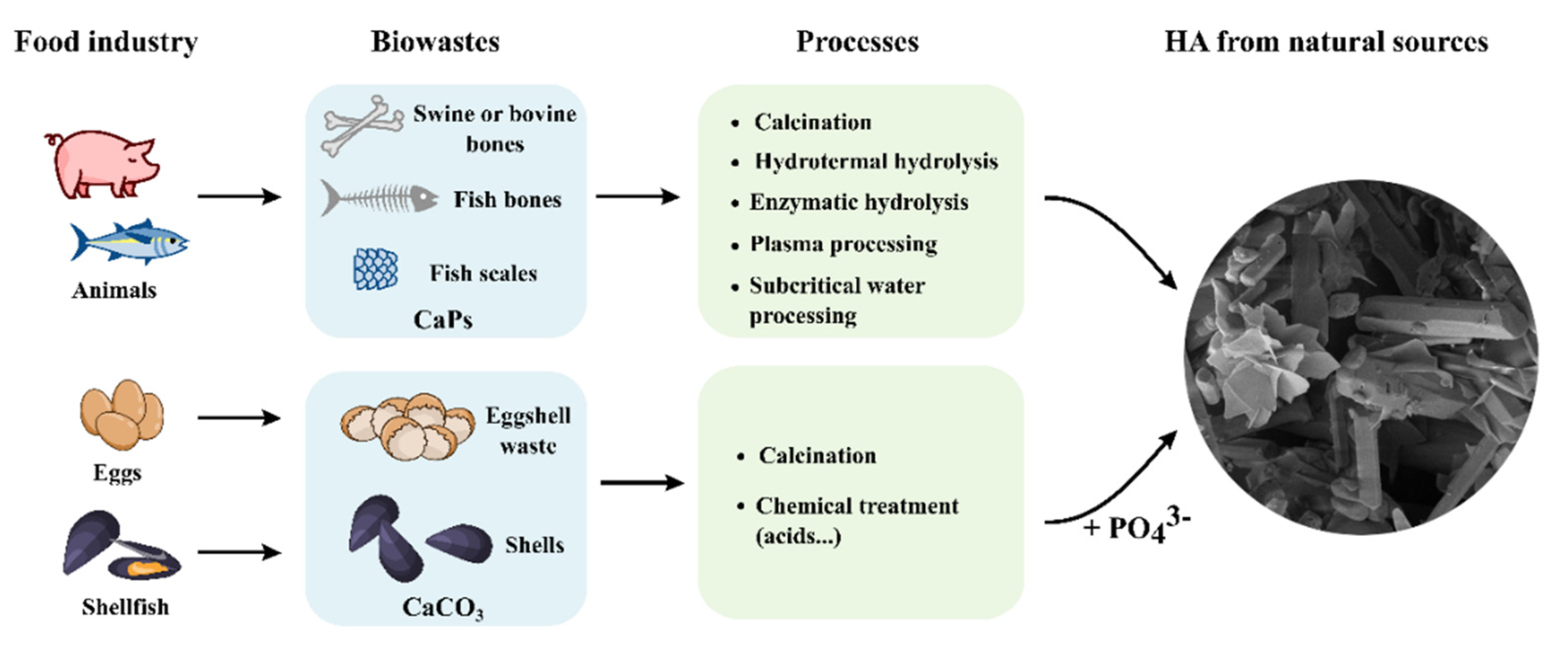
The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives
Abstract
:1. Introduction
2. Calcium Phosphates
2.1. Amorphous Calcium Phosphate
2.2. Hydroxyapatite
2.3. Octacalcium Phosphate
2.4. Tricalcium Phosphate
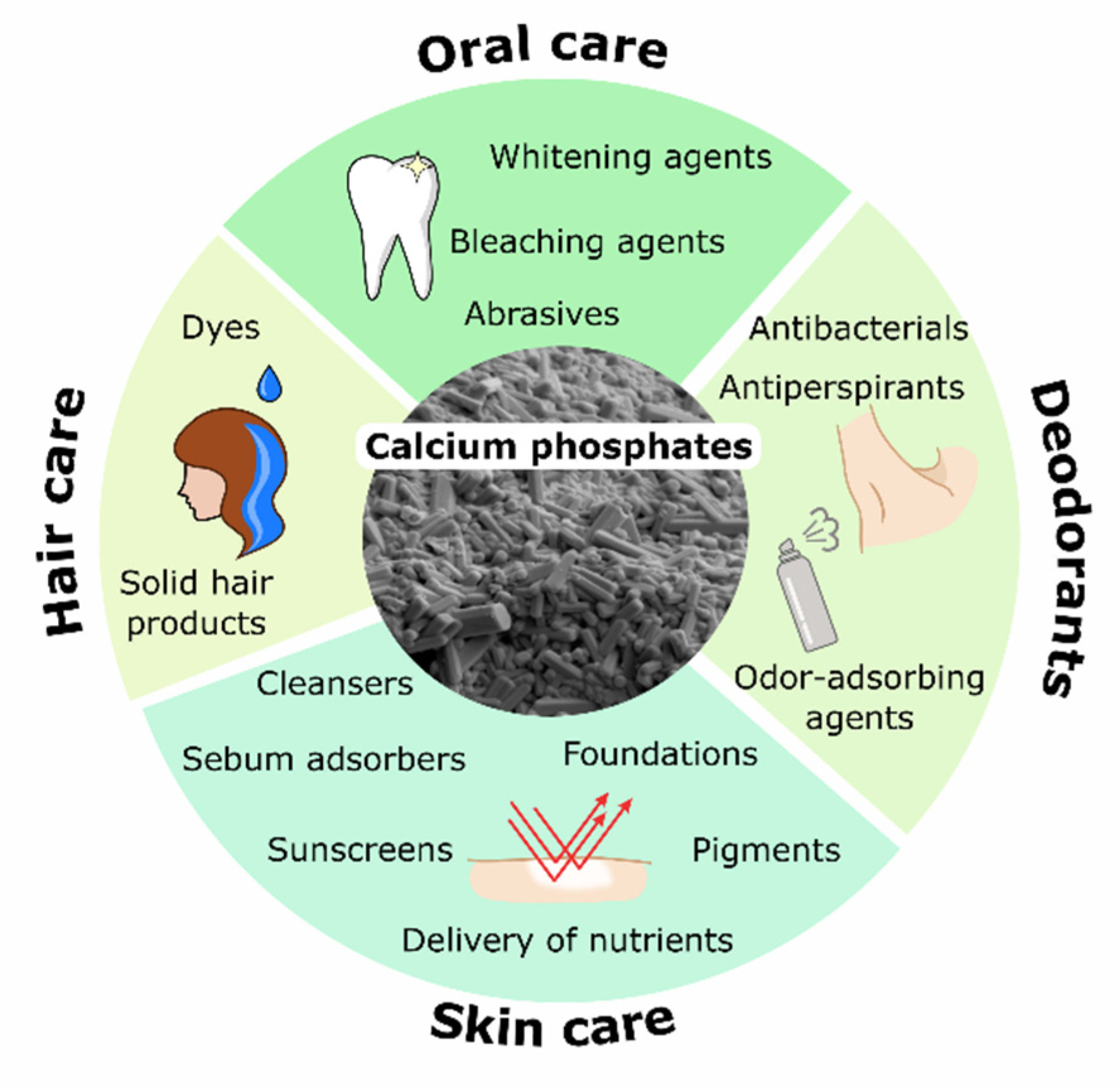
3. Applications of Calcium Phosphates in Cosmetics
3.1. Oral Care
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [132] | Set for tooth bleaching | Lion Corp | 2000 | Product for tooth whitening by stain abrasion. CaP: HA, fluorine or carbonate doped HA, TCP, OCP |
| [133] | Manufacturing method of hydroxyl apatite | Nippon Zettoc Co., Ltd. | 2001 | Coating varnish for tooth whitening. CaP: TCP + hydrogen phosphate ions |
| [140] | Rapid temporary tooth whitening composition | Colgate Palmolive Co | 2005 | Adhesive for tooth whitening by HA adhesion. CaP: HA |
| [125] | Dentifrice composition | Sangi Co., Ltd. | 2005 | Toothpaste for tooth whitening. CaP: HA |
| [141] | Dental whitening compositions | Discus Dental LLC | 2006 | Product for tooth whitening by stain abrasion and for desensitization and remineralization. CaP: HA, TCP, OCP |
| [142] | Instant tooth whitening with silicone resin and silicone adhesive | Colgate-Palmolive Company | 2006 | Product for tooth whitening. CaP: HA |
| [143] | Dental Whitening Compositions | Discus Dental LLC | 2008 | Product for tooth whitening by stain abrasion and for desensitization and remineralization. CaP: HA, TCP, OCP |
| [131] | Pastes for improving tooth using micro hydroxyapatite powders | Pusan National University Cooperation Foundation Ryu Su Chak | 2009 | Toothpaste for tooth whitening. CaP: HA |
| [148] | Oral composition | Sangi Co., Ltd. | 2005 | Product for tooth whitening and for remineralization. CaP: HA |
| [149] | Dental colorant | Zakrytoe Aktsionernoe Obshchestvo Opytno-ehksperimental’nyj Zavod “Vladmiva” | 2011 | Resin varnish for tooth dyeing. CaP: HA |
| [144] | Teeth whitening composition and method | OCSLabo—Oral Care Science Lab Sagl | 2011 | Product for tooth whitening by bleaching. CaP: HA |
| [145] | Compositions and methods for altering the color of teeth | Colgate Palmolive Co | 2007 | Product for tooth whitening by coating. CaP: HA |
| [126] | Total effect toothpaste and preparation method thereof | Yiwu Aishang Commodity Co., LTD. | 2011 | Toothpaste for tooth whitening. CaP: strontium doped HA, TCP |
| [150] | Mineral-enzyme complex for fortifying and whitening tooth enamel, oral hygiene composition and toothpaste | Belous, Elena Yurievna; Galimova, Anna Zufarovna; Maltabar, Svetlana Alekseevna; Obshchstvo S Ogranichennoj Otvetstvennostyu “Splat-Kosmetika” | 2014 | Product for tooth whitening by bleaching and for remineralization. CaP: HA |
| [151] | Brush structure with health-care effect | Nakata Tomoko; Tanaka Fumiko; Tanaka Kimiko | 2014 | Toothbrush bristle with whitening and antibacterial ingredients. CaP: HA |
| [146] | Tooth-whitening compositions comprising silicone polymer and methods therefor | Colgate Palmolive Co | 2005, 2014 | Product for tooth whitening. CaP: HA |
| [127] | Toothpaste for simultaneously cleaning, whitening, and restoring teeth and preparation method of toothpaste | Masson Group Co., Ltd. | 2013 | Toothpaste for tooth whitening by abrasion and remineralization. CaP: HA |
| [128] | Dentifrice composition comprising sintered hydroxyapatite | Glaxo Group Limited | 2015 | Toothpaste for tooth whitening by stain abrasion. CaP: HA (sintered) |
| [152] | Natural bacteriostatic tooth whitening powder and preparation method thereof | Qingdao Bright Medicine Hall Medical Treatment Co., Ltd. | 2016 | Powder for tooth whitening by stain abrasion. CaP: HA |
| [153] | Whitening gel composition based on natural agents | Universitatea “Babes Bolyai”—Institutul de cercetari in chimie “Raluca Ripan” Cluj-Napoca | 2017 | Gel for tooth whitening by bleaching. CaP: HA |
| [147] | Mineral-enzyme complex for strengthening and whitening tooth enamel, oral hygiene composition, and toothpaste | Elena Yurievna Belous, Svetlana Alekseevna Maltabar, Anna Zufarovna Galimova | 2017 | Product for tooth whitening by bleaching and for remineralization. CaP: HA |
| [134] | Tooth coating agent and compositions thereof | Medice Co., Ltd. | 2018 | Product for tooth whitening by coating. CaP: HA |
| [135] | Tooth cold-light whitening composition and application thereof | Jilin dengtaike Dentistry material Co., Ltd. | 2018 | Product for tooth whitening by bleaching and for remineralization. CaP: HA |
| [129] | Whitening toothpaste | Foshan Yuan Po Xin Technology Co., Ltd. | 2018 | Toothpaste for tooth whitening. CaP: HA |
| [130] | Whitening tooth paste capable of effectively removing stains on teeth and preparation method thereof | Anhui Wanchun Daily Chemical Co., Ltd. | 2019 | Toothpaste for tooth whitening. CaP: HA |
| [136] | Dental care product for tooth whitening | Credentis AG | 2016 | Product for tooth whitening by stain abrasion and for desensitization and remineralization. CaP: HA |
| [137] | Method for teeth cleaning by means of a composition in the form of powder based on hydroxyapatite | Mectron SPA | 2019 | Product for tooth whitening by air polishing. CaP: silica particles containing HA |
| [138] | Mineral-enzyme complex for strengthening and whitening tooth enamel, oral hygiene composition, and toothpaste | Obshchestvo S Ogranichennoj Otvetstvennostyu “Splat-Kosmeticka” | 2014 | Product for tooth whitening by bleaching and for remineralization. CaP: HA |
| [139] | Pearl whitening and refreshing toothpaste and preparation method thereof | Henan Shuiantinglan Cosmetics Co., Ltd. | 2020 | Product for tooth whitening by stain abrasion. CaP: HA |
3.2. Skin Care
3.2.1. Skin Protection—Sunscreen (UV Protection)
Synthetic Calcium Phosphates as Sunscreens
Natural Calcium Phosphates as Sunscreens
Patents about Calcium Phosphates as Sunscreens
3.2.2. Skin Cleaners
3.2.3. Skin Beautifying—(Make-Up)
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [286] | Powder cosmetics | Kose Corp | 2004 | Cosmetics powder product to be loaded on sponges and mats. CaP: HA and zinc oxide on a flaky powder |
| [278] | Cosmetics paper | Shiseido Co., Ltd. | 2002 | Sebum absorbing paper. CaP: HA |
| [279] | Solid powder cosmetics | Kose Corp | 2005 | Cosmetics powder product with make-up persistence and UV shielding. CaP: HA as sandwich between zinc oxide and platy powder |
| [297] | Therapeutic calcium phosphate particles in use for aesthetic or cosmetics medicine, and methods of manufacture and use | Biosante Pharmaceuticals, Inc. | 2006 | Anti-age cosmetics for topical application. CaP: non-disclosed |
| [223] | Coated powdery material and cosmetics containing the same | Pola Chem Ind INC | 2006 | Powder material for make-up of UV-shielding product. CaP: HA as layer with titanium oxide, alumina, silica |
| [295] | Stick-line cosmetics | Shiseido Co., Ltd. | 2007 | Stick-like cosmetics for lips. CaP: HA |
| [287] | Makeup cosmetics | Club Cosmetics Co., Ltd., Sekisui Plastics Co., Ltd., | 2007 | Make-up cosmetics product. CaP: ACP-coated glass flakes |
| [288] | Powdery cosmetics | Shiseido Co., Ltd. | 2008 | Cosmetics powder product as foundation or make-up base. CaP: HA or HA composites |
| [289] | Solid powder cosmetics | Pola Chem Ind INC | 2008 | Cosmetics powder product. CaP: HA coating on sericite |
| [280] | Cosmetics | Clover Cosmake: Kk, Sekisui Plastics Co., Ltd. | 2009 | Product for suppressing smearing of make-up. CaP: dicalcium phosphate mixed with resin particles |
| [294] | Cosmetics composition for lips | Amorepacific Corporation | 2010 | Cosmetics product for lips. CaP: HA |
| [298] | Topical formulations comprising hydroxyapatite particles for stimulation and maintenance of collagen fibers | Laboratory Skin Care, Inc. | 2010 | Product for stimulation and maintenance of collagen fibers of skin. CaP: HA (sintered) |
| [281] | Bright pigment and cosmetics composition using the same | Nippon Sheet Glass Co., Ltd. | 2010 | Bright pigment and stabilizer for make-up. CaP: HA |
| [296] | Collagen production enhancer | SofSera Corp | 2012 | Product for stimulation and maintenance of collagen fibers of skin. CaP: HA |
| [301] | Two-in-one mixed curative effect type cosmetics | Zhou Qinghai | 2013 | Whitening and freckle-removing product. CaP: not disclosed |
| [290] | Cosmetics material and cosmetics | Horie Kako Co., Ltd.; Kinki University; Sofusera Co., Ltd. | 2015 | Cream or make-up cosmetic products. CaP: HA (sintered) |
| [282] | Composite particle and a cosmetics composition containing the same | Chanel Perfume Beauty Company | 2015 | Product for suppressing smearing of make-up and sebum adsorption. CaP: ACP coating of resin particles |
| [287] | Makeup cosmetics | Shiseido Co., Ltd. | 2016 | Make-up colored product. CaP: HA |
| [302] | Preparation method and application of fibroblast growth factor covering lipide calcium phosphate nanoparticles | Guangzhou Jipeng Biotechnology Co., Ltd., Medical and Biological Technology Research and Development Center Jinan Univ G | 2016 | Product for drug delivery to the skin. CaP: non-disclosed lipid-coated CaP nanoparticles |
| [299] | Calcium carbonate complex | Kotegawa Sangyo Kk | 2017 | Oil adsorbing product. CaP: HA coating of calcium carbonate |
| [291] | Makeup cosmetics | Shiseido Co., Ltd. | 2018 | Make-up colored product. CaP: HA |
| [300] | Stable O/W-type pickering emulsion by using hydroxyapatite nano particles and preparation method thereof | Xuchang University | 2018 | Pickering emulsion stabilizer. CaP: HA |
| [303] | Collagen production promoting agent | Sofsera Corp | 2018 | Product for stimulation and maintenance of collagen fibers of skin. CaP: HA (sintered) |
| [283] | Powder cosmetics and makeup method | Mikimoto Seiyaku KK | 2018 | Product for make-up stabilization. CaP: HA |
| [292] | Preparation method of whitening and moisturizing cream containing bismuth oxychloride | Guangzhou Lakel Stem Cell Research Institute | 2020 | Whitening moisturizing cream. CaP: HA |
| [293] | Calcium phosphate cerium phosphor | Sakai Chem Ind Co., Ltd. | 2020 | Phosphorescent cosmetics product. CaP: Non-disclosed cerium doped CaP |
| [284] | Cosmetics | Kose Corp | 2020 | Base material for cosmetics stabilization. CaP: HA-zinc oxide composite |
| [285] | Composition with makeup maintaining and oil controlling effects and cosmetics | Guangdong Bawei Biotechnology Co ltd | 2020 | Base material for make-up stabilization and sebum adsorption. CaP: HA |
3.3. Hair Care
3.4. Deodorants
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosmetics Overview. Available online: https://www.fda.gov/industry/regulated-products/cosmeticss-overview (accessed on 20 October 2021).
- Matwiejczuk, N.; Galicka, A.; Brzóska, M.M. Review of the safety of application of cosmetic products containing parabens. J. Appl. Toxicol. 2019, 40, 176–210. [Google Scholar] [CrossRef]
- Mesko, M.F.; Novo, D.L.R.; Costa, V.C.; Henn, A.S.; Flores, E.M.M. Toxic and potentially toxic elements determination in cosmetics used for make-up: A critical review. Anal. Chim. Acta 2019, 1098, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Dávila, F. Beauty and the Body: The Origins of Cosmetics. Plast. Reconstr. Surg. 2000, 105, 1196–1204. [Google Scholar] [CrossRef]
- Karim-Cooper, F. Cosmetics. In The Cambridge Guide to the Worlds of Shakespeare; Cambridge University Press: New York, NY, USA, 2019; pp. 113–117. [Google Scholar] [CrossRef]
- de Ágredos Pascual, M.L.V.; Gamberini, M.C.; Walter, P. Perfumes and Cosmetics. In The Encyclopedia of Archaeological Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Murube, J. Ocular Cosmetics in Ancient Times. Ocul. Surf. 2013, 11, 2–7. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, H. The research status of cosmeticss and the establishment of biological beautiology. Life Sci. J. 2020, 17, 5–11. [Google Scholar]
- Bilal, M.; Iqbal, H.M. An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—A review. Sci. Total Environ. 2019, 670, 555–568. [Google Scholar] [CrossRef]
- Lim, D.S.; Roh, T.; Kim, M.K.; Kwon, Y.C.; Choi, S.M.; Kwack, S.J.; Kim, K.B.; Yoon, S.; Kim, H.S.; Lee, B.-M. Non-cancer, cancer, and dermal sensitization risk assessment of heavy metals in cosmetics. J. Toxicol. Environ. Health Part A 2018, 81, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- Germany Reduces Heavy Metal Limits in Cosmeticss. Available online: https://www.cosmeticssdesign-europe.com/Article/2017/07/25/Germany-reduces-heavy-metal-limits-in-cosmeticss?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright (accessed on 2 August 2021).
- Guidance on Heavy Metal Impurities in Cosmetics—Canada.ca. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guidance-heavy-metal-impurities-cosmeticss.html (accessed on 2 August 2021).
- Bashir, A.; Lambert, P. Microbiological study of used cosmetic products: Highlighting possible impact on consumer health. J. Appl. Microbiol. 2019, 128, 598–605. [Google Scholar] [CrossRef]
- Feizi, R.; Jaafarzadeh, N.; Akbari, H.; Jorfi, S. Evaluation of lead and cadmium concentrations in lipstick and eye pencil cosmetics. Environ. Health Eng. Manag. 2019, 6, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.D.; Alam, M.N. Cosmetics and their associated adverse effects: A review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Mehmood, S.; Iqbal, H.M.N. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, M.; Masand, N.; Sahoo, J.; Patil, V.M. Risk Assessment of Cosmetic Preservatives Using QSAR. Int. J. Quant. Struct. Relatsh. 2020, 5, 44–62. [Google Scholar] [CrossRef]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- McLean, F.C.; Hinrichs, M.A. The formation and behavior of colloidal calcium phosphate in the blood. Am. J. Physiol. Content 1938, 121, 580–588. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Sun, R.; Åhlén, M.; Tai, C.-W.; Bajnóczi, É.G.; De Kleijne, F.; Ferraz, N.; Persson, I.; Strømme, M.; Cheung, O. Highly Porous Amorphous Calcium Phosphate for Drug Delivery and Bio-Medical Applications. Nanomaterials 2019, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, Z.; Qing, F.; Hong, Y.; Zhang, X. Applications of calcium phosphate nanoparticles in porous hard tissue engineering scaffolds. Nano 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; McCarthy, H.; Montufar, E.; Ginebra, M.-P.; Wilson, D.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; A Reynolds, M. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.-P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Boehm, A.V.; Meininger, S.; Gbureck, U.; Müller, F.A. Self-healing capacity of fiber-reinforced calcium phosphate cements. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Makkar, P.; Kang, H.J.; Padalhin, A.R.; Faruq, O.; Lee, B. In-vitro and in-vivo evaluation of strontium doped calcium phosphate coatings on biodegradable magnesium alloy for bone applications. Appl. Surf. Sci. 2020, 510, 145333. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, J.; Lu, X.; Li, Z.; Wang, K.; Ren, F.; Wang, M.; Wang, Q.; Qian, B. Controllable phase transformation of fluoridated calcium phosphate ultrathin coatings for biomedical applications. J. Alloys Compd. 2020, 847, 155920. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Leòn, B.; Jahnsen, J.A. Thin Calcium Phosphate Coatings for Medical Implants; León, B., Jansen, J., Eds.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-77718-4. [Google Scholar]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- De Groot, K.; Wolke, J.G.C.; A Jansen, J. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Velino, C.; Carella, F.; Adamiano, A.; Sanguinetti, M.; Vitali, A.; Catalucci, D.; Bugli, F.; Iafisco, M. Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis. Front. Bioeng. Biotechnol. 2019, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Adamiano, A.; Wu, V.; Carella, F.; Lamura, G.; Canepa, F.; Tampieri, A.; Iafisco, M.; Uskoković, V. Magnetic calcium phosphates nanocomposites for the intracellular hyperthermia of cancers of bone and brain. Nanomedicine 2019, 14, 1267–1289. [Google Scholar] [CrossRef]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Degli Esposti, L.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degli Esposti, L.; Carella, F.; Adamiano, A.; Tampieri, A.; Iafisco, M. Calcium phosphate-based nanosystems for advanced targeted nanomedicine. Drug Dev. Ind. Pharm. 2018, 44, 1223–1238. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Ideia, P.; Degli Esposti, L.; Miguel, C.C.; Adamiano, A.; Iafisco, M.; Castilho, P.C. Extraction and characterization of hydroxyapatite-based materials from grey triggerfish skin and black scabbardfish bones. Int. J. Appl. Ceram. Technol. 2020, 18, 235–243. [Google Scholar] [CrossRef]
- Scialla, S.; Carella, F.; Dapporto, M.; Sprio, S.; Piancastelli, A.; Palazzo, B.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Piccirillo, C. Mussel Shell-Derived Macroporous 3D Scaffold: Characterization and Optimization Study of a Bioceramic from the Circular Economy. Mar. Drugs 2020, 18, 309. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [Green Version]
- Drouet, C.; Bosc, F.; Banu, M.; Largeot, C.; Combes, C.; Dechambre, G.; Estournes, C.; Raimbeaux, G.; Rey, C. Nanocrystalline apatites: From powders to biomaterials. Powder Technol. 2009, 190, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.; Shore, R.C.; Wood, S.R.; Brookes, S.J.; Smith, D.A.M.; Wright, J.T.; Connell, S.; Kirkham, J. Subunit Structures in Hydroxyapatite Crystal Development in Enamel: Implications for Amelogenesis Imperfecta. Connect. Tissue Res. 2003, 44, 65–71. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Saito, M.; Uchino, T.; Senna, M.; Iafisco, M.; Prat, M.; Rimondini, L.; Otsuka, M. Preparation of injectable auto-forming alginate gel containing simvastatin with amorphous calcium phosphate as a controlled release medium and their therapeutic effect in osteoporosis model rat. J. Mater. Sci. Mater. Electron. 2012, 23, 1291–1297. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.-B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Central J. 2011, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Boskey, A.; Posner, A. Magnesium stabilization of amorphous calcium phosphate: A kinetic study. Mater. Res. Bull. 1974, 9, 907–916. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.G.G.; Bisaz, S.; Termine, J.D.; Posner, A.S. Influence of pyrophosphate on the transformation of amorphous to crystalline calcium phosphate. Calcif. Tissue Int. 1968, 2, 49–59. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous calcium phosphate materials: Formation, structure and thermal behaviour. J. Eur. Ceram. Soc. 2018, 39, 1642–1649. [Google Scholar] [CrossRef]
- Yengopal, V.; Mickenautsch, S. Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): A meta-analysis. Acta Odontol. Scand. 2009, 67, 321–332. [Google Scholar] [CrossRef]
- Iafisco, M.; Degli Esposti, L.; Rodriguez, G.B.R.; Carella, F.; Gomez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2015, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Kumta, P.N.; Sfeir, C.; Lee, D.-H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2019, 383, 121139. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O. Octacalcium phosphate: Osteoconductivity and crystal chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef]
- Komlev, V.S.; Fadeeva, I.V.; Fomin, A.S.; Shvorneva, L.I.; Ferro, D.; Barinov, S.M. Synthesis of octacalcium phosphate by precipitation from solution. Dokl. Chem. 2010, 432, 178–182. [Google Scholar] [CrossRef]
- Monma, H. Preparation of octacalcium phosphate by the hydrolysis of α-tricalcium phosphate. J. Mater. Sci. 1980, 15, 2428–2434. [Google Scholar] [CrossRef]
- Kotani, S.; Fujita, Y.; Kitsugi, T.; Nakamura, T.; Yamamuro, T.; Ohtsuki, C.; Kokubo, T. Bone bonding mechanism of β-tricalcium phosphate. J. Biomed. Mater. Res. 1991, 25, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, R.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Famery, R.; Richard, N.; Boch, P. Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994, 20, 327–336. [Google Scholar] [CrossRef]
- Xue, W.; Dahlquist, K.; Banerjee, A.; Bandyopadhyay, A.; Bose, S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J. Mater. Sci. Mater. Electron. 2008, 19, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate Cements and Concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.Y.; Lee, J.G.; Degli Esposti, L.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.-R.; Adamiano, A. Synergistic Release of Crop Nutrients and Stimulants from Hydroxyapatite Nanoparticles Functionalized with Humic Substances: Toward a Multifunctional Nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef] [Green Version]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Degli Esposti, L.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal conversion of fish bones into fertilizers and biostimulants for plant growth—A low tech valorization process for the development of circular economy in least developed countries. J. Environ. Chem. Eng. 2020, 9, 104815. [Google Scholar] [CrossRef]
- Adamiano, A.; Fellet, G.; Vuerich, M.; Scarpin, D.; Carella, F.; Piccirillo, C.; Jeon, J.-R.; Pizzutti, A.; Marchiol, L.; Iafisco, M. Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy. Molecules 2021, 26, 2810. [Google Scholar] [CrossRef]
- Espacenet. Available online: https://worldwide.espacenet.com/?locale=en_EP (accessed on 20 October 2021).
- Dorozhkin, S.V. Dental applications of calcium orthophosphates (CaPO4). J. Dent. Res. 2019, 1, 24–54. [Google Scholar]
- Enax, J.; Epple, M. Synthetic Hydroxyapatite as a Biomimetic Oral Care Agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar]
- LeGeros, R.Z. Apatites in biological systems. Prog. Cryst. Growth Charact. 1981, 4, 1–45. [Google Scholar] [CrossRef]
- Bosch, J.T.; Coops, J. Tooth Color and Reflectance as Related to Light Scattering and Enamel Hardness. J. Dent. Res. 1995, 74, 374–380. [Google Scholar] [CrossRef]
- Algarni, A.A.; Ungar, P.S.; Lippert, F.; Martínez-Mier, E.A.; Eckert, G.J.; González-Cabezas, C.; Hara, A.T. Trend-analysis of dental hard-tissue conditions as function of tooth age. J. Dent. 2018, 74, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Epple, M.; Meyer, F.; Enax, J. A Critical Review of Modern Concepts for Teeth Whitening. Dent. J. 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathoo, S.A. The chemistry and mechanisms of extrinsic and intrinsic discoloration. J. Am. Dent. Assoc. 1997, 128, 6S–10S. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J. Mechanisms of Stain Formation on Teeth, in Particular Associated with Metal Ions and Antiseptics. Adv. Dent. Res. 1995, 9, 450–456. [Google Scholar] [CrossRef]
- Alkahtani, R.; Stone, S.; German, M.; Waterhouse, P. A review on dental whitening. J. Dent. 2020, 100, 103423. [Google Scholar] [CrossRef]
- Joiner, A. Whitening toothpastes: A review of the literature. J. Dent. 2010, 38, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Thickett, E.; Cobourne, M.T. New developments in tooth whitening. The current status of external bleaching in orthodontics. J. Orthod. 2009, 36, 194–201. [Google Scholar] [CrossRef]
- Lewis, R.; Dwyer-Joyce, R.; Pickles, M. Interaction between toothbrushes and toothpaste abrasive particles in simulated tooth cleaning. Wear 2004, 257, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Lippert, F. An Introduction to Toothpaste—Its Purpose, History and Ingredients. Toothpastes 2013, 23, 1–14. [Google Scholar] [CrossRef]
- White, D.J. Development of an improved whitening dentifrice based upon “stain-specific soft silica” technology. J. Clin. Dent. 2001, 12, 25–29. [Google Scholar]
- Fearon, J. Tooth whitening: Concepts and controversies. J. Ir. Dent. Assoc. 2007, 53, 132–140. [Google Scholar]
- Akal, N.; Over, H.; Olmez, A.; Bodur, H. Effects of carbamide peroxide containing bleaching agents on the morphology and subsurface hardness of enamel. J. Clin. Pediatr. Dent. 2001, 25, 293–296. [Google Scholar]
- Mondelli, R.F.L.; Gabriel, T.R.C.G.; Rizzante, F.A.P.; Magalhães, A.C.; Bombonatti, J.F.S.; Ishikiriama, S.K. Do different bleaching protocols affect the enamel microhardness? Eur. J. Dent. 2015, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Markovic, L.; Jordan, R.A.; Lakota, N.; Gaengler, P. Micromorphology of Enamel Surface After Vital Tooth Bleaching. J. Endod. 2007, 33, 607–610. [Google Scholar] [CrossRef]
- Pinto, C.F.; De Oliveira, R.; Cavalli, V.; Giannini, M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz. Oral Res. 2004, 18, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Maier, M.; Gieren, A.-K.; Eliav, E. Tooth sensitivity during and after vital tooth bleaching: A systematic review on an unsolved problem. Quintessence Int. 2015, 46, 881–897. [Google Scholar] [CrossRef]
- Barkhordar, R.A.; Kempler, D.; Plesh, O. Effect of nonvital tooth bleaching on microleakage of resin composite restorations. Quintessence Int. 1997, 28, 341–344. [Google Scholar] [PubMed]
- Cullen, D.R.; Nelson, J.A.; Sandrik, J.L. Peroxide bleaches: Effect on tensile strength of composite resins. J. Prosthet. Dent. 1993, 69, 247–249. [Google Scholar] [CrossRef]
- De Boer, P.; Duinkerke, A.; Arends, J. Influence of Tooth Paste Particle Size and Tooth Brush Stiffness on Dentine Abrasion in vitro. Caries Res. 1985, 19, 232–239. [Google Scholar] [CrossRef]
- Harte, D.; Manly, R. Four Variables Affecting Magnitude of Dentrifice Abrasiveness. J. Dent. Res. 1976, 55, 322–327. [Google Scholar] [CrossRef]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening Effects of a Novel Oral Care Gel with Biomimetic Hydroxyapatite: A 4-Week Observational Pilot Study. Biomimetics 2020, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Dabanoglu, A.; Wood, C.; García-Godoy, F.; Kunzelmann, K.-H. Whitening effect and morphological evaluation of hydroxyapatite materials. Am. J. Dent. 2009, 22, 23–29. [Google Scholar] [PubMed]
- Hojabri, N.; Kaisarly, D.; Kunzelmann, K.-H. Adhesion and whitening effects of P11-4 self-assembling peptide and HAP suspension on bovine enamel. Clin. Oral Investig. 2020, 25, 3237–3247. [Google Scholar] [CrossRef]
- Jin, J.; Xu, X.; Lai, G.; Kunzelmann, K.-H. Efficacy of tooth whitening with different calcium phosphate-based formulations. Eur. J. Oral Sci. 2013, 121, 382–388. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, S.H.; Jang, S.; Kim, K.N.; Kwon, H.; Park, Y.D. Tooth Whitening Effect of Toothpastes Containing Nano-Hydroxyapatite. Key Eng. Mater. 2006, 309–311, 541–544. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Park, D.-O.; Jung, Y.-S.; Song, K.-B. Evaluation of the whitening and remineralization effects of a mixture of amorphous calcium phosphate, hydroxyapatite and tetrasodium pyrophosphate on bovine enamel. J. Korean Acad. Oral Health 2016, 40, 92. [Google Scholar] [CrossRef] [Green Version]
- Sarembe, S.; Enax, J.; Morawietz, M.; Kiesow, A.; Meyer, F. In Vitro Whitening Effect of a Hydroxyapatite-Based Oral Care Gel. Eur. J. Dent. 2020, 14, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Flessa, H.-P.; Xu, X.; Kunzelmann, K.-H. Hydroxyapatite and Self-Assembling Peptide Matrix for Non-Oxidizing Tooth Whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar] [PubMed]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Mellgren, T.; Jefferies, S.; Xia, W.; Engqvist, H. A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres. Dent. J. 2016, 5, 3. [Google Scholar] [CrossRef]
- Mellgren, T.; Qin, T.; Öhman-Mägi, C.; Zhang, Y.; Wu, B.; Xia, W.; Engqvist, H. Calcium Phosphate Microspheres as a Delivery Vehicle for Tooth-Bleaching Agents. J. Dent. Res. 2017, 97, 283–288. [Google Scholar] [CrossRef]
- Khurana, R.; Tredwin, C.J.; Weisbloom, M.; Moles, D.R. A clinical evaluation of the individual repeatability of three commercially available colour measuring devices. Br. Dent. J. 2007, 203, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, O.; Burrow, M.; Tyas, M. Effects of conditioners on microshear bond strength to enamel after carbamide peroxide bleaching and/or casein phosphopeptide–amorphous calcium phosphate (CPP–ACP) treatment. J. Dent. 2007, 35, 862–870. [Google Scholar] [CrossRef]
- Giniger, M.; Macdonald, J.; Ziemba, S.; Felix, H. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. J. Am. Dent. Assoc. 2005, 136, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.D.; Ram, S.M.; Shetty, O.; Chand, P.; Yadav, R. Efficacy of casein phosphopeptide-amorphous calcium phosphate to prevent stain absorption on freshly bleached enamel: An in vitro study. J. Conserv. Dent. 2010, 13, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kwon, H.; Kim, B.I. Effect of nano-carbonate apatite to prevent re-stain after dental bleaching in vitro. J. Dent. 2011, 39, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.G.G.; De Vasconcelos, A.A.M.; Borges, B.C.D.; Vitoriano, J.D.O.; Alves-Junior, C.; Machado, C.T.; Dos Santos, A.J.S. Efficacy of in-office bleaching techniques combined with the application of a casein phosphopeptide-amorphous calcium phosphate paste at different moments and its influence on enamel surface properties. Microsc. Res. Tech. 2012, 75, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kiminori, A.; Tsutomu, I.; Shuji, S. Dentifrice Composition. JP2005320321A, 17 November 2005. [Google Scholar]
- Zhao, G. Total Effect Toothpaste and Preparation Method Thereof. CN102204875A, 29 August 2012. [Google Scholar]
- Jin, L.; Yiping, L. Toothpaste for Simultaneously Cleaning, Whitening and Restoring Teeth and Preparation Method of Toothpaste. CN103006445A, 8 October 2014. [Google Scholar]
- Lucas, R.A.; Smith, A.J.; Wang, C. Dentifrice Composition Comprising Sintered Hydroxyapatite. WO2015044156A1, 2 April 2015. [Google Scholar]
- Hua, D. Whitening Toothpaste. CN108478495A, 4 September 2018. [Google Scholar]
- Liu, N. Whitening Tooth Paste Capable of Effectively Removing Stains on Teeth and Preparation Method Thereof. CN109125178A, 4 January 2019. [Google Scholar]
- Ryu, S.C. Pastes for Improving Tooth Using Micro Hydroxyapatite Powders. KR20090035929A, 13 April 2009. [Google Scholar]
- Noriko, T.; Masahito, A.; Takashi, W. Set for Tooth Bleaching. JP2002193775A, 10 July 2002. [Google Scholar]
- Sugiyama, S.; Hirai, T. Manufacturing Method of Hydroxyl Apatite. JP2001348212A, 19 September 2001. [Google Scholar]
- Su, K.B.; Heon, K.S.; Wan, K.G.; Sun, L.H. Tooth Coating Agent and Compositions Thereof. CN108125827A, 8 June 2018. [Google Scholar]
- Ma, R.; Mu, Q.; Wu, H.; Yan, P.; Yin, R. Tooth Cold-light Whitening Composition and Application Thereof. CN108451793A, 28 August 2018. [Google Scholar]
- Hug, M.; Kunzelmann, K.-H.; Lysek, D.A. Dental Care Product for Tooth Whitening. CN105555255A, 12 July 2019. [Google Scholar]
- Colletti, C.; De Faveri, S.; Lelli, M.; Minutoli, S.; Roveri, N.; Sivieri, M. Method for Teeth Cleaning by Means of a Composition in the Form of Powder Based on Hydroxyapatite. WO2019224693A1, 28 November 2019. [Google Scholar]
- Belous, E.Y.; Galimova, A.Z.; Maltabar, S.A. Mineral-Enzyme Complex for Strengthening and Whitening Tooth Enamel, Oral Hygiene Composition, and Toothpaste. CA2882558A1, 27 April 2014. [Google Scholar]
- Chen, L. Pearl Whitening and Refreshing Toothpaste and Preparation Method Thereof. CN112006963A, 1 December 2020. [Google Scholar]
- Sayed, I.; Suman, C.; Michael, P. Rapid Temporary Tooth Whitening Composition. US2005036959A1, 17 February 2005. [Google Scholar]
- Hayman, R.; Quan, N.; MacDonald, J. Dental Whitening Compositions. US2006115437A1, 1 June 2006. [Google Scholar]
- Sayed, I.; Suman, K.C.; Suryakant, P.; Michael, P. Instant Tooth Whitening with Silicone Resin and Silicone Adhesive. WO2006071677A2, 24 August 2006. [Google Scholar]
- Hayman, R.; Quan, N.; MacDonald, J. Dental Whitening Compositions. US2008050408A1, 28 February 2008. [Google Scholar]
- Marino, E. Teeth Whitening Composition and Method. EP2399568A1, 28 December 2011. [Google Scholar]
- Prithwiraj, M.; Suman, K.C.; Sayed, I.; Pashkovski, E.; Prencipe, M. Compositions and Methods for Altering the Color of Teeth. CA2613796A1, 1 May 2012. [Google Scholar]
- Prithwiraj, M.; Suman, K.C.; Sayed, I.; Pashkovski, E.; Prencipe, M. Tooth-Whitening Compositions Comprising Silicone Polymer and Methods Therefor. CA2562098C, 8 July 2014. [Google Scholar]
- Belous, E.Y.; Maltabar, S.A.; Galimova, A.Z. Mineral-Enzyme Complex for Strengthening and Whitening Tooth Enamel, Oral Hygiene Composition, and Toothpaste. US2017119647A1, 4 May 2017. [Google Scholar]
- Kiminori, A.; Muneteru, S.; Shuji, S. Oral Composition. JP2005314266A, 15 May 2010. [Google Scholar]
- Vladimirovich, C.V.; Petrovich, C.V.; Fedorovna, P.V. Dental Colourant. RU2429814C1, 27 September 2011. [Google Scholar]
- Belous, E.Y.; Galimova, A.Z.; Maltabar, S.A. Mineral-Enzime Complex for Fortifying and Whitening Tooth Enamel, Oral Hygiene Composition and Toothpaste. WO2014031035A1, 27 February 2014. [Google Scholar]
- Nakata, T.; Tanaka, F.; Tanaka, K. Brush Structure with Health-Care Effect. TW201412269A, 1 April 2014. [Google Scholar]
- Chao, H.; Yuqiang, L.; Hongmei, L.; Zhenchao, P.; Nannan, W.; Maoli, Y.; Qianqian, Z. Natural Bacteriostatic Tooth Whitening Powder and Preparation Method Thereof. CN106176284A, 7 December 2016. [Google Scholar]
- Moldovan, M.; Prejmerean, C.; Prodan, D.; Cuc, S.; Dudea, D.; Sarosi, C.; Silaghi-Dumitrescu, L. Whitening Gel Composition Based on Natural Agents. RO131614A2, 30 July 2017. [Google Scholar]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control. 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Marks, R. The stratum corneum barrier: The final frontier. J. Nutr. 2004, 134, 2017S–2021S. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Boelsma, E.; van de Vijver, L.P.; Goldbohm, R.A.; Klöpping-Ketelaars, I.A.; Hendriks, H.F.; Roza, L. Human skin condition and its associations with nutrient concentrations in serum and diet. Am. J. Clin. Nutr. 2003, 77, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Park, K. Role of Micronutrients in Skin Health and Function. Biomol. Ther. 2015, 23, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Johnson, A.W. Overview: Fundamental skin care--protecting the barrier. Dermatol. Ther. 2004, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abels, C.; Angelova-Fischer, I. Skin Care Products: Age-Appropriate Cosmetics. Curr. Probl. Dermatol. 2018, 54, 173–182. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Mbeng, W.O. Plants used for cosmetics in the Eastern Cape Province of South Africa: A case study of skin care. Pharmacogn. Rev. 2018, 12, 139. [Google Scholar] [CrossRef]
- Shuji, S.; Keiichiro, K.; Kiminori, A. Ubiquinone-Containing Composition. JP2007161640A, 19 September 2012. [Google Scholar]
- Roveri, N.; Lelli, M.; Masetti, M.; Petraroia, S.; Begotti, S. Compositions Comprising Polyesters of Biological Origin and Biocompatible Inorganic Compounds, and Uses Thereof in the Cosmetics Field. US2019159997A1, 30 May 2019. [Google Scholar]
- Lee, H.J.; Nam, H.Y.; Lee, S.C. pH A Hybrid Nanoparticle Comprising Calcium Phosphate a Preparation Method Thereof and a Smart Delivery Vehicle for Loading and Delivery of Bioactive Agents. KR20190131797A, 27 November 2019. [Google Scholar]
- Jieyao, L.; Wenhan, L.; Yufei, W.; Naiyu, X.; Xueqin, Z.; Le, Z.; Wei, Z.; Yanqing, Z. Compound Composed of Aliphatic Polycarbonate and Inorganic Compound and Application of Compound in Related Fields of Cosmetics. CN111904892A, 10 November 2020. [Google Scholar]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Baldermann, C.; Weiskopf, D. Verhaltens- und Verhältnisprävention Hautkrebs. Der Hautarzt 2020, 71, 572–579. [Google Scholar] [CrossRef]
- Green, A.C.; Williams, G.; Logan, V.; Strutton, G.M. Reduced Melanoma After Regular Sunscreen Use: Randomized Trial Follow-Up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Pols, J.; Williams, G.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged Prevention of Squamous Cell Carcinoma of the Skin by Regular Sunscreen Use. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2546–2548. [Google Scholar] [CrossRef] [Green Version]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Blasco, J. (Eds.) Sunscreens in Coastal Ecosystems. In The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020; Volume 94, ISBN 978-3-030-56076-8. [Google Scholar]
- Serpone, N. Sunscreens and their usefulness: Have we made any progress in the last two decades? Photochem. Photobiol. Sci. 2021, 20, 189–244. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Pillai, S.K.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2019, 96, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a Source of Hydrogen Peroxide Production in Coastal Waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2018, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Fivenson, D.; Sabzevari, N.; Qiblawi, S.; Blitz, J.; Norton, B.B.; Norton, S.A. Sunscreens: UV filters to protect us: Part 2-Increasing awareness of UV filters and their potential toxicities to us and our environment. Int. J. Women’s Dermatol. 2020, 7, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Siller, A.; Blaszak, S.C.; Lazar, M.; Harken, E.O. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast. Surg. Nurs. 2018, 38, 158–161. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2020, 755, 142486. [Google Scholar] [CrossRef]
- Berardesca, E.; Zuberbier, T.; Viera, M.S.; Marinovich, M. Review of the safety of octocrylene used as an ultraviolet filter in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Rigano, L.; Lionetti, N. Nanobiomaterials in galenic formulations and cosmeticss. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 121–148. [Google Scholar]
- Bennat, C.; Goymann, M. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int. J. Cosmet. Sci. 2000, 22, 271–283. [Google Scholar] [CrossRef]
- Smijs, T.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety Evaluation of Sunscreen Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned Skin: An In Vitro and In Vivo Study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sá, R.G.; Arantes, T.M.; de Macedo, E.F.; Dona’, L.M.; Pereira, J.C.; Hurtado, C.R.; Varghese, R.J.; Oluwafemi, O.S.; Tada, D.B. Photoprotective activity of zirconia nanoparticles. Colloids Surf. B Biointerfaces 2021, 202, 111636. [Google Scholar] [CrossRef]
- Osmond, M.J.; McCall, M. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2009, 4, 15–41. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small Amounts of Zinc from Zinc Oxide Particles in Sunscreens Applied Outdoors Are Absorbed through Human Skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Millington, K.R.; Osmond, M.J.; McCall, M.J. Detecting free radicals in sunscreens exposed to UVA radiation using chemiluminescence. J. Photochem. Photobiol. B Biol. 2014, 133, 27–38. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Yu, W.W.; Oliva, B.L.; Contreras, E.Q.; Colvin, V.L. Photochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredients. J. Photochem. Photobiol. A Chem. 2013, 263, 24–33. [Google Scholar] [CrossRef]
- Tucci, P.; Porta, G.; Agostini, M.; Dinsdale, D.; Iavicoli, I.; Cain, K.; Finazzi-Agró, A.; Melino, G.; Willis, A. Metabolic effects of TiO2 nanoparticles, a common component of sunscreens and cosmetics, on human keratinocytes. Cell Death Dis. 2013, 4, e549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Sunscreen drug products for over-the-counter human use: Proposed rule. Fed. Regist. 2019, 84, 6204–6275. [Google Scholar]
- Narla, S.; Lim, H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem. Photobiol. Sci. 2019, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, Y.; Guo, C.; Hu, W.; Liu, K.; Wang, Y.; Zhu, T. Occurrence and behavior of four of the most used sunscreen UV filters in a wastewater reclamation plant. Water Res. 2007, 41, 3506–3512. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2015, 70, 265–288. [Google Scholar] [CrossRef]
- Nery, E.M.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. A short review of alternative ingredients and technologies of inorganic UV filters. J. Cosmet. Dermatol. 2020, 20, 1061–1065. [Google Scholar] [CrossRef]
- Pal, A.; Hadagalli, K.; Bhat, P.; Goel, V.; Mandal, S. Hydroxyapatite—A promising sunscreen filter. J. Aust. Ceram. Soc. 2019, 56, 345–351. [Google Scholar] [CrossRef]
- Amin, R.M.; Elfeky, S.; Verwanger, T.; Krammer, B. A new biocompatible nanocomposite as a promising constituent of sunscreens. Mater. Sci. Eng. C 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.; Pullar, R.; da Cruz, I.B.; Jorge, R.; Pintado, M.; Castro, P. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2012, 33, 103–110. [Google Scholar] [CrossRef]
- Coelho, T.M.; Nogueira, E.S.; Steimacher, A.; Medina, A.; Weinand, W.R.; Lima, W.M.; Baesso, M.L.; Bento, A.C. Characterization of natural nanostructured hydroxyapatite obtained from the bones of Brazilian river fish. J. Appl. Phys. 2006, 100, 094312. [Google Scholar] [CrossRef]
- Hadagalli, K.; Shenoy, S.; Shakya, K.R.; G, M.; Tarafder, K.; Mandal, S.; Basu, B. Effect of Fe3+ substitution on the structural modification and band structure modulated UV absorption of hydroxyapatite. Int. J. Appl. Ceram. Technol. 2020, 18, 332–344. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Piccirillo, C.; Tobaldi, D.M.; Pullar, R.; Labrincha, J.; Ferreira, M.; Castro, P.; Pintado, M.M. Effect of preparation and processing conditions on UV absorbing properties of hydroxyapatite-Fe2O3 sunscreen. Mater. Sci. Eng. C 2016, 71, 141–149. [Google Scholar] [CrossRef]
- Piccirillo, C.; Fernández-Arias, M.; Boutinguiza, M.; Tobaldi, D.M.; Del Val, J.; Pintado, M.M.; Pou, J. Increased UV absorption properties of natural hydroxyapatite-based sunscreen through laser ablation modification in liquid. J. Am. Ceram. Soc. 2018, 102, 3163–3174. [Google Scholar] [CrossRef]
- Piccirillo, C.; Rocha, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. A hydroxyapatite—Fe2O3 based material of natural origin as an active sunscreen filter. J. Mater. Chem. B 2014, 2, 5999–6009. [Google Scholar] [CrossRef]
- Cunha, C.S.; Castro, P.J.; Sousa, S.; Pullar, R.; Tobaldi, D.M.; Piccirillo, C.; Pintado, M.M. Films of chitosan and natural modified hydroxyapatite as effective UV-protecting, biocompatible and antibacterial wound dressings. Int. J. Biol. Macromol. 2020, 159, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, T.S.; O De Souza, S.; De Sousa, E.M.B. Effect of Zn2+, Fe3+ and Cr3+ addition to hydroxyapatite for its application as an active constituent of sunscreens. J. Phys. Conf. Ser. 2010, 249, 12012. [Google Scholar] [CrossRef]
- de Araujo, T.; de Souza, S.; Miyakawa, W.; de Sousa, E. Phosphates nanoparticles doped with zinc and manganese for sunscreens. Mater. Chem. Phys. 2010, 124, 1071–1076. [Google Scholar] [CrossRef]
- Rozaini, M.Z.H.; Hamzah, H.; Mohtar, N.F.; Razali, M.H.; Ibrahim, N.H. Characterization of Photoprotective Hydroxiapatite from Fringescale sardinella (Valenciennes, 1847) Bones as Natural Sunscreen for Cosmeceutical Treatments. Key Eng. Mater. 2018, 792, 67–73. [Google Scholar] [CrossRef]
- Rozaini, M.Z.H.; Hamzah, H.; Wai, C.P.; Razali, M.H.; Osman, U.M.; Anuar, S.T.; Soh, S.K.C.; Ghazali, S.R.B.; Ibrahim, N.H.; Fei, L.C.; et al. Calcium Hydroxyapatite-Based Marine Origin: Novel Sunscreen Materials for Cosmeceutical Treatments. Orient. J. Chem. 2018, 34, 2770–2776. [Google Scholar] [CrossRef] [Green Version]
- Ghazali, S.R.; Rosli, N.H.; Hassan, L.S.; Rozaini, M.Z.H.; Hamzah, H. Biocompatibility of Hydroxyapatite (HAp) derived from clamshell as active ingredients in sunscreen product. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012059. [Google Scholar] [CrossRef]
- Pyo, E.; Kim, Y.; Park, J.B.; Kwon, K.-Y. A Silver-doped Hydroxyapatite for an Active Sunscreen Material. Bull. Korean Chem. Soc. 2016, 37, 1395–1396. [Google Scholar] [CrossRef]
- Nishikawa, H. Thermal behavior of hydroxyapatite in structural and spectrophotometric characteristics. Mater. Lett. 2001, 50, 364–370. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Lee, S.; Woo, D.K.; Byun, J.-H.; Kwon, K.-Y. Synthesis and Morphological Characterization of Calcium Phosphates Prepared under Different NaOH Concentrations. Bull. Korean Chem. Soc. 2014, 35, 2241–2242. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Kaur, A.; Grenèche, J.-M.; Nedelec, J.-M.; Renaudin, G. Atomic scale modeling of iron-doped biphasic calcium phosphate bioceramics. Acta Biomater. 2017, 50, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Nedelec, J.-M.; Renaudin, G. On the effect of temperature on the insertion of zinc into hydroxyapatite. Acta Biomater. 2012, 8, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef] [PubMed]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2011, 32, 478–486. [Google Scholar] [CrossRef]
- Hernández-Cocoletzi, H.; Salinas, R.A.; Águila-Almanza, E.; Rubio-Rosas, E.; Chai, W.S.; Chew, K.W.; Mariscal-Hernández, C.; Show, P.L. Natural hydroxyapatite from fishbone waste for the rapid adsorption of heavy metals of aqueous effluent. Environ. Technol. Innov. 2020, 20, 101109. [Google Scholar] [CrossRef]
- Granito, R.N.; Renno, A.C.; Yamamura, H.; de Almeida, M.C.; Ruiz, P.L.M.; Ribeiro, D.A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Pu’Ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.I.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, M.; Suzuki, S. Microstructural Development of Natural Hydroxyapatite Originated from Fish-Bone Waste through Heat Treatment. J. Am. Ceram. Soc. 2004, 85, 1315–1317. [Google Scholar] [CrossRef]
- Nishimura, H.; Kuroda, A. Coated Powdery Material and Cosmetics Containing the Same. JP2006241012A, 14 September 2006. [Google Scholar]
- Anazawa, N.; Araseki, M.; Kumei, T.; Satonaka, K. Sunscreen Cosmetics. JP2009155332A, 16 July 2009. [Google Scholar]
- Joon-Byeong, C.; Cho, J. Organic-Inorganic Composite Powder, a Preparation Method Thereof, and a Use of the Same. KR20110062501A, 10 June 2011. [Google Scholar]
- Kalichem Italia SRL. Sunscreen Product Comprising Hydroxyapatite as Physical Filterrch Results. CN102361623A, 11 December 2013. [Google Scholar]
- Laboratory Skin Care Inc. Sunscreen Compositions Comprising Uniform, Rigid, Spherical, Nanoporous Calcium Phosphate Particles and Methods of Making and Using the Same. US2016015606A1, 21 January 2016. [Google Scholar]
- Universidade Catolica Portuguesa. UV-Filters, Method of Producing the Same and Their Use in Compositions, in Particular Sunscreens. US10314772B2, 11 June 2019. [Google Scholar]
- Tampieri, A.; Sandri, M.; Sprio, S. Physical Solar Filter Consisting of Substituted Hydroxyapatite in an Organic Matrix. WO2017153888A1, 14 September 2017. [Google Scholar]
- Kwon, K.-Y.; Choi, W.-G.; Jeong, K.; Young-Yong, K.; Pyo, E. Hydroxyapatite-Transition Metal Composite Preparation Method Thereof and Meterial for Blocking Ultraviolet Rays and Visible Rays Comprising the Same. KR101879395B1, 18 July 2018. [Google Scholar]
- Iafisco, M.; Adamiano, A.; Piccirillo, C. Physical Sunscreen Comprising Hydroxyapatite or Modified Hydroxyapatite Obtained from Fisheries and Aquaculture Waste, Process for its Production and Photoprotective Compositions Comprising It. WO2020193750A1, 1 October 2020. [Google Scholar]
- Hunan Yujia Cosmetics Manufacturing Co., Ltd. Calcium Phosphate-Folic Acid Composite Particles, Preparation Method and Application Thereof. CN112125291A, 25 December 2020. [Google Scholar]
- Corazza, M.; Lauriola, M.M.; Zappaterra, M.; Bianchi, A.; Virgili, A. Surfactants, skin cleansing protagonists. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Cleansers. J. Cosmet. Dermatol. 2017, 17, 8–14. [Google Scholar] [CrossRef]
- Fowler, J.F.; Eichenfield, L.F.; Elias, P.M.; Horowitz, P.; McLeod, R.P. The chemistry of skin cleansers: An overview for clinicians. Semin. Cutan. Med. Surg. 2013, 32, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Sawant, P. Potential Use of Spin Traps to Control ROS in Antipollution Cosmetics—A Review. Cosmetics 2018, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Kong, B.J.; Kwon, S.S.; Jang, H.N.; Park, S.N. Preparation and characterization of W/O microemulsion for removal of oily make-up cosmetics. Int. J. Cosmet. Sci. 2014, 36, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Ananthapadmanabhan, K.P.; Moore, D.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Seweryn, A.; Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.U. CXCVI—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Ascenso, A.; Simões, S.; Almeida, A.; Ribeiro, H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert Opin. Drug Deliv. 2016, 13, 1093–1107. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, B.; Carr, A.J.; Yu, X.; McClements, D.J.; Bhatia, S.R. Emulsions stabilized by inorganic nanoclays and surfactants: Stability, viscosity, and implications for applications. Inorganica Chim. Acta 2020, 508, 119566. [Google Scholar] [CrossRef] [PubMed]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, A.E.; Gauckler, L.J. Stabilization of Foams with Inorganic Colloidal Particles. Langmuir 2006, 22, 10983–10988. [Google Scholar] [CrossRef]
- Lb, N.; Almeida, L.; Marques, M.J.; Soares, G.; Ramakrishna, S. Emulsions Stabilization for Topical Application. Biomater. Med Appl. 2018, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Liang, Y.; He, Y. On the Pickering emulsions stabilized by calcium carbonate particles with various morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, L.-H.; Gao, J.; Cui, Z.-G.; Binks, B. Effect of trace impurities in triglyceride oils on phase inversion of Pickering emulsions stabilized by CaCO3 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 417, 126–132. [Google Scholar] [CrossRef]
- Cui, Z.-G.; Cui, C.-F.; Zhu, Y.; Binks, B.P. Multiple Phase Inversion of Emulsions Stabilized by in Situ Surface Activation of CaCO3 Nanoparticles via Adsorption of Fatty Acids. Langmuir 2011, 28, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Nunes, A.; Martins, A.M.; Carvalheira, J.; Prazeres, P.; Gonçalves, L.; Marques, A.; Lucas, A.; Ribeiro, H.M. Pickering Emulsions Stabilized by Calcium Carbonate Particles: A New Topical Formulation. Cosmetics 2020, 7, 62. [Google Scholar] [CrossRef]
- Hirota, K.; Nishihara, K. Creamy Apatite Face Cleanser. JP2002068962A, 8 March 2002. [Google Scholar]
- Kiminori, A.; Fujita, K.; Kikukawa, K. Cosmetics. JP2001302454A, 31 October 2001. [Google Scholar]
- Takei, M. Skin Care Preparation. JP2002080358A, 19 March 2002. [Google Scholar]
- Tetsuto, S.; Niide, C.; Kawamoto, K. Skin Care Preparation. JP2003267834A, 25 September 2003. [Google Scholar]
- Takamasa, H.; Tetsuto, S.; Okada, K.; Ono, E. Sebum Secretion Control Kit. JP2004107244A, 8 April 2004. [Google Scholar]
- Takeshi, A.; Masaaki, H. Sebum Adsorbing Powder and Use Thereof. JP2004315467A, 11 November 2004. [Google Scholar]
- Kumei, T. Porous Particle of Synthetic Resin Bonded with Hydroxy Apatite Particle, External Preparation and Cosmetics. JP2005041827A, 17 February 2005. [Google Scholar]
- Kumei, T. Synthetic Resin Porous Particle Combined with Hydroxyapatite Particle, External Preparation, and Cosmetics. JP2005112847A, 28 April 2005. [Google Scholar]
- Sun, Z. Application Method of Nano Calcium Phosphate Like Salt for Cosmetics Product. CN1813657A, 9 August 2008. [Google Scholar]
- Yagi, K.; Ono, K.; Saeki, T. Cosmetics. JP2007001970A, 11 January 2007. [Google Scholar]
- Taniguchi, T. Make-Up Composition. CN1921821A, 28 February 2002. [Google Scholar]
- Fuji, N.; Mori, T.; Sakuyama, H. Cosmetics for Cleaning Skin. JP2008137896A, 19 June 2006. [Google Scholar]
- Fuji, N.; Mori, T.; Sakuyama, H. Cosmetics for Cleansing Skin. JP2009062309A, 26 March 2009. [Google Scholar]
- Fuji, N.; Iwashita, S. Skin-Cleaning Agent Composition. JP2010260801A, 18 November 2010. [Google Scholar]
- Nishimei, S. Cosmetics and Cleansing Agent Having Detox Function. JP2011020928A, 3 February 2011. [Google Scholar]
- Tate, Y.; Sakaida, K.; Tsuji, N. Gommage Cosmetics Material. JP2012148984A, 9 August 2012. [Google Scholar]
- Gao, X.; Chen, J.; Pang, C. Biomass Nerve Soothing Facial Mask Liquid. CN109106619A, 1 January 2019. [Google Scholar]
- Kosmala, A.; Wilk, I.; Kassolik, K. Influence of makeup on the well-being and self-esteem of women. Nurs. Public Health 2019, 9, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Al-Samydai, A.; Abu Hajleh, M.N.; Othman, M.A.; Marie, D.; Altatar, E.; Taher, H.; Hamdan, R.; Alharairy, R.; Yousif, R.O.; Al—Samydai, M. Make-Up Effects: Psychological and Sociological Perspective. Int. J. Pharm. Res. 2021, 13. [Google Scholar] [CrossRef]
- Korichi, R.; Pelle-De-Queral, D.; Gazano, G.; Aubert, A. Why women use makeup: Implication of psychological traits in makeup functions. J. Cosmet. Sci. 2008, 59, 127–137. [Google Scholar] [PubMed]
- Fan, H.; Zhou, H.; Ma, C.; Huang, Y.; Li, Y.; Xia, Q. A Novel Method for the Improved Skin Whitening Effect Based on Nanostructured Lipid Carrier. Mol. Cryst. Liq. Cryst. 2014, 593, 232–242. [Google Scholar] [CrossRef]
- Nordin, F.N.M.; Aziz, A.; Zakaria, Z.; Radzi, C.W.J.W.M. A systematic review on the skin whitening products and their ingredients for safety, health risk, and the halal status. J. Cosmet. Dermatol. 2020, 20, 1050–1060. [Google Scholar] [CrossRef]
- Tang, K.; Lu, S.-Y.; Ma, D.-L.; Leung, C.-H.; Lee, S.-S.; Lin, S.-W.; Wang, H.-M.D. A Review on Common Ingredients of Periocular Cosmetics and Their Hazards. Curr. Org. Chem. 2015, 19, 30–38. [Google Scholar] [CrossRef]
- Benson, H.A.; Newton, A.-F.; Leite-Silva, V.R.; Leonardi, G.R. Powders in Cosmetic Formulations. In Cosmetic Formulation; CRC Press: Boca Raton, FL, USA, 2019; pp. 209–220. [Google Scholar]
- Johnson, N.F. Inhalation Toxicity of Talc. J. Aerosol Med. Pulm. Drug Deliv. 2021, 34, 79–107. [Google Scholar] [CrossRef]
- Hildick-Smith, G.Y. The biology of talc. Occup. Environ. Med. 1976, 33, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, T.; Steffen, J.E.; Tran, T.H.; Egilman, D.S. A Review of the Talc Industry’s Influence on Federal Regulation and Scientific Standards for Asbestos in Talc. New Solut. J. Environ. Occup. Health Policy 2021, 31, 152–169. [Google Scholar] [CrossRef]
- Fiume, M.M.; Boyer, I.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Talc as Used in Cosmetics. Int. J. Toxicol. 2015, 34, 66S–129S. [Google Scholar] [CrossRef]
- Bamford, E.; Grahn, A.; Århammar, C.; Ajaxon, I.; Annerén, C. Mesoporous magnesium carbonate for use in powder cosmetics. Int. J. Cosmet. Sci. 2020, 43, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shiseido Co., Ltd. Cosmetics Paper. KR100814997B1, 20 December 2002. [Google Scholar]
- Tanaka, S.; Motoi, Y. Solid Powder Cosmetics. JP2005298475A, 27 October 2005. [Google Scholar]
- Saeki, T.; Matsuno, T. Cosmetics. JP2009013111A, 22 January 2009. [Google Scholar]
- Kitamura, T. Bright Pigment and Cosmetics Composition Using the Same. US2010129412A1, 27 May 2010. [Google Scholar]
- Ito, H.; Yamaki, H.; Kuromiya, T.; Nagahiro, C.; Ishimori, F. Composite Particle and a Cosmetics Composition Containing the Same. CN105008434A, 28 October 2015. [Google Scholar]
- Tate, Y. Powder Cosmetics and Makeup Method. JP2018123087A, 9 August 2018. [Google Scholar]
- Kose Corp. Cosmetics. JP2020075870A, 21 May 2020. [Google Scholar]
- Shu, X. Composition with Makeup Maintaining and Oil Controlling Effects and Cosmetics. CN111529431A, 14 August 2020. [Google Scholar]
- Kawasaki, A.; Matsushita, A. Powder Cosmetics. JP2004262794A, 24 September 2004. [Google Scholar]
- Club Cosmetics Co., Ltd.; Sekisui Plastics Co., Ltd.; Shiseido Co., Ltd. Makeup Cosmetics 2007. JP2007217319A, 30 August 2007. [Google Scholar]
- Kanamaru, T.; Yagi, K.; Kurahashi, T. Powdery Cosmetics. JP2008184399A, 14 August 2008. [Google Scholar]
- Miyazawa, M. Solid Powder Cosmetics. JP2008297240A, 11 December 2008. [Google Scholar]
- Furuzono, T.; Horie, S.; Kawabe, K.K. Cosmetics Material and Cosmetics. JP2015024970A, 5 February 2015. [Google Scholar]
- Oohashi, S.; Hiruma, Y. Makeup Cosmetics. US2018008527A1, 11 January 2008. [Google Scholar]
- Zhang, Y. Preparation Method of Whitening and Moisturizing Cream Containing Bismuth Oxychloride. CN110934755A, 31 March 2020. [Google Scholar]
- Ogata, N.; Koizumi, T.; Sako, E.; Mori, K. Calcium Phosphate Cerium Phosphor. JP2020050733A, 2 April 2020. [Google Scholar]
- Lee, W.Y.; Kim, E.J.; Go, S.Y. Cosmetics Composition for Lips. KR101061344B1, 31 August 2011. [Google Scholar]
- Yagi, K.; Hosokawa, K.; Ono, K. Stick-Line Cosmetics. JP2007051110A, 1 March 2007. [Google Scholar]
- Kogai, Y.; Kawabe, K.K. Collagen Production Enhancer. EP2438922A1, 11 April 2012. [Google Scholar]
- Biosante Pharmaceuticals, I. Therapeutic Calcium Phosphate Particles in Use for Aesthetic or Cosmetics Medicine, and Methods of Manufacture and Use. WO2006050368A2, 3 May 2007. [Google Scholar]
- Mansouri, Z. Topical Formulations Comprising Hydroxyapatite Particles for Stimulation and Maintenance of Collagen Fibers. WO2010050980A1, 6 May 2010. [Google Scholar]
- Sugawara, K.; Abe, Y.; Ishii, S. Calcium Carbonate Complex. JP2017190256A, 19 October 2016. [Google Scholar]
- Xuchang University. Stable O/W-type Pickering Emulsion by Using Hydroxyapatite Nano Particles and Preparation Method Thereof. CN107595647A, 19 October 2018. [Google Scholar]
- Zhou, Q. Two-in-One Mixed Curative Effect Type Cosmetics. CN103126912A, 5 June 2013. [Google Scholar]
- Guangzhou Jiyuan Bio-technology Co., Ltd.; Medical and Biological Technology Research and Development Center; Jinan Univ. G. Preparation Method and Application of Fibroblast Growth Factor Covering Lipide Calcium Phosphate Nanoparticles. CN105878047A, 23 December 2014. [Google Scholar]
- Kokayu, Y.; Aoi, N.; Nomi, D.; Kawabe, K.K. Collagen Production Promoting Agent. JP2018033639A, 8 March 2008. [Google Scholar]
- Miranda-Vilela, A.L.; Botelho, A.J.; Muehlmann, L.A. An overview of chemical straightening of human hair: Technical aspects, potential risks to hair fibre and health and legal issues. Int. J. Cosmet. Sci. 2013, 36, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2013, 53, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Costa, C.; Gomes, A.; Matamá, T.; Cavaco-Paulo, A. Human Hair and the Impact of Cosmetics Procedures: A Review on Cleansing and Shape-Modulating Cosmetics. Cosmetics 2016, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Rei Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Alessandrini, A.; Piraccini, B. Essential of Hair Care Cosmetics. Cosmetics 2016, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Da Gama, R.M.; Baby, A.R.; Velasco, M.V.R. In Vitro Methodologies to Evaluate the Effects of Hair Care Products on Hair Fiber. Cosmetics 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.F.R.G.; Pichler, J.; Adriano, A.R.; Cecato, P.; de Almeida, A.M. The shampoo pH can affect the hair: Myth or Reality? Int. J. Trichol. 2014, 6, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, J.; Landriscina, A.; Friedman, A. Nanotechnology-Based Cosmetics for Hair Care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Inorganic nanoparticles for targeted drug delivery. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–373. [Google Scholar]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Liao, Y.; Pathak, J.L.; Li, H.; Liu, Y. Biomimetic Calcium Phosphate Coating as a Drug Delivery Vehicle for Bone Tissue Engineering: A Mini-Review. Coatings 2020, 10, 1118. [Google Scholar] [CrossRef]
- Taisne, L.; Park, M.S.; Seo, E.J. Temporary Hair Dye Cosmetics Composition. KR20150006400A, 16 January 2015. [Google Scholar]
- Yumiko, S. Hair Dye Composition. JP2013075865A, 25 April 2013. [Google Scholar]
- Dormont, L.; Bessière, J.-M.; Cohuet, A. Human Skin Volatiles: A Review. J. Chem. Ecol. 2013, 39, 569–578. [Google Scholar] [CrossRef]
- Gautschi, M.; Natsch, A.; Schröder, F. Biochemistry of Human Axilla Malodor and Chemistry of Deodorant Ingredients. Chim. Int. J. Chem. 2007, 61, 27–32. [Google Scholar] [CrossRef]
- Laden, K. Antiperspirants and Deodorants; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9780429161247. [Google Scholar]
- Quartrale, R.P. The mechanism of antiperspirant action in eccrine sweat glands. In Antiperspirants Deodorants; Marcel Dekker Inc.: New York, NY, USA, 1988; pp. 89–110. ISBN 978-0824778934. [Google Scholar]
- Quatrale, R.P.; Waldman, A.; Rogers, J.; Felger, C. The mechanism of antiperspirant action by aluminum salts. I. The effect of cellophane tape stripping on aluminum salt-inhibited eccrine sweat glands. J. Soc. Cos. Chem. 1981, 32, 67–73. [Google Scholar]
- Wulf, R.J. Safety of Antiperspirant Salts. In Antiperspirants and Deodorants; CRC Press: Boca Raton, FL, USA, 1999; pp. 231–248. [Google Scholar]
- Pineau, A.; Fauconneau, B.; Sappino, A.-P.; Deloncle, R.; Guillard, O. If exposure to aluminium in antiperspirants presents health risks, its content should be reduced. J. Trace Elem. Med. Biol. 2014, 28, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Fergus, D.J.; Savage, A.M.; Ehlers, M.; Menninger, H.L.; Dunn, R.R.; Horvath, J.E. The effect of habitual and experimental antiperspirant and deodorant product use on the armpit microbiome. PeerJ 2016, 4, e1605. [Google Scholar] [CrossRef] [Green Version]
- Callewaert, C.; Hutapea, M.; Van De Wiele, T.; Boon, N. Deodorants and antiperspirants affect the axillary bacterial community. Arch. Dermatol. Res. 2014, 306, 701–710. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, Y.H.C. Surface Chemical Characteristics and Adsorption Properties of Apatite. In Adsorption on and Surface Chemistry of Hydroxyapatite; Springer US: Boston, MA, USA, 1984; pp. 129–149. [Google Scholar]
- Okazaki, M.; Yoshida, Y.; Yamaguchi, S.; Kaneno, M.; Elliott, J. Affinity binding phenomena of DNA onto apatite crystals. Biomaterials 2001, 22, 2459–2464. [Google Scholar] [CrossRef]
- Benaziz, L.; Barroug, A.; Legrouri, A.; Rey, C.; Lebugle, A. Adsorption of O-Phospho-L-Serine and L-Serine onto Poorly Crystalline Apatite. J. Colloid Interface Sci. 2001, 238, 48–53. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 2002, 23, 2817–2823. [Google Scholar] [CrossRef]
- Fleming, D.E.; Van Bronswijk, W.; Ryall, R.L. A comparative study of the adsorption of amino acids on to calcium minerals found in renal calculi. Clin. Sci. 2001, 101, 159–168. [Google Scholar] [CrossRef]
- Tsortos, A.; Nancollas, G.H. The Role of Polycarboxylic Acids in Calcium Phosphate Mineralization. J. Colloid Interface Sci. 2002, 250, 159–167. [Google Scholar] [CrossRef]
- Nishida, H.; Kimata, M.; Ogata, T.; Kawai, T. Malodors adsorption behavior of metal cation incorporated hydroxyapatite. J. Environ. Chem. Eng. 2017, 5, 2815–2819. [Google Scholar] [CrossRef]
- Nishida, H.; Kanno, I.; Kimata, M.; Ogata, T.; Kawai, T. Effect of chemical modification of hydroxyapatite/zeolite composite on malodors adsorption behavior. Phosphorus Res. Bull. 2019, 35, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Onoda, H.; Nakanishi, H.; Takenaka, A. Preparation of calcium phosphate with corbicula shells. J. Ecotechnol. Res. 2012, 16, 85–89. [Google Scholar]
- Rastrelli, G.; Rastrelli, F.; Tosti, G.; Deola, M.; Rigano, L. Safer sweat control. Hydroxyapatite as an Alternative AP/DEO ‘Active’. Cosmet. Toilet. 2020, 135, 58–64. [Google Scholar]
- Minoru, U.; Masaaki, M.; Kazuo, I.; Takuta, S.; Muneteru, S.; Shuji, S. Antimicrobial Fine Particle, Method for Producing the Same and Cosmetics or Antimicrobial Insecticide Containing the Antimicrobial Fine Particle. JP2005298418A, 27 October 2005. [Google Scholar]
- Shinji, I.; Kazuyoshi, B.; Keiko, I.; Kazuo, I. Non-Aqueous Powder Aerosol. JP2005314283A, 10 November 2005. [Google Scholar]
- Yasuyuki, S. Bactericidal/Deodorizing Agent. JP2007302586A, 13 February 2013. [Google Scholar]
- Masaaki, H. Body Odor Suppressing Agent and Cosmetics Product Compounded Therewith. JP2011178721A, 15 September 2011. [Google Scholar]
- Tianjin Zhongtian Jingke Technology Co., Ltd. Bentonite Deodorant. CN109010889A, 18 December 2018. [Google Scholar]
- Rastrelli, G.; Rastrelli, F. Antiperspirant and Deodorant Compositions. WO2020201103A1, 8 October 2020. [Google Scholar]


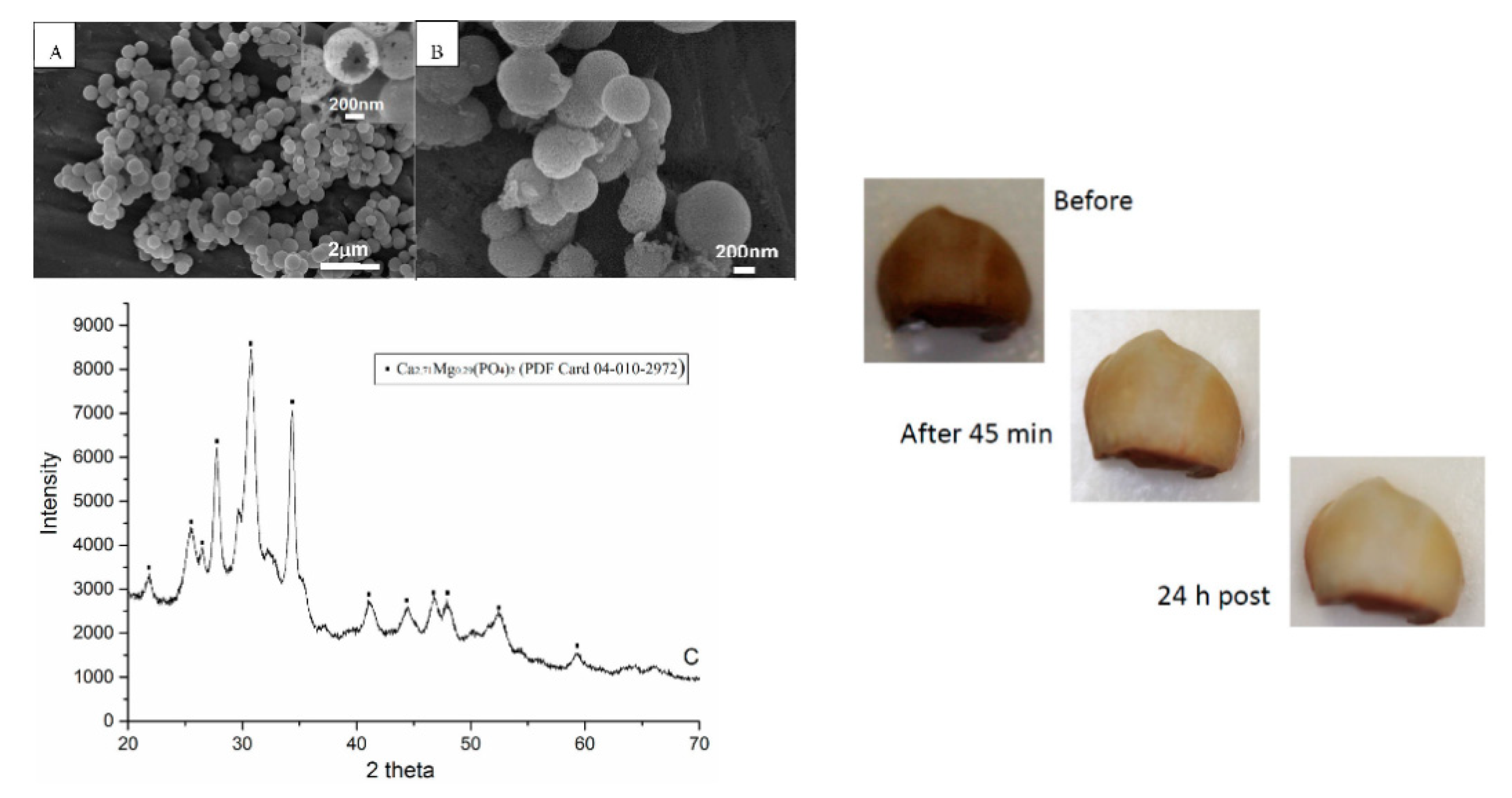
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [164] | Ubiquinone-containing composition | Sangi Co | 2007 | Base material for ubiquinone stabilization. CaP: HA |
| [165] | Compositions comprising polyesters of biological origin and biocompatible inorganic compounds, and uses thereof in the cosmetics field | Bio On SpA | 2019 | Base material for delivery of ingredients to the skin. CaP: HA composite with polymers |
| [166] | A pH hybrid nanoparticle comprising calcium phosphate a preparation method thereof and a smart delivery vehicle for loading and delivery of bioactive agents | University-Industry cooperation group of Kyung Hee University | 2019 | Product for skin. CaP: non-disclosed composite particle with CaP and polymers |
| [167] | Compound composed of aliphatic polycarbonate and inorganic compound and application of compound in related fields of cosmetics | Zhongkai University of Agriculture and Engineering | 2020 | Base material for delivery of ingredients to the skin. CaP: non-disclosed composite particle with HA and polycarbonate |
| Ref. | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [223] | Coated powdery material and cosmetics containing the same | Pola Chem Ind INC | 2006 | Powder for UV absorption. CaP: HA-coating on a multilayer material |
| [224] | Sunscreen cosmetics | Fancl Corp | 2009 | Sunscreen for UV absorption. CaP: HA |
| [225] | Organic-inorganic composite powder, a preparation method thereof, and a use of the same | Woongjin Coway Co., Ltd. | 2011 | Powder for UV absorption. CaP: HA-coating on a hollow particle |
| [226] | Sunscreen product comprising hydroxyapatite as physical filter | Kalichem Italia SRL | 2012 | Product for solar radiation protection. CaP: HA |
| [227] | Sunscreen Compositions Comprising Uniform, Rigid, Spherical, Nano porous Calcium Phosphate Particles and Methods of Making and Using the Same | Laboratory Skin Care Inc. | 2016 | Product for solar radiation protection. CaP: not disclosed |
| [228] | UV-filters, method of producing the same and their use in compositions, in particular sunscreens | Universidade Catolica Portuguesa | 2017 | Product for UV absorption in sunscreen and textiles. CaP: iron-doped HA + iron oxide |
| [229] | Physical solar filter consisting of substituted hydroxyapatite in an organic matrix | Consiglio Nazionale Delle Ricerche | 2017 | Product for solar radiation protection. CaP: HA doped with titanium and iron |
| [230] | Hydroxyapatite-transition metal composite preparation method thereof and material for blocking ultraviolet rays and visible rays comprising the same | Industry-Academic Cooperation foundation Gyeongsang National University | 2018 | Product for UV and Vis shielding. CaP: iron-doped HA + iron oxide |
| [231] | Physical sunscreen comprising hydroxyapatite or modified hydroxyapatite obtained from fisheries and aquaculture waste, process for its production and photoprotective compositions comprising it | Consiglio Nazionale Delle Ricerche | 2020 | Product for solar radiation protection and photoprotective boost effect. CaP: HA derived from natural sources doped with metal ions, TCP + metal oxides |
| [232] | Calcium phosphate-folic acid composite particles, preparation method and application thereof | Hunan Yujia Cosmetics Manufacturing Co ltd | 2020 | Product for UV absorption and scattering. CaP: non-disclosed composite with folic acid |
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [249] | Creamy apatite face cleanser | Kazushi Hirota, Katsunari Nishihara | 2002 | Skin cleaner cream. CaP: HA |
| [250] | Cosmetics | Sangi Co | 2001 | Product for skin renewal. CaP: amorphous HA |
| [251] | Skin care preparation | Noevir Co., Ltd. | 2002 | Product for skin renewing. CaP: HA |
| [252] | Skin care preparation | Fancl Corp | 2003 | Product for sebum control. CaP: HA |
| [253] | Sebum secretion control kit | Fancl Corp | 2004 | Product for sebum control. CaP: HA |
| [254] | Sebum adsorbing powder and use thereof | Miyoshi Kasei Inc. | 2004 | Powder for sebum adsorption and deodorant. CaP: zinc oxide-coated HA |
| [255] | Porous particle of synthetic resin bonded with hydroxy apatite particle, external preparation and cosmetics | Fancl Corp | 2005 | Product for sebum adsorption. CaP: HA coating on resin particles |
| [256] | Synthetic resin porous particle combined with hydroxyapatite particle, external preparation, and cosmetics | Fancl Corp | 2005 | Product for sebum adsorption. CaP: HA coating on resin particles |
| [257] | Application method of nano calcium phosphate like salt for cosmetics product | Sun Zhenlin | 2006 | Product for skin cleansing. CaP: TCP, HA, OCP, calcium pyrophosphates |
| [258] | Cosmetics | Sekisui Plastics Co., Ltd., Shiseido Co., Ltd., | 2007 | Product for sebum adsorption. CaP: ACP coating of mica particles |
| [259] | Make-up composition | Procter & Gamble | 2007 | Product for sebum control. CaP: HA coating of zinc oxide |
| [260] | Cosmetics for cleaning skin | Mandom Corp | 2008 | Product for skin cleansing, sebum removal, and skin smoothing. CaP: surface-modified HA |
| [261] | Cosmetics for cleansing skin | Mandom Corp | 2009 | Product for skin cleansing, sebum removal, and skin smoothing. CaP: surface-modified HA |
| [262] | Skin-cleaning agent composition | Mandom Corp | 2010 | Product for skin cleansing. CaP: HA |
| [263] | Cosmetics and cleansing agent having detox function | Kankyo Hozen Kenkyusho: Kk, | 2011 | Product for skin cleansing, and deodorant. CaP: non-disclosed CaP coating on titanium dioxide |
| [264] | Gommage cosmetics material | Mikimoto Pharmaceut Co., Ltd. | 2012 | Gommage product with sebum control. CaP: HA |
| [265] | Biomass nerve soothing facial mask liquid | Gao Xinwen | 2019 | Liquid for facial mask. CaP: HA |
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [315] | Temporary hair dye cosmetics composition | Cosmax INC | 2015 | Solid temporary hair dye product. CaP: HA |
| [316] | Hair dye composition | Kuriya Yumi: Kk | 2013 | Temporary hair dye product. CaP: HA particles adsorbed on titanium dioxide powder |
| Ref | Title | Applicants | Publication Year | Description |
|---|---|---|---|---|
| [254] | Sebum adsorbing powder and use thereof | Miyoshi Kasei Inc. | 2004 | Powder for sebum and fatty acids adsorption and deodorization. CaP: zinc oxide-coated HA |
| [336] | Antimicrobial fine particle, method for producing the same and cosmetics or antimicrobial insecticide containing the antimicrobial fine particle | Kyowa Industrial Co., Ltd., Sangi Co., Ltd., Suzuki Yushi Kogyo Kk | 2005 | Deodorant, antimicrobial, antiperspirant, sebum absorbing product. CaP: HA |
| [337] | Non-aqueous powder aerosol | Nivea Kao KK | 2005 | Deodorizing powder, specifically for low MW fatty acids. CaP: HA |
| [338] | Bactericidal/deodorizing agent | Asahi Shokai: Kk | 2007 | Deodorant and sterilizing product. CaP: silver-HA composite |
| [339] | Body odor suppressing agent and cosmetics product compounded therewith | Miyoshi Kasei Inc. | 2011 | Deodorizing powder. CaP: HA |
| [340] | Bentonite deodorant | Tianjin Zhongtian Jingke Technology Co., Ltd. | 2018 | Deodorant product. CaP: HA |
| [341] | Antiperspirant and deodorant compositions | Kalichem S.r.l. | 2020 | Antiperspirant and deodorant product. CaP: HA doped with aluminum, zinc, magnesium, zirconium, titanium, copper, silver, or iron |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carella, F.; Degli Esposti, L.; Adamiano, A.; Iafisco, M. The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives. Materials 2021, 14, 6398. https://doi.org/10.3390/ma14216398
Carella F, Degli Esposti L, Adamiano A, Iafisco M. The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives. Materials. 2021; 14(21):6398. https://doi.org/10.3390/ma14216398
Chicago/Turabian StyleCarella, Francesca, Lorenzo Degli Esposti, Alessio Adamiano, and Michele Iafisco. 2021. "The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives" Materials 14, no. 21: 6398. https://doi.org/10.3390/ma14216398
APA StyleCarella, F., Degli Esposti, L., Adamiano, A., & Iafisco, M. (2021). The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives. Materials, 14(21), 6398. https://doi.org/10.3390/ma14216398









