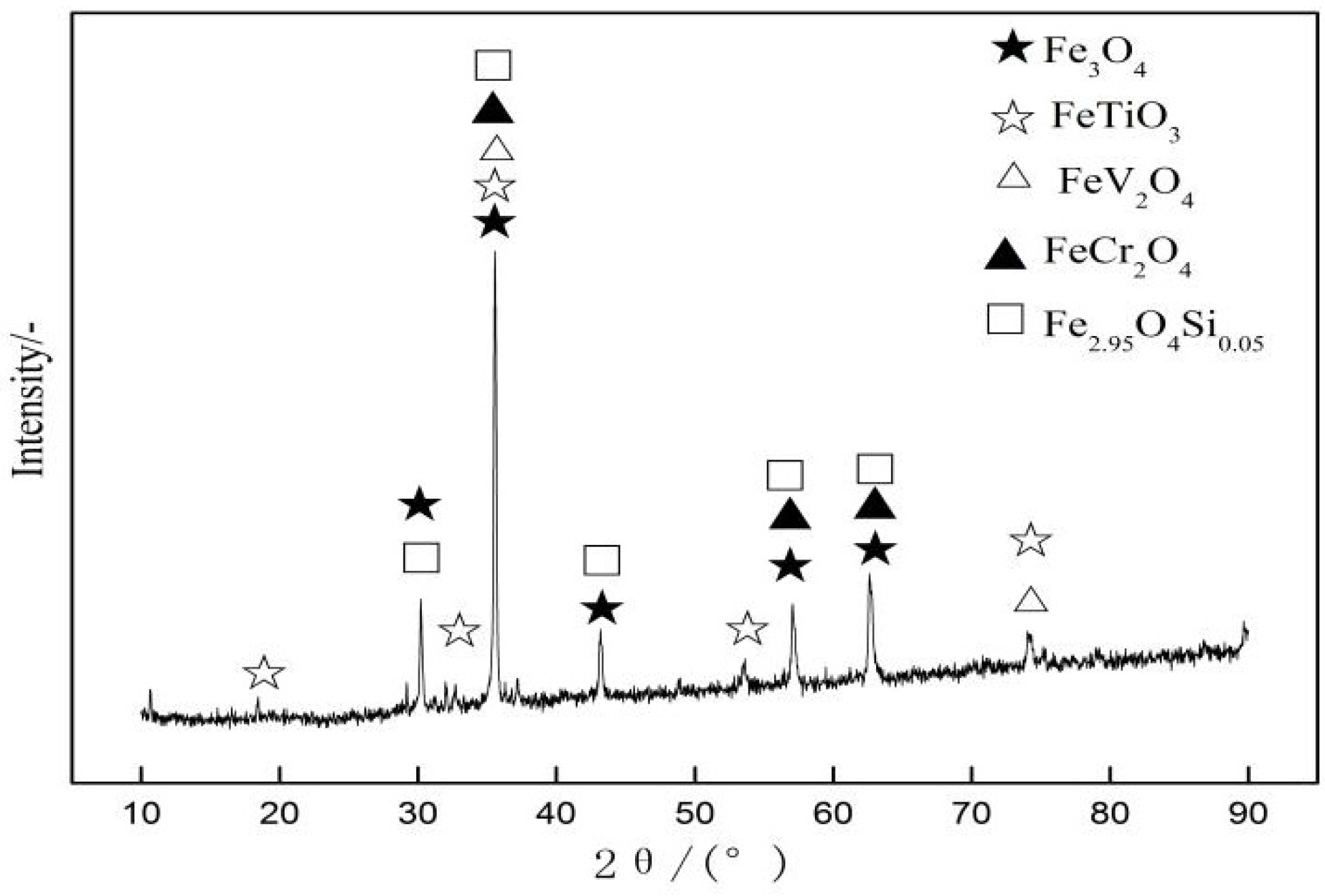
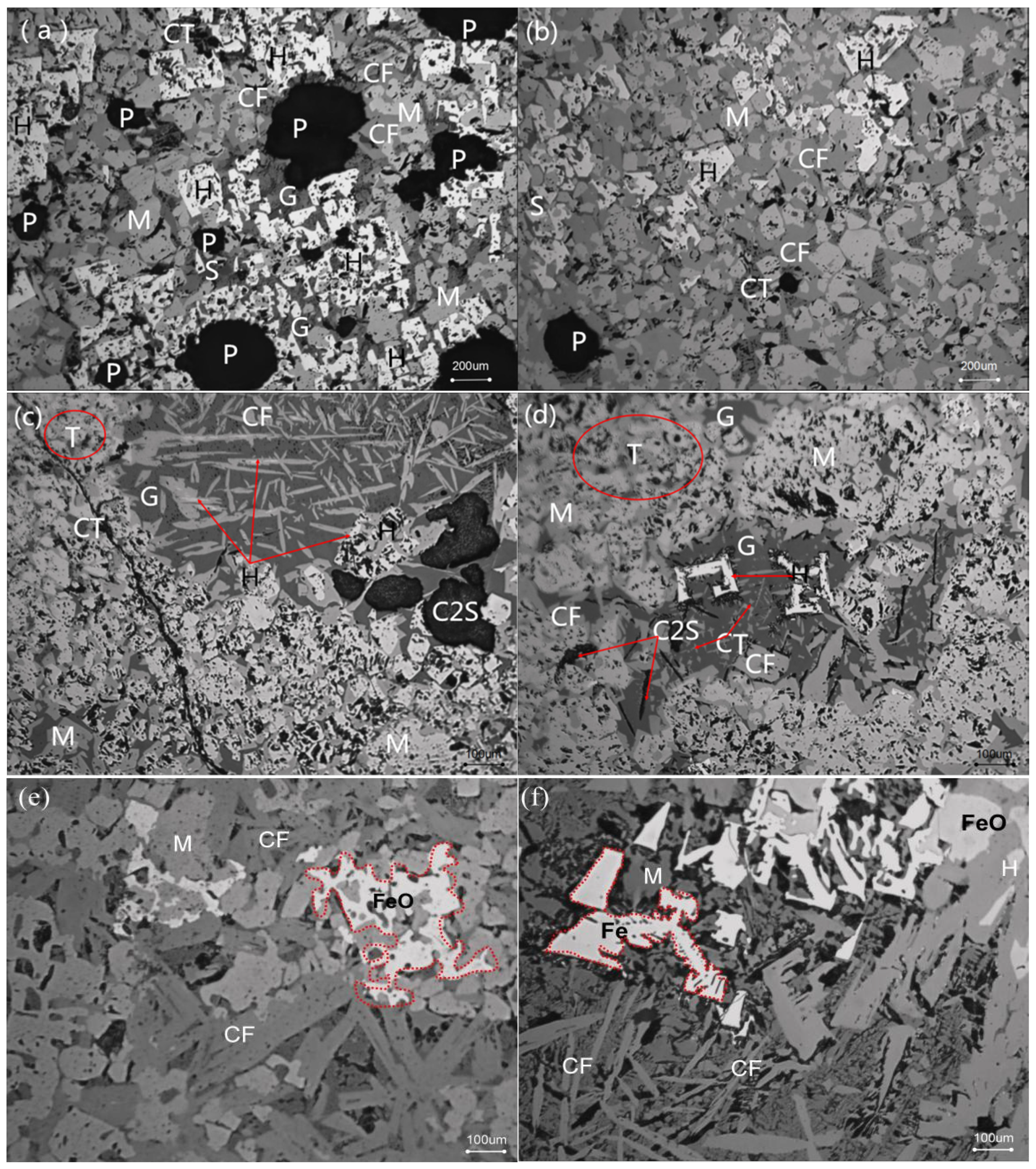
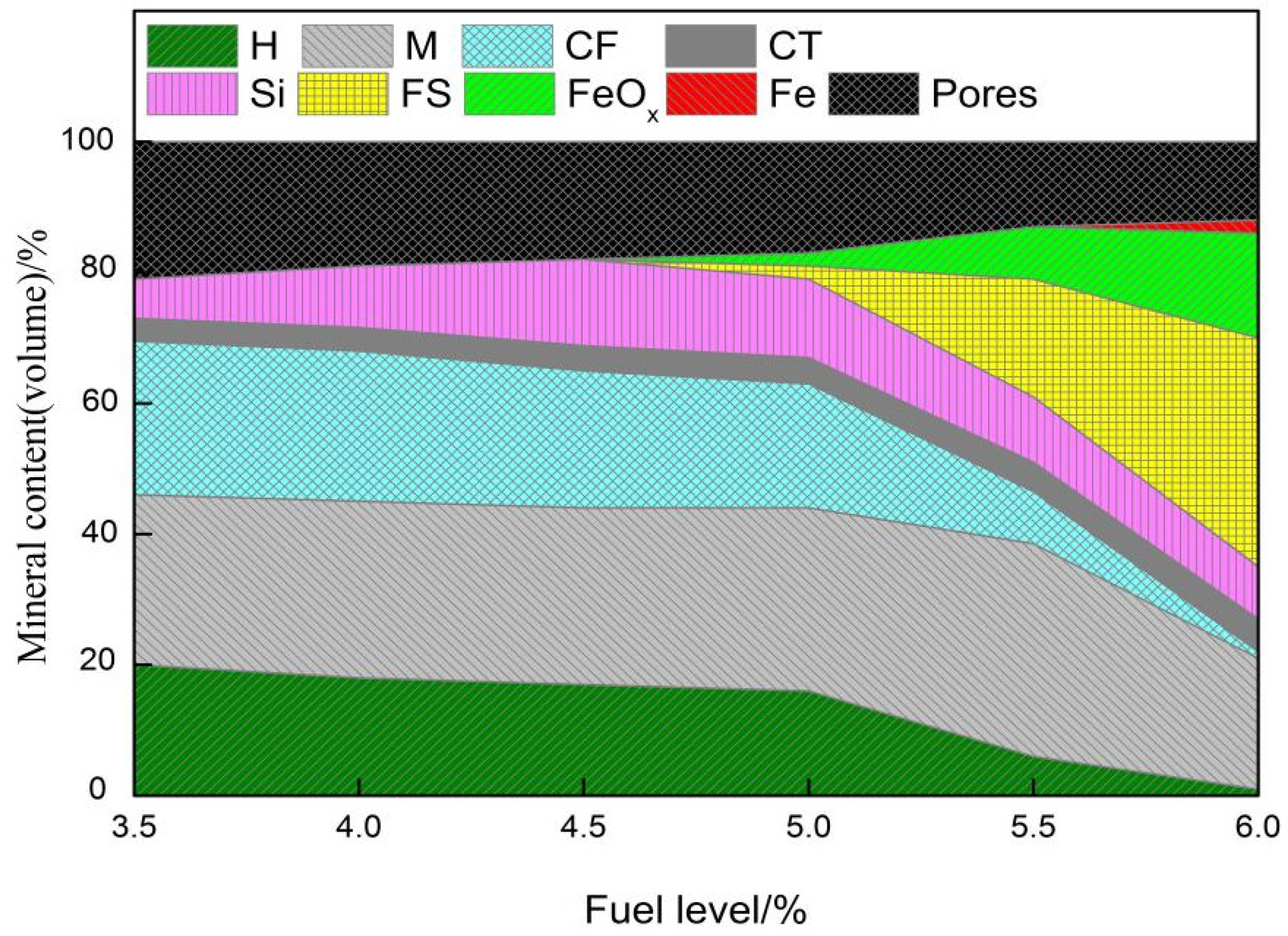
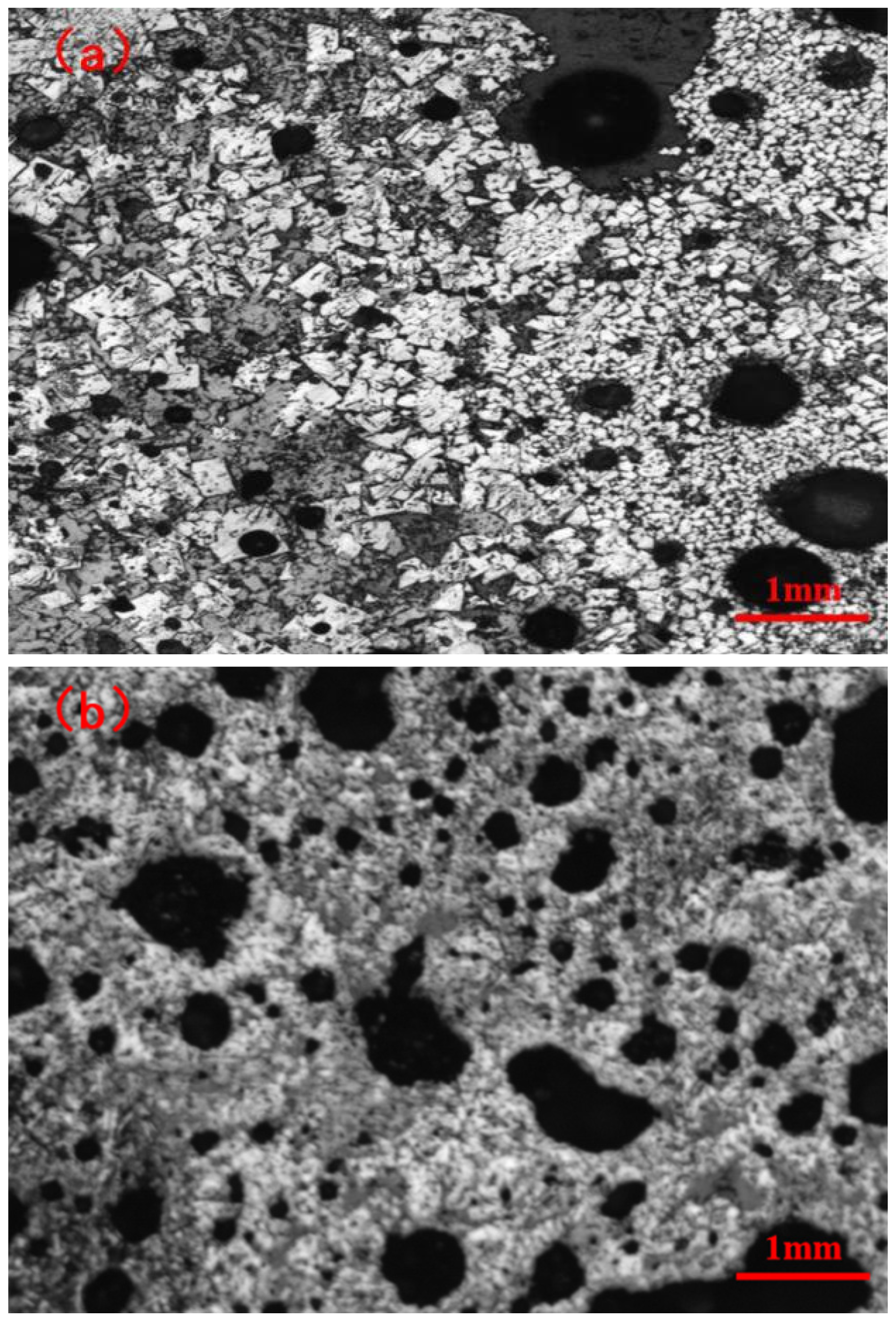
3.2. Influence of Fuel Level on Mineralogy and Microstructure
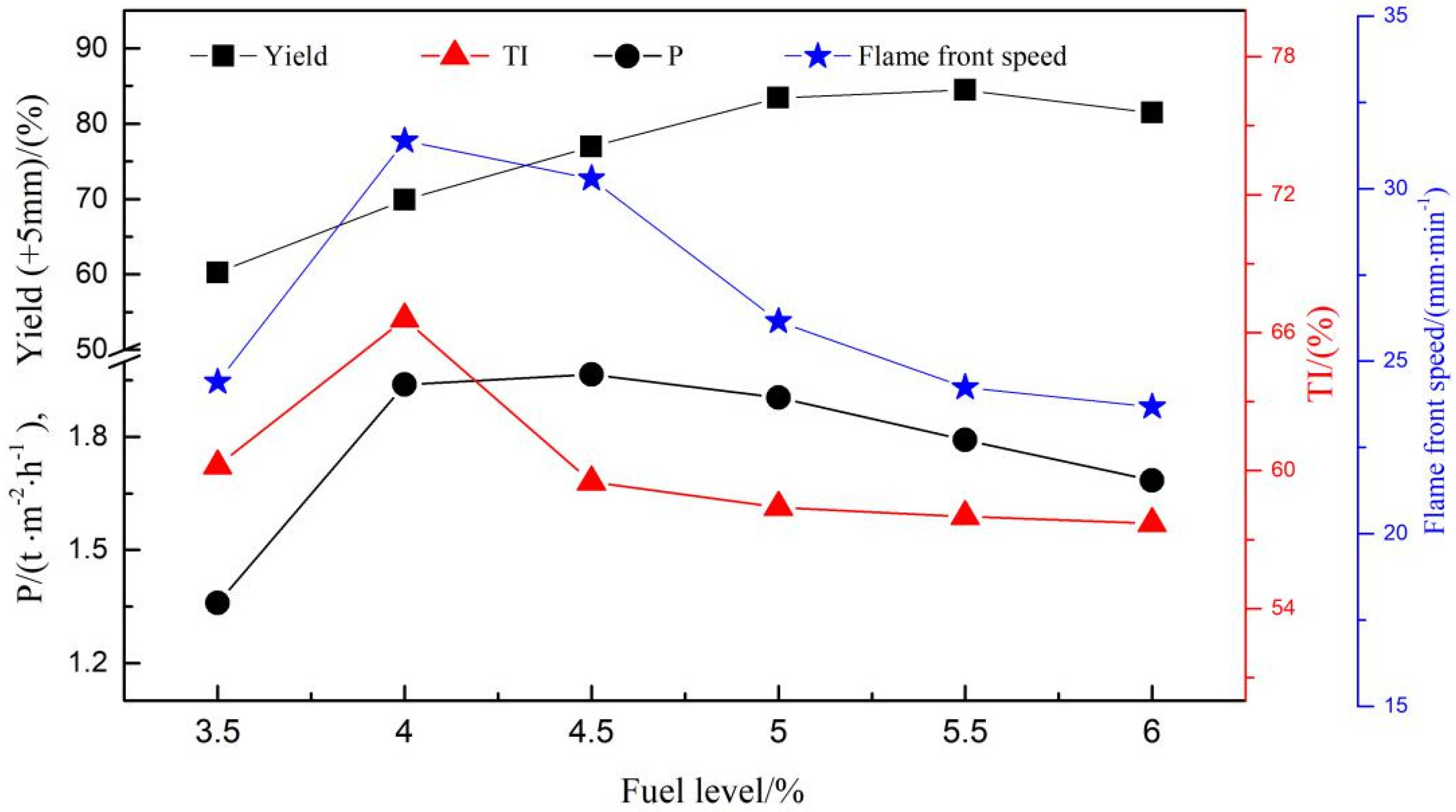
3.3. Operation Parameters of Sintering Process
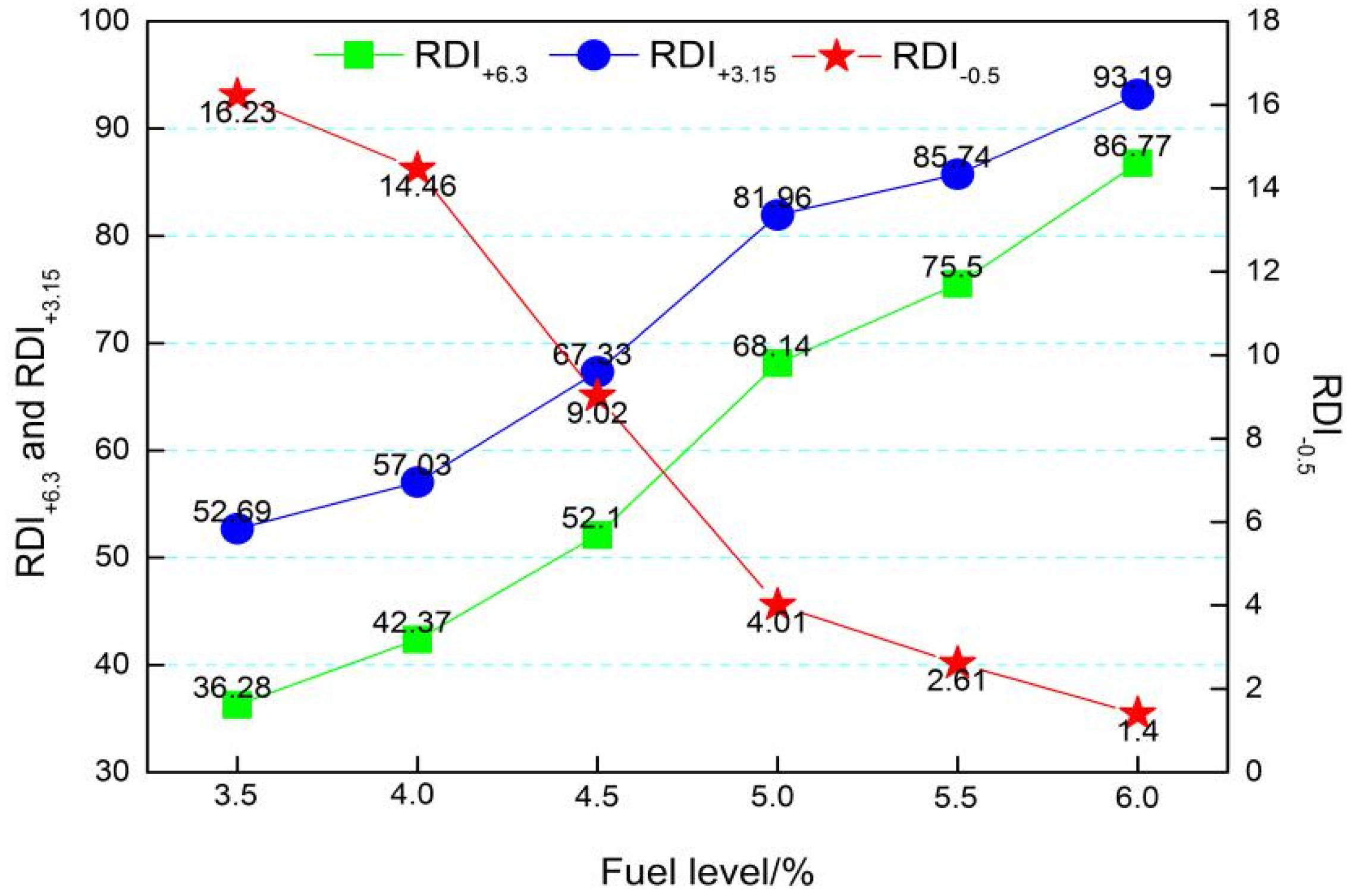
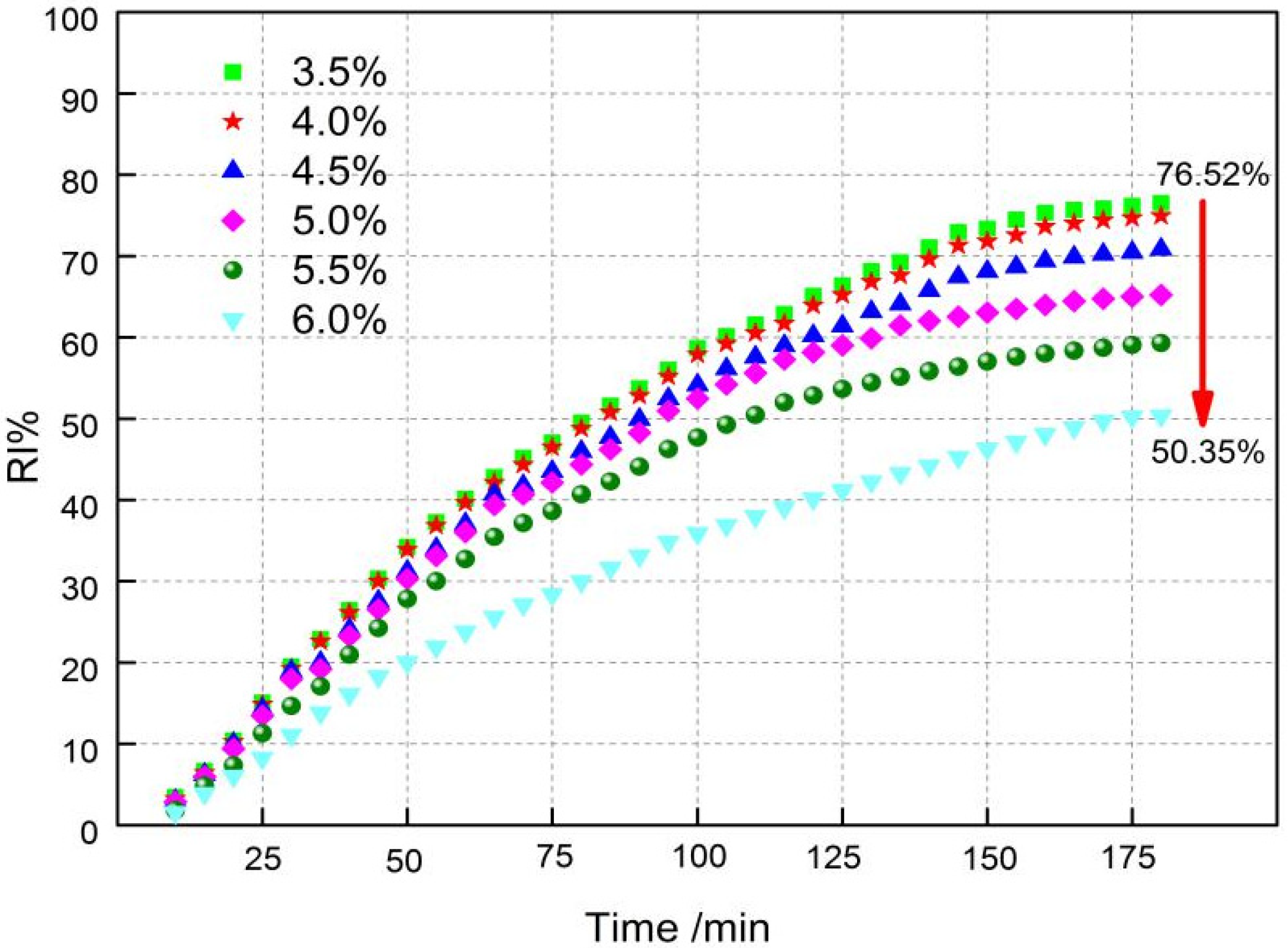
3.4. Effects of Fuel Level Onmetallurgical Properties
3.5. Comprehensive Index
4. Discussion
5. Conclusions
- Changes in temperature and atmosphere in the combustion zone caused by fuel combustion in turn affect the sintering process operation parameters. By increasing the fuel level from 3.5% to 4.0%, the flame front speed, TI, yield and productivity increase and then decrease, with the highest flame front speed and TI at 4% fuel level, the highest yield at 5.5% fuel level, and the highest productivity at 4.5% fuel level.
- The different fuel levels in the sinter mix affect the type and quantity of minerals in the sinter, as well as the microstructure of the minerals and thus the metallurgical properties of the sinter. With the increase in fuel level (3.5−6.0%), RDI+3.15 increased from 52.69% to 93.19%, RI decreased from 76.52% to 50.35%, the softening zone shifted, and the softening zone ΔT widened from 108.5 °C to 114 °C.
- With the increase in fuel level (3.5−6.0%), the contents of FeO, SiO2, and MgO increased, while TiO2 had a drop. Meanwhile, the number of pores and calcium ferrite and hematite decreased but had an increase in silicate (glassy, dicalcium silicate, olivine, etc.). In addition, in the fuel level ranged from 3.5% to 5.5% and magnetite content increased as fuel level gradually increased, but then had a drop. Moreover, when the fuel level increased to greater than 5.0%, FeOx and fayalite increased quickly and a small amount of metallic iron appeared under the fuel level of 6.0%.
- The comprehensive index of the effect of fuel level on RVT sinter properties and productivity was evaluated through the comprehensive index method, combined with the indicators and significance coefficient of PANGGANG. The optimal fuel level was determined to be 4.0% under current production conditions and indicators for the highest value of the comprehensive index.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhou, M.; Chu, M.S.; Xue, X.X. Sintering test of high-chromium vanadium-titanium magnetite. J. Northeast. Univ. Nat. Sci. Ed. 2013, 3, 383–387. [Google Scholar]
- Zhou, M.; Jiang, T.; Yang, S.T.; Xue, X.X. Vanadium–Titanium magnetite ore blend optimization for sinter strength based on iron ore basic sintering characteristics. Int. J. Miner. Process. 2015, 142, 125–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Chu, M.S.; Xue, X.X.; Jiang, M.F. Sintering basic characteristics of imported high-chromium vanadium-titanium magnetite. Iron Steel 2012, 12, 23–28. [Google Scholar]
- Takano, C.; Zambrano, A.P.; Nogueira, A.E.A.; Mourao, M.B.; Iguchi, Y. Chromites reduction reaction mechanisms in carbon-chromites composite agglomerates at 1773K. ISIJ Int. 2007, 47, 1585–1589. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.Y.; Zou, X.L.; Lu, X.G.; Xie, X.L.; Zheng, K.; Xiao, W.; Cheng, H.W.; Li, G.S. Reductive kinetics of panzhihua ilmenite with hydrogen. Trans. Nonferrous Met. Soc. 2016, 26, 3266–3273. [Google Scholar] [CrossRef]
- Cheng, G.J.; Xue, X.X.; Gao, Z.X.; Jiang, T.; Yang, H.; Duan, P.N. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets. ISIJ Int. 2016, 56, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Jena, B.C.; Dresler, W.; Reilly, I.G. Extraction of titanium, vanadium and iron from titanomagnetite deposits at pipestone lake, manitoba, canada. Miner. Eng. 1995, 8, 159–168. [Google Scholar] [CrossRef]
- Cheng, G.J.; Gao, Z.X.; Yang, H.; Xue, X.X. Effect of calcium oxide on the crushing strength, reduction, and smelting performance of high-chromium vanadium–titanium magnetite pellets. Metals 2017, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Zhou, M.; Jiang, T.; Xue, X.X.; Sun, C.Y. Isothermal reduction kinetics of chromium-bearing vanadium–titanium sinter reduced with CO gas at 1173 K. JOM-US 2019, 71, 2812–2820. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Jiang, T.; Xue, X.X. Isothermal reduction kinetics and mineral phase of chromium-bearing vanadium–titanium sinter reduced with CO gas at 873–1273 K. Int. J. Min. Met. Mater. 2018, 25, 145–152. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, T.; Yang, S.T.; Ma, K.; Xue, X.X. Optimization utilization of vanadium-titanium iron ore in sintering based on orthogonal method. Metalurgija 2016, 55, 581–584. [Google Scholar]
- Zhou, M.; Yang, S.T.; Jiang, T.; Xue, X.X. Influence of mgo in form of magnesite on properties and mineralogy of high chromium, vanadium, titanium magnetite sinters. Ironmak. Steelmak. 2017, 42, 217–225. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Jiang, T.; Wang, Y.J.; Xue, X.X. Study on Sintering Characteristics of Ultra-Poor Vanadium-Titanium Magnetite. Minerals 2021, 11, 515. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Tang, W.D.; Jiang, T.; Xue, X.X.; Zhang, W.J. Influence of coke ratio on the sintering behavior of high-chromium vanadium–titanium magnetite. Minerals 2017, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Tang, W.D.; Zhou, M.; Jiang, T.; Xue, X.X.; Zhang, W.J. Effects of dolomite on mineral compositions and metallurgical properties of chromium-bearing vanadium–titanium magnetite sinter. Minerals 2017, 7, 210. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.T.; Zhou, M.; Jiang, T.; Wang, Y.J.; Xue, X.X. Effect of basicity on sintering behavior of low-titanium vanadium–titanium magnetite. Trans. Nonferrous Met. Soc. 2015, 25, 2087–2094. [Google Scholar] [CrossRef]
- Glazer, A.M. The Classification of tilted octahedral in perovskites. Struct. Sci. 1972, 28, 3384–3392. [Google Scholar]
- Glazer, A.M. The Simple ways of determining perovskite Strueture. Found. Crystallogr. 1975, 31, 756–762. [Google Scholar]
- Umadevi, T.; Nelson, K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of magnesia on iron ore sinter properties and productivity. Ironmak. Steelmak. 2009, 36, 515–520. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, J.L.; Wu, L.S.; Su, B.X.; Xing, X.D.; Zhu, G.Y. Effect of TiO2 on equilibrium phase sinter at oxygen partial pressure of 5 × 1003 atm. Ironmak. Steelmak. 2013, 41, 132–137. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Dwarapudi, S.; Ranjan, M. Influence of oxide and silicate melt phases on the RDI of iron ore pellets suitable for shaft furnace of direct reduction process. ISIJ Int. 2010, 50, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Guo, X.M.; Huang, X.J. Effects of temperature and atmosphere on sintering process of iron ores. J. Iron Steel Res. Int. 2012, 19, 1–6. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Effect of oxygen partial pressure on the formation mechanisms of complex Ca-rich ferrites. ISIJ Int. 2013, 53, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.S.; Ren, G.; Sun, Y.Q.; Lv, Q. The effect of carbon content on the mineral composition and microstructure of Chenggang’s vanadium-titanium sinter. Iron Steel 2010, 10, 13–17. [Google Scholar] [CrossRef]
- Fan, X.H.; Yu, Z.Y.; Gan, M.; Li, W.Q.; Ji, Z.Y. Influence of O2 content in circulating flue gas on iron ore sintering. J. Iron Steel Res. Int. 2013, 20, 1–6. [Google Scholar] [CrossRef]
- Jiang, D.J.; He, M.G.; Gan, Q.; He, Q. The effect of feo on the properties of sinter under high alkalinity conditions. China Metall. 2008, 18, 14–21. [Google Scholar]
- Lovel, R.R.; Vining, K.R.; Amico, M.D. The influence of fuel reactivity on iron ore sintering. ISIJ Int. 2009, 49, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Choi, S.; Choi, E.S.; Ri, D.W.; Kim, S. Combustion characteristics in an iron ore sintering bed—Evaluation of fuel substitution. Combust. Flame. 2006, 145, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, T.; Roy, A.K.; Mahapatra, P.C.; Prabhu, M.; Ra, N.M. Influence of magnesia on iron ore sinter properties and productivity—Use of dolomite and dunite. Steel Res. Int. 2009, 80, 800–807. [Google Scholar]
- Panigrahy, S.C.; Verstraeten, P.; Dilewijns, J. Influence of mgo addition on mineralogy of iron ore sinter. Metall. Mater. Trans. B 1984, 15, 23–32. [Google Scholar] [CrossRef]



| Raw Materials | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | TiO2 | V2O5 | Cr2O3 | H2O |
|---|---|---|---|---|---|---|---|---|---|---|
| RVT ore | 61.54 | 28.51 | 0.32 | 2.37 | 1.21 | 2.89 | 5.17 | 1.01 | 0.50 | 1.01 |
| Magnetite A | 62.87 | 25.79 | 0.46 | 5.16 | 1.03 | 3.40 | 0.27 | |||
| Magnetite B | 64.38 | 26.86 | 0.05 | 5.38 | 0.18 | 3.64 | 0 | |||
| Magnetite C | 61.85 | 23.11 | 1.36 | 3.36 | 3.44 | 2.45 | 0 | |||
| Shaft furnace dust | 62.47 | 0.33 | 8.46 | 0.61 | 0.93 | 0 | ||||
| Magnesite | 0 | 1.21 | 3.55 | 42.24 | 0 | 2.01 | ||||
| Quicklime | 0 | 80.1 | 5.02 | 1.10 | 0 | 0 |
| Fixed Carbon | Total Sulfur | Volatile | Ash (14.00) | ∑ | |||||
|---|---|---|---|---|---|---|---|---|---|
| FeO | CaO | SiO2 | MgO | Al2O3 | Others | ||||
| 84.98 | 0.48 | 1.49 | 0.14 | 0.5 | 7.52 | 0.14 | 2.77 | 2.93 | 100.00 |
| Item | Parameter | Item | Parameter | Item | Parameter |
|---|---|---|---|---|---|
| Sinter bed depth | 500 mm | Sintering pot diameter | 150 mm | Granulation time | 12 min |
| Ignition vacuum | 5.0 kPa | Sintering vacuum | 10.0 kPa | Ignition time | 2 min |
| Ignition temperature | 1100 °C | Moisture | 7.5 ± 0.3% | Fuel level in blend mix | 3.5–6.0% |
| Return fines content | 14.0% | Height of hearth layer | 20 mm | Basicity(R = CaO/SiO2) | 2.25 |
| Item | TFe | FeO | CaO | SiO2 | MgO | Al2O3 | TiO2 | V2O5 | Cr2O3 |
|---|---|---|---|---|---|---|---|---|---|
| NO.1 Fuel-3.5% | 54.46 | 6.86 | 12.10 | 5.37 | 1.65 | 2.96 | 1.47 | 0.279 | 0.108 |
| NO.2 Fuel-4.0% | 54.71 | 8.46 | 12.62 | 5.71 | 1.66 | 3.01 | 1.49 | 0.269 | 0.115 |
| NO.3 Fuel-4.5% | 54.85 | 9.26 | 12.08 | 5.77 | 1.65 | 3.01 | 1.48 | 0.275 | 0.114 |
| NO.4 Fuel-5.0% | 54.61 | 10.56 | 12.23 | 5.79 | 1.68 | 2.97 | 1.44 | 0.285 | 0.110 |
| NO.5 Fuel-5.5% | 55.23 | 12.26 | 12.50 | 5.88 | 1.69 | 3.01 | 1.42 | 0.264 | 0.102 |
| NO.6 Fuel-6.0% | 54.89 | 18.14 | 12.42 | 5.81 | 1.72 | 3.02 | 1.42 | 0.273 | 0.104 |
| Items | Zi1 | Zi2 | Zi3 | Zi4 | Zi5 | ||
|---|---|---|---|---|---|---|---|
| NO.1 Fuel = 3.5% | 1.359 | 60.2 | 52.69 | 76.52 | 3.5 | 296.60 | 100.00 |
| NO.2 Fuel = 4.0% | 1.939 | 66.6 | 57.03 | 74.95 | 4.0 | 335.55 | 138.95 |
| NO.3 Fuel = 4.5% | 1.965 | 59.5 | 67.33 | 70.84 | 4.5 | 312.21 | 115.61 |
| NO.4 Fuel = 5.0% | 1.904 | 58.4 | 81.96 | 65.23 | 5.0 | 307.51 | 110.91 |
| NO.5 Fuel = 5.5% | 1.792 | 58.0 | 85.74 | 59.32 | 5.5 | 297.94 | 101.34 |
| NO.6 Fuel = 6.0% | 1.684 | 57.7 | 93.19 | 50.35 | 6.0 | 288.91 | 92.31 |
| 0.606 | 8.9 | 40.5 | 26.17 | 2.5 | Result: NO.2 > NO.3 > NO.4 > NO.5 > NO.1 > NO.6 | ||
| 20 | 30 | 20 | 15 | 15 | |||
| 33.00 | 3.37 | 0.494 | 0.573 | 6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Men, J.; Yang, S.; Zhou, M. Influence of Fuel Level on Properties, Productivity, and Mineralogy of Russian Vanadiferous Titanomagnetite Sinter. Materials 2021, 14, 6258. https://doi.org/10.3390/ma14216258
Li J, Men J, Yang S, Zhou M. Influence of Fuel Level on Properties, Productivity, and Mineralogy of Russian Vanadiferous Titanomagnetite Sinter. Materials. 2021; 14(21):6258. https://doi.org/10.3390/ma14216258
Chicago/Turabian StyleLi, Jiahao, Jingwei Men, Songtao Yang, and Mi Zhou. 2021. "Influence of Fuel Level on Properties, Productivity, and Mineralogy of Russian Vanadiferous Titanomagnetite Sinter" Materials 14, no. 21: 6258. https://doi.org/10.3390/ma14216258





