Synthesis and Effect of the Structure of Bithienyl-Terminated Surfactants for Dielectric Layer Modification in Organic Transistor
Abstract
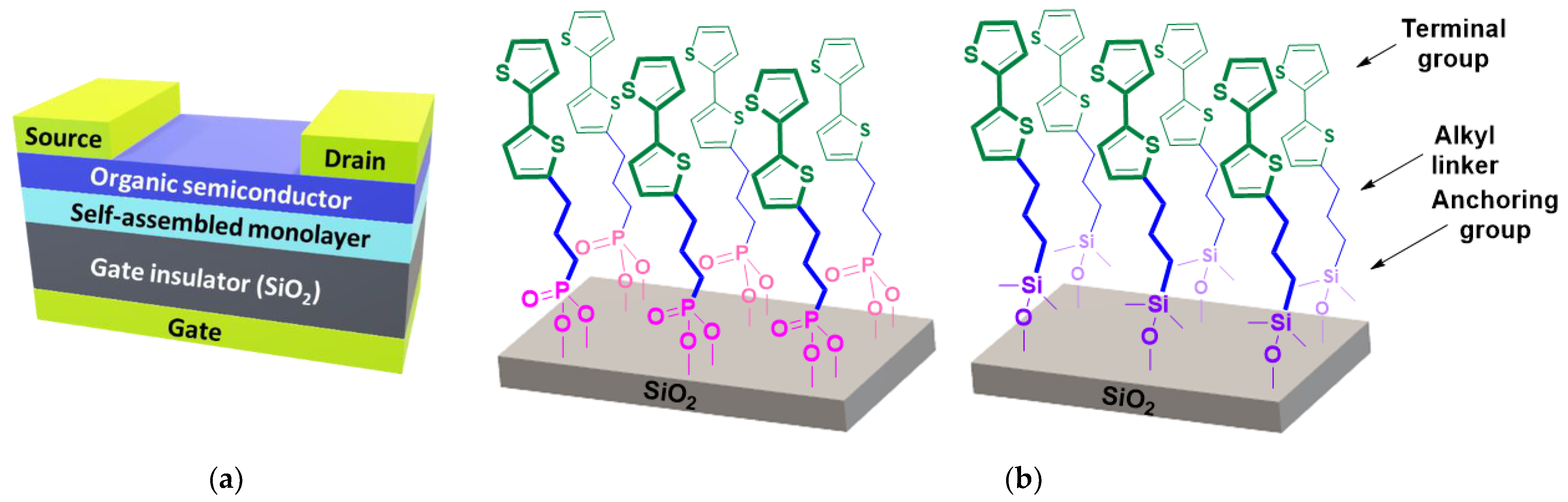
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment
2.2.1. Synthesis of ω-Bromoalk-1-enes (1e–f)
2.2.2. General Procedure for Preparation of 5-(Alk-ω-en-1-yl)-2,2′-bithiophenes (2a–f)
2.2.3. General Procedure for 2-[ω-(2,2′-Bithiophen-5-yl)alkyl]dioxaphospholane 2-oxides (3a–f)
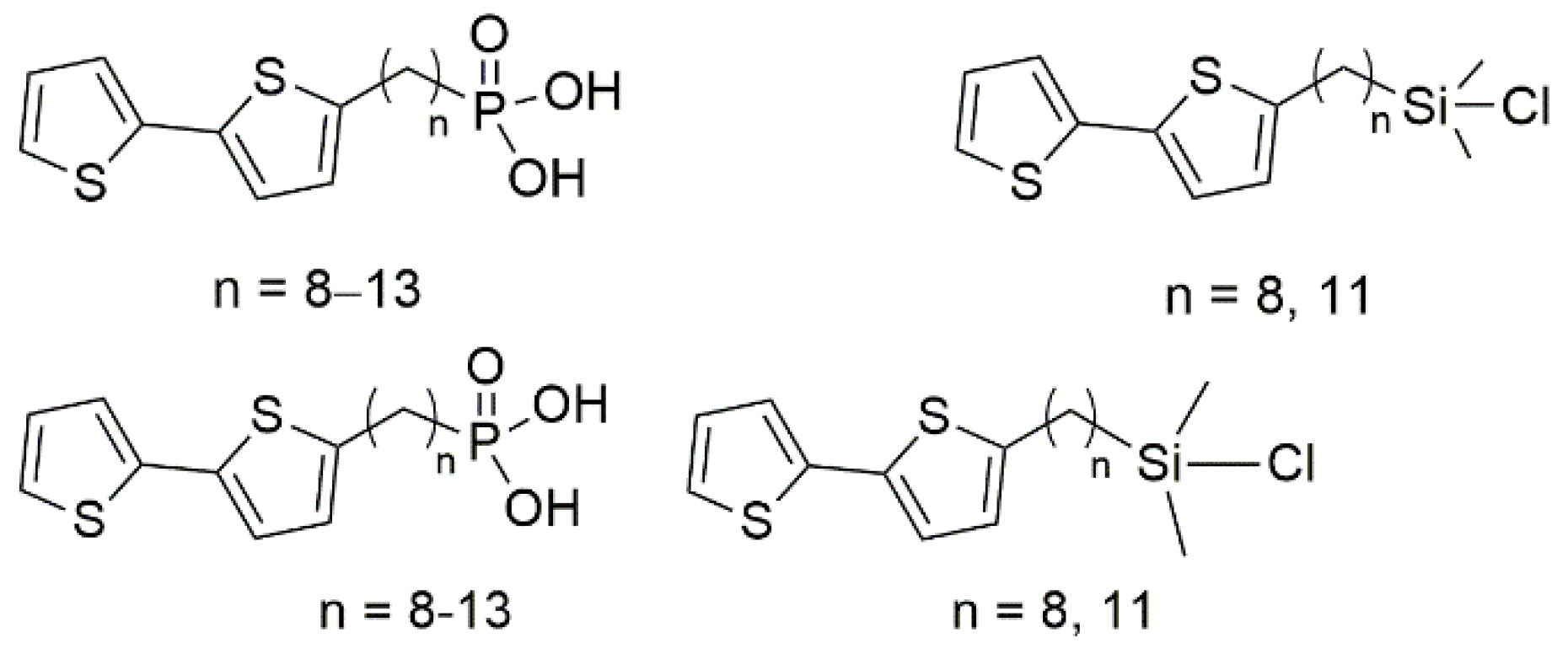
2.2.4. General Synthesis of ω-(2,2′-Bithiophen-5-yl)alkylphosphonic Acids TTCnP
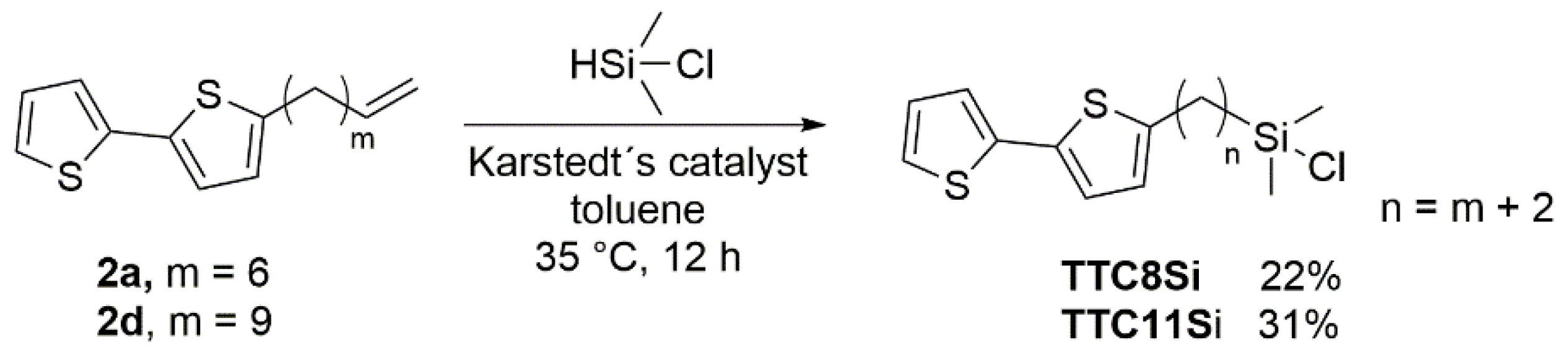
2.2.5. General Synthesis of ω-(2,2′-Bithiophen-5-yl)alkylchlorodimethylsilanes TTCnSi
2.3. Device Fabrication
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of Surfactants
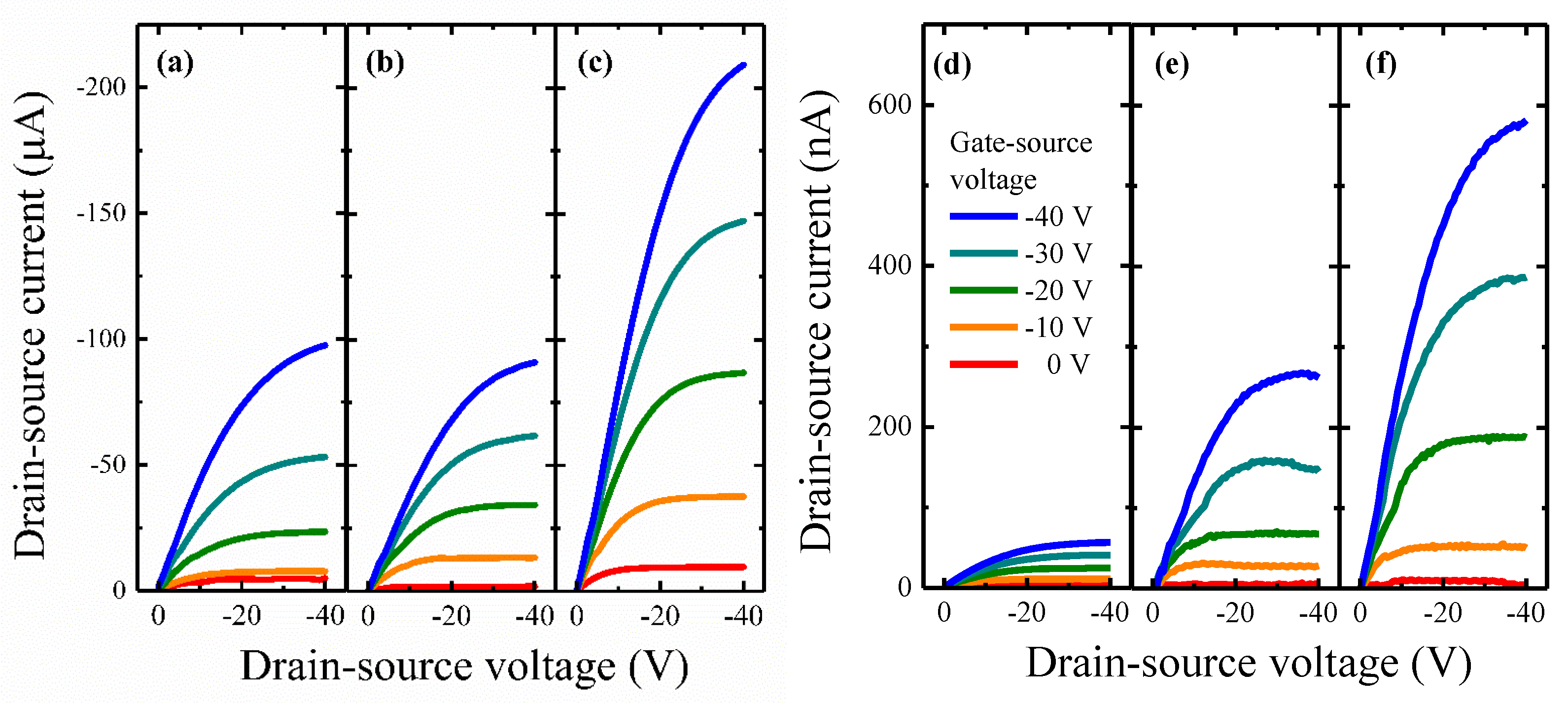
3.2. OFET Performance
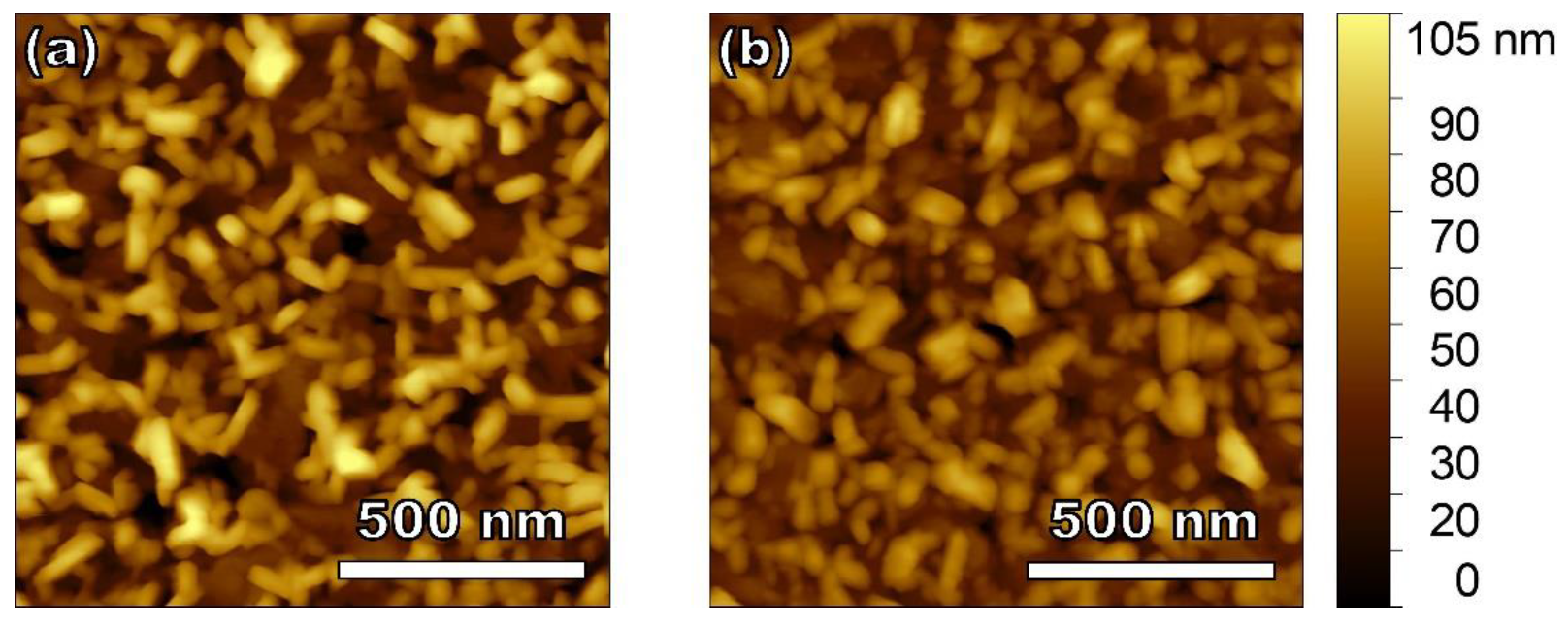
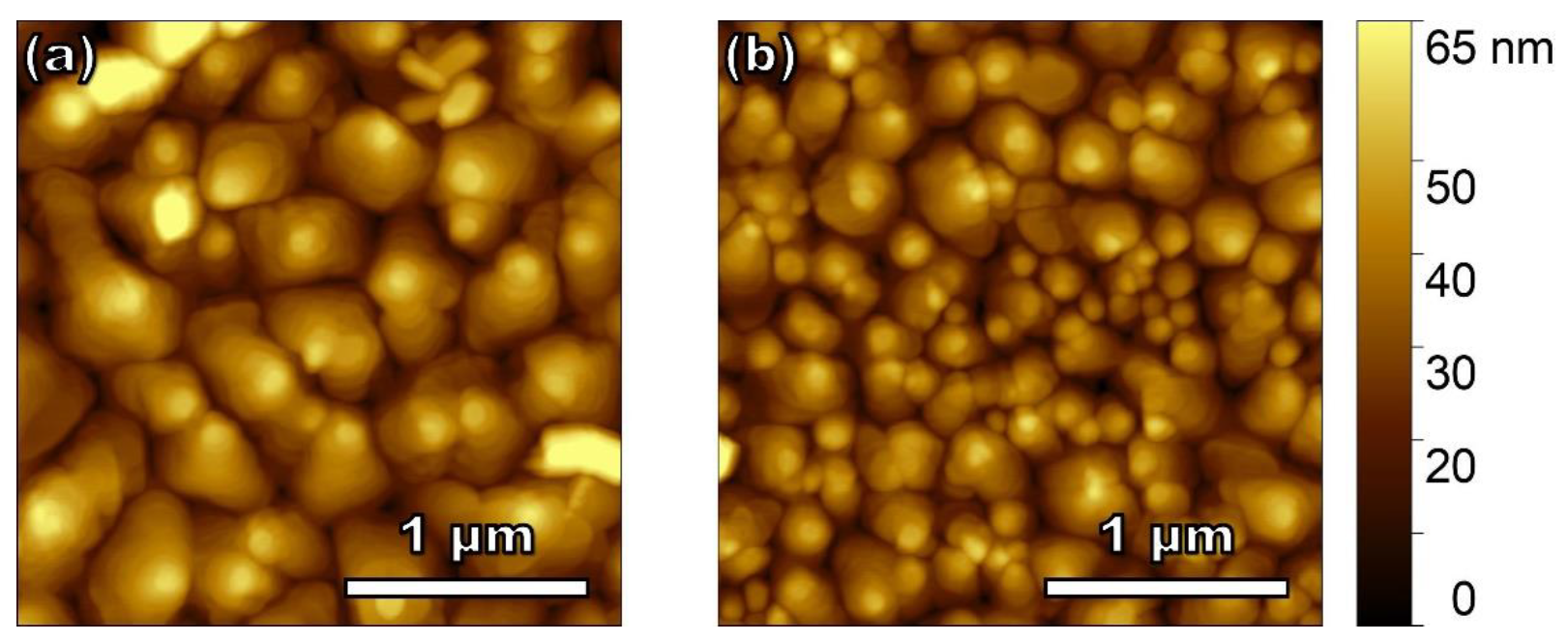
3.3. Surface Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahlman, M.; Fabiano, S.; Gueskine, V.; Simon, D.; Berggren, M.; Crispin, X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Ogier, S.; Ng, T.N.; Caironi, M.; Perinot, A.; Li, L.; Zhao, J.; Tang, W.; Sporea, R.A.; et al. Current Status and Opportunities of Organic Thin-Film Transistor Technologies. IEEE Trans. Electron Devices 2017, 64, 1906–1921. [Google Scholar] [CrossRef]
- Choi, H.H.; Cho, K.; Frisbie, C.D.; Sirringhaus, H.; Podzorov, V. Critical assessment of charge mobility extraction in FETs. Nat. Mater. 2017, 17, 2–7. [Google Scholar] [CrossRef]
- Flesch, H.G.; Mathijssen, S.G.J.; Gholamrezaie, F.; Moser, A.; Neuhold, A.; Novák, J.; Ponomarenko, S.A.; Shen, Q.; Teichert, C.; Hlawacek, G.; et al. Microstructure and phase behavior of a quinquethiophene-based self-assembled monolayer as a function of temperature. J. Phys. Chem. C 2011, 115, 22925–22930. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, Y.; Liu, Y. Recent progress in organic field-effect transistor-based integrated circuits. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Mottaghi, M.; Horowitz, G. Field-induced mobility degradation in pentacene thin-film transistors. Org. Electron. 2006, 7, 528–536. [Google Scholar] [CrossRef]
- Liu, D.; Miao, Q. Recent progress in interface engineering of organic thin film transistors with self-assembled monolayers. Mater. Chem. Front. 2018, 2, 11–21. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Comini, E. The role of self-assembled monolayers in electronic devices. J. Mater. Chem. C 2020, 8, 3938–3955. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.H.; Cho, J.H.; Hwang, M.; Jang, Y.; Cho, K. Effect of the phase states of self-assembled monolayers on pentacene growth and thin-film transistor characteristics. J. Am. Chem. Soc. 2008, 130, 10556–10564. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, D.H.; Cho, J.H.; Park, Y.D.; Kim, J.S.; Cho, K. Enhancement of interconnectivity in the channels of pentacene thin-film transistors and its effect on field-effect mobility. Adv. Funct. Mater. 2006, 16, 1859–1864. [Google Scholar] [CrossRef]
- Bent, S.F. Heads or tails: Which is more important in molecular self-assembly? ACS Nano 2007, 1, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Chiodi, M.; Gavioli, L.; Beccari, M.; Di Castro, V.; Cossaro, A.; Floreano, L.; Morgante, A.; Kanjilal, A.; Mariani, C.; Betti, M.G. Interaction strength and molecular orientation of a single layer of pentacene in organic-metal interface and organic-organic heterostructure. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77. [Google Scholar] [CrossRef]
- Reig, M.; Bagdziunas, G.; Ramanavicius, A.; Puigdollers, J.; Velasco, D. Interface engineering and solid-state organization for triindole-based p-type organic thin-film transistors. Phys. Chem. Chem. Phys. 2018, 20, 17889–17898. [Google Scholar] [CrossRef]
- Bujaldón, R.; Puigdollers, J.; Velasco, D. Towards the bisbenzothienocarbazole core: A route of sulfurated carbazole derivatives with assorted optoelectronic properties and applications. Materials 2021, 14, 3487. [Google Scholar] [CrossRef]
- Borshchev, O.V.; Ponomarenko, S.A. Self-assembled organic semiconductors for monolayer field-effect transistors. Polym. Sci.-Ser. C 2014, 56, 32–46. [Google Scholar] [CrossRef]
- Filo, J.; Mišicák, R.; Cigáň, M.; Weis, M.; Jakabovič, J.; Gmucová, K.; Pavúk, M.; Dobročka, E.; Putala, M. Oligothiophenes with the naphthalene core for organic thin-film transistors: Variation in positions of bithiophenyl attachment to the naphthalene. Synth. Met. 2015, 202, 73–81. [Google Scholar] [CrossRef]
- Mišicák, R.; Novota, M.; Weis, M.; Cigáň, M.; Šiffalovič, P.; Nádaždy, P.; Kožíšek, J.; Kožíšková, J.; Pavúk, M.; Putala, M. Effect of alkyl side chains on properties and organic transistor performance of 2,6-bis(2,2′-bithiophen-5-yl)naphthalene. Synth. Met. 2017, 233, 1–14. [Google Scholar] [CrossRef]
- Feriancová, L.; Cigáň, M.; Gmucová, K.; Kožíšek, J.; Nádaždy, V.; Putala, M. Effect of electron-withdrawing groups on molecular properties of naphthyl and anthryl bithiophenes as potential n-type semiconductors. New J. Chem. 2021, 45, 9794–9804. [Google Scholar] [CrossRef]
- Haensch, C.; Schubert, U.S.; Hoeppener, S. Chemical modification of self-assembled silane based monolayers by surface reactions. Chem. Soc. Rev. 2010, 39, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Krzykawska, A.; Szwed, M.; Ossowski, J.; Cyganik, P. Odd-Even Effect in Molecular Packing of Self-Assembled Monolayers of Biphenyl-Substituted Fatty Acid on Ag(111). J. Phys. Chem. C 2018, 122, 919–928. [Google Scholar] [CrossRef]
- Stoliar, P.; Kshirsagar, R.; Massi, M.; Annibale, P.; Albonetti, C.; De Leeuw, D.M.; Biscarini, F. Charge injection across self-assembly monolayers in organic field-effect transistors: Odd-even effects. J. Am. Chem. Soc. 2007, 129, 6477–6484. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 5th ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2003; ISBN 9780123821614. [Google Scholar]
- Christoffers, J.; Oertling, H. Intramolecular Michael reactions by iron(III) catalysis. Tetrahedron 2001, 56, 1339–1344. [Google Scholar] [CrossRef]
- Johnson, D.K.; Donohoe, J.; Kang, J. Dilithium tetrachlorocuprate catalyzed coupling of allylmagnesium bromide with a,co-dihaloalkanes. Synth. Commun. 1994, 24, 1557–1564. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Borshchev, O.V.; Meyer-Friedrichsen, T.; Pleshkova, A.P.; Setayesh, S.; Smits, E.C.P.; Mathijssen, S.G.J.; De Leeuw, D.M.; Kirchmeyer, S.; Muzafarov, A.M. Synthesis of monochlorosilyl derivatives of dialkyloligothiophenes for self-assembling monolayer field-effect transistors. Organometallics 2010, 29, 4213–4226. [Google Scholar] [CrossRef][Green Version]
- Han, L.B.; Mirzaei, F.; Zhao, C.Q.; Tanaka, M. High reactivity of a five-membered cyclic hydrogen phosphonate leading to development of facile palladium-catalyzed hydrophosphorylation of alkenes. J. Am. Chem. Soc. 2000, 122, 5407–5408. [Google Scholar] [CrossRef]
- McKenna, C.E.; Higa, M.T.; Cheung, N.H.; McKenna, M.C. The facile dealkylation of phosphonic acid dialkyl esters by bromotrimethylsilane. Tetrahedron Lett. 1977, 18, 155–158. [Google Scholar] [CrossRef]
- McKenna, C.E.; Schmidhuser, J. Functional selectivity in phosphonate ester dealkylation with bromotrimethylsilane. J. Chem. Soc. Chem. Commun. 1979, 739. [Google Scholar] [CrossRef]
- Reichwein, J.F.; Pagenkopf, B.L. New mixed phosphonate esters by transesterification of pinacol phosphonates and their use in aldehyde and ketone coupling reactions with nonstabilized phosphonates. J. Org. Chem. 2003, 68, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Mayer, A.C.; Malliaras, G.G.; Nickel, B.; Scoles, G.; Kazimirov, A.; Kim, H.; Headrick, R.L.; Islam, Z. Structure of pentacene thin films. Appl. Phys. Lett. 2004, 85, 4926–4928. [Google Scholar] [CrossRef]
- Käfer, D.; Ruppel, L.; Witte, G. Growth of pentacene on clean and modified gold surfaces. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 75, 085309. [Google Scholar] [CrossRef]
- Cheng, H.L.; Mai, Y.S.; Chou, W.Y.; Chang, L.R.; Liang, X.W. Thickness-dependent structural evolutions and growth models in relation to carrier transport properties in polycrystalline pentacene thin films. Adv. Funct. Mater. 2007, 17, 3639–3649. [Google Scholar] [CrossRef]
- Ruiz, R.; Choudhary, D.; Nickel, B.; Toccoli, T.; Chang, K.C.; Mayer, A.C.; Clancy, P.; Blakely, J.M.; Headrick, R.L.; Iannotta, S.; et al. Pentacene thin film growth. Chem. Mater. 2004, 16, 4497–4508. [Google Scholar] [CrossRef]
- Nickel, B.; Barabash, R.; Ruiz, R.; Koch, N.; Kahn, A.; Feldman, L.C.; Haglund, R.F.; Scoles, G. Dislocation arrangements in pentacene thin films. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 70, 125401. [Google Scholar] [CrossRef]
- Kakudate, T.; Yoshimoto, N.; Saito, Y. Polymorphism in pentacene thin films on Si O2 substrate. Appl. Phys. Lett. 2007, 90, 130–133. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feriancová, L.; Kmentová, I.; Micjan, M.; Pavúk, M.; Weis, M.; Putala, M. Synthesis and Effect of the Structure of Bithienyl-Terminated Surfactants for Dielectric Layer Modification in Organic Transistor. Materials 2021, 14, 6345. https://doi.org/10.3390/ma14216345
Feriancová L, Kmentová I, Micjan M, Pavúk M, Weis M, Putala M. Synthesis and Effect of the Structure of Bithienyl-Terminated Surfactants for Dielectric Layer Modification in Organic Transistor. Materials. 2021; 14(21):6345. https://doi.org/10.3390/ma14216345
Chicago/Turabian StyleFeriancová, Lucia, Iveta Kmentová, Michal Micjan, Milan Pavúk, Martin Weis, and Martin Putala. 2021. "Synthesis and Effect of the Structure of Bithienyl-Terminated Surfactants for Dielectric Layer Modification in Organic Transistor" Materials 14, no. 21: 6345. https://doi.org/10.3390/ma14216345
APA StyleFeriancová, L., Kmentová, I., Micjan, M., Pavúk, M., Weis, M., & Putala, M. (2021). Synthesis and Effect of the Structure of Bithienyl-Terminated Surfactants for Dielectric Layer Modification in Organic Transistor. Materials, 14(21), 6345. https://doi.org/10.3390/ma14216345






