An Acylhydrazone-Based Fluorescent Sensor for Sequential Recognition of Al3+ and H2PO4−
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Equipment
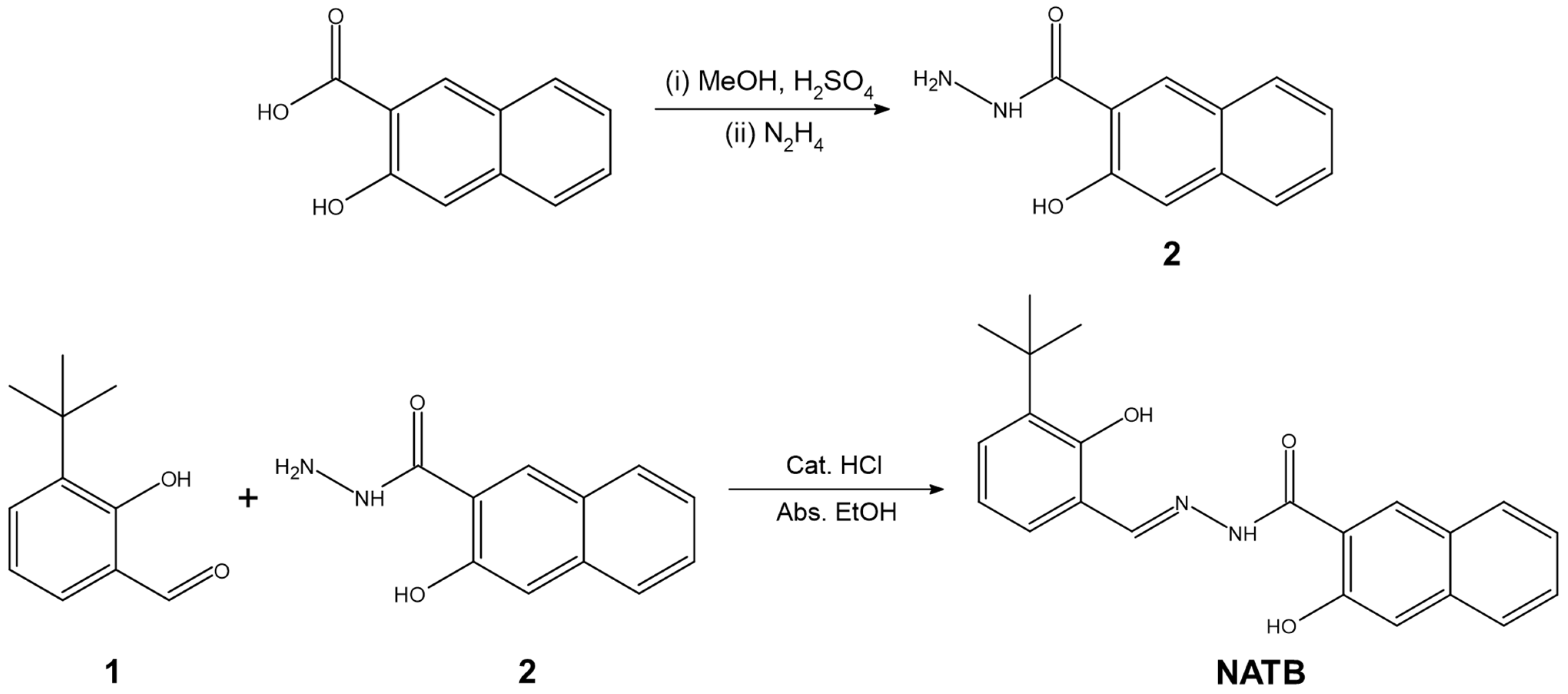
2.2. Synthesis of N′-[(E)-(3-tert-butyl-2-hydroxyphenyl)methylidene]-3-hydroxynaphthalene-2-carbohydrazide (NATB)
2.3. Preparation of Spectroscopic Experiments
2.4. Competitive Experiments
2.5. Determination of Association Constant (K)
2.6. Calculations
3. Results and Discussion
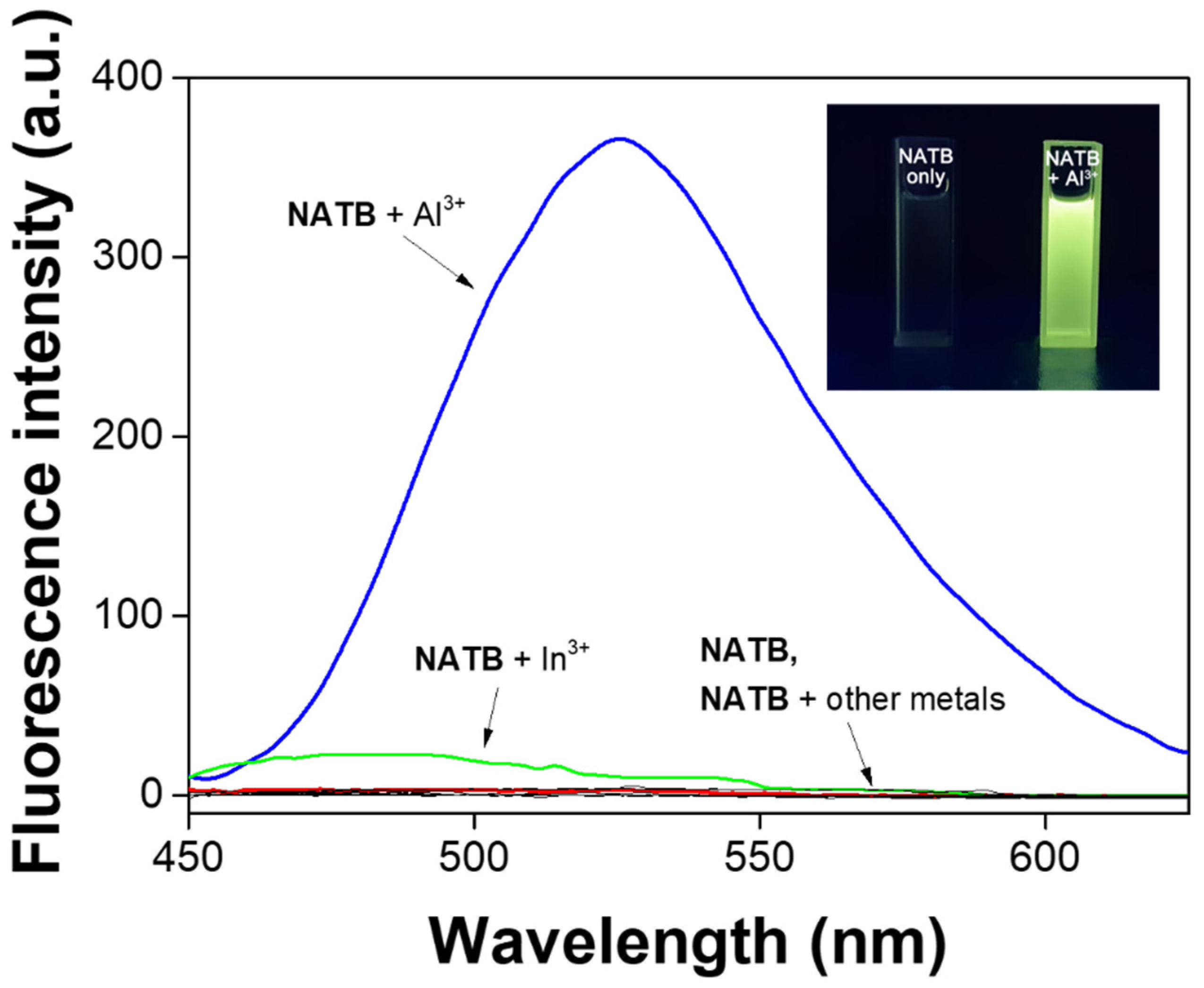
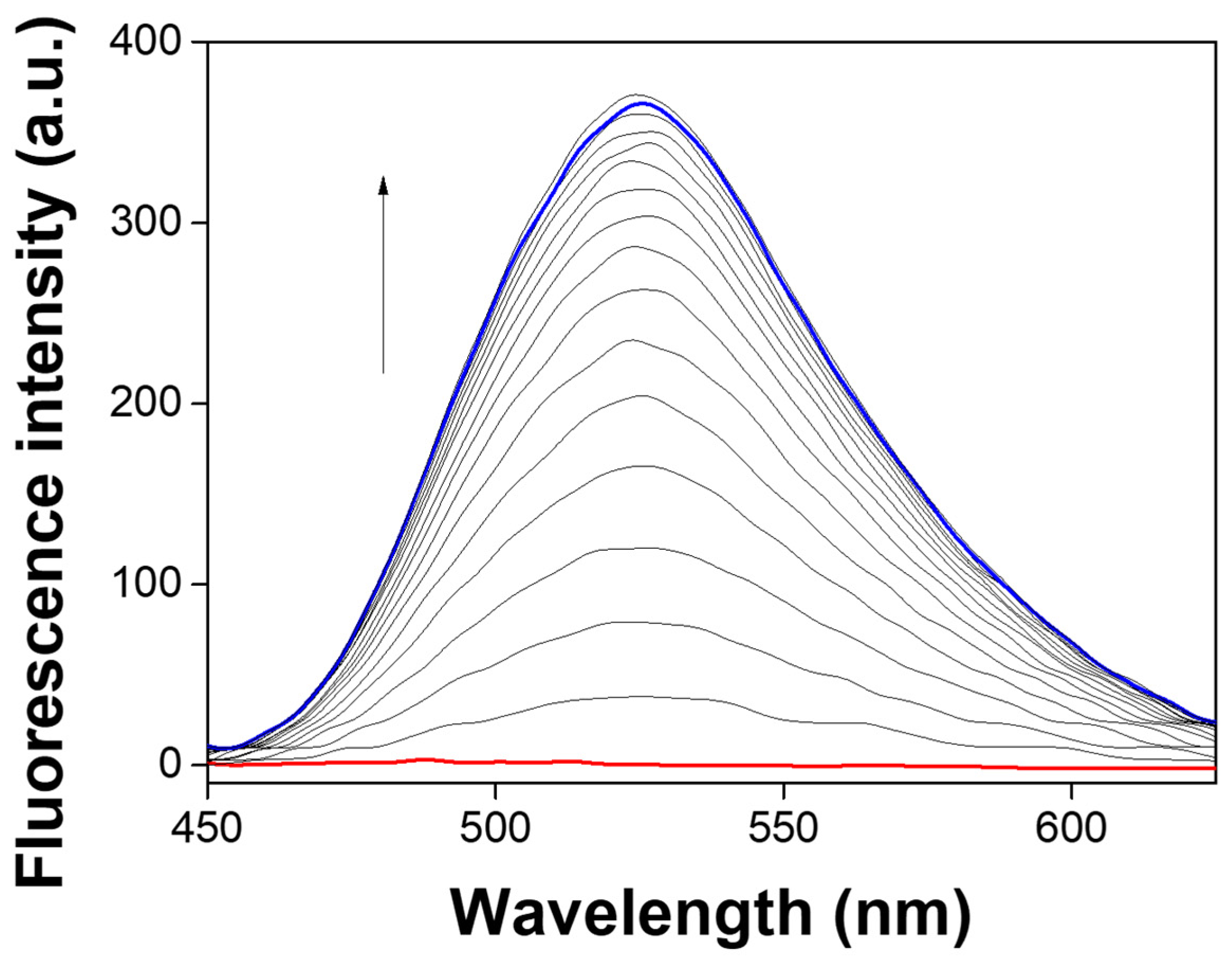
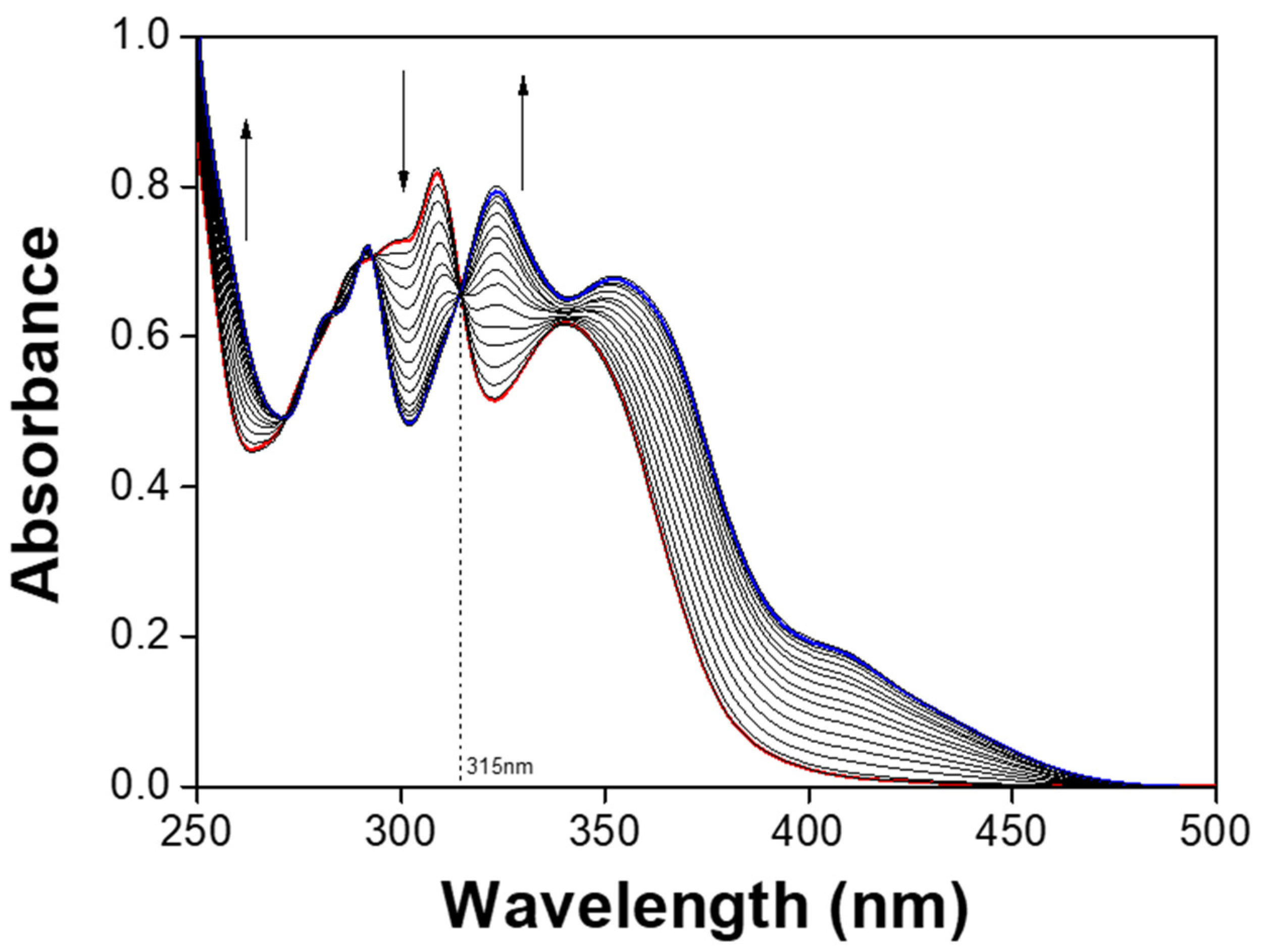
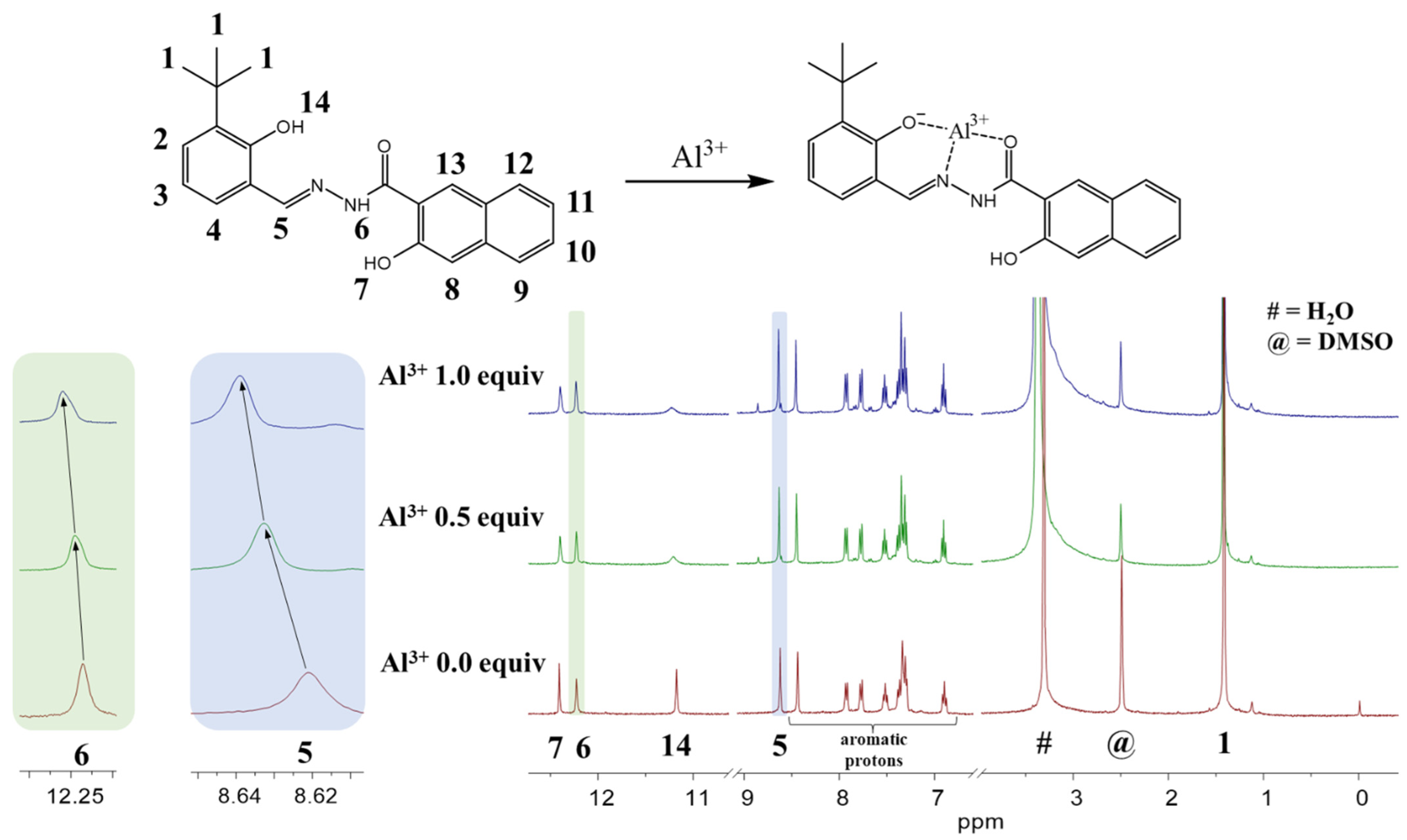
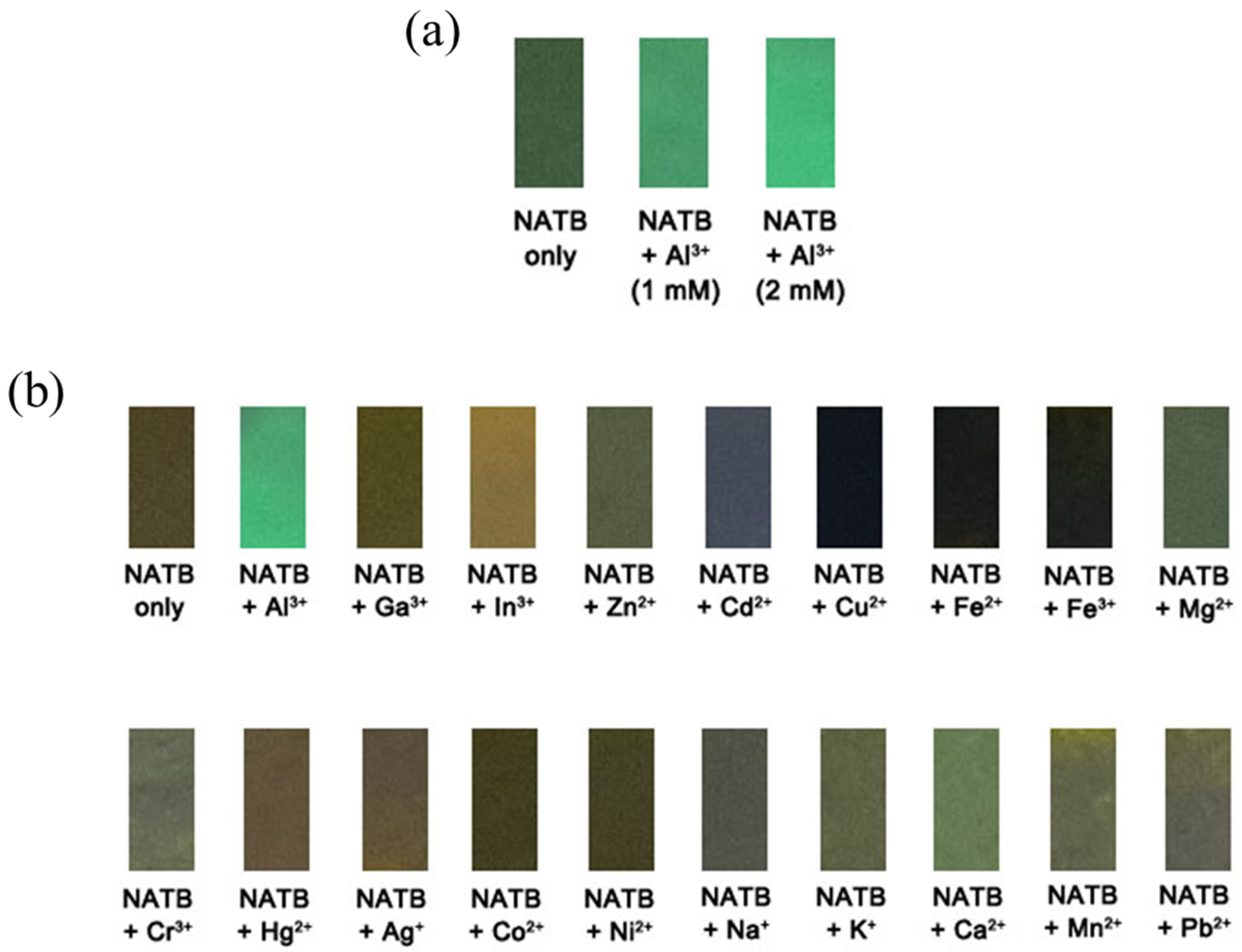
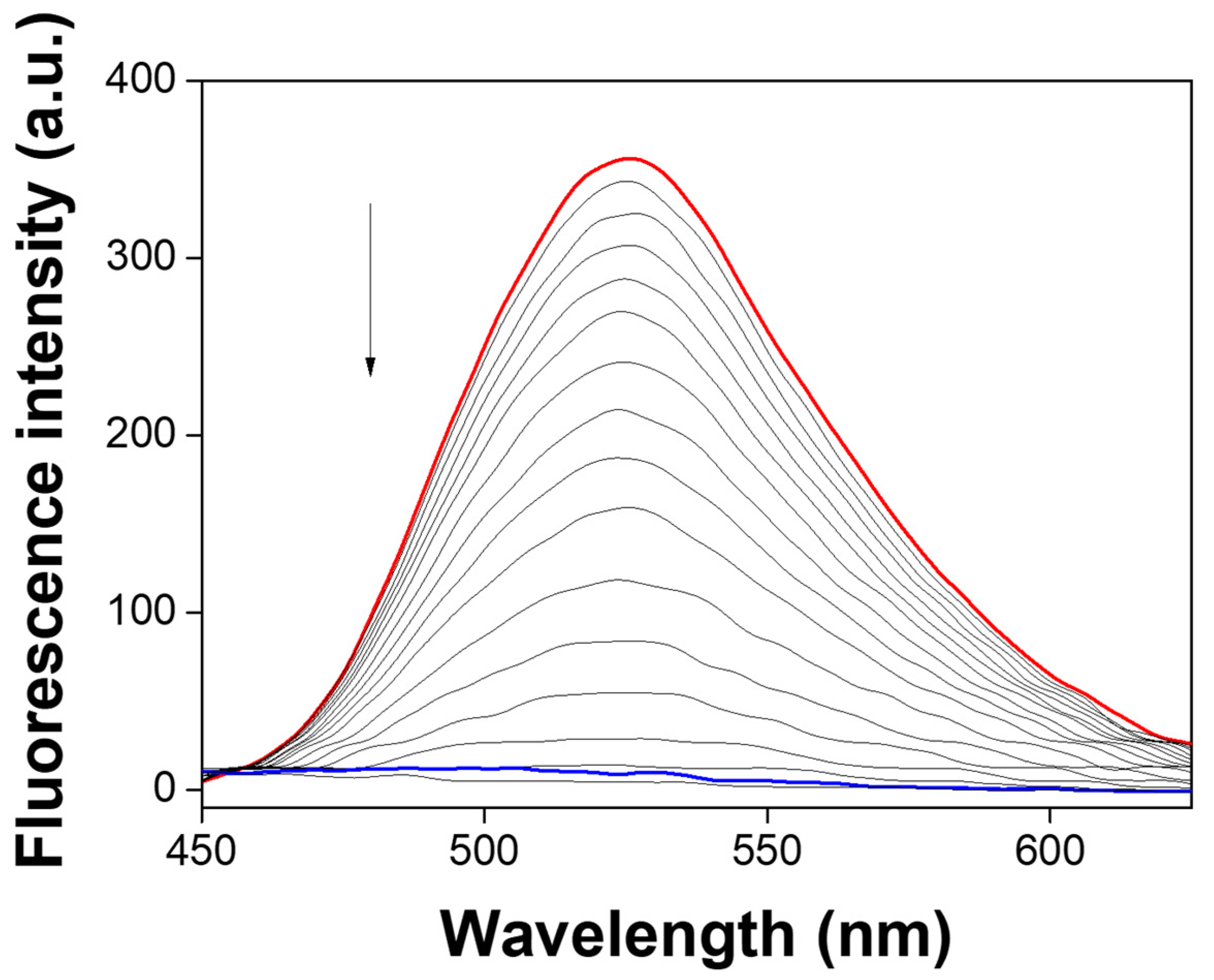
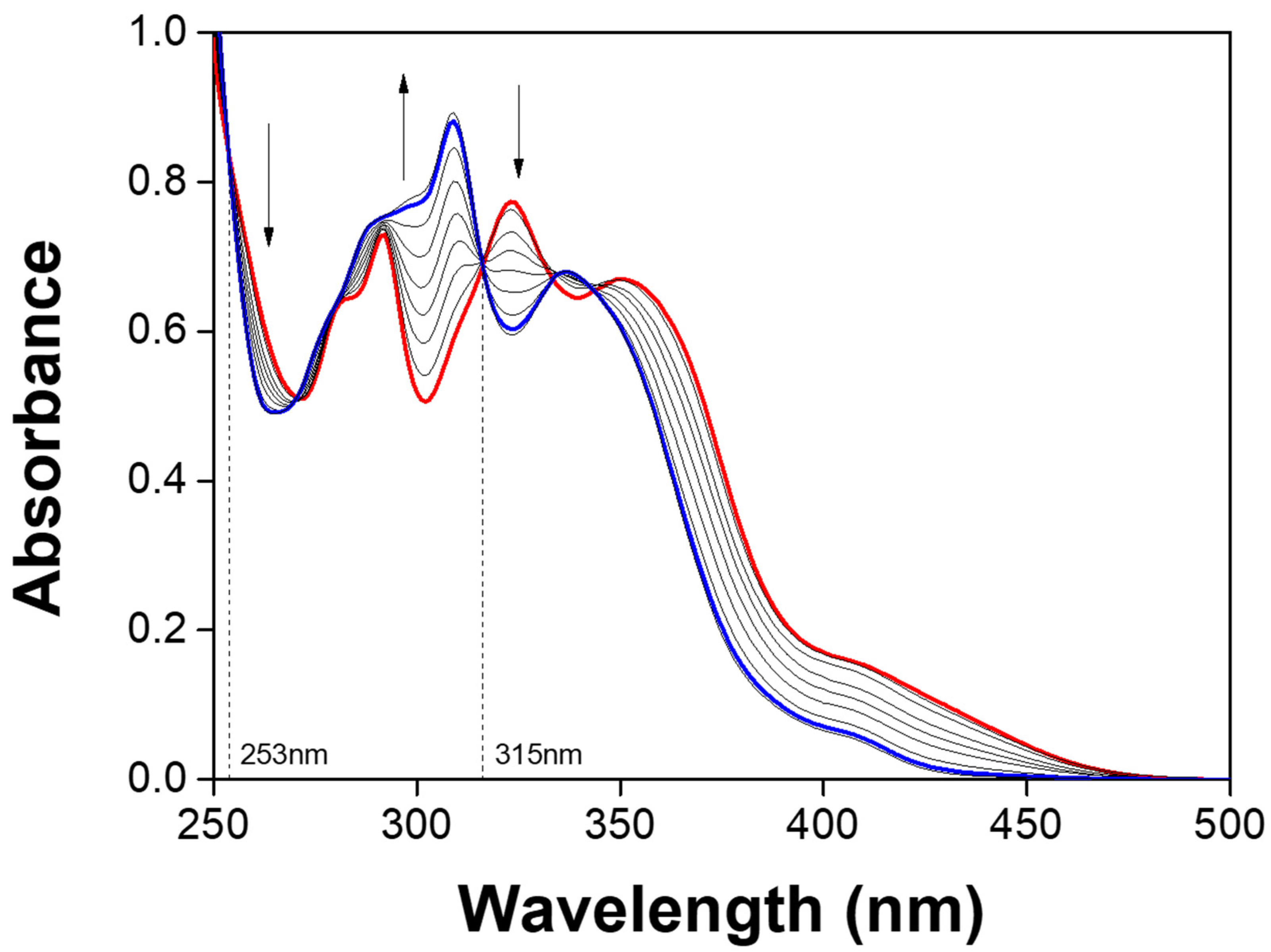
3.1. Spectroscopic Examination of NATB to Al3+
3.2. Calculations
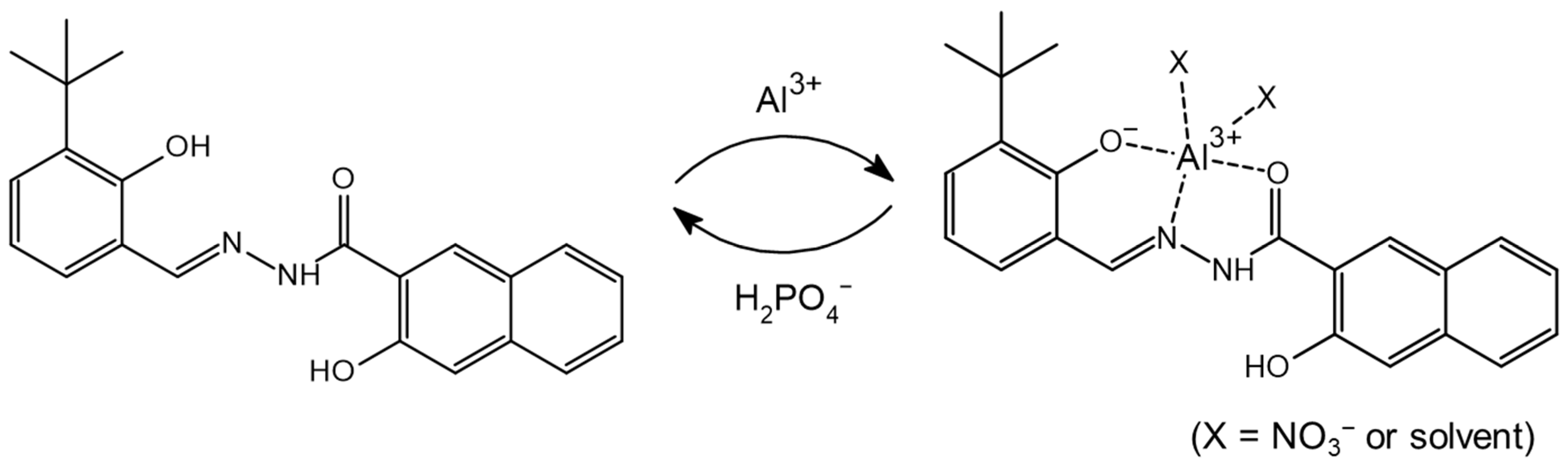
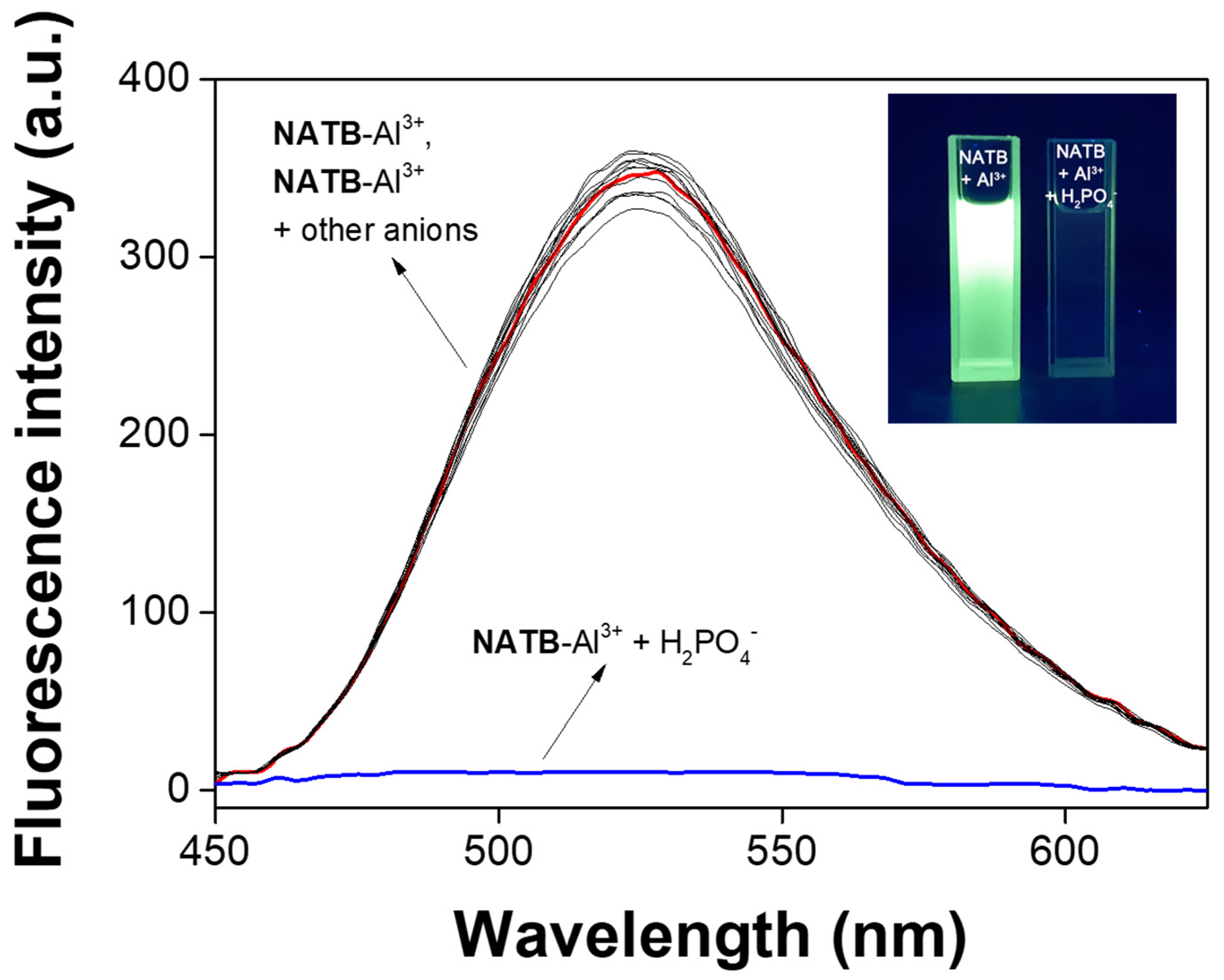
3.3. Spectroscopic Examination of NATB-Al3+ to H2PO4−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pungut, N.A.S.; Saad, H.M.; Sim, K.S.; Tan, K.W. A turn on fluorescent sensor for detecting Al3+ and colorimetric detection for Cu2+: Synthesis, cytotoxicity and on-site assay kit. J. Photochem. Photobiol. A Chem. 2021, 414, 113290. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. Pyrrolidine constrained bipyridyl-dansyl click fluoroionophore as selective Al3+sensor. Chem. Commun. 2010, 46, 4499–4501. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.Y.; Kang, J.H.; Kim, M.S.; Kim, A.; Kim, C. Colorimetric detection of Fe3+/2+ and fluorescent detection of Al3+ in aqueous media: Applications and DFT calculations. J. Coord. Chem. 2018, 71, 2401–2414. [Google Scholar] [CrossRef]
- Kaur, R.; Saini, S.; Kaur, N.; Singh, N.; Jang, D.O. Rhodamine-based fluorescent probe for sequential detection of Al3+ ions and adenosine monophosphate in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117523. [Google Scholar] [CrossRef]
- Cheah, P.W.; Heng, M.P.; Izati, A.; Ng, C.H.; Tan, K.W. Rhodamine B conjugate for rapid colorimetric and fluorimetric detection of aluminium and tin ions and its application in aqueous media. Inorg. Chim. Acta 2020, 512, 119901. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem. Commun. 2012, 48, 1039–1041. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, Y.-F.; Zhuo, J.-B.; Lin, C.-X. Synthesis and characterization of cyclophane: The highly selective recognition of Fe3+ in aqueous solution and H2PO4− in acetonitrile solution. Tetrahedron Lett. 2018, 59, 1059–1064. [Google Scholar] [CrossRef]
- Singh, A.; Nishith, U.; Trivedi, D.R. Spectroscopic studies of colorimetric receptors for detection of biologically important inorganic F−, AcO− and H2PO4− anions in organo-aqueous medium: Real-life application. Inorg. Chem. Commun. 2020, 115, 107874. [Google Scholar] [CrossRef]
- Goswami, S.; Maity, S.; Maity, A.C.; Das, A.K.; Khanra, K.; Mandal, T.K.; Bhattacharyya, N. A macrocyclic piperazine linked extremely Zn2+ selective fluorescent chemosensor with bio-imaging and for H2PO4− sensing. Tetrahedron Lett. 2014, 55, 5993–5997. [Google Scholar] [CrossRef]
- Cao, C.; You, X.; Feng, L.; Luo, G.; Yue, G.; Ji, X. Synthesis of new chromogenic sensors containing thiourea and selective detection for F–, H2PO4–, and Ac– anions. Can. J. Chem. 2020, 98, 659–666. [Google Scholar] [CrossRef]
- Choi, J.H.; Pandith, A.; Chakradhar, D.; Kim, H.R.; Kim, H.S. Al3+-Morpholine-appended Anthracene Ensemble as a Dual Photonic Switch for H2PO4− and CN− Ions and its Biological Applications. Bull. Korean Chem. Soc. 2019, 40, 138–145. [Google Scholar] [CrossRef]
- Pandith, A.; Uddin, N.; Choi, C.H.; Kim, H.S. Highly selective imidazole-appended 9,10-N,N’-diaminomethylanthracene fluorescent probe for switch-on Zn2+ detection and switch-off H2PO4− and CN− detection in 80% aqueous DMSO, and applications to sequential logic gate operations. Sens. Actuators B Chem. 2017, 247, 840–849. [Google Scholar] [CrossRef]
- Theetharappan, M.; Neelakantan, M.A. A Water-Soluble Schiff Base Turn-on Fluorescent Chemosensor for the Detection of Al3+ and Zn2+ Ions at the Nanomolar Level: Application in Live-Cell Imaging. J. Fluoresc. 2021, 31, 1277–1290. [Google Scholar] [CrossRef]
- Chandra, R.; Manna, A.K.; Sahu, M.; Rout, K.; Patra, G.K. Simple salicylaldimine-functionalized dipodal bis Schiff base chromogenic and fluorogenic chemosensors for selective and sensitive detection of Al3+ and Cr3+. Inorg. Chim. Acta 2020, 499, 119192. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, S.Y.; Han, J.; Lim, M.H.; Kim, C. Thiophene and diethylaminophenol-based “turn-on” fluorescence chemosensor for detection of Al3+ and F− in a near-perfect aqueous solution. Tetrahedron 2017, 73, 2690–2697. [Google Scholar] [CrossRef]
- Wang, M.; Lu, L.; Song, W.; Wang, X.; Sun, T.; Zhu, J.; Wang, J. AIE-active Schiff base compounds as fluorescent probe for the highly sensitive and selective detection of Al3+ ions. J. Lumin. 2021, 233, 117911. [Google Scholar] [CrossRef]
- Park, S.M.; Saini, S.; Park, J.E.; Singh, N.; Jang, D.O. A benzothiazole-based receptor for colorimetric detection of Cu2+ and S2− ions in aqueous media. Tetrahedron Lett. 2021, 73, 153115. [Google Scholar] [CrossRef]
- So, H.; Cho, H.; Lee, H.; Tran, M.C.; Kim, K.T.; Kim, C. Detection of zinc (II) and hypochlorite by a thiourea-based chemosensor via two emission channels and its application in vivo. Microchem. J. 2020, 155, 104788. [Google Scholar] [CrossRef]
- Feng, X.; Fu, Y.; Jin, J.; Wu, J. A highly selective and sensitive fluorescent sensor for relay recognition of Zn2+ and HSO4−/H2PO4− with “on-off” fluorescent responses. Anal. Biochem. 2018, 563, 20–24. [Google Scholar] [CrossRef]
- Du, K.; Niu, S.; Qiao, L.; Dou, Y.; Zhu, Q.; Chen, X.; Zhang, P. A highly selective ratiometric fluorescent probe for the cascade detection of Zn2+ and H2PO4− and its application in living cell imaging. RSC Adv. 2017, 7, 40615–40620. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.M.; Kim, M.S.; Lee, M.; Lim, M.H.; Kim, C. Single fluorescent chemosensor for multiple targets: Sequential detection of Al3+ and pyrophosphate and selective detection of F− in near-perfect aqueous solution. N. J. Chem. 2017, 41, 15590–15600. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Feng, E.; Fan, C.; Pu, S. A highly selective sequential recognition probe for Zn2+ and HSO4−/H2PO4− based on a diarylethene chemosensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119052. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Deswal, N.; Joshi, A.; Ramachandran, C.N.; Kumar, R. Triazole-appended pyrano[2,3-c]pyrazolone based colorimetric chemosensors for recognition of Fe3+ ions and their molecular logic gate behavior. Anal. Methods 2019, 11, 3230–3243. [Google Scholar] [CrossRef]
- Qu, W.J.; Yan, G.T.; Ma, X.L.; Wei, T.B.; Lin, Q.; Yao, H.; Zhang, Y.M. “Cascade recognition” of Cu2+ and H2PO4− with high sensitivity and selectivity in aqueous media based on the effect of ESIPT. Sens. Actuators B Chem. 2017, 242, 849–856. [Google Scholar] [CrossRef]
- Li, S.; Cao, D.; Meng, X.; Hu, Z.; Li, Z.; Yuan, C.; Zhou, T.; Han, X.; Ma, W. A novel schiff base fluorescent probe based on coumarin and benzothiazole for sequential detection of Al3+ and PPi and its applicability in live cell imaging. J. Photochem. Photobiol. A Chem. 2020, 392, 112427. [Google Scholar] [CrossRef]
- Fu, J.; Li, B.; Mei, H.; Chang, Y.; Xu, K. Fluorescent schiff base probes for sequential detection of Al3+ and F− and cell imaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117678. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Liu, T.T.; Fu, H.; Li, N.N.; Xing, Z.Y.; Yang, F. A Naphthalimide-Based Fluorescence “Off-on-Off” Chemosensor for Relay Detection of Al3+ and ClO−. Front. Chem. 2019, 7, 549. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.X.; Lai, J.P.; Sun, H.; Wu, W.Z. A simple, highly selective and ultra-sensitive “off-on-off” fluorescent chemosensor for successive detection of aluminum ion and phosphate in water samples. Microchem. J. 2019, 151, 104195. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, X.P.; Liang, G.Y.; Zhong, K.P.; Yao, H.; Wei, T.B.; Lin, Q. A water-soluble fluorescent chemosensor based on Asp functionalized naphthalimide for successive detection Fe3+ and H2PO4−. Can. J. Chem. 2018, 96, 363–370. [Google Scholar] [CrossRef]
- Meng, X.; Li, S.; Ma, W.; Wang, J.; Hu, Z.; Cao, D. Highly sensitive and selective chemosensor for Cu2+ and H2PO4− based on coumarin fluorophore. Dyes Pigments 2018, 154, 194–198. [Google Scholar] [CrossRef]
- Purkait, R.; Das Mahapatra, A.; Chattopadhyay, D.; Sinha, C. An azine-based carbothioamide chemosensor for selective and sensitive turn-on-off sequential detection of Zn(II) and H2PO4−, live cell imaging and INHIBIT logic gate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, V.; Upadhyay, K.K. An Al3+ and H2PO4−/HSO4− selective conformational arrest and bail to a pyrimidine-naphthalene anchored molecular switch. Analyst 2013, 138, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Anu, D.; Naveenm, P.; Rajamanikandan, R.; Kaveri, M.V. Development of hydrazide based fluorescence probe for detection of Al3+ ions and application in live cell image. J. Photochem. Photobiol. A Chem. 2021, 405, 112921. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Shen, S.; Yan, Q.; Xia, G.; Wang, H. A squaraine-based fluorescence turn on chemosensor with ICT character for highly selective and sensitive detection of Al3+ in aqueous media and its application in living cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117590. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, L.; Bai, L.; Chen, A.; Li, N.; Cheng, L.; Liu, W.; Sun, X.; Tao, F.; Wang, L.; et al. A new water-soluble polymer fluorescent chemosensor with thiophene Schiff base site for selectively sensing Al3+ ions. Tetrahedron 2021, 79, 131888. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Wang, J.; Jing, X.; Wang, S.; Xiao, L.; Li, L. A fluorescent light-up probe for selective detection of Al3+ and its application in living cell imaging. Inorg. Chim. Acta 2020, 500, 119231. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, Y.; Han, S.F.; Wang, D.; Zheng, J.Q.; Zheng, X.J.; Jin, L.P. A novel acylhydrazone-based derivative as dual-mode chemosensor for Al3+, Zn2+ and Fe3+ and its applications in cell imaging. Sens. Actuators B Chem. 2017, 244, 914–921. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, Y.; Wang, C.; Lu, L.; Sun, T.; Wang, M.; Tang, Y.; Shan, D.; Wen, S.; Zhu, J. A novel coumarin-based fluorescence chemosensor for Al3+ and its application in cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 105–110. [Google Scholar] [CrossRef]
- Das, A.K.; Goswami, S. 2-Hydroxy-1-naphthaldehyde: A versatile building block for the development of sensors in supramolecular chemistry and molecular recognition. Sens. Actuators B Chem. 2017, 245, 1062–1125. [Google Scholar] [CrossRef]
- Immanuel David, C.; Bhuvanesh, N.; Jayaraj, H.; Thamilselvan, A.; Parimala Devi, D.; Abiram, A.; Prabhu, J.; Nandhakumar, R. Experimental and Theoretical Studies on a Simple S-S-Bridged Dimeric Schiff Base: Selective Chromo-Fluorogenic Chemosensor for Nanomolar Detection of Fe2+ & Al3+ Ions and Its Varied Applications. ACS Omega 2020, 5, 3055–3072. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Dolai, M.; Dhara, A.; Ghosh, A.; Mabhai, S.; Misra, A.; Dey, S.; Jana, A. Solvent-Regulated Fluorimetric Differentiation of Al3+ and Zn2+ Using an AIE-Active Single Sensor. J. Phys. Chem. A 2021, 125, 1490–1504. [Google Scholar] [CrossRef]
- Singh, R.; Das, G. “Turn-on” Pb2+ sensing and rapid detection of biothiols in aqueous medium and real samples. Analyst 2019, 144, 567–572. [Google Scholar] [CrossRef]
- Yang, R.; Li, K.; Wang, K.; Zhao, F.; Li, N.; Liu, F. Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal. Chem. 2003, 75, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, T.G.; Bok, K.H.; Han, J.; Lim, M.H.; Kim, C. Colorimetric detection of Fe3+ and Fe2+ and sequential fluorescent detection of Al3+ and pyrophosphate by an imidazole-based chemosensor in a near-perfect aqueous solution. Dyes Pigments 2017, 139, 136–147. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, D.; Kim, C. An Acylhydrazone-Based Fluorescent Sensor for Sequential Recognition of Al3+ and H2PO4−. Materials 2021, 14, 6392. https://doi.org/10.3390/ma14216392
Choe D, Kim C. An Acylhydrazone-Based Fluorescent Sensor for Sequential Recognition of Al3+ and H2PO4−. Materials. 2021; 14(21):6392. https://doi.org/10.3390/ma14216392
Chicago/Turabian StyleChoe, Donghwan, and Cheal Kim. 2021. "An Acylhydrazone-Based Fluorescent Sensor for Sequential Recognition of Al3+ and H2PO4−" Materials 14, no. 21: 6392. https://doi.org/10.3390/ma14216392





