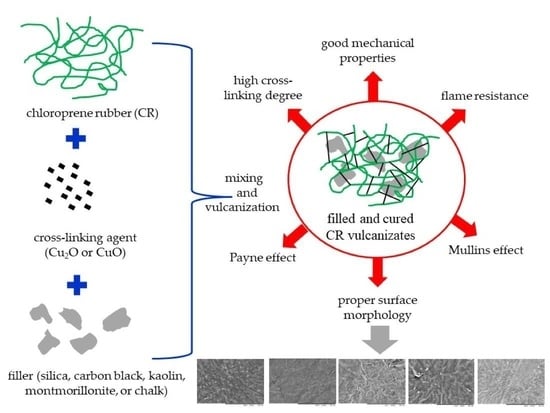
The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
- precipitated silica Arsil (Z. Ch. Rudniki S.A., Rudniki, Poland) with a bulk density of ~150 g/dm3 and pureness of >95%;
- technical chalk (POCH S.A., Gliwice, Poland) with a density of 2.71 g/cm3 and grain size of <10 µm (99.7%);
- technical kaolin (POCH S.A., Gliwice, Poland) with a density of 2.60 g/cm3 and an average grain size of 1,3 µm;
- nanofiller NanoBent, MMT (ZGM “Zębiec”, Zębiec, Poland), montmorillonite modified by ammonium salt-containing hydroxyl groups, with an average grain size from 20–60 µm (81%), ≤20 µm (19%), and a layer spacing from 3.8–3.9 nm;
- carbon black, CB (Chemical Worldwide Business Sp. z o. o., Słupca, Poland), with a bulk destiny of 380 g/dm3 and a surface area of 78 m2/g.
2.2. Research Methods
3. Results and Discussion
3.1. Morphology of Cross-Linking Agents and Fillers
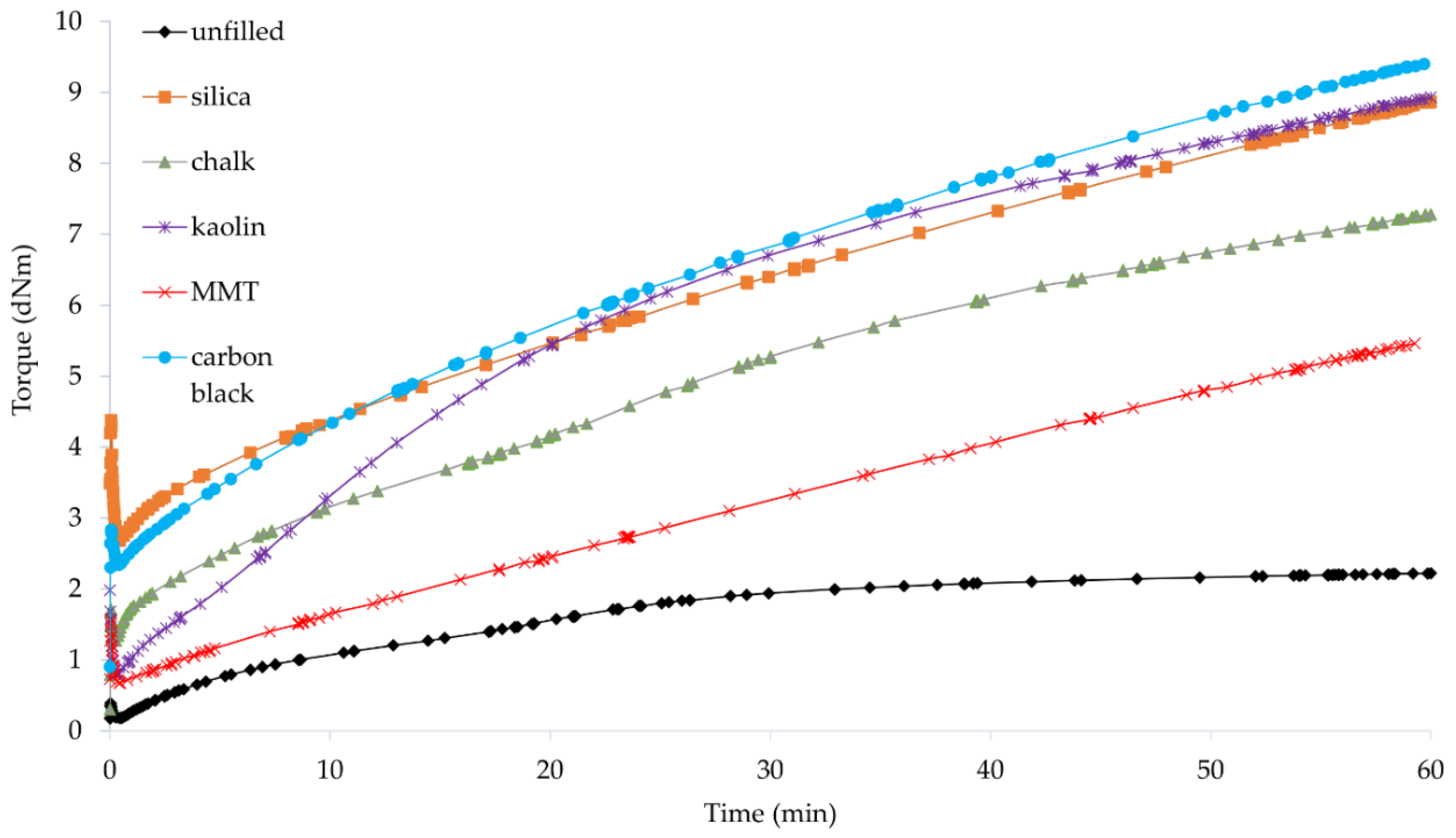
3.2. Vulcametric Parameters of Filled CR Compositions
3.3. Equilibrium Swelling of Filled CR Vulcanizates
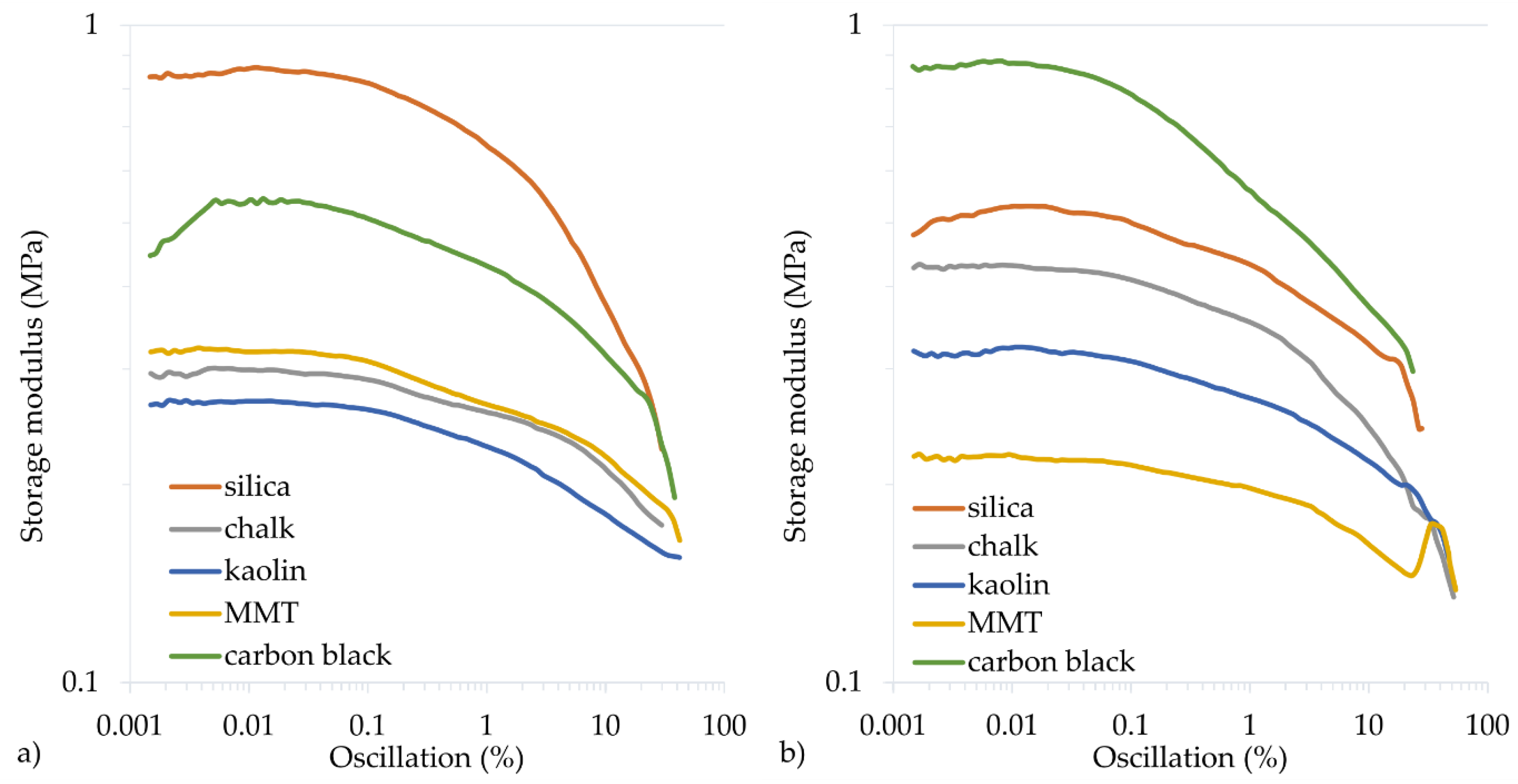
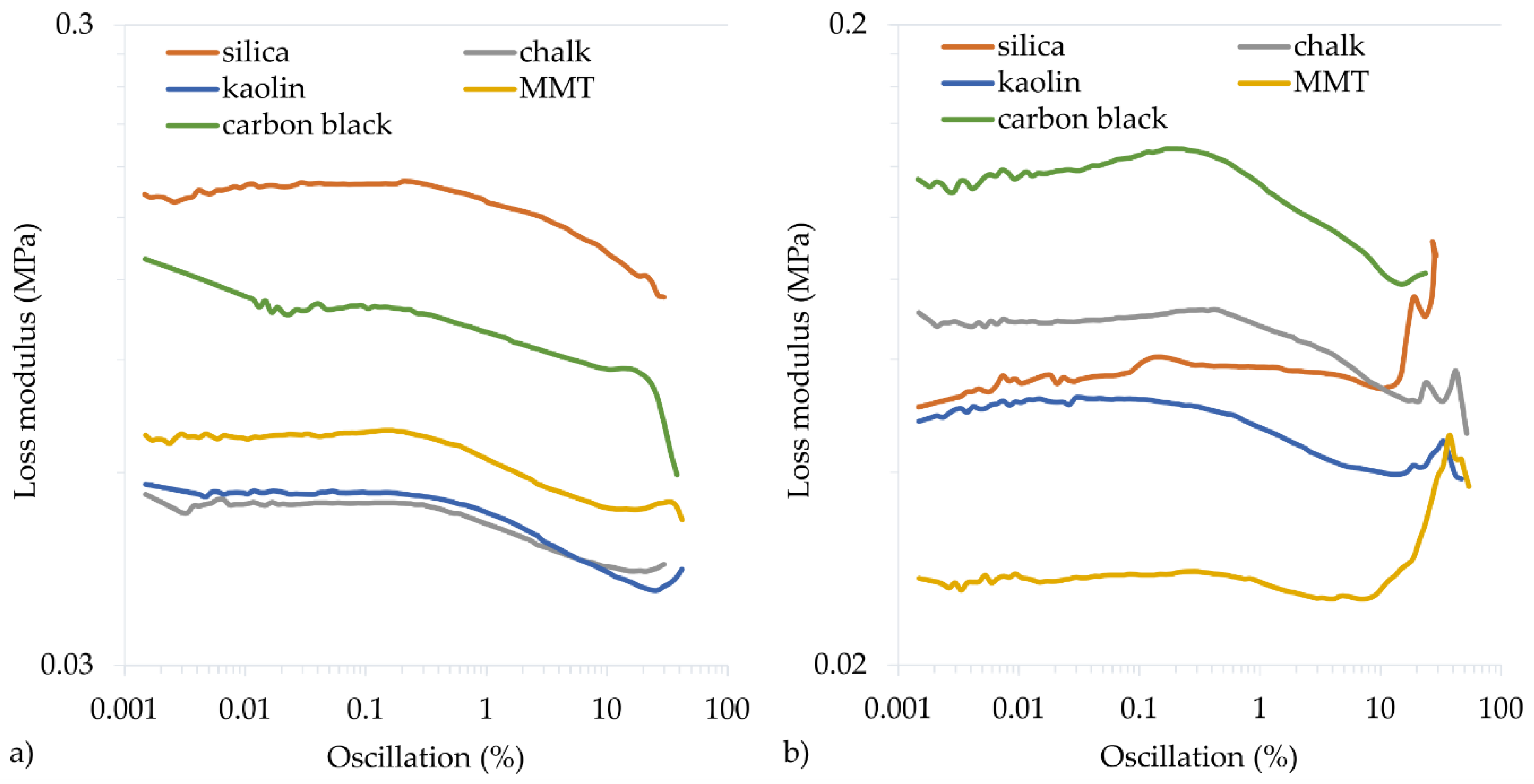
3.4. Mechanical and Dynamical Properties of Filled CR Vulcanizates
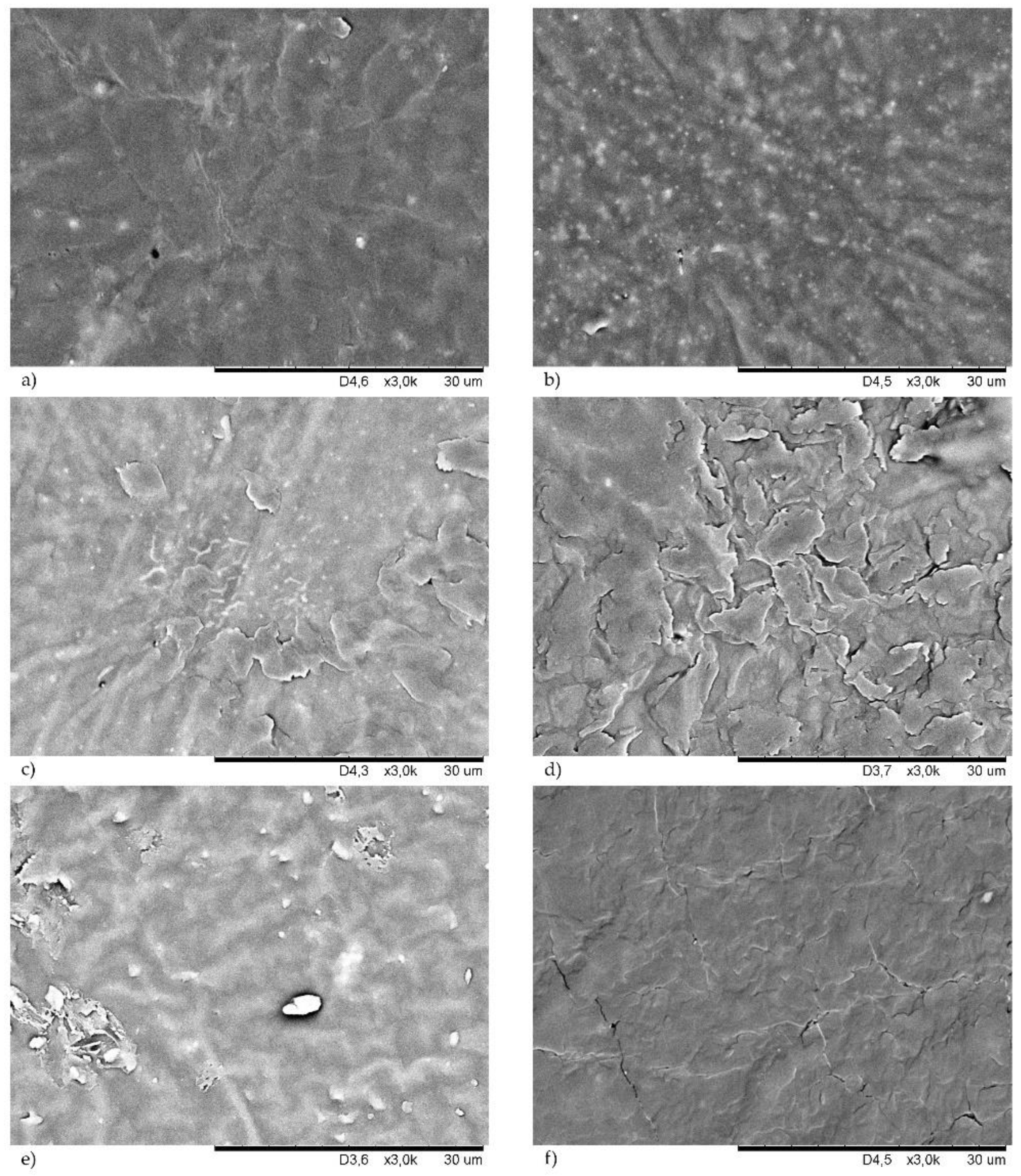
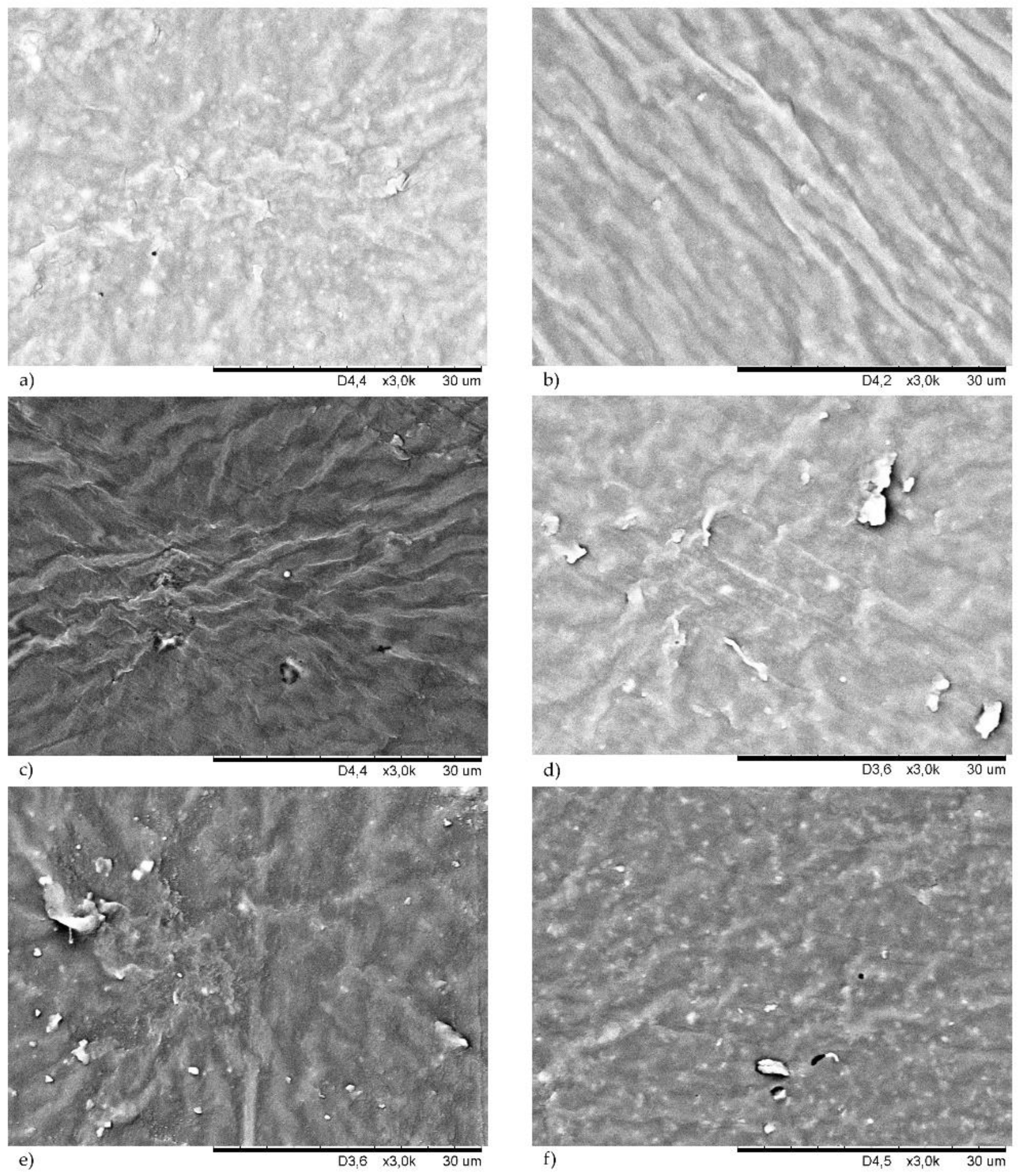
3.5. Morphology of Filled CR Vulcanizates
3.6. Flammability of Filled CR Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, P.; Nunes-Pereira, J.; Oliveira, J.; Silva, J.; Moreira, J.A.; Carabineiro, S.A.C.; Buijnsters, J.G.; Lanceros-Mendez, S. High-performance graphene-based carbon nanofiller/polymer composites for piezoresistive sensor applications. Compos. Sci. Technol. 2017, 153, 241–252. [Google Scholar] [CrossRef]
- Tessema, A.; Zhao, D.; Moll, J.; Xu, S.; Yang, R.; Li, C.; Kumar, S.K.; Kidane, A. Effect of filler loading, geometry, dispersion and temperature on thermal conductivity of polymer nanocomposites. Polym. Test. 2017, 57, 101–106. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Ishida, H. A review on the very high nanofiller-content nanocomposites: Their preparation methods and properties with high aspect ratio fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Dios, J.R.; García-Astrain, C.; Costa, P.; Viana, J.C.; Lanceros-Méndez, S. Carbonaceous Filler Type and Content Dependence of the Physical-Chemical and Electromechanical Properties of Thermoplastic Elastomer Polymer Composites. Materials 2019, 12, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroni, L.G.; Araujo, J.R.; Felisberti, M.I.; Nogueira, A.F. Nanocomposites based on MWCNT and styrene–butadiene–styrene block copolymers: Effect of the preparation method on dispersion and polymer–filler interactions. Compos. Sci. Technol. 2012, 72, 1487–1492. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Fillers. ChemTec Publishing: Toronto, ON, Canada, 1999; pp. 63–692. [Google Scholar]
- Nanda, M.; Tripathy, D.K. Physico-mechanical and electrical properties of conductive carbon black reinforced chlorosulfonated polyethylene vulcanizates. Express Polym. Lett. 2008, 2, 855–865. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Salim, Z.A.; Salim, A.; Hassan, A.; Ismail, H. A review on hybrid fillers in rubber composites. Polym. Plast. Technol. Eng. 2018, 6, 523–539. [Google Scholar] [CrossRef]
- Gaczyński, R. (Ed.) Guma. Poradnik Inżyniera i Technika; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 1981; pp. 147–148. [Google Scholar]
- White, J.R.; De, S.K. Rubber Technologiest’s Handbook; RAPRA Technology LTD.: Piastów, Poland, 2001; pp. 127–161. [Google Scholar]
- Rothon, R. (Ed.) Fillers for Polymer Applications. In Polymers and Polymeric Composites: A Reference Series; Chapter 6; Springer International Publishing: Cham, Switzerland, 2017; pp. 125–146. [Google Scholar] [CrossRef]
- Suryasarathi, B.; Mahanwar, P. Effect of flyash on the mechanical, thermal, dielectric, rheological and morphological properties of filled Nylon 6. J. Min. Mater. Charact. Eng. 2004, 3, 65–89. [Google Scholar]
- Jovanović, S.; Samaržija-Jovanović, S.; Marković, G.; Jovanović, V. Mechanical properties and thermal aging behaviour of polyisoprene/polybutadiene/styrene-butadiene rubber ternary blend reinforced with carbon black. Compos. Part B Eng. 2016, 98, 126–133. [Google Scholar] [CrossRef]
- Vijayan, D.; Mathiazhagan, A.; Joseph, R. Aluminium trihydroxide: Novel reinforcing filler in polychloroprene rubber. Polymer 2017, 132, 143–156. [Google Scholar] [CrossRef]
- Odlyhal, M.; Cohen, N.S.; Foster, G.M.; Aliev, A.; Verdonck, E.; Grandy, D. Dynamic mechanical analysis (DMA) 13C solid state NMR and micro-thermomechanical studies of historical parchment. J. Therm. Anal. Calorim. 2003, 71, 939–950. [Google Scholar] [CrossRef]
- Marković, G.; Samaržija-Jovanović, S.; Jovanowić, V.; Marinowić-Cincowić, M. Thermal stability of CR/CSM rubber blends filled with nano- and micro-silica particles. J. Therm. Anal. Calorim. 2010, 100, 881–888. [Google Scholar] [CrossRef]
- Roychoudhury, A.; De, P.P.; Roychoudhury, N.; Vidal, A. Chemical interaction between chlorosulfonated polyethylene and silica: Effect of surface modifications of silica. Rubber Chem. Technol. 1995, 68, 815–823. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; De, P.P.; Tripathy, D.K.; De, S.K. Interaction between carboxylated nitrile rubber and precipitated silica: Role of (3-aminopropyl) triethoxysilane. Rubber Chem. Technol. 1996, 69, 637–647. [Google Scholar] [CrossRef]
- Manna, A.K.; De, P.P.; Tripathy, D.K.; De, S.K.; Peiffer, D.G. Bonding between precipitated silica and epoxidized natural rubber in the presence of silane coupling agent. J. Appl. Polym. Sci. 1999, 74, 389–398. [Google Scholar] [CrossRef]
- Sae-oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Dependence of mechanical and aging properties of chloroprene rubber on silica and ethylene thiourea loadings. Eur. Polym. J. 2007, 43, 185–193. [Google Scholar] [CrossRef]
- Mostafa, A.; Abouel-Kasem, A.; Bayoumi, M.R.; El-Sebaie, M.G. Effect of carbon black loading on the swelling and compression set behavior of SBR and NBR rubber compounds. Mater. Des. 2009, 30, 1561–1568. [Google Scholar] [CrossRef]
- Leblance, J.L. Rubber-filler interactions and rheological properties in filled compounds. Prog. Polym. Sci. 2002, 27, 627–687. [Google Scholar] [CrossRef]
- Prasertsri, S.; Rattanasom, N. Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: Mechanical and dynamic properties. Polym. Test. 2012, 31, 593–605. [Google Scholar] [CrossRef]
- Dębek, C. Influence of silanes on mechanical properties of SBS composites filled with montmorillonite intercalated by water soluble polymers. Elastomery 2016, 2, 18–23. [Google Scholar]
- Fan, Q.; Han, G.; Cheng, W.; Tian, H.; Wang, D.; Xuan, L. Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites. Polymers 2018, 10, 896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedia, Y.; Fathi-Achachloueia, B.; Yousefi, A.R. Physical and mechanical properties of hybrid montmorillonite/zincoxide reinforced carboxymethyl cellulose nanocomposites. Int. J. Biol. Macromol. 2018, 108, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mahaling, R.N.; Stöckelhuber, K.W.; Heinrich, G. Reinforcement and migration of nanoclay in polychloroprene/ethylene-propylene-diene-monomer rubber blends. Compos. Sci. Technol. 2011, 71, 276–281. [Google Scholar] [CrossRef]
- Roy, K.; Alam, M.N.; Mandal, S.K.; Debnath, S.C. Development of a suitable nanostructured cure activator system for polychloroprene rubber anocomposites with enhanced curing, mechanical and thermal properties. Polym. Bull. 2016, 73, 191–207. [Google Scholar] [CrossRef]
- Kobędza, P.; Smejda-Krzewicka, A.; Strzelec, K. The use of copper oxides as cross-linking substances for chloroprene rubber and study of the vulcanizates properties. Part I. Materials 2021, 14, 5535. [Google Scholar] [CrossRef]
- Ślusarski, L.; Janowska, G.; Szkodziński, A.; Niedomagała, M.; Harwaziński, A. System for Measuring the Oxygen Index of Plastics, Natural Materials or Rubber. Polish Patent No. PL 129411, 30 April 1987. [Google Scholar]
- Soroceanu, A.; Stiubianu, G.T. Siloxane matrix molecular weight influences the properties of nanocomposites based on metal complexes and dielectric elastomer. Materials 2021, 14, 3352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, K.; Xiong, Y. Multiple intermolecular interaction to improve the abrasion resistance and wet skid resistance of eucommia ulmoides gum/styrene butadiene rubber composite. Materials 2021, 14, 5246. [Google Scholar] [CrossRef] [PubMed]
- Słubik, A.; Smejda-Krzewicka, A.; Strzelec, K. Curing behaviors, mechanical and dynamic properties of composites containing chloroprene and butadiene rubbers crosslinked with nano-iron(III) oxide. Polymers 2021, 13, 853. [Google Scholar] [CrossRef] [PubMed]
- Suntako, R. Effect of modified silica fume using MPTMS for the enhanced EPDM foam insulation. Polymers 2021, 13, 2996. [Google Scholar] [CrossRef] [PubMed]
- Surya, I.; Waesateh, K.; Saiwari, S.; Ismail, H.; Othman, N.; Hayeemasae, N. Potency of urea-treated halloysite nanotubes for the simultaneous boosting of mechanical properties and crystallization of epoxidized natural rubber composites. Polymers 2021, 13, 3068. [Google Scholar] [CrossRef] [PubMed]
- Bunriw, W.; Harnchana, V.; Chanthad, C.; Huynh, V.N. Natural rubber-TiO2 nanocomposite film for triboelectric nanogenerator application. Polymers 2021, 13, 2213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Song, M.; Lee, D.-J.; Park, S.-S. Silicone rubber composites reinforced by carbon nanofillers and their hybrids for various applications: A review. Polymers 2021, 13, 2322. [Google Scholar] [CrossRef] [PubMed]
- Smejda-Krzewicka, A.; Rzymski, W.M.; Kowalski, D. Tin oxide cross-linking of chloroprene rubber. Polimery 2015, 60, 186–191. [Google Scholar] [CrossRef]
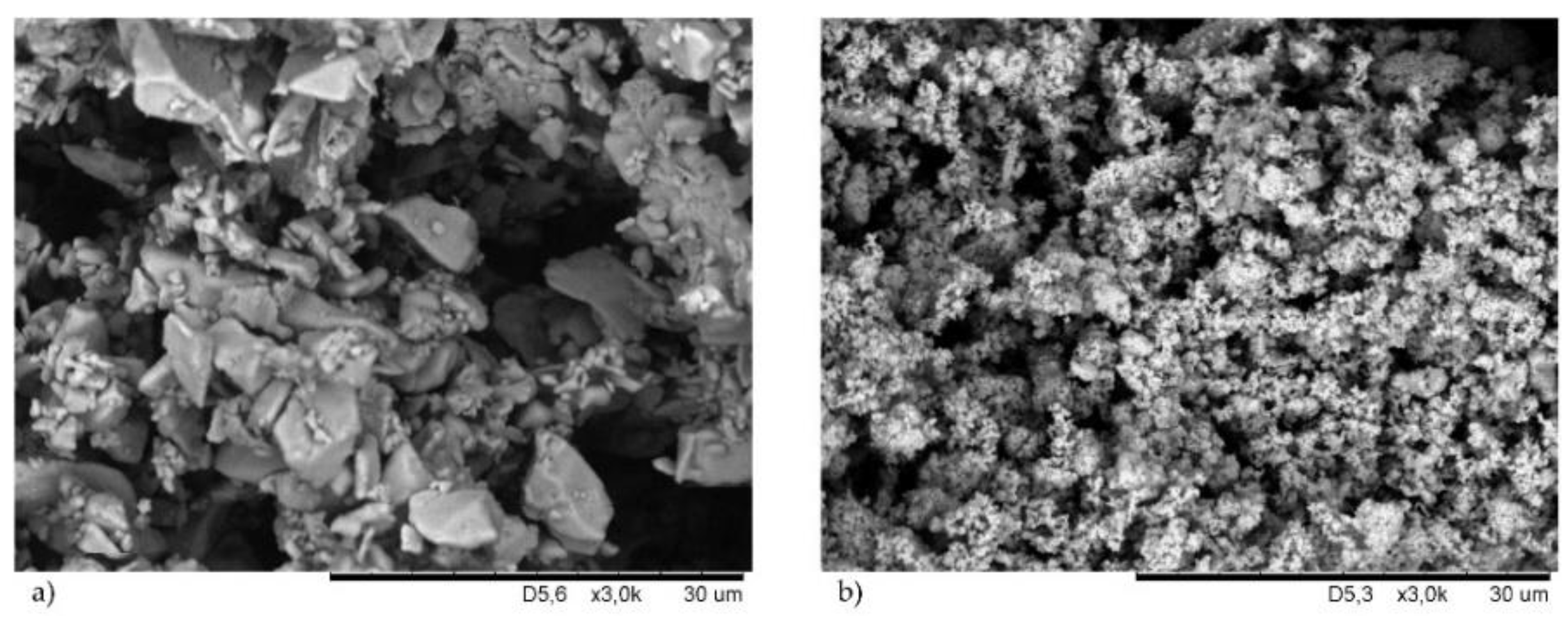


 Cu2O as a cross-linking agent,
Cu2O as a cross-linking agent,  CuO as a cross-linking agent.
CuO as a cross-linking agent.
 Cu2O as a cross-linking agent,
Cu2O as a cross-linking agent,  CuO as a cross-linking agent.
CuO as a cross-linking agent.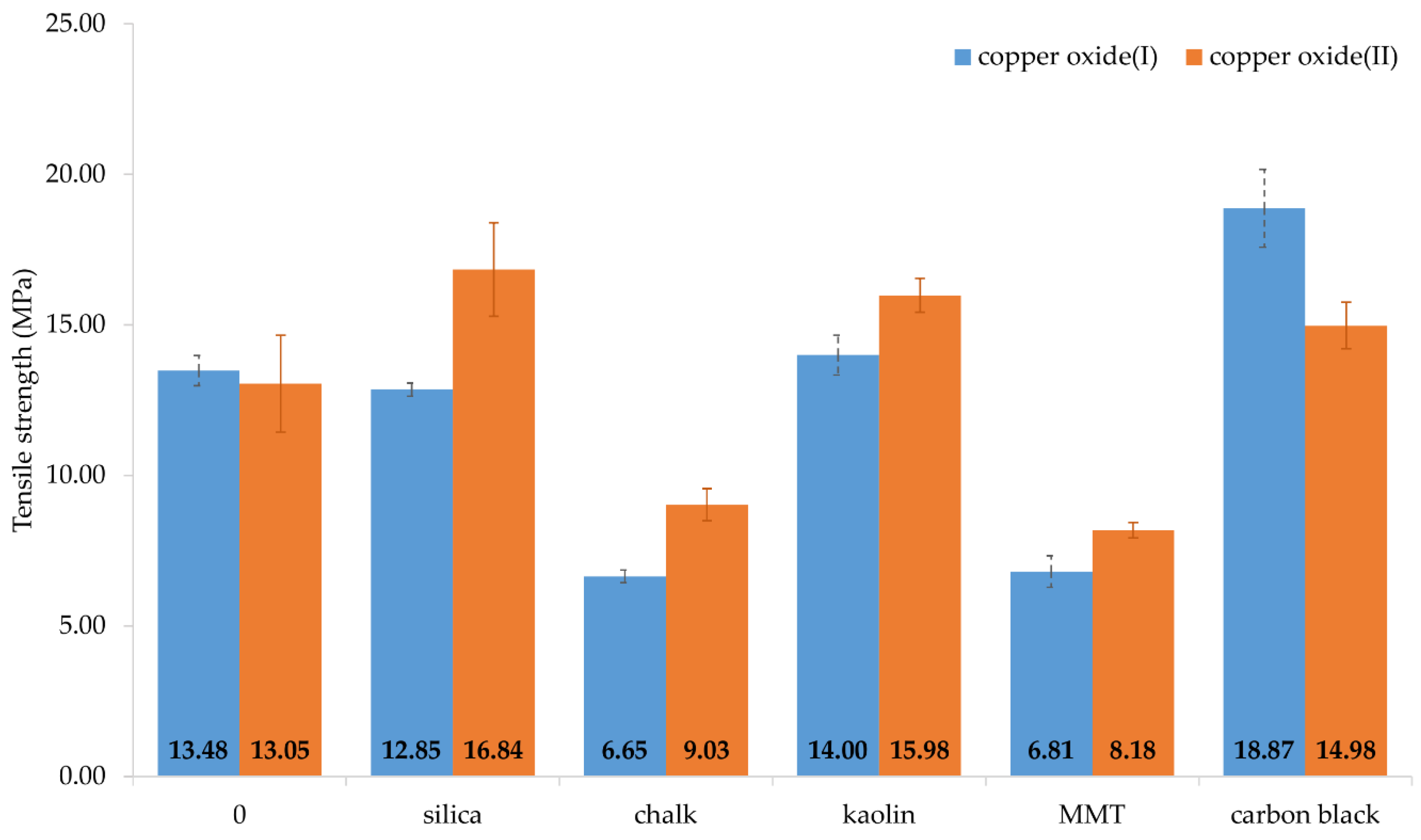
| Component Content (phr) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| SA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cu2O | 3 | - | 3 | - | 3 | - | 3 | - | 3 | - |
| CuO | - | 3 | - | 3 | - | 3 | - | 3 | - | 3 |
| Silica | 30 | 30 | - | - | - | - | - | - | - | - |
| Chalk | - | - | 30 | 30 | - | - | - | - | - | - |
| Kaolin | - | - | - | - | 30 | 30 | - | - | - | - |
| MMT | - | - | - | - | - | - | 5 | 5 | - | - |
| CB | - | - | - | - | - | - | - | - | 30 | 30 |
| Symbol | Cu2O/Si | CuO/Si | Cu2O/Ch | CuO/Ch | Cu2O/Ka | CuO/Ka | Cu2O/MMT | CuO/MMT | Cu2O/CB | CuO/CB |
| Symbol | t02 (min) | t90 (min) | Mmin (dN · m) | ΔM45 (dN · m) | CRI (min−1) |
|---|---|---|---|---|---|
| Cu2O/Si | 1.3 | 51.8 | 2.68 | 4.96 | 1.98 |
| CuO/Si | 1.1 | 39.7 | 3.09 | 19.71 | 2.59 |
| Cu2O/Ch | 0.6 | 58.8 | 1.27 | 5.11 | 1.72 |
| CuO/Ch | 1.7 | 35.6 | 1.01 | 11.57 | 2.95 |
| Cu2O/Ka | 1.5 | 47.6 | 0.79 | 7.11 | 2.16 |
| CuO/Ka | 1.1 | 34.3 | 1.09 | 12.89 | 3.01 |
| Cu2O/MMT | 2.6 | 53.0 | 0.67 | 3.75 | 1.98 |
| CuO/MMT | 1.1 | 29.9 | 0.76 | 9.57 | 3.47 |
| Cu2O/CB | 1.5 | 50.7 | 2.34 | 6.04 | 2.03 |
| CuO/CB | 0.8 | 34.0 | 2.28 | 19.98 | 3.01 |
| Symbol | QVT (cm3/cm3) | QVH (cm3/cm3) | QwT (mg/mg) | QwH (mg/mg) | αc |
|---|---|---|---|---|---|
| Cu2O/Si | 6.99 ± 0.14 | 0.44 ± 0.15 | 4.94 ± 0.10 | 0.24 ± 0.09 | 0.14 |
| CuO/Si | 3.87 ± 0.07 | 1.00 ± 0.01 | 2.74 ± 0.05 | 0.55 ± 0.01 | 0.26 |
| Cu2O/Ch | 6.05 ± 0.07 | 0.29 ± 0.05 | 4.28 ± 0.06 | 0.16 ± 0.03 | 0.17 |
| CuO/Ch | 4.89 ± 0.11 | 1.00 ± 0.02 | 3.46 ± 0.08 | 0.55 ± 0.01 | 0.20 |
| Cu2O/Ka | 15.89 ± 0.39 | 0.48 ± 0.03 | 11.24 ± 0.28 | 0.26 ± 0.02 | 0.06 |
| CuO/Ka | 4.43 ± 0.06 | 1.02 ± 0.02 | 3.14 ± 0.05 | 0.56 ± 0.01 | 0.23 |
| Cu2O/MMT | 10.68 ± 0.09 | 0.31 ± 0.04 | 7.55 ± 0.07 | 0.17 ± 0.03 | 0.09 |
| CuO/MMT | 4.85 ± 0.11 | 0.54 ± 0.03 | 3.43 ± 0.08 | 0.30 ± 0.02 | 0.21 |
| Cu2O/CB | 10.92 ± 0.11 | 0.38 ± 0.03 | 7.73 ± 0.08 | 0.21 ± 0.02 | 0.09 |
| CuO/CB | 3.34 ± 0.04 | 1.00 ± 0.05 | 2.37 ± 0.03 | 0.56 ± 0.03 | 0.30 |
| Symbol | Se100 (MPa) | Se200 (MPa) | Se300 (MPa) | TSb (MPa) | Eb (%) | ∆W1 (N · mm) | ME (%) |
|---|---|---|---|---|---|---|---|
| Cu2O/Si | 3.32 ± 0.06 | 5.71 ± 0.20 | 8.54 ± 0.39 | 12.85 ± 0.22 | 483 ± 26 | 163 | 58.7 |
| CuO/Si | 2.02 ± 0.10 | 3.60 ± 0.07 | 5.47 ± 0.30 | 16.84 ± 1.55 | 869 ± 47 | 265 | 39.9 |
| Cu2O/Ch | 0.65 ± 0.04 | 0.79 ± 0.04 | 0.98 ± 0.04 | 6.65 ± 0.21 | 708 ± 23 | 71 | 25.9 |
| CuO/Ch | 1.12 ± 0.06 | 1.60 ± 0.09 | 2.05 ± 0.12 | 9.03 ± 0.53 | 1125 ± 39 | 92 | 19.6 |
| Cu2O/Ka | 1.33 ± 0.06 | 2.01 ± 0.07 | 2.86 ± 0.09 | 14.00 ± 0.66 | 710 ± 11 | 11 | 38.7 |
| CuO/Ka | 1.74 ± 0.01 | 2.93 ± 0.05 | 4.05 ± 0.10 | 15.98 ± 0.56 | 1123 ± 25 | 201 | 32.2 |
| Cu2O/MMT | 0.73 ± 0.06 | 0.92 ± 0.09 | 1.16 ± 0.15 | 6.81 ± 0.52 | 713 ± 16 | 60 | 29.7 |
| CuO/MMT | 0.83 ± 0.01 | 1.12 ± 0.01 | 1.36 ± 0.02 | 8.18 ± 0.26 | 1290 ± 10 | 54 | 13.8 |
| Cu2O/CB | 1.53 ± 0.23 | 4.22 ± 0.22 | 8.53 ± 1.32 | 18.87 ± 1.29 | 484 ± 31 | 197 | 48.4 |
| CuO/CB | 3.14 ± 0.05 | 6.30 ± 0.08 | 10.1 ± 0.20 | 14.98 ± 0.77 | 445 ± 41 | 295 | 37.0 |
| Symbol | ∆G′ (MPa) | G′max (MPa) | G″max (MPa) |
|---|---|---|---|
| Cu2O/Si | 0.636 | 0.862 | 0.171 |
| CuO/Si | 0.287 | 0.530 | 0.092 |
| Cu2O/Ch | 0.127 | 0.301 | 0.056 |
| CuO/Ch | 0.298 | 0.433 | 0.072 |
| Cu2O/Ka | 0.114 | 0.269 | 0.058 |
| CuO/Ka | 0.169 | 0.324 | 0.052 |
| Cu2O/MMT | 0.158 | 0.323 | 0.070 |
| CuO/MMT | 0.084 | 0.222 | 0.046 |
| Cu2O/CB | 0.358 | 0.549 | 0.137 |
| CuO/CB | 0.585 | 0.882 | 0.128 |
| Symbol | OI (%) | tb (s) | Observation |
|---|---|---|---|
| Cu2O * | 37.3 | 175 | The sample burned completely, an orange flame with a blue glow covered the entire sample volume, a smoking flame, a medium amount of soot, after burning the sample retained shape but was brittle, it was easy to break, |
| CuO * | 37.5 | 187 | The sample burned completely, an orange flame with a blue glow covered the entire sample volume, a smoking flame, a medium amount of soot, after burning the sample retained shape but was brittle, it was easy to break. |
| Cu2O/Si | >37.5 | 23 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a small amount of soot. |
| CuO/Si | >37.5 | <5 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a small amount of soot. |
| Cu2O/Ch | >37.5 | 218 | The sample burned completely, an orange flame with a blue glow covered the entire sample volume, a smoking flame, a medium amount of soot, sample burned completely, there were no residues after combustion of the sample. |
| CuO/Ch | >37.5 | 239 | The sample burned completely, an orange flame with a blue glow covered the entire sample volume, a smoking flame, a medium amount of soot, sample burned completely, there were no residues after combustion of the sample. |
| Cu2O/Ka | >37.5 | <5 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a small amount of soot. |
| CuO/Ka | >37.5 | 41 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a small amount of soot. |
| Cu2O/MMT | >37.5 | 204 | The sample did not burn completely, an orange flame with a blue glow covered almost the entire sample volume, a smoking flame, a medium amount of soot. |
| CuO/MMT | >37.5 | 216 | The sample burned completely, an orange flame with a blue glow covered the entire sample volume, a smoking flame, a medium amount of soot, the sample retained shape after burning. |
| Cu2O/CB | >37.5 | 60 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a very large amount of soot. |
| CuO/CB | >37.5 | 60 | The sample did not burn completely, an orange flame with a blue glow layer covered the sample, a smoking flame, a very large amount of soot. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smejda-Krzewicka, A.; Kobędza, P.; Strzelec, K.; Adamus-Włodarczyk, A. The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products. Materials 2021, 14, 6528. https://doi.org/10.3390/ma14216528
Smejda-Krzewicka A, Kobędza P, Strzelec K, Adamus-Włodarczyk A. The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products. Materials. 2021; 14(21):6528. https://doi.org/10.3390/ma14216528
Chicago/Turabian StyleSmejda-Krzewicka, Aleksandra, Piotr Kobędza, Krzysztof Strzelec, and Agnieszka Adamus-Włodarczyk. 2021. "The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products" Materials 14, no. 21: 6528. https://doi.org/10.3390/ma14216528
APA StyleSmejda-Krzewicka, A., Kobędza, P., Strzelec, K., & Adamus-Włodarczyk, A. (2021). The Use of Copper Oxides as Cross-Linking Substances for Chloroprene Rubber and Study of the Vulcanizates Properties. Part II. The Effect of Filler Type on the Properties of CR Products. Materials, 14(21), 6528. https://doi.org/10.3390/ma14216528