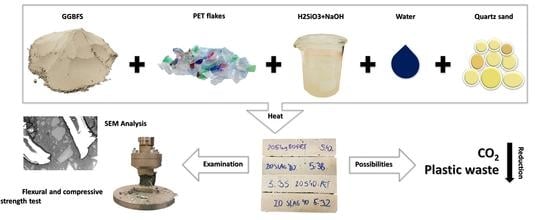
Strength Characteristics of Alkali-Activated Slag Mortars with the Addition of PET Flakes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
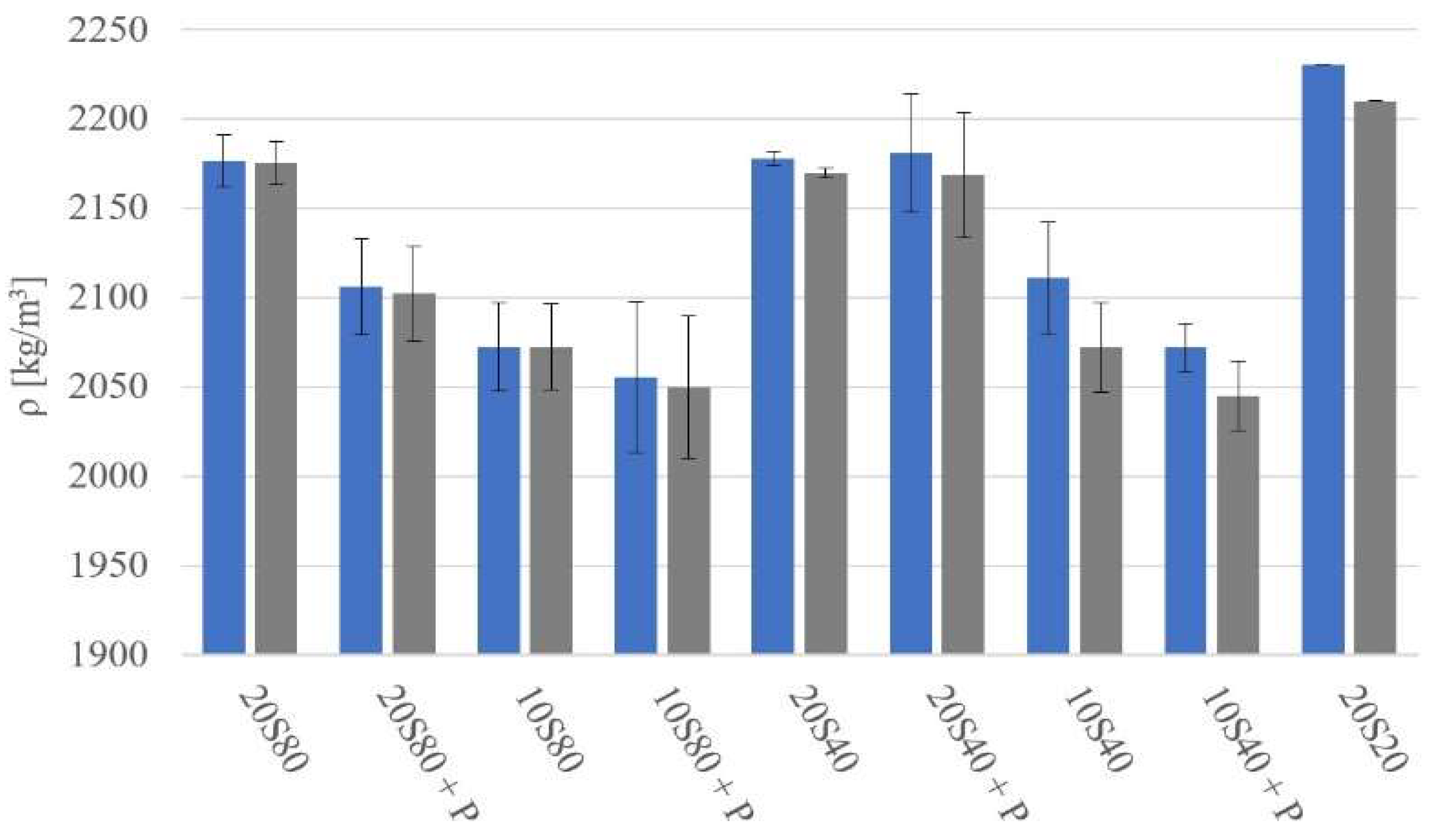
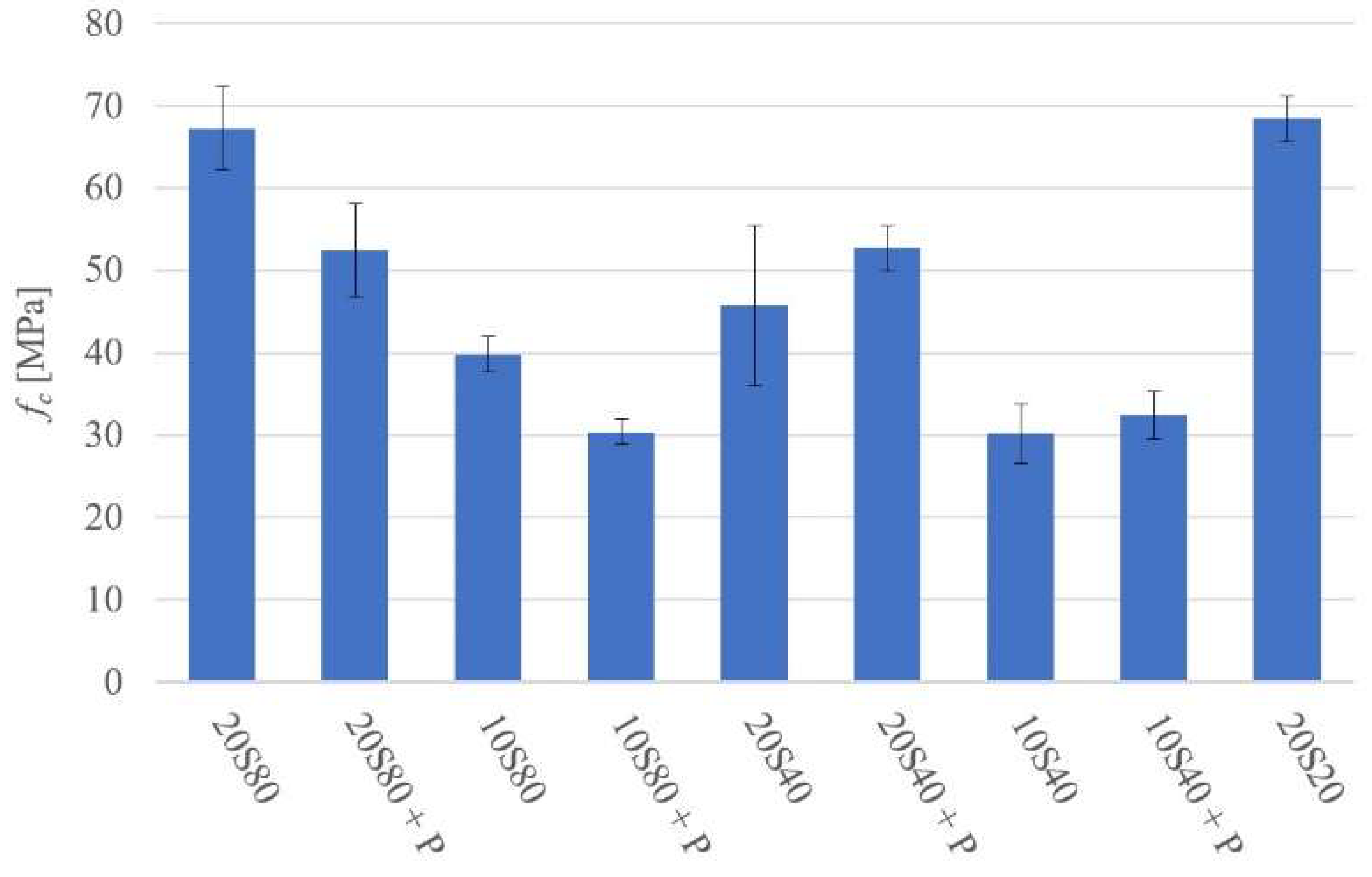
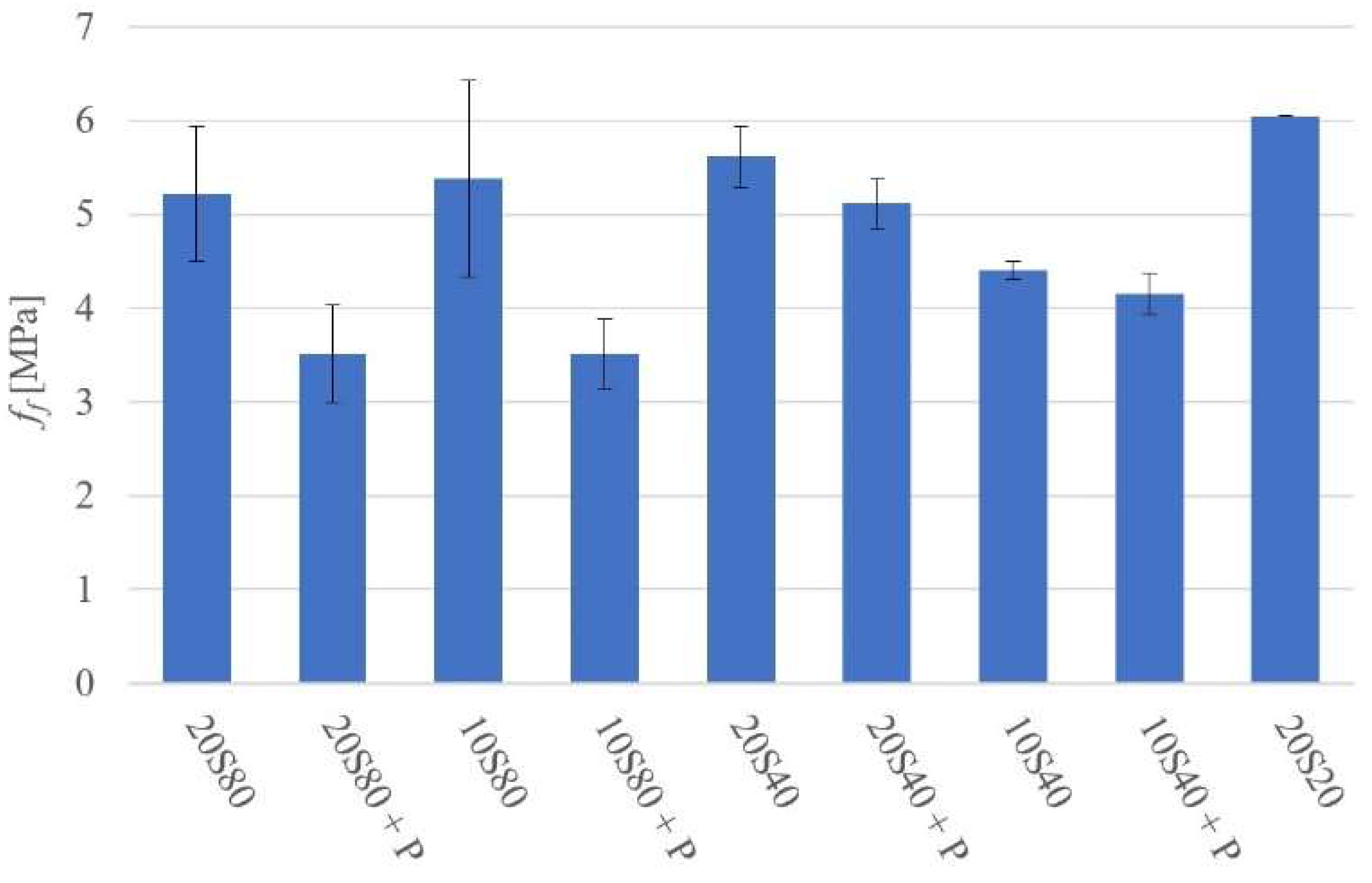
- Curing at 20 °C and 80 °C produced matrixes with higher density in comparison to mortars cured at higher temperatures; it also resulted in higher compressive strength values.
- Curing at 40 °C produced matrixes with lower strength values.
- All tested mortars could be assigned an ordinary concrete strength class ranging from C20/25 to C50/60.
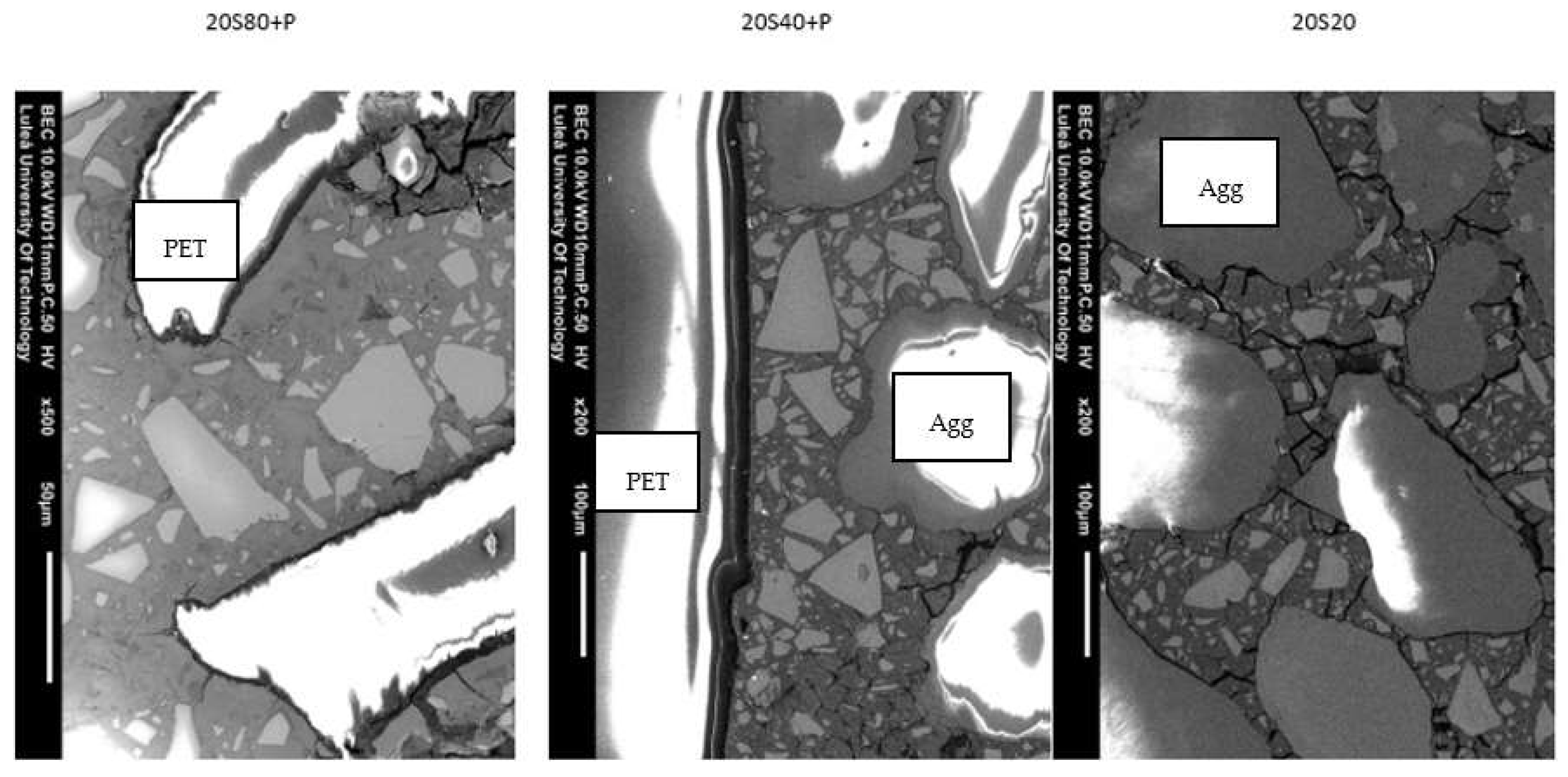
- The addition of PET generally lowered both the compressive and flexural strength values and introduced an interfacial transition zone with a presumably higher water-to-cement ratio, more portlandite, and less anhydrous GGBFS.
- Matrixes cured at 80 °C showed more microcracking.
- The PET particles had no effect on the hydration processes, mainly due to their chemical inactivity and large size, which excluded possible nucleation sites for the hydration to occur.
- The PET had a very weak bond with the binder matrix, leading to pull-out under tension.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef] [Green Version]
- USGS. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020; ISBN 9781411343627.
- Klugman, S.; Stripple, H.; Lönnqvist, T.; Sandberg, E.; Krook-Riekkola, A. A Climate Neutral Swedish Industry—An Inventory of Technologies; IVL Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2019; ISBN 9789178831319. [Google Scholar]
- GUS. Produkcja Wyrobów Przemysłowych w 2019 r; 2019. Available online: https://stat.gov.pl/obszary-tematyczne/przemysl-budownictwo-srodki-trwale/przemysl/produkcja-wyrobow-przemyslowych-w-2019-roku,3,17.html (accessed on 12 July 2020).
- GUS Ochrona Środowiska 2018. 2018; 219. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-w-2018-roku,12,1.html (accessed on 12 July 2020).
- Bumanis, G.; Vitola, L.; Pundiene, I.; Sinka, M.; Bajare, D. Gypsum, Geopolymers, and Starch-Alternative Binders for Bio-Based Building Materials: A Review and Life-Cycle Assessment. Sustainability 2020, 12, 5666. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J.; Bernal, S.A. Alkali-Activated Materials; Springer: Berlin/Heidelberg, Germany, 2014; Volume 13, ISBN 9789400776715. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Binder Chemistry—Blended Systems and Intermediate Ca Content; Springer: Dordrecht, The Netherlands, 2014; Volume 13, ISBN 9789400776715. [Google Scholar]
- Ballekere Kumarappa, D.; Peethamparan, S.; Ngami, M. Autogenous Shrinkage of Alkali Activated Slag Mortars: Basic Mechanisms and Mitigation Methods. Cem. Concr. Res. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, Y.; Zheng, W.; Huang, W. Effect of the Activator on the Performance of Alkali-Activated Slag Mortars with Pottery Sand as Fine Aggregate. Constr. Build. Mater. 2019, 197, 83–90. [Google Scholar] [CrossRef]
- Alasady, A.; Provis, J.; Cwirzen, A. Alkali-Activation of a High MgO GGBS—Fresh and Hardened Properties. Mag. Concr. Res. 2017, 70, 1–24. [Google Scholar] [CrossRef]
- Humad, A.M.; Provis, J.L.; Cwirzen, A. Effects of Curing Conditions on Shrinkage of Alkali-Activated High-MgO Swedish Slag Concrete. Front. Mater. 2019, 6, 287. [Google Scholar] [CrossRef]
- Patel, Y.J.; Shah, N. Development of Self-Compacting Geopolymer Concrete as a Sustainable Construction Material. Sustain. Environ. Res. 2018, 28, 412–421. [Google Scholar] [CrossRef]
- Çelikten, S.; Sarıdemir, M.; Özgür Deneme, İ. Mechanical and Microstructural Properties of Alkali-Activated Slag and Slag + fly Ash Mortars Exposed to High Temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the Properties of Geopolymer Mortar and Concrete with Mineral Admixtures: A Review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si + Ca) and Metakaolin (Si + Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Ou, Z. Effect of Alkali Dosage on Alkali-Silica Reaction in Sodium Hydroxide Activated Slag Mortars. Constr. Build. Mater. 2017, 143, 16–23. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Batayneh, M.; Marie, I.; Asi, I. Use of Selected Waste Materials in Concrete Mixes. Waste Manag. 2007, 27, 1870–1876. [Google Scholar] [CrossRef]
- Thorneycroft, J.; Orr, J.; Savoikar, P.; Ball, R.J. Performance of Structural Concrete with Recycled Plastic Waste as a Partial Replacement for Sand. Constr. Build. Mater. 2018, 161, 63–69. [Google Scholar] [CrossRef]
- Nestlé, Green Antz Address Waste Plastic Laminates as Corporations, LGUs Join Up. Available online: https://www.nestle.com.ph/media/newsfeed/nestle-green-antz-address-waste-plastic-laminates-as-corporations (accessed on 12 July 2020).
- Gu, L.; Ozbakkaloglu, T. Use of Recycled Plastics in Concrete: A Critical Review. Waste Manag. 2016, 51, 19–42. [Google Scholar] [CrossRef]
- Dipta, I.A.; Alam, A.; Islam, J. Rahat A Study of Green Lightweight Concrete Using Polyethylene Terephthalate (Pet) As Aggregate Green Lightweight Concrete Using Polyethylene Terephthalate (Pet) As Aggregate. In Proceedings of the International Conference on Engineering Research, Innovation and Education 2017 ICERIE 2017, Sylhet, Bangladesh, 13–15 January 2017; pp. 120–126. [Google Scholar]
- Boiny, H.U.; Alshkane, Y.M.; Rafiq, S.K. Mechanical Properties of Cement Mortar by Using Polyethylene. In Proceedings of the 5th National and 1st International Conference on Modern Materials and Structures in Civil Engineering, Terhan, Iran, 26–27 October 2016. [Google Scholar]
- da Silva, A.M.; de Brito, J.; Veiga, R. Incorporation of Fine Plastic Aggregates in Rendering Mortars. Constr. Build. Mater. 2014, 71, 226–236. [Google Scholar] [CrossRef]
- Hannawi, K.; Kamali-Bernard, S.; Prince, W. Physical and Mechanical Properties of Mortars Containing PET and PC Waste Aggregates. Waste Manag. 2010, 30, 2312–2320. [Google Scholar] [CrossRef]
- Safi, B.; Saidi, M.; Aboutaleb, D.; Maallem, M. The Use of Plastic Waste as Fine Aggregate in the Self-Compacting Mortars: Effect on Physical and Mechanical Properties. Constr. Build. Mater. 2013, 43, 436–442. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Dolati-Milehsara, S.; Lotfi-Omran, O.; Sadeghi-Nik, A. The Combined Effects of Waste Polyethylene Terephthalate (PET) Particles and Pozzolanic Materials on the Properties of Selfcompacting Concrete. J. Clean. Prod. 2016, 112, 2363–2373. [Google Scholar] [CrossRef]
- Ulu, C.; Akçaözoglu, S.; Akçaözoǧlu, S.; Ulu, C. Recycling of Waste PET Granules as Aggregate in Alkali-Activated Blast Furnace Slag/Metakaolin Blends. Constr. Build. Mater. 2014, 58, 31–37. [Google Scholar] [CrossRef]
- Freire, M.T.D.A.; Damant, A.P.; Castle, L.; Reyes, F.G.R. Thermal Stability of Polyethylene Terephthalate (PET): Oligomer Distribution and Formation of Volatiles. Packag. Technol. Sci. 1999, 12, 29–36. [Google Scholar] [CrossRef]
- Kubba, Z.; Fahim Huseien, G.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of Curing Temperatures and Alkaline Activators on Compressive Strength and Porosity of Ternary Blended Geopolymer Mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors Affecting the Mechanical Properties of Alkali Activated Ground Granulated Blast Furnace Slag Concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Cwirzen, A.; Engblom, R.; Punkki, J.; Habermehl-Cwirzen, K. Effects of Curing: Comparison of Optimised Alkali-Activated PC-FA-BFS and PC Concretes. Mag. Concr. Res. 2014, 66, 315–323. [Google Scholar] [CrossRef]
- Cwirzen, A.; Penttala, V. Aggregate-Cement Paste Transition Zone Properties Affecting the Salt-Frost Damage of High-Performance Concretes. Cem. Concr. Res. 2005, 35, 671–679. [Google Scholar] [CrossRef]
- Benosman, A.S.; Mouli, M.; Taibi, H.; Belbachir, M.; Senhadji, Y. Resistance of Polymer (PET)—Mortar Composites to Aggressive Solutions. Int. J. Eng. Res. Afr. 2011, 5, 1–15. [Google Scholar] [CrossRef]
- Hanumantha Rao, C.; Dinesh, Y. Strength Characteristics of Fibre Reinforced Concrete Using Recycled PET. Int. J. Civil. Eng. Technol. 2017, 8, 92–99. [Google Scholar]
- Saikia, N.; de Brito, J. Mechanical Properties and Abrasion Behaviour of Concrete Containing Shredded PET Bottle Waste as a Partial Substitution of Natural Aggregate. Constr. Build. Mater. 2014, 52, 236–244. [Google Scholar] [CrossRef]
- Ferreira, L.; de Brito, J.; Saikia, N. Influence of Curing Conditions on the Mechanical Performance of Concrete Containing Recycled Plastic Aggregate. Constr. Build. Mater. 2012, 36, 196–204. [Google Scholar] [CrossRef]
- Choi, Y.W.; Moon, D.J.; Kim, Y.J.; Lachemi, M. Characteristics of Mortar and Concrete Containing Fine Aggregate Manufactured from Recycled Waste Polyethylene Terephthalate Bottles. Constr. Build. Mater. 2009, 23, 2829–2835. [Google Scholar] [CrossRef]
- Choi, Y.W.; Moon, D.J.; Chung, J.S.; Cho, S.K. Effects of Waste PET Bottles Aggregate on the Properties of Concrete. Cem. Concr. Res. 2005, 35, 776–781. [Google Scholar] [CrossRef]
- Dębska, B.; Lichołai, L. The Effect of the Type of Curing Agent on Selected Properties of Epoxy Mortar Modified with PET Glycolisate. Constr. Build. Mater. 2016, 124, 11–19. [Google Scholar] [CrossRef]
- Aattache, A.; Mahi, A.; Soltani, R.; Mouli, M.; Benosman, A.S. Experimental Study on Thermo-Mechanical Properties of Polymer Modified Mortar. Materials Design 2013, 52, 459–469. [Google Scholar] [CrossRef]
- Badache, A.; Benosman, A.S.; Senhadji, Y.; Mouli, M. Thermo-Physical and Mechanical Characteristics of Sand-Based Lightweight Composite Mortars with Recycled High-Density Polyethylene (HDPE). Constr. Build. Mater. 2018, 163, 40–52. [Google Scholar] [CrossRef]
- Kocot, A. Impact of Artificial Waste on the Strength of Cementitious Composites. Proceedings 2019, 34, 11. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yue, X.; Liu, X.; Tong, Y. Properties of Self-Compacting Lightweight Concrete Containing Recycled Plastic Particles. Constr. Build. Mater. 2015, 84, 444–453. [Google Scholar] [CrossRef]




| References | PET Type | Size [mm] | Amount [%] | Composite |
|---|---|---|---|---|
| [22] | Crushed | 2.36–19 | 0, 10, 20, 30 vol. of aggregate | Concrete |
| [23] | Fibres | width < 1, length 8, 16 | 0, 0.5, 1.0, 1.5, 2.0 mass of mix | Mortar |
| [24] | Flakes, pellet | 1–2 | 0, 5, 10, 15 vol. of sand | Mortar |
| [25] | Flakes | 1.6–10 | 3, 10, 20, 50 vol. of sand | Mortar |
| [26] | Crushed | 0.5–5 | 10, 20, 30, 50 mass of sand | Mortar |
| [27] | Flakes | 0–9.5 | 5, 10, 15 mass of sand | Concrete |
| Oxide Type | Content [%] |
|---|---|
| SiO2 | 37.9 |
| Al2O3 | 13.2 |
| Na2O | 0.5 |
| K2O | 0.6 |
| CaO | 38.5 |
| MgO | 7.8 |
| Fe2O3 | 0.4 |
| Mix | PET Flakes [wt. %] | Activator [wt. %] | Curing Temperature [°C] |
|---|---|---|---|
| 20S80 | 0 | 20 | 80 |
| 20S80 + P | 5 | 20 | 80 |
| 20S40 | 0 | 20 | 40 |
| 20S40 + P | 5 | 20 | 40 |
| 10S80 | 0 | 10 | 80 |
| 10S80 + P | 5 | 10 | 80 |
| 10S40 | 0 | 10 | 40 |
| 10S40 + P | 5 | 10 | 40 |
| 20S20 (reference) | 0 | 20 | 20 |
| Spectrum | Carbon | Oxygen | Sodium | Magnesium | Aluminium | Silicon | Calcium | Ca/Si | Al/Si |
|---|---|---|---|---|---|---|---|---|---|
| D0 5198 | 38.11 | 52.07 | 2.10 | 1.42 | 4.60 | 1.69 | 0.37 | 0.31 | |
| D0 5199 | 37.49 | 52.77 | 1.85 | 1.28 | 5.05 | 1.56 | 0.31 | 0.25 | |
| D0 5200 | 28.84 | 58.89 | 3.04 | 0.84 | 0.67 | 5.60 | 2.11 | 0.38 | 0.12 |
| Sample | Binder Matrix [%] | Microcracks [%] | Other Particles [%] | Aggregates [%] |
|---|---|---|---|---|
| 20S40.1 | 43.0 | 1.2 | 32.8 | 23.0 |
| 20S40.2 | 41.8 | 3.3 | 29.7 | 25.2 |
| 20S40.3 | 42.0 | 3.1 | 28.6 | 26.3 |
| 20S80.1 | 38.7 | 4.1 | 30.0 | 27.2 |
| 20S80.2 | 53.8 | 4.1 | 28.7 | 13.4 |
| 20S80.3 | 75.2 | 2.3 | 22.5 | 0.0 |
| 20S40P.1 | 69.0 | 1.5 | 11.4 | 18.1 |
| 20S40P.2 | 84.7 | 1.2 | 14.1 | 0.0 |
| 20S80P.1 | 54.3 | 2.4 | 43.3 | 0.0 |
| 20S80P.2 | 45.6 | 6.0 | 22.0 | 26.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocot, A.; Ćwirzeń, A.; Ponikiewski, T.; Katzer, J. Strength Characteristics of Alkali-Activated Slag Mortars with the Addition of PET Flakes. Materials 2021, 14, 6274. https://doi.org/10.3390/ma14216274
Kocot A, Ćwirzeń A, Ponikiewski T, Katzer J. Strength Characteristics of Alkali-Activated Slag Mortars with the Addition of PET Flakes. Materials. 2021; 14(21):6274. https://doi.org/10.3390/ma14216274
Chicago/Turabian StyleKocot, Agnieszka, Andrzej Ćwirzeń, Tomasz Ponikiewski, and Jacek Katzer. 2021. "Strength Characteristics of Alkali-Activated Slag Mortars with the Addition of PET Flakes" Materials 14, no. 21: 6274. https://doi.org/10.3390/ma14216274
APA StyleKocot, A., Ćwirzeń, A., Ponikiewski, T., & Katzer, J. (2021). Strength Characteristics of Alkali-Activated Slag Mortars with the Addition of PET Flakes. Materials, 14(21), 6274. https://doi.org/10.3390/ma14216274